Abstract
The goal of this study was to determine the distribution of unbound amprenavir in the central nervous system (CNS) of rats. The concentration of unbound amprenavir in the extracellular fluid of the brain and the blood was examined in the presence and absence of the MDR modulator GF120918 by microdialysis. The brain-to-blood ratio of amprenavir in the absence and presence of GF120918 was found to be significantly different (P < 0.003; 0.076 and 0.617, respectively). The use of the MDR modulator GF120918 could potentially increase the penetration of human immunodeficiency virus protease inhibitors into the CNS.
The viral load of the human immunodeficiency virus (HIV) in plasma can be reduced to levels below the limit of detection by a combination of nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, and human immunodeficiency virus (HIV) protease inhibitors (PIs) (13). Although HIV load is reduced in plasma, it is recognized that the virus is still able to survive and infect cells throughout the body. A major concern of HIV therapy is the persistence of the virus either in reservoirs where antiviral drugs do not readily penetrate (e.g., central nervous system [CNS]) or in HIV-infected cells that undergo slow turnover (e.g., memory cells of the immune system) (11). An indicator of HIV infection in the CNS is the prevalence of HIV-associated dementia, a cognitive and motor impairment observed in HIV-positive patients (14).
P-glycoprotein, the product of the MDR1 gene, is thought to be one reason for the low levels of HIV PIs in the CNS (2, 15). P-glycoprotein is located at the blood-brain barrier and can diminish the rate of entry of drugs into the CNS. MDR1 and the functionally equivalent rodent mdr1a gene products actively transport the HIV PIs indinavir, saquinavir, ritonavir, nelfinavir, and amprenavir (8, 9, 12). The extent of the distribution of PIs in the CNS is significantly greater in mdr1a−/− knockout mice (8) and in mice treated with the P-glycoprotein modulators valspodar (PSC-833), LY-335979 (6), and GF120918 (12). The hypothesized clinical significance of this efflux is viral survival in the CNS due to subtherapeutic concentrations of the PIs.
Amprenavir is extensively bound to plasma proteins (10). Determining the concentration of unbound parent amprenavir in the brain and blood would more accurately reflect the mechanism of CNS distribution, since only the free drug concentration is measured. In the previously mentioned studies only the total amount of drug (i.e., both free and bound) was measured, and therefore, changes in protein binding could have altered the distribution in the CNS. Microdialysis is a technique that determines the concentration of an unbound compound in blood or extracellular fluid (7), and it has been used extensively to determine the distribution of endogenous and exogenous compounds in the brain (3, 5, 16, 17), including stavudine, a nucleoside reverse transcriptase inhibitor (18). In the present study microdialysis was used to determine if the P-glycoprotein modulator GF120918 would increase the concentration of free amprenavir in the CNS of male Sprague-Dawley rats. Retrodialysis was used as a marker of probe recovery (4).
Drugs and reagents.
Amprenavir, [14C]amprenavir, and GF120918 were generous gifts from Glaxo Wellcome (Research Triangle, N.C.). Acetonitrile (Fisher Scientific, Pittsburgh, Pa.) was of high-pressure liquid chromatography grade. Polyethylene glycol 400 (PEG-400; Union Carbide, Danbury, Conn.) was used as a solvent for the intravenous administration of amprenavir. Hydroxypropylmethylcellulose (Aldrich Chemical Co., Milwaukee, Wis.) and Tween 80 (City Chemical Corporation) were used in the oral formulation of GF120918. All other chemicals were obtained from commercial sources and were of analytical grade.
Probes, artificial cerebrospinal fluid, and drug solution.
CMA/12 microdialysis probes (CMA/Microdialysis, Acton, Mass.) were used to determine the in vitro recovery and loss of amprenavir. CMA/12 and CMA/20 probes were inserted in the frontal cortex of the CNS and the left jugular vein, respectively. Artificial cerebrospinal fluid (dialysate) consisted of Na (155 mM), K (2.9 mM), Ca (2 mM), Mg (0.7 mM), Cl (138 mM), HCO3 (25 mM), and glucose (6.0 mM) at pH 7.35. Before each experiment a dosing solution of amprenavir, 536 mg ml−1 kg of body weight−1, in PEG-400 was made. Also before each experiment a dialysate solution consisting of 6.82 μCi of [14C]amprenavir per liter was prepared.
Animals.
Ten adult male Sprague-Dawley rats (250 to 350 g; Harlan Laboratories, Indianapolis, Ind.) were used. Animals were maintained under a 12-h-light-12-h-dark cycle and had access to food and water ad libitum. All animal research was conducted in accordance with guidelines of the University of Kentucky Institutional Animal Care and Use Committee.
Retrodialysis.
In vivo recovery was assessed by retrodialysis (4). 14C-labeled amprenavir was added to the dialysate. The dialysate was then perfused through the probe, and a 10-μl sample of effluent dialysate was taken. The amount of amprenavir recovered from the brain or blood was determined by the fraction of 14C-labeled amprenavir lost through the dialysate. The “actual” in vivo concentrations of amprenavir from the CMA/12 and CMA/20 probes were determined by taking the effluent dialysate concentration and dividing it by the fraction lost during retrodialysis.
In vitro recovery was determined by inserting a CMA/12 microdialysis probe (n = 3) into a dialysate solution of amprenavir (1,000 ng/ml) while perfusing the probe with 14C-labeled dialysate. Recovery was determined as the concentration of amprenavir in the effluent dialysate divided by the concentration of amprenavir in the solution. Concurrently, retrodialysis with 14C-labeled amprenavir was used to determine the fraction lost, as discussed above.
Microdialysis. (i) Treatment group.
Five rats were dosed orally with 250 mg of GF120918 kg−1 in suspension (0.5% hydroxypropylmethylcellulose, 1% Tween 80) for 4 consecutive days. On the third day the left femoral vein was cannulated with PE-50 tubing (Becton Dickinson and Company, Cockeysville, Md.) and silastic tubing (Dow Corning Corp., Midland, Mich.). A CMA/20 microdialysis probe was placed in the jugular vein, and a CMA/12 microdialysis probe guide was placed in the frontal cortex (3 mm anterior and 3 mm lateral from bregma) and secured with screws and dental cement (CMA/Microdialysis). On the fourth day, a CMA/12 microdialysis probe was placed in the probe guide. The animal was placed in an awake animal system (Bioanalytical Systems Inc.) and had free access to food and water throughout the experiment. Approximately 2 h after the fourth daily dose of GF120918 the rat was given a constant intravenous infusion of amprenavir (26.8 mg h−1 kg−1, 0.05 ml h−1) in PEG-400. Radiolabeled dialysate was perfused through the probes at a rate of 1 μl min−1. Samples were taken from the CMA/12 and CMA/20 probes every 45 min over 5.25 h.
(ii) Control group.
Five rats were treated identically to the treatment group with the exception that the animals were given the suspension vehicle without GF120918. Both groups had free access to food and water.
Analysis of amprenavir.
[14C]amprenavir was analyzed with a Packard Tri-Carb liquid scintillation analyzer (Packard Instrument Co., Meriden, Conn,). Amprenavir was analyzed with a reverse-phase (C18 microbore column; Bioanalytical Systems Inc.) microbore high-pressure liquid chromatography system (Bioanalytical Delivery System [Bioanalytical Systems Inc.] and CMA/200 sample injector [CMA/Microdialysis]) with a mobile phase consisting of 60% H2O and 40% (vol/vol) acetonitrile at a flow rate of 0.1 ml min−1. Amprenavir was detected with a fluorescence detector (Bioanalytical Systems Inc.) with an excitation and an emission wavelength of 244 and 340 nm, respectively. A 10-μl sample was injected and had a retention time of 6.85 min. The limit of detection was 100 ng/ml. Intraday and interday assay precision values (percent coefficient of variation) for the high, medium, and low control samples were less than 10%. The intraday and interday accuracy values were between 90 and 110%.
Statistical analysis.
The brain-to-blood ratio (BBR) was calculated by dividing the “actual” concentration in the brain by the “actual” concentration in plasma. A two-way analysis of variance was used to examine the effect of time and treatment on BBR. A Student t test was used to compare the pooled BBRs for the control and treatment groups. A P value of <0.05 was considered statistically significant.
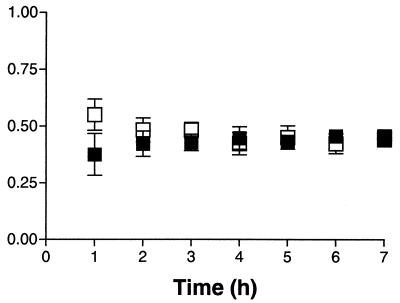
Retrodialysis is one commonly used method to determine recovery. Figure 1 shows the results of the in vitro recovery of amprenavir and loss of [14C]amprenavir from CMA/12 microdialysis probes (n = 3). There was no significant difference between in vitro recovery and loss after 1 h (P = 0.36), and therefore, it is assumed that the in vivo loss would be a valid marker of in vivo recovery. A minimum of 20 h between the insertion of the microdialysis probe and the start of the amprenavir infusion was used, since previous literature has shown that the integrity of the blood-brain barrier was not compromised after this allotted time (1).
FIG. 1.
In vitro recovery of amprenavir (▪) and the loss of [14C]amprenavir (□) from CMA/12 microdialysis probes (means ± standard errors of the means; n = 3).
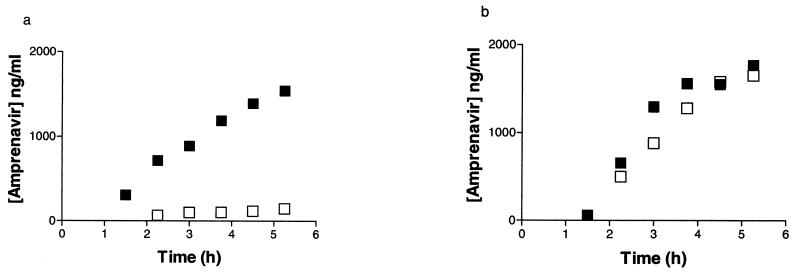
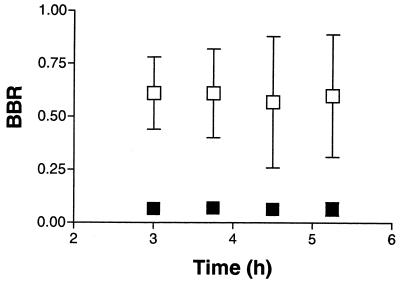
Figure 2 shows the concentration-time profiles of unbound amprenavir in blood and brain in a control and a treated animal. Systemic steady-state concentrations of unbound drug were not achieved during the time course of this study, suggesting a long half-life. No significant difference was observed in the unbound amprenavir concentrations in plasma at each time point for the control and treated groups (P = 0.20). Although inconclusive, this lack of a difference in the concentration of unbound amprenavir in plasma between control rats and rats treated with GF120918 suggests that there was no influence of GF120918 on the intrinsic clearance of amprenavir. Additional studies were performed to assess the effect that the microdialysis procedure has on the systemic profile of amprenavir. The plasma unbound drug concentration profiles paralleled those seen in the microdialysis studies, suggesting that the observations were not an artifact of the microdialysis procedure itself. Figure 3 shows the time profile of the BBR of amprenavir in the control and treated groups. The BBR could not be determined prior to the third hour since the unbound drug concentrations in the CNS were lower than the limit of quantification. No significant difference was observed in the BBR with respect to time between the control and the treated groups (P = 0.80); therefore, the BBR values at 3, 3.75, 4.5, and 5.25 h were pooled for each of the two groups. There was a significant increase (P < 0.003) in the average BBR of amprenavir with rats treated with GF120918 compared to that for control rats (0.617 and 0.076, respectively).
FIG. 2.
(a) Concentration-time profiles of unbound amprenavir (26.8 mg h−1 kg−1) in plasma (▪) and CNS (□) in a rat treated with vehicle (suspension for 4 consecutive days). (b) Concentration-time profiles of unbound amprenavir (26.8 mg h−1 kg−1) in plasma (▪) and CNS (□) in a rat treated with GF120918 (250 mg kg−1 day−1 for 4 consecutive days).
FIG. 3.
BBR time profile of amprenavir (26.8 mg h−1 kg−1) in rats treated with GF120918 (□) (250 mg kg−1 day−1 for 4 consecutive days) and rats treated with vehicle (▪) (suspension for 4 consecutive days) (means ± standard errors of the means; n = 5).
As a result, the unbound drug concentration, and thus the effective concentration, of this PI was increased in the extracellular fluid of the CNS with the use of a P-glycoprotein modulator. This increase was similar to the increase in the BBR (13-fold, based on total radioactivity) seen in whole-body autoradiography studies in mice at 2 h postdose (12). The coadministration of a P-glycoprotein modulator with a PI could have a significant effect on decreasing HIV viral load in the CNS. The decrease in viral load could potentially have an effect on the HIV-associated dementia observed in many HIV-positive individuals.
Acknowledgments
This work was supported by Glaxo Wellcome, and J.E.E. was supported by NIEHS training grant ES07266.
REFERENCES
- 1.Allen, D. D., P. A. Crooks, and R. A. Yokel. 1992. 4-Trimethylammonium antipyrine: a quaternary ammonium nonradionuclide marker for blood-brain barrier integrity during in vivo microdialysis. J. Pharmacol. Toxicol. Methods 28:129-135. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy, W. T. 1996. P-glycoproteins and multidrug resistance. Annu. Rev. Pharmacol. Toxicol. 36:161-183. [DOI] [PubMed] [Google Scholar]
- 3.Borg, N., and L. Stahle. 1998. Pharmacokinetics and distribution over the blood-brain barrier of zalcitabine (2′,3′-dideoxycytidine) and BEA005 (2′,3′-dideoxy-3′-hydroxymethylcytidine) in rats, studied by microdialysis. Antimicrob. Agents Chemother. 42:2174-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouw, M. R., and M. Hammarlund-Udenaes. 1998. Methodological aspects of the use of a calibrator in in vivo microdialysis—further development of the retrodialysis method. Pharm. Res. 15:1673-1679. [DOI] [PubMed] [Google Scholar]
- 5.Burgio, D. E., M. P. Gosland, and P. J. McNamara. 1996. Modulation effects of cyclosporine on etoposide pharmacokinetics and CNS distribution in the rat utilizing microdialysis. Biochem. Pharmacol. 51:987-992. [DOI] [PubMed] [Google Scholar]
- 6.Choo, E. F., B. Leake, C. Wandel, H. Imamura, A. J. Wood, G. R. Wilkinson, and R. B. Kim. 2000. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab. Dispos. 28:655-660. [PubMed] [Google Scholar]
- 7.Elmquist, W. F., and R. J. Sawchuk. 1997. Application of microdialysis in pharmacokinetic studies. Pharm. Res. 14:267-288. [DOI] [PubMed] [Google Scholar]
- 8.Kim, R. B., M. F. Fromm, C. Wandel, B. Leake, A. J. J. Wood, D. M. Roden, and B. R. Wilkinson. 1998. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J. Clin. Investig. 101:289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, C. G., M. M. Gottesman, C. O. Cardarelli, M. Ramachandra, K. T. Jeang, S. V. Ambudkar, I. Pastan, and S. Dey. 1998. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry 37:3594-3601. [DOI] [PubMed] [Google Scholar]
- 10.Livington, D. J., S. Pazhanisamy, D. J. Porter, J. A. Partaledis, R. D. Tung, and G. R. Painter. 1995. Weak binding of VX-478 to human plasma proteins and implications for anti-human immunodeficiency virus therapy. J. Infect. Dis. 172:1238-1245. [DOI] [PubMed] [Google Scholar]
- 11.Pierson, T., J. McArthur, and R. F. Siliciano. 2000. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu. Rev. Immunol. 18:665-708. [DOI] [PubMed] [Google Scholar]
- 12.Polli, J. W., J. L. Jarrett, S. D. Studenberg, J. E. Humphreys, S. W. Dennis, K. R. Brouwer, and J. L. Woolley. 1999. Role of P-glycoprotein on the CNS disposition of amprenavir (141W94), an HIV protease inhibitor. Pharm. Res. 16:1206-1212. [DOI] [PubMed] [Google Scholar]
- 13.Rana, K. Z., and M. N. Dudley. 1999. Human immunodeficiency virus protease inhibitors. Pharmacotherapy 19:35-59. [DOI] [PubMed] [Google Scholar]
- 14.Swindells, S., J. Zheng, and H. E. Gendelman. 1999. HIV-associated dementia: new insights into disease pathogenesis and therapeutic interventions. AIDS Patient Care STDs 13:153-163. [DOI] [PubMed] [Google Scholar]
- 15.van Asperen, J., U. Mayer, O. van Tellingen, and J. H. Beijnen. 1997. The functional role of P-glycoprotein in the blood-brain barrier. J. Pharm. Sci. 86:881-884. [DOI] [PubMed] [Google Scholar]
- 16.Wang, Q., H. Yang, D. W. Miller, and W. F. Elmquist. 1995. Effect of the P-glycoprotein inhibitor, cyclosporin A, on the distribution of rhodamine-123 to the brain: an in vivo microdialysis study in freely moving rats. Biochem. Biophys. Res. Commun. 211:719-726. [DOI] [PubMed] [Google Scholar]
- 17.Wang, Y., and R. J. Sawchuk. 1995. Zidovudine transport in the rabbit brain during intravenous and intracerebroventricular infusion. J. Pharm. Sci. 84:871-876. [DOI] [PubMed] [Google Scholar]
- 18.Yang, Z., R. C. Brundage, R. H. Barbhaiya, and R. J. Sawchuk. 1997. Microdialysis studies of the distribution of stavudine into the central nervous system in the freely-moving rat. Pharm. Res. 14:865-872. [DOI] [PubMed] [Google Scholar]