Abstract
Drug delivery methods can impact efficacy as much as the nature of the drug itself. Microparticles made of biodegradable polymers such as poly(D,L-lactide-co-glycolide) and poly(lactic acid) (PLA) have been studied extensively for controlled release of diverse drugs. By using a modified solvent extraction/evaporation method called precision particle fabrication (PPF), uniform microparticles such as single-wall microspheres, double-wall microspheres and liquid-core microcapsules have been fabricated with precise control of their geometric structures. By producing particles of uniform size, which has crucial impact on drug release behaviors, PPF-fabricated microparticles provide unique insights about drug release mechanism. Using small-molecule and macromolecule model drugs, our group demonstrated that physicochemical properties of the polymers and drugs and structural properties of the matrix can greatly impact drug distribution within microparticles, particle erosion and drug release rates. By careful selection of particle size and shell thickness, uniform microparticles can achieve “zero-order”, pulsatile or tandem release of drugs.
Keywords: Biodegradable polymers, precision particle fabrication, double-wall microspheres, liquid-core microcapsules, controlled release
Introduction
The IMS Institute for Healthcare Informatics has projected that in 2014 global spending on prescription drugs will top $1 trillion even as growth slows in Europe and North America, and the global pharmaceutical industry may reach $1.2 trillion by the end of 2017 (Rickwood et al., 2013). The method of drug delivery can impact efficacy as much as the nature of the drug itself, and for many years researchers have sought to develop effective systems that can target drugs to specific body sites and/or precisely control drug release rates for prolonged time (Langer, 1998). To produce desired results, the plasma concentration of drug should be maintained within the therapeutic window, which consists of a lower bound, the minimum effective concentration (MEC), and an upper bound, the minimum toxic concentration (MTC). With conventional oral dosing and parenteral injections, peaks and valleys typically appear in the plasma concentration profiles, which can lead to side effects and the need for frequent administration. Controlled release devices, in contrast, may maintain drug concentration within the therapeutic window for a prolonged time with reduced dosage frequency, thus increasing efficacy and patient compliance. Furthermore, multiple drugs can be incorporated into one delivery depot, which may facilitate synergistic therapy strategies.
Biodegradable polymers have been utilized in numerous devices as a means to deliver drugs in a controlled and minimally invasive manner (Park et al., 2005; Varde and Pack, 2004). Compared to non-biodegradable polymers which may pose problems of toxicity and are difficult to remove, biodegradable polymer devices have attracted much attention since the early 1970s (Ha and Gardella, 2005; Sinha and Trehan, 2003). Specifically, spherical biodegradable microparticles such as monolithic microspheres (single-wall microspheres), double-wall microspheres with a core and shell composed of two different materials, and microcapsules with liquid cores have been employed for controlled release of various therapeutics (Anderson and Shive, 2012; Singh et al., 2010; Tamber et al., 2005; Wang et al., 2007). Microparticles exhibiting sizes ranging from a few to several hundred microns have received much attention in academia and industry. For example, microparticles approximately 1–5 μm in diameter would be ideal for passive targeting of professional antigen-presenting cells (Evora et al., 1998; Wattendorf et al., 2008). Microparticles 10–20 μm in diameter could be used to target the tortuous capillary bed of tumor tissues by chemo-embolization (Dass and Burton, 1999; Salem et al., 2005). Microparticles 1–5 μm in diameter and highly porous particles 5–20 μm in diameter are effective pulmonary drug delivery vehicles (Langer, 1998). Finally, microparticles 10–100 μm in diameter can serve as intramuscular or subcutaneous depots as they are small enough for syringe injection yet large enough to avoid uptake by phagocytic cells.
Due to the simplicity and versatility of such microparticle devices, many products have been commercialized. For example, Trelstar® injectable microspheres (triptorelin pamoate) and Lupron® depot (leuprolide acetate) for prostate cancer, Sandostatin LAR® depot (octreotide acetate) for acromegaly, Risperdal® Consta® depot (risperidone) for the treatment of schizophrenia as well as for the longer-term treatment of Bipolar I disorder, and Vivitrol® depot (naltrexone) for alcohol dependence are all commercialized implantable or injectable biodegradable microparticle devices (Malik et al., 2007).
Fabrication methods of biodegradable microparticles include solvent extraction/evaporation (Bindschaedler et al., 1988), polymer extrusion (Poncelet et al., 1994), spray drying (Pavanetto et al., 1993), coacervation or precipitation (Kawashima et al., 1989), electrospray (Almería et al., 2010), particle replication in non-wetting templates (PRINT) technology (Gratton et al., 2007) and microfluidic flow-focusing (Xu et al., 2009). Common features of these methods include: (i) the biodegradable polymer should be dissolved in a suitable solvent such as dichloromethane or ethyl acetate; (ii) therapeutics are co-dissolved with polymer, suspended as solid particulate or dissolved in an immiscible solvent (e.g., aqueous) and emulsified with polymer solution; (iii) the drug-polymer solution, suspension or emulsion is disrupted to produce droplets from which the solvent is removed to form the hardened particles. A key limitation of these biodegradable microparticle production methods, however, is the difficulty of controlling particle sizes. Since outer diameter, shell thickness, and size distributions have great influence on drug release rate and localizing behaviors, uniform or monodisperse microparticle systems are often preferred.
Several techniques to produce uniform microparticles have been reported. Sugiura et al. fabricated uniform solid lipid microspheres using a temperature-controlled microchannel emulsification process (Sugiura et al., 2000). Radulescu et al. developed a technology for fabricating uniform polymer microspheres by dispensing polymeric material from an orifice of a drop-on-demand ink jet printhead while the orifice was immersed in a solvent extraction media (Radulescu and Wawro, 2003). Desimone et al. pioneered a technique known as particle replication in non-wetting templates (PRINT) based on the exploitation of the low surface energy of novel fluoropolymeric molds. The molds are derived from liquid perfluoropolyether (PFPE) precursors, which can be photochemically cross-linked at room temperature. The resulting elastomeric solids enable high-resolution imprint lithography and fabrication of uniform organic particles of varying shapes (Gratton et al., 2007; Rolland et al., 2004). Although PRINT is highly versatile in terms of particle size and shape, fabrication requires clean-room facilities for creation of molds, and scale-up appears to be more complicated. Bohmer et al. adopted ink-jet printing technology and fabricated uniform polymer microspheres as well as hollow-core microcapsules (Böhmer et al., 2006). Microfluidic methods have also been proven effective in fabricating uniform microparticles. Taking advantage of the periodic and predictable breakup of immiscible fluids, discrete and monodisperse droplets can be formed in T-junction or flow-focusing channels (Hung et al., 2010; Shum et al., 2008; Teh et al., 2008; Xu et al., 2009; Zhang et al., 2009). This paper presents a review of uniform microparticle systems fabricated by a unique technique called precision particle fabrication and the study of drug release behaviors from these vehicles.
Precision Particle Fabrication
Precision particle fabrication (PPF) is a technology developed to produce uniform particles of a variety of materials (Foster et al., 1977; Gilliard et al., 1981; Kim et al., 1991; Kim et al., 1989) and adapted for fabrication of controlled-release microparticle systems comprising biodegradable polymers. The main apparatus of PPF (Fig. 1(A),(B)) is based on passing a fluid containing the sphere-forming material(s) and any drug to be encapsulated through a small (10–100 μm) orifice to form a smooth, cylindrical stream. To break the stream into uniform droplets, the nozzle is acoustically excited by a piezoelectric transducer driven by a wave generator at a defined frequency. By employing an annular flow of a non-solvent phase, called the carrier stream, surrounding the polymer-drug jet to provide additional “drag” force, microparticle size and shape can be further controlled; particles even smaller than the nozzle openings can be generated. The particle size (rd) can be controlled by jet radius (rj), solution flow rate (vj) and acoustic wave frequency (f). By changing the flow rates of the polymer and carrier streams and the acoustic wave frequency droplet sizes vary as predicted in equation (1) (Rayleigh, 1879, 1882). Thus, we can control the particle size to within one micron (Berkland, 2003).
Fig. 1.
Schematic of PPF apparatus for fabrication of single-wall microspheres (SWMS) (A) and double-wall microspheres (DWMS)/liquid-core microcapsules (MC) (B); PPF double nozzle system (C) and triple nozzle system (D).
| (1) |
The nozzle system is the key part of PPF. To produce single-wall microspheres (SWMS), a hypodermic needle was used as the innermost nozzle carrying the drug-polymer solution, which was surrounded by a second glass nozzle for forming the non-solvent carrier stream. For fabrication of double-wall microspheres (DWMS) and liquid-core microcapsules (MC), a triple nozzle system was employed comprising the double-nozzle system surrounded by a larger glass nozzle. The core phase (polymer, aqueous or oil stream) passes through the inner metal nozzle, the shell-phase polymer stream passes through the inner glass nozzle, and the outer glass nozzle was for the non-solvent carrier stream (Fig. 1(C),(D)).
Uniform microparticle systems for controlled release of small-molecule drugs
Compared to conventional dosage forms, biodegradable polymeric matrices provide enhanced delivery for small-molecule drugs, yet an important limitation of these matrices has been the difficulty of designing systems exhibiting precisely controlled release rates. Because microparticle size is a primary determinant of small-molecule drug release rate, uniform microparticle systems are preferred to better control drug release rates as well as study other factors which might contribute to release rates.
Controlled release of small molecules from uniform SWMS
Uniform biodegradable polymer SWMS systems have been first produced using PPF by Berkland et al. (Berkland et al., 2001). Bulk eroding polyesters such as poly(D,L-lactide-co-glycolide) (PLG) or poly(lactic acid) (PLA) and surface eroding polyanhydrides such as poly(sebacic anhydride) (PSA) or poly[(1,6-bis-carboxyphenoxy) hexane)] (PCPH) have been used as matrix materials. As a first demonstration of the capabilities of the technology, rhodamine and piroxicam were co-dissolved with PLG solutions to fabricate uniform SWMS (Berkland et al., 2002). In order to examine the effect of microsphere diameter on drug release kinetics, rhodamine-containing SWMS with outer diameter of 20, 40 and 65 μm as well as piroxicam-containing microspheres with outer diameter of 10, 50 and 100 μm were fabricated (Fig. 2). Rhodamine and piroxicam release profiles showed that release rates increased with decreasing particle size, likely due to the increased surface area-to-volume ratio compared to larger particles. Particles less than 50 μm in diameter exhibited concave downward release profiles typical of diffusion-controlled release, while larger microspheres exhibited sigmoidal release profiles (Fig. 3). Furthermore, microsphere erosion rate increased with particle size, due to the effect of autocatalytic polymer degradation (acidic byproducts of PLG degradation accumulate to a greater degree with increasing microsphere size because of longer diffusion length and can accelerate matrix degradation) (Berkland et al., 2003).
Fig. 2.
Scanning electron micrographs of (A) 40 μm, (C) 20 μm rhodamine-loaded SWMS and (B) 50 μm and (D) 10 μm piroxicam-loaded SWMS and (E) their size distributions. Scale bar=100 μm; applies to panels A–D. Adapted from (Berkland et al., 2002)
Fig. 3.

Effect on SWMS size and drug loading on (A) rhodamine and (B) piroxicam release rates. Adapted from (Berkland et al., 2002)
The initial spatial distribution of drug within the particles was also controlled by particle size and played a significant role in controlling the release rates (Fig. 4). Rhodamine is highly water soluble (~8 mg/mL) and was localized near the particle surface and away from the hydrophobic PLG core. Piroxicam, in contrast, is only poorly water soluble (<100 μg/mL) and partitioned toward the PLG core. The degree of drug redistribution varied significantly with microsphere size, however; the initial distribution of both drugs was more uniform as particle size decreased. It appeared that the more rapid hardening of smaller particles (due to shorter solvent diffusion distance) tended to kinetically trap the drugs before redistribution could take place. This trend was also observed upon encapsulation of rhodamine, p-nitroaniline and piroxicam in PSA microspheres (Berkland et al., 2004b). PSA provides an even more hydrophobic matrix than PLG. Surface localization of rhodamine was sufficiently dramatic to eliminate the effect of particle size on release rates; all rhodamine was localized within a few microns of the surface even in the smallest microspheres. p-Nitroaniline is slightly less water-soluble (~1 mg/mL) and was more evenly distributed. Piroxicam was also distributed uniformly in small PSA SWMS but partitioned to the surface of larger particles, which resulted in a surprising increase in release rate with increasing particle size. Taken together, these results emphasize the importance of the interplay between the polymer degradation rate, drug-polymer, particle size and polymer precipitation kinetics on the drug distribution within and release from polymer SWMS.
Fig. 4.
Laser scanning confocal microscopy cross section through the midline of 10, 20, 40, 65 and 100 μm rhodamine-loaded PLG SWMS (top row), revealing increasing surface distribution of rhodamine as microsphere diameter increases. Cross section of 10, 50, and 100 μm piroxicam-loaded microspheres (bottom row) reveal increasing amounts of piroxicam in the microsphere interior as diameter increases. Adapted from (Berkland et al., 2003)
To help understand the competing factors affecting small-molecule drug release from uniform SWMS and to better design controlled release systems, Raman et al. (Raman et al., 2005) developed a mathematical model of drug release from uniform PLG SWMS. The model accounted for the dependence of drug diffusivity on polymer molecular weight and the variation of polymer molecular weight with time due to degradation. The model also included the non-uniform initial drug distribution. The model produced a good fit of the experimental data, including the sigmoidal profiles characteristic of larger particles, and could be a useful tool to predict small-molecule release from SWMS.
To investigate the generality of modulating small-molecule drug release kinetics using uniform SWMS, Berkland et al. (Berkland et al., 2002) mixed appropriate proportions of uniform SWMS of different sizes. Multiple linear combinations of 10, 50 and 100 μm SWMS were examined computationally to identify combinations resulting in linear drug release. Various formulations were experimentally confirmed to exhibit approximate “zero-order” release (Fig. 5). Such constant release rates cannot be achieved using PLG SWMS with conventionally broad size distributions.
Fig. 5.
(A) Piroxicam release from 3:1, 1:1 and 1:3 (w/w) mixtures of 10 μm/15% SWMS and 50 μm/15% SWMS; (B) Piroxicam release from 1:6.1, 1:11.5 and 1:39 (w/w) mixtures of 10 μm/20% SWMS and 50 μm/10% SWMS. Adapted from (Berkland et al., 2002)
Controlled release of small molecules from uniform DWMS
Although uniform SWMS can provide enhanced control of small-molecule drug release rates, there is need for more versatile delivery vehicles. DWMS composed of two distinct polymers as core and shell materials with varied outer diameter and shell thickness are one such approach. PPF is capable of producing DWMS by passing the core-forming material through the innermost nozzle and the shell-forming material through the annular nozzle. The ultimate orientation of the materials, however, is governed by the thermodynamics of the system. Under certain conditions, the relative magnitudes of the core-shell, core-water and shell-water interfacial tensions may cause the two polymer layers to invert positions (i.e., the intended core material becomes the shell), or Janus-like particles with a three-phase contact line may result (Berkland et al., 2004c). Pollauf et al. (Pollauf and Pack, 2006) developed thermodynamic predictions of polymer-polymer immiscibility, drug-polymer interaction energies and polymer-solution interfacial tensions that lead to well-formed DWMS with desired morphologies and drug partitioning. They found formation of well-defined core–shell particles is controlled by polymer–polymer immiscibility and preferential spreading of the shell material on the core. For example, by choosing appropriate solvents and polymer concentrations, drug may be selectively localized in the core of DWMS comprising two very similar polymers (PLG and polylactofate), and DWMS with highly hydrophobic PCPH shells encapsulating PLG cores may be produced.
Berkland et al. (Berkland et al., 2004a) reported the release of piroxicam from DWMS of varying shell thickness. By keeping piroxicam-loaded core phase PLG flow rate constant and gradually increasing drug-free shell phase poly(L-lactide) (PLL) flow rates, DWMS with constant PLG core diameter and incrementally increasing PLL shell thickness were fabricated (Fig. 6). At lower PLL:PLG ratio (Fig. 6(C)), a significant percentage of particles exhibited only partial engulfment of the PLG core by PLL. At 50:50 PLL:PLG ratio, fully formed DWMS were produced.
Fig. 6.
Size distributions of (A) DWMS with varying PLL shell thickness and (B) monodisperse PLG and PLL SWMS. Confocal fluorescence and SEM (insets) of (C) PLL:PLG 40:60 and (D) PLL:PLG 50:50 DWMS. In SEMs, PLG was selectively dissolved with EtAc to image the PLL. In vitro piroxicam release from uniform PLG, PLL SWMS and PLL(PLG) DWMS (E). Adapted from (Berkland et al., 2004a)
The shapes of the piroxicam release profiles clearly depended on the ratio of PLL:PLG and thus the PLL shell thickness. As the PLL shell thickness increased from ~2 to 10 μm, a significant reduction in initial burst compared with piroxicam release from pure PLG SWMS and increasing duration of release were observed. With appropriately thick drug-free PLL shells, “zero-order” release could be achieved (Fig. 6(E)). PLL degrades very slowly and as a result no piroxicam release was observed from PLL SWMS (PLL:PLG 100:0) over the course of 30 days. Similar results were achieved employing DWMS comprising poly(D,L-lactide) (PDLL) shells surround PLG cores (Pollauf et al., 2005). SEM confirmed that the slowly degrading PDLL shell was almost intact and the faster degrading PLG core was completely degraded after 90 days of in vitro release (Fig. 7).
Fig. 7.
Scanning electron micrographs of piroxicam-loaded PDLL(PLG) DWMS after 90 days of in vitro release showing particle exterior morphology (first column), particle cross-section (second column), and wall cross-section (third column). PDLL:PLG ratios of (A–C) 0.5:1; (D–F) 0.75:1; (G–I) 1.5:1; (J–L) 2.25:1; and (M–O) 3:1. Scale bar=50 μm (first column), 20 μm (second column), 1.5 μm (C) and 5 μm (F, I, L and O). Adapted from (Pollauf et al., 2005)
Xu et al. (Xu et al., 2013) studied the release of a small-molecule anti-cancer drug, doxorubicin, from uniform PPF-fabricated DWMS composed of poly(D,L-lactide) (PDLL) drug-free shell and doxorubicin-loaded PLG core. Interestingly, doxorubicin release from DWMS with same particle size and shell thickness but different PDLL shell molecular weight were similar, suggesting that although drug-free PDLL shells were able to postpone drug release rates from DWMS, only varying PDLL molecular weight, without varying drug-free shell thickness, had limited influence on small-molecule release.
Uniform microparticle systems for controlled release of macromolecules
Macromolecules such as proteins, polysaccharides and DNA are rapidly developing as specific and potent therapeutics. However, their formulation and delivery face many challenges. For example, many macromolecular therapeutics require stringent control of the in vivo concentration and localization (Fu et al., 2003; Ron et al., 1993). Uniform microparticles for macromolecule release can provide better encapsulation and well-controlled release rate as well as provide the opportunity to explore the mechanisms controlling macromolecule release profiles.
Controlled release of macromolecular drugs from uniform SWMS
Berkland et al. (Berkland et al., 2007a) examined the encapsulation and release of two model macromolecules, dextran (Mw 70 kDa) and bovine serum albumin (BSA, Mw 67 kDa), from uniform PLG SWMS produced by PPF. Macromolecules were encapsulated via a double emulsion technique wherein a primary aqueous solution of dextran or BSA was emulsified into an oil phase of PLG (40% w/v) dissolved in DCM at a 1:10 v/v ratio. The macromolecules were encapsulated at 2.5% w/w PLG resulting in high encapsulation efficiency (actual drug loading/theoretical drug loading×100%) for dextran (88–97%) and acceptable encapsulation efficiency for BSA (64–66%).
Release of hydrophilic macromolecules from PLG SWMS most often exhibits a tri-phasic shape consisting of an initial burst due to macromolecules attached to the surface of microparticles or near the periphery, a lag period of very slow release due to low diffusivity of water-soluble macromolecules through the relatively dense and hydrophobic polymer, and finally steady release governed by higher effective diffusivity through water-filled pores developed within eroding microparticles (Badri Viswanathan et al., 2001; Batycky et al., 1997; Kim and Park, 2004). Berkland et al. (Berkland et al., 2007a) studied the intraparticle distribution and in vitro release of dextran and BSA from uniform PLG SWMS of varying size (Fig. 8(A)). Confocal fluorescence micrographs revealed that each model drug (fluorescein isothiocyanate-labeled dextran, and sulforhodamine B-labeled BSA) was evenly distributed throughout the PLG matrix (Fig. 8(B)). An interesting observation was the relatively small initial burst. Surprisingly, the duration of the lag phase decreased and the overall rate of release increased with increasing SWMS size (Fig. 8(C, D)) due to autocatalytic PLG degradation. The degree of autocatalysis is governed by accumulation of acidic degradation products and increased with particle size due to the decreased surface area-to-volume ratio. Thus the rates of erosion and swelling increased with particle size, countering the effects of surface area-to-volume ratio on drug diffusion and increasing the overall rate of drug release.
Fig. 8.
(A) Size distributions of dextran and BSA-loaded PLG SWMS; (B) Confocal fluorescence micrographs of dextran and BSA within PLG SWMS. In vitro release of (C) dextran and (D) BSA from uniform PLG SWMS. (C): 31 μm (closed circles), 44 μm (open circles), and 80 μm (triangles). (D): 34 μm (closed circles), 47 μm (open circles), and 85 μm (triangles). Adapted from (Berkland et al., 2007a)
Uniform biodegradable SWMS have also been investigated to deliver naked plasmid DNA (pDNA). Passive targeting of microspheres encapsulating pDNA based on size via phagocytosis to professional antigen presenting cells has been demonstrated (Hedley et al., 1998), and active targeting by coating particle surfaces with cell-specific ligands is also possible (Eniola and Hammer, 2005; Jang and Shea, 2006). Thus, particle size can be crucial not only for pDNA release rate, but also for particle targeting. Varde et al. (Varde and Pack, 2007) fabricated uniform PLG SWMS encapsulating pDNA together with magnesium hydroxide using PPF. By adding magnesium hydroxide as an antacid excipient, the “initial burst” was reduced and SWMS displayed a more homogenous surface coverage of smaller but more numerous pores, which were likely due to dissolution of magnesium hydroxide from near the surface of nascent microspheres during particle hardening process (Fig. 9(A)–(D)). Further, Varde et al. demonstrated the final amount of pDNA release was primarily affected by SWMS size, not antacid concentration, and found complete DNA release from smaller SWMS, probably due to the higher surface area-to-volume ratio which led to fast diffusion of acidic degradation byproduct out of the particles. For larger SWMS, higher release amount with Mg(OH)2 suggested that the antacid was effective in neutralizing the acidic microclimate during PLG matrix degradation/erosion (Fig. 9(E)).
Fig. 9.
SEM images of SWMS prior to release: (A) 47 μm non-Mg(OH)2, (B) 80 μm non- Mg(OH)2, (C) 47 μm with 3% Mg(OH)2, (D) 80 μm with 3% Mg(OH)2 and pDNA in vitro release (E). Scale bar=10 μm, 2 μm on inset. Adapted from (Varde and Pack, 2007)
Controlled release of macromolecular drugs from uniform DWMS
As with small molecules, DWMS composed of two different polymers as core and shell materials can provide better encapsulation and controlled release of macromolecules. By adding a drug-free polymer shell, DWMS can tailor macromolecule release profiles not only by overall particle diameter, but also by changing the shell thickness. Xia et al. (Xia et al., 2013b) investigated uniform DWMS with different polymer composition and organic solvent configuration (solvent combination for dissolving core and shell polymers). DWMS were fabricated comprising protein-loaded PLG and protein-free PDLL of varying molecular weight as core and shell materials, respectively. Uniform DWMS were fabricated with overall diameter ~55 μm, calculated core diameters ~35 μm and shell thickness ~10 μm. PLG SWMS with ~35 μm diameter, without PDLL drug-free shell, were fabricated as controls. Importantly, the solvents used in fabrication and polymer molecular weight were critical for BSA loading, encapsulation efficiency, initial distribution and release rate. Using PDLL in EtAc to form the shell and PLG in DCM for the core (denoted as EtAc(DCM)), DWMS hardened quickly due to rapid extraction of the more water-soluble EtAc, and exhibited higher loading and encapsulation efficiency compared to DCM(DCM) DWMS. (Attempts to use EtAc in the core-forming solution were not successful.) In addition, rapid hardening could prevent BSA encapsulated in PLG cores from moving toward the matrix-aqueous media boundary. DWMS formed with low molecular weight PLG cores exhibited a relatively concentrated BSA core and dense PDLL drug free shell (Fig. 10(A)–(F)). With increasing core PLG molecular weight, BSA distributed throughout the matrix, likely due to higher hydrophobicity of the cores which pushed BSA toward the periphery. The BSA release rate from DWMS with drug-free PDLL shell was significantly slower compared to SWMS without PDLL shell (Fig. 10(G)). Higher molecular weight PDLL in the shell did not decrease BSA release rate significantly.
Fig. 10.
Confocal fluorescence micrographs (for each pair, left: fluorescence; right: merged fluorescence and transmitted light) of BSA-loaded SWMS (A, B) and BSA-loaded DWMS (C and D, EtAc(DCM), PLG Mw 4.2 kDa, PDLL Mw 43 kDa; E and F, EtAc(DCM), PLG Mw 4.2 kDa, PDLL Mw 106 kDa. In vitro release profiles of BSA from DWMS/shell-free SWMS control (G): DWMS, EtAc(DCM), PDLL 43, 106 kDa, PLG 4.2 kDa; SWMS PLG 4.2 kDa. Scale bar=50 μm. Adapted from (Xia et al., 2013b)
Subsequently, Xia et al. (Xia et al., 2013a) investigated the effect of the PDLL shell thickness with constant BSA-loaded PLG core diameter by changing PDLL/PLG mass ratio. The BSA loading gradually decreased as shell thickness increased. BSA encapsulation efficiency was relatively constant at 50%, however, showing that the shell thickness increase did not provide better protein encapsulation. BSA was concentrated in the core PLG area, and drug-free PDLL shells were observed from confocal fluorescence microscopy (Fig. 11(A)–(F)). Approximately 35% of the total protein was released in the first week. The rate of the subsequent release decreased with increasing shell thickness, and “zero-order” release was achieved when PDLL drug-free shell thickness increased from 6.3 μm to 13.9 μm (Fig. 11(G)).
Fig. 11.
Confocal fluorescence micrographs of uniform BSA-loaded DWMS (for each pair, left: fluorescence; right: merged fluorescence and transmitted light): (A, B), EtAc(DCM), PDLL/PLG 1.09; (C, D), EtAc(DCM), PDLL/PLG 2.14; (E, F), EtAc(DCM), PDLL/PLG 3.04. In vitro release of BSA from DWMS with different PDLL shell thickness (G): PDLL/PLG=1.09, shell thickness=6.3 μm; PDLL/PLG=2.14, shell thickness=10.6 μm; PDLL/PLG=3.04, shell thickness=13.9 μm. Scale bar=50 μm. Adapted from (Xia et al., 2013a)
Controlled release of macromolecular drugs from uniform liquid-core MC
Continuous delivery may not be optimal for all drugs, and pulsatile delivery is preferred in many cases (Sauder et al., 1984; Schwartz, 2001). Pulsatile release is the rapid release of therapeutic during a relatively short time window (Grayson et al., 2003; Roy and Shahiwala, 2009). This naturally occurring mechanism has been mimicked for the development of pulsatile release systems to improve therapeutic efficacy. Liquid-core biodegradable MC can be a promising vehicle for pulsatile drug release. With careful selection of PPF fabrication parameters, Berkland et al. (Berkland et al., 2007b) produced liquid-core MC of highly uniform size and shell thickness for first time (Fig. 12).
Fig. 12.

(A) Coulter Multisizer size distributions for different polymer, oil, and aqueous core MC; (B) Scanning electron micrograph depicting the uniformity and surface morphology of ~115 μm canola oil core/PLG shell MC (oil core not visible). (C) Optical micrograph of ~110 μm MC encapsulating an aqueous core containing 100 mg/mL dextran and 10 mg/mL BSA with a PLG shell. Adapted from (Berkland et al., 2007b)
Xia et al. (Xia and Pack, 2014) subsequently fabricated uniform BSA-loaded liquid-core MC with varied PLG shell thickness. BSA solution was emulsified with canola oil as the DWMS core. It was found that higher PLG molecular weight (88 kDa) resulted in higher liquid-core encapsulation, and thus higher protein loading and encapsulation efficiency; attempts to produce particles with lower molecular PLG resulted in escape of the oil phase from the PLG shell. By varying PLG solution flow rates with constant liquid-core phase flow rate, MC with varied shell thickness were fabricated. BSA release showed an initial phase of slow release (10–20%), and a burst release of the remaining BSA within a short time window (less than 5 days). Importantly, the time at which the burst occurred increased with shell thickness (Fig. 13). SEM images showed that the microcapsules collapsed at times corresponding to the onset of burst release observed in the in vitro release profiles (Fig. 14). These results demonstrate that uniform liquid-core MC can provide pulsatile drug release and the onset of “burst release” time can be controlled by varied PLG shell thickness.
Fig. 13.

In vitro release profiles of BSA from liquid-core MC of PLG shell flow rate at 30, 40 and 50 mL/h, (calculated PLG shell thickness: 14.7, 16.5 and 19.0 μm). Adapted from (Xia, 2013)
Fig. 14.
SEM images of microcapsules degradation/erosion study with different PLG (Mw 88 kDa) shell flow rate at 30, 40 and 50 mL/h (calculated PLG shell thickness: 14.7, 16.5 and 19.0 μm). Adapted from (Xia and Pack, 2014)
Uniform microparticle systems for synergistic drug therapy
PPF may also be employed to fabricate particles designed to deliver two or more therapeutics simultaneously. Xu et al. (Xu et al., 2012) employed uniform DWMS with PDLL/PLL and PLG as shell and core materials, respectively. Chitosan–DNA nanoparticles containing the gene encoding the p53 tumor suppressor protein (chi-p53) were encapsulated in the PDLL/PLL shell phase while doxorubicin was encapsulated in the PLG core. Several formulations of uniform DWMS loaded with chitosan-p53 nanoparticles and doxorubicin were fabricated with outer diameter of ~74 μm and shell thickness ~15 μm. The dual-drug loaded DWMS exhibited an early release of chitosan-p53 nanoparticles from the PDLL/PLL shell layer, followed by a sustained release of doxorubicin from the PLG core (Fig. 15). These results demonstrated that the PPF method was capable of producing DWMS encapsulating dual agents for combined modality treatment, such as gene therapy and chemotherapy.
Fig. 15.
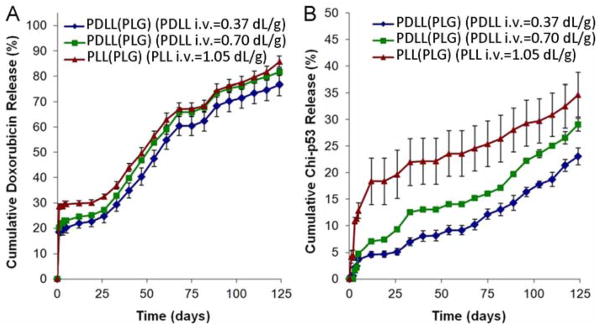
(A) Doxorubicin and (B) chitosan-p53 nanoparticles release rate from PDLL/PLL(PLG) DWMS. Adapted from (Xu et al., 2012)
Conclusions
Biodegradable microparticles will continue to play an important and versatile role for controlled release drug delivery. Uniform biodegradable microparticles fabricated by precision particle fabrication technology provide unprecedented control of particle size, a critical factor for drug release from polymer depots, allowing us to explore different drug release mechanisms. By manipulating structural properties such as overall diameter and shell thickness, “zero-order” and pulsatile release profiles have been achieved. Although several challenges regarding drug stability and bioactivity during depot fabrication and release remain, uniform biodegradable microparticles are regarded as highly promising vehicles for advanced drug delivery.
HIGHLIGHTS.
Precision particle fabrication (PPF) provides uniform polymer micrspheres.
PPF provides microcapsules with precisely controlled shell thickness.
Particle size controls small molecule and macromolecule release rates.
PPF microparticles can achieve “zero-order”, pulsatile or tandem release of drugs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almería B, Deng W, Fahmy TM, Gomez A. Controlling the morphology of electrospray-generated PLGA microparticles for drug delivery. Journal of Colloid and Interface Science. 2010;343:125–133. doi: 10.1016/j.jcis.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Advanced Drug Delivery Reviews. 2012;64(Supplement):72–82. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- Badri Viswanathan N, Patil SS, Pandil JK, Lele AK, Kulkarni MG, Mashelkar RA. Morphological changes in degrading PLGA and P(DL)LA microspheres: implications for the design of controlled release systems. Journal of Microencapsulation. 2001;18:783–800. doi: 10.1080/02652040110065440. [DOI] [PubMed] [Google Scholar]
- Batycky RP, Hanes J, Langer R, Edwards DA. A theoretical model of erosion and macromolecular drug release from biodegrading microspheres. Journal of Pharmceutical Sciences. 1997;86:1464–1477. doi: 10.1021/js9604117. [DOI] [PubMed] [Google Scholar]
- Berkland C. Control of micro- and nano- sphere size distributions: implications in drug delivery, Chemical and Biomolecular Engineering. University of Illinois at Urbana-Champaign; Urbana-Champaign: 2003. [Google Scholar]
- Berkland C, Cox A, Kim K, Pack DW. Three-month, zero-order piroxicam release from monodispersed double-walled microspheres of controlled shell thickness. Journal of Biomedical Materials Research Part A. 2004a;70:576–584. doi: 10.1002/jbm.a.30114. [DOI] [PubMed] [Google Scholar]
- Berkland C, Kim K, Pack DW. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. Journal of Controlled Release. 2001;73:59–74. doi: 10.1016/s0168-3659(01)00289-9. [DOI] [PubMed] [Google Scholar]
- Berkland C, Kim K, Pack DW. PLG microsphere size controls drug release rate through several competing factors. Pharmaceutical Research. 2003;20:1055–1062. doi: 10.1023/a:1024466407849. [DOI] [PubMed] [Google Scholar]
- Berkland C, King M, Cox A, Kim K, Pack DW. Precise control of PLG microsphere size provides enhanced control of drug release rate. Journal of Controlled Release. 2002;82:137–147. doi: 10.1016/s0168-3659(02)00136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkland C, Kipper MJ, Narasimhan B, Kim KK, Pack DW. Microsphere size, precipitation kinetics and drug distribution control drug release from biodegradable polyanhydride microspheres. J Control Release. 2004b;94:129–141. doi: 10.1016/j.jconrel.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Berkland C, Pollauf E, Pack DW, Kim K. Uniform double-walled polymer microspheres of controllable shell thickness. Journal of Controlled Release. 2004c;96:101–111. doi: 10.1016/j.jconrel.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Berkland C, Pollauf E, Raman C, Silverman R, Kim K, Pack DW. Macromolecule release from monodisperse PLG microspheres: control of release rates and investigation of release mechanism. Journal of Pharmaceutical Sciences. 2007a;96:1176–1191. doi: 10.1002/jps.20948. [DOI] [PubMed] [Google Scholar]
- Berkland C, Pollauf E, Varde N, Pack DW, Kim KK. Monodisperse liquid-filled biodegradable microcapsules. Pharmaceutical Research. 2007b;24:1007–1013. doi: 10.1007/s11095-006-9197-9. [DOI] [PubMed] [Google Scholar]
- Bindschaedler C, Leong K, Mathiowitz E, Langer R. Polyanhydride microsphere formulation by solvent extraction. Journal of Pharmaceutical Sciences. 1988;77:696–698. doi: 10.1002/jps.2600770811. [DOI] [PubMed] [Google Scholar]
- Böhmer MR, Schroeders R, Steenbakkers JAM, de Winter SHPM, Duineveld PA, Lub J, Nijssen WPM, Pikkemaat JA, Stapert HR. Preparation of monodisperse polymer particles and capsules by ink-jet printing. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2006;289:96–104. [Google Scholar]
- Dass CR, Burton MA. Microsphere-mediated targeted gene therapy of solid tumors. Drug Delivery. 1999;6:243–252. doi: 10.1080/107175400266740. [DOI] [PubMed] [Google Scholar]
- Eniola AO, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes II: effect of degradation on targeting activity. Biomaterials. 2005;26:661–670. doi: 10.1016/j.biomaterials.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Evora C, Soriano I, Rogers RA, Shakesheff KN, Hanes J, Langer R. Relating the phagocytosis of microparticles by alveolar macrophages to surface chemistry: the effect of 1,2-dipalmitoylphosphatidylcholine. Journal of controlled release : official journal of the Controlled Release Society. 1998;51:143–152. doi: 10.1016/s0168-3659(97)00149-1. [DOI] [PubMed] [Google Scholar]
- Foster CA, Kim K, Turnbull RJ, Hendricks CD. Apparatus for producing uniform solid spheres of hydrogen. Review of Scientific Instruments. 1977;48:625–631. [Google Scholar]
- Fu K, Harrell R, Zinski K, Um C, Jaklenec A, Frazier J, Lotan N, Burke P, Klibanov AM, Langer R. A potential approach for decreasing the burst effect of protein from PLGA microspheres. Journal of Pharmaceutical Sciences. 2003;92:1582–1591. doi: 10.1002/jps.10414. [DOI] [PubMed] [Google Scholar]
- Gilliard RP, Kim K, Turnbull RJ. Spherical hydrogen pellet generator for magnetic confinement fusion research. Review of Scientific Instruments. 1981;52:183–190. [Google Scholar]
- Gratton SEA, Pohlhaus PD, Lee J, Guo J, Cho MJ, DeSimone JM. Nanofabricated particles for engineered drug therapies: A preliminary biodistribution study of PRINT™ nanoparticles. Journal of Controlled Release. 2007;121:10–18. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson ACR, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, Langer R. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nature Materials. 2003;2:767–772. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- Ha CS, Gardella JA. Surface Chemistry of Biodegradable Polymers for Drug Delivery Systems. Chemical Reviews. 2005;105:4205–4232. doi: 10.1021/cr040419y. [DOI] [PubMed] [Google Scholar]
- Hedley ML, Curley J, Urban R. Microspheres containing plasmid-encoded antigens elicit cytotoxic T-cell responses. Nature medicine. 1998;4:365–368. doi: 10.1038/nm0398-365. [DOI] [PubMed] [Google Scholar]
- Hung LH, Teh SY, Jester J, Lee AP. PLGA micro/nanosphere synthesis by droplet microfluidic solvent evaporation and extraction approaches. Lab on a chip. 2010;10:1820–1825. doi: 10.1039/c002866e. [DOI] [PubMed] [Google Scholar]
- Jang JH, Shea LD. Intramuscular delivery of DNA releasing microspheres: Microsphere properties and transgene expression. Journal of Controlled Release. 2006;112:120–128. doi: 10.1016/j.jconrel.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Niwa T, Handa T, Takeuchi H, Iwamoto T, Itoh K. Preparation of controlled-release microspheres of ibuprofen with acrylic polymers by a novel quasi-emulsion solvent diffusion method. Journal of Pharmaceutical Sciences. 1989;78:68–72. doi: 10.1002/jps.2600780118. [DOI] [PubMed] [Google Scholar]
- Kim HK, Park TG. Comparative study on sustained release of human growth hormone from semi-crystalline poly(L-lactic acid) and amorphous poly(D,L-lactic-co-glycolic acid) microspheres: morphological effect on protein release. Journal of Controlled Release. 2004;98:115–125. doi: 10.1016/j.jconrel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Kim K, Jang KY, Upadhye RS. Hollow silica spheres of controlled size and porosity by sol-gel processing. Journal of the American Ceramic Society. 1991;74:1987–1992. [Google Scholar]
- Kim NK, Kim K, Payne DA, Upadhye RS. Fabrication of hollow silica aerogel spheres by a droplet generation method and sol-gel processing. Journal of Vacuum Science & Technology A. 1989;7:1181–1184. [Google Scholar]
- Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- Malik DK, Baboota S, Ahuja A, Hasan S, Ali J. Recent advances in protein and peptide drug delivery systems. Current Drug Delivery. 2007;4:141–151. doi: 10.2174/156720107780362339. [DOI] [PubMed] [Google Scholar]
- Park J, Ye M, Park K. Biodegradable Polymers for Microencapsulation of Drugs. Molecules. 2005;10:146–161. doi: 10.3390/10010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavanetto F, Genta I, Giunchedi P, Conti B. Evaluation of spray drying as a method for polylactide and polylactide-co-glycolide microsphere preparation. Journal of Microencapsulation. 1993;10:487–497. doi: 10.3109/02652049309015325. [DOI] [PubMed] [Google Scholar]
- Pollauf EJ, Kim KK, Pack DW. Small-molecule release from poly(D,L-lactide)/poly(D,L-lactide-co-glycolide) composite microparticles. Journal of Pharmaceutical Sciences. 2005;94:2013–2022. doi: 10.1002/jps.20408. [DOI] [PubMed] [Google Scholar]
- Pollauf EJ, Pack DW. Use of thermodynamic parameters for design of double-walled microsphere fabrication methods. Biomaterials. 2006;27:2898–2906. doi: 10.1016/j.biomaterials.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Poncelet D, Neufeld R, Bugarski B, Amsden BG, Zhu J, Goosen MFA. A parallel plate electrostatic droplet generator: parameters affecting microbead size. Applied Microbiology and Biotechnology. 1994;42:251–255. [Google Scholar]
- Radulescu D, Wawro D. Uniform paclitaxel loaded biodegradable microspheres manufactured by ink-jet printing. 11 th International Symposium and Exhibition on Recent Advantages in Drug-Delivery Systems, Controlled Release Society; Salt Lake City UT. 2003. pp. 1–5. [Google Scholar]
- Raman C, Berkland C, Kim K, Pack DW. Modeling small-molecule release from PLG microspheres: effects of polymer degradation and nonuniform drug distribution. Journal of Controlled Release. 2005;103:149–158. doi: 10.1016/j.jconrel.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Rayleigh L. Proceedings of the London Mathematical Society; 1879. p. 10. [Google Scholar]
- Rayleigh L. Philosophical Magazine. 1882. p. 14. [Google Scholar]
- Rickwood S, Kleinrock M, Nunez-Gaviria M. The global use of medicines: outlook through 2017. IMS Institute of Healthcare Informatics; 2013. [Google Scholar]
- Rolland JP, Hagberg EC, Denison GM, Carter KR, De Simone JM. High-Resolution Soft Lithography: Enabling Materials for Nanotechnologies. Angewandte Chemie International Edition. 2004;43:5796–5799. doi: 10.1002/anie.200461122. [DOI] [PubMed] [Google Scholar]
- Ron E, Turek T, Mathiowitz E, Chasin M, Hageman M, Langer R. Controlled release of polypeptides from polyanhydrides. Proceedings of the National Academy of Sciences. 1993;90:4176–4180. doi: 10.1073/pnas.90.9.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P, Shahiwala A. Multiparticulate formulation approach to pulsatile drug delivery: Current perspectives. Journal of Controlled Release. 2009;134:74–80. doi: 10.1016/j.jconrel.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Salem R, Lewandowski RJ, Atassi B, Gordon SC, Gates VL, Barakat O, Sergie Z, Wong CO, Thurston KG. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. Journal of Vascular and Interventional Radiology. 2005;16:1627–1639. doi: 10.1097/01.RVI.0000184594.01661.81. [DOI] [PubMed] [Google Scholar]
- Sauder SE, Frager M, Case GD, Kelch RP, Marshall JC. Abnormal patterns of pulsatile luteinizing hormone secretion in women with hyperprolactinemia and amenorrhea: responses to bromocriptine. The Journal of clinical endocrinology and metabolism. 1984;59:941–948. doi: 10.1210/jcem-59-5-941. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Editorial: pulsatile hormone patterns governing transcription factor function. Physiology of episodic GH secretion. Endocrinology. 2001;142:4595–4598. doi: 10.1210/endo.142.11.8564. [DOI] [PubMed] [Google Scholar]
- Shum HC, Kim JW, Weitz DA. Microfluidic fabrication of monodisperse biocompatible and biodegradable polymersomes with controlled permeability. Journal of the American Chemical Society. 2008;130:9543–9549. doi: 10.1021/ja802157y. [DOI] [PubMed] [Google Scholar]
- Singh MN, Hemant KS, Ram M, Shivakumar HG. Microencapsulation: a promising technique for controlled drug delivery. Research in pharmaceutical sciences. 2010;5:65–77. [PMC free article] [PubMed] [Google Scholar]
- Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. Journal of Controlled Release. 2003;90:261–280. doi: 10.1016/s0168-3659(03)00194-9. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Nakajima M, Tong J, Nabetani H, Seki M. Preparation of monodispersed solid lipid microspheres using a microchannel emulsification technique. Journal of Colloid and Interface Science. 2000;227:95–103. doi: 10.1006/jcis.2000.6843. [DOI] [PubMed] [Google Scholar]
- Tamber H, Johansen P, Merkle HP, Gander B. Formulation aspects of biodegradable polymeric microspheres for antigen delivery. Advanced Drug Delivery Reviews. 2005;57:357–376. doi: 10.1016/j.addr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Teh SY, Lin R, Hung LH, Lee AP. Droplet microfluidics. Lab on a chip. 2008;8:198–220. doi: 10.1039/b715524g. [DOI] [PubMed] [Google Scholar]
- Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert Opinion on Biological Therapy. 2004;4:35–51. doi: 10.1517/14712598.4.1.35. [DOI] [PubMed] [Google Scholar]
- Varde NK, Pack DW. Influence of particle size and antacid on release and stability of plasmid DNA from uniform PLGA microspheres. Journal of Controlled Release. 2007;124:172–180. doi: 10.1016/j.jconrel.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ye W, Zheng Y, Liu X, Tong Z. Fabrication of drug-loaded biodegradable microcapsules for controlled release by combination of solvent evaporation and layer-by-layer self-assembly. International Journal of Pharmaceutics. 2007;338:165–173. doi: 10.1016/j.ijpharm.2007.01.049. [DOI] [PubMed] [Google Scholar]
- Wattendorf U, Coullerez G, Vörös J, Textor M, Merkle HP. Mannose-based molecular patterns on stealth microspheres for receptor-specific targeting of human antigen-presenting cells. Langmuir. 2008;24:11790–11802. doi: 10.1021/la801085d. [DOI] [PubMed] [Google Scholar]
- Xia Y. Uniform biodegradable microparticle systems for protein delivery, Chemical and Biomolecular Engineering. University of Illionis at Urbana-Champaign; Urbana-Champaign: 2013. [Google Scholar]
- Xia Y, Pack DW. Pulsatile protein release from monodisperse liquid-core microcapsules of controllable shell thickness. Pharm Res. 2014 doi: 10.1007/s11095-014-1412-5. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Ribeiro PF, Pack DW. Controlled protein release from monodisperse biodegradable double-wall microspheres of controllable shell thickness. Journal of Controlled Release. 2013a;172:707–714. doi: 10.1016/j.jconrel.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Xu Q, Wang CH, Pack DW. Protein encapsulation in and release from monodisperse double-wall polymer microspheres. Journal of Pharmaceutical Sciences. 2013b;102:1601–1609. doi: 10.1002/jps.23511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Chin SE, Wang C, Pack DW. Mechanism of drug release from double-walled PDLLA(PLGA) microspheres. Biomaterials. 2013;34:3902–3911. doi: 10.1016/j.biomaterials.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Hashimoto M, Dang TT, Hoare T, Kohane DS, Whitesides GM, Langer R, Anderson DG. Preparation of monodisperse biodegradable polymer microparticles using a microfluidic flow-focusing device for controlled drug delivery. Small. 2009;5:1575–1581. doi: 10.1002/smll.200801855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Xia Y, Wang C, Pack DW. Monodisperse double-walled microspheres loaded with chitosan-p53 nanoparticles and doxorubicin for combined gene therapy and chemotherapy. Journal of Controlled Release. 2012;163:130–135. doi: 10.1016/j.jconrel.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Liang Q, Ma S, Mu X, Hu P, Wang Y, Luo G. On-chip manipulation of continuous picoliter-volume superparamagnetic droplets using a magnetic force. Lab on a chip. 2009;9:2992–2999. doi: 10.1039/b906229g. [DOI] [PubMed] [Google Scholar]













