SUMMARY
We showed previously that protein kinase C (PKC) is required for phagocytosis of apoptotic leukocytes by murine alveolar (AMø) and peritoneal macrophages (PMø), and that such phagocytosis is markedly lower in AMø compared to PMø. In this study, we examined the roles of individual PKC isoforms in phagocytosis of apoptotic thymocytes by these two Mø populations. By immunoblotting, AMø expressed equivalent PKC η but lower amounts of other isoforms (α, βI, βII, δ, ε, μ, ζ), with the greatest difference in βII expression. A requirement for PKCβII for phagocytosis was demonstrated collectively by phorbol 12-myristate 13-acetate-induced depletion of PKC βII, by dose-response to PKC inhibitor Ro-32-0432, and by use of PKC βII myristylated peptide as a blocker. Exposure of PMø to phosphatidylserine (PS) liposomes specifically induced translocation of PKC βII and other isoforms to membranes and cytoskeleton. Both AMø and PMø expressed functional PS receptor, blockade of which inhibited PKC βII translocation. Our results indicate that murine tissue Mø require PKC βII for phagocytosis of apoptotic cells, which differs from the PKC isoform requirement previously described in Mø phagocytosis of other particles, and imply that a crucial action of the PS receptor in this process is PKC βII activation.
INTRODUCTION
Phagocytosis, the uptake of large particles (>0.5 μm) via actin-dependent mechanisms (1), is the obligatory means of clearing apoptotic cells during development and in resolving inflammation (2). Only macrophages can efficiently clear the large numbers of apoptotic leukocytes produced during waning immune responses (3-6). Indeed, the efficiency of this process is evidenced by the fact that apoptotic cells are rarely observed in vivo (7); one exception is in the lungs of mice, where apoptotic lymphocytes are easily demonstrable both in health and inflammation (8). This defect in clearance is consistent with the finding that the principal resident lung phagocytes, alveolar macrophages (AMø), exhibit markedly lower capacity for phagocytosis of apoptotic leukocytes, either compared to inflammatory lung Mø (in rabbits) (9), or to resident peritoneal Mø (PMø) (in mice) (10). In the latter system, no disparity between AMø and PMø was detected using three other particle types (10,11). Murine AMø also exhibited a relative deficit in phagocytosis of apoptotic cells in vivo (10). We have recently found that human AMø also show much lower phagocytosis of apoptotic cells than of other particles in vitro 4. Contrasting the properties of these two types of resident tissue Mø could aid in defining the molecular basis of apoptotic cell recognition, which is poorly understood.
Recognition of apoptotic cells is initiated through at least two pathways. Using a 70 kDa glycosylated type II transmembrane protein called PS-R' (12), Mø and other cell types recognize externalized phosphatidylserine (PS), which translocates from the inner to the outer leaflet of the cell membrane early in apoptosis (13-17). Recognition of externalized PS has been suggested to be both necessary and sufficient to generate a signal for ingestion (13,18). More recently, the Mø-specific receptor tyrosine kinase Mer has been identified as critical for the phagocytosis of apoptotic cells by murine Mø (19). How signaling from these two receptors leads to apoptotic cell phagocytosis is undefined. A host of other Mø cell-surface receptors {reviewed in (20)} have also been implicated in clearance of apoptotic cells, but they appear to be involved principally in adhesion rather than in recognition of cell death (21). Moreover, even though we (10) and others (22) have identified a number of differences in expression of adhesion molecules between murine AMø and PMø, blocking experiments using monoclonal antibodies (mAbs) or arginine-glycine-aspartic acid-serine (RGDS) tetrapeptide have not supported any identified adhesion receptor, including several integrins, as being responsible for the functional difference in phagocytosis (10).
An alternative explanation for disparity between Mø types in apoptotic cell phagocytosis would be differences in post-receptor signal transduction. A logical candidate for such a difference is protein kinase C (PKC), because we and others have shown that it is required for apoptotic cell clearance (11,23). PKC comprises a family of related serine/threonine kinases divided into three groups on the basis of structure and cofactor requirements (24). Activation of PKC requires phosphorylation on serines/threonines, displacement of its auto-inhibitory pseudo-substrate domain, and translocation to specific cytoskeletal and intracellular membrane sites of action (25). Activation of the conventional group (cPKC) (α, βI, βII, and γ) is calcium-, and diacylglycerol (DAG)-dependent. Activation of the novel group (nPKC) (ε, δ, η and θ) also depends on binding of DAG, but is calcium-independent. The atypical group (aPKC) (i/λ, and ζ) cannot be activated by calcium or DAG. All PKC family members bind PS on the cytosolic leaflet of the cell membrane, but aPKCs require additional incompletely-defined lipid activators(24). Another isoform, PKC μ (often called PKD in the mouse), does not fit into any of the major groups. PKC μ contains two unique hydrophobic domains in its amino-terminus, and is phospholipid-dependent but calcium-insensitive (26). Individual cell types usually express several PKC isoforms, each of which appears to mediate unique functions (27). Even the 50 amino acid difference in the alternatively spliced forms of PKC β (βI and βII) appears to be responsible for the unique role of each PKC β isozyme (28,29). Thus, differences between murine AMø and PMø in PKC isoform expression or function could explain the functional difference between these two cell types in apoptotic cell phagocytosis.
We recently showed (11) that phagocytosis of apoptotic thymocytes by murine AMø and PMø was reduced by the nonspecific PKC inhibitor staurosporine, by Gö 6976, but only incompletely by calphostin C. Gö 6976 has been reported to act as a partially selective inhibitor of the cPKC α and βI isoforms (30), whereas calphostin C has greater activity against nPKCs than cPKCs (31). However, current data on the specificity of these inhibitors are too inconclusive (32) to allow us to predict with certainty which isoforms are involved. Therefore, in this study we used six approaches to further define the PKC isoform(s) involved in Mø phagocytosis of apoptotic thymocytes. First, because the pattern of PKC isoforms in primary murine tissue Mø has not been described, we analyzed PKC isoform expression using isotype-specific antibodies and Western blotting. Second, we tested the effect of overnight exposure of Mø to phorbol 12-myristate 13-acetate (PMA), which depletes cPKC and nPKC isoforms by interacting with their DAG-binding sites, on AMø and PMø phagocytosis of apoptotic thymocytes. Third, we employed the isoform-selective inhibitors, rottlerin and Ro-32-0432. Fourth, we tested the effect of myristylated blocking peptides against PKC βI, PKC βII, and PKC α on phagocytosis of apoptotic thymocytes by AMø and PMø. Fifth, we examined the effect of PS liposomes, as models for apoptotic thymocytes, on translocation of PKC isoforms to cell membranes and cytoskeleton; apoptotic thymocytes could not be used because they themselves express multiple PKC isoforms. Finally, we studied Mø expression of PS-R' and the relationship between PS-R' stimulation and PKC translocation. Collectively, our results indicate a requirement for PKC βII in Mø phagocytosis of apoptotic thymocytes, show that an antibody against PS-R' blocks translocation of PKC βII in response to PS liposomes, and suggest that relative deficiency of PKC βII and possibly other PKC isoforms may partially explain the functional difference in apoptotic cell clearance by AMø.
EXPERIMENTAL PROCEDURES
Reagents
Rottlerin and Ro-32-0432 were purchased from Calbiochem Novabiochem Corp. (San Diego, CA). PMA, PBS, RPMI 1640, FBS, HEPES, pyruvate and penicillin/streptomycin were obtained from Life Technologies (Rockville, MD). Dimethyl sulfoxide dexamethasone, 2-mercaptoethanol, sodium deoxycholate, glycerol, NaCl, Tris HCl, Triton X-100, Tween 20, L-α-phosphatidylinositol (PI), L-α-PS, and phosphatase inhibitor cocktail II were purchased from Sigma (St. Louis, MO). Antibodies and blocking peptides for PKC isoforms and horseradish peroxidase (HRP)-conjugated anti-rabbit IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Complete mini-protease inhibitor tablets and the LDH cytotoxicity detection kit were purchased from Roche (Indianapolis, IN). SDS, 0.2 μm polyvinylidene difluoride (PVDF) membrane, non-fat dry milk blocker, and 10% Ready Acrylamide gels were obtained from BioRad (Hercules, CA). Supersignal West Femto Maximum Sensitivity substrate was obtained from Pierce (Rockford, IL). Kodak X-Omat AR film and 8-well Lab-Tek slides were obtained from Fisher Scientific (Chicago, IL). mAb 217 (anti-murine PS-R; rat IgM) was generously provided by Dr. Valerie Fadok (National Jewish Medical Center; Denver, CO) as a culture supernatant; in selected experiments, a commercial preparation of this mAb (Cascade BioScience; Winchester, MA) was used. Myristylated PKC peptides were synthesized by Biosource Quality Controlled Biochemicals (Hopkinton, MA) at 90% purity, as confirmed by high-pressure liquid chromatography and mass spectroscopy performed by the manufacturer.
Mice
All experiments were performed using pathogen-free C57BL/6 female mice purchased from Charles River Laboratories Inc. (Wilmington, MA) at 7-8 weeks of age and used at 8-14 weeks of age. Mice were housed in the Animal Care Facility at the Ann Arbor VA Medical Center, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care. This study complied with the NIH “Guide for the Care and Use of Laboratory Animals” (Dept. of Health, Education, & Welfare Publication No. 80-32) and followed a protocol approved by the Animal Care Subcommittee of the local Institutional Review Board.
Isolation and culture of Mø
Mice were euthanized by asphyxia in a high CO2 environment, which we have previously shown does not impair the capacity of AMø to ingest apoptotic thymocytes (10). Resident AMø and PMø were harvested and cultured as previously described (10). Mø were isolated by adherence onto tissue culture plates (for protein isolation) or 8-well Lab-Tek slides (for phagocytosis assay) for 2-4 h at 37° C in 5% CO2 in complete medium (RPMI 1640 containing 10% heat-inactivated FBS, 25 mM HEPES, 2 mM L-glutamine, 1 mM pyruvate, 100 units/ml penicillin/streptomycin, 55 μM 2-mercaptoethanol. Nonadherent cells were removed by gentle washing. In experiments in which PKC localization was analyzed by Western blotting, complete medium was replaced with serum-free medium for 1 h.
Preparation of apoptotic thymocytes
Thymuses were harvested from normal mice and minced to yield a single cell suspension. To induce apoptosis, thymocytes were resuspended in complete medium to a concentration of 1 × 106 cells/ml and incubated for 6 h in complete medium containing 1 μM dexamethasone. This treatment yields a population with a low percentage (mean 13.4%) contamination of late apoptotic or necrotic cells (10,11).
Western analysis of PKC isozymes
Resident AMø and PMø from normal mice were isolated and plated at 1.0 × 106 cells/ml on 100 mm × 15 mm plates in media containing 10% serum, and incubated for 2 h at 37° C and 5% CO2. Next, Mø were washed and solubilized in ice cold lysis buffer consisting of 1.0% Triton X-100, 20 mM Tris HCl pH 8.0, 150 mM NaCl, 0.1% SDS, 0.5% Na Deoxycholate, and 10% glycerol with protease inhibitors (complete mini-tablet) and phosphatase inhibitor cocktail II (1:100) for 30 min on ice. After sonicating for 3 s and centrifuging at 13,800 × g for 3 min, 7 μg of protein per sample was run on a 10% acrylamide gel under reducing conditions and transferred to a PVDF membrane using 25 mM Tris, 192 mM glycine, 20% methanol. Blots were blocked with 5% nonfat dry milk in PBS (blocker), incubated with the appropriate anti-PKC isoform antibody (1:1000 dilution in blocker), and washed five times for 5 min each with PBS containing 0.1% Tween 20. Blots were incubated with HRP-conjugated goat anti-rabbit IgG (diluted 1:10,000 in PBS containing 5% nonfat dry milk), and chemiluminescence signal was developed by adding a peroxidase/luminol-based substrate (Supersignal Femto reagent, Pierce). The identity of PKC isoforms on Western blots was verified using isoform-specific blocking peptides. No bands were seen when blots were stained using HRP-conjugated goat anti-rabbit IgG alone.
To analyze the subcellular distribution of PKC isozymes, Mø were isolated and plated at 1.0 × 106 cells/ml on 30 mm × 15 mm plates in media containing 10% serum, and incubated for 2 h at 37° C and 5% CO2. The medium was then removed and replaced with serum-free medium for 1 h followed by washing and scraping in cold PBS. Next, Mø were sonicated for 10 s in 50 mM Tris pH 8.0, 9 mM EDTA, and then centrifuged at 100,000 × g for 45 min. The supernatant was collected and defined as the cytosolic fraction. The pellet, defined as the membrane and cytoskeleton fractions, was solubilized in ice cold lysis buffer (defined above) for 30 min on ice. This solubilized pellet fraction was sonicated for 3 s and then centrifuged at 13,800 × g for 3 min. Equal amounts of protein (3-7 μg) were run on a 10% SDS-PAGE gel under reducing conditions, transferred to the PVDF membrane, and stained as described above.
Preparation of liposomes
To produce liposomes, PS or the negatively-charged control lipid PI were dried under N2, and resuspended in serum-free medium by vortexing. Liposome size was determined by Coulter counter analysis to be in a similar range as apoptotic thymocytes, 2-3.2 μm. In these experiments, Mø were incubated for 1 h in serum-free medium, liposomes in serum-free medium were added to Mø monolayers in a final PS or PI concentration of 0.11 mM, and were co-incubated for the indicated time at 37° C and 5% CO2. Cells were washed and scraped in cold PBS, sonicated for 10 s in 50 mM Tris pH 8.0, 9 mM EDTA and centrifuged at 100,000 × g for 45 min.
RNA preparation and RT-PCR
Total RNA was isolated from adherent AMø and PMø using TRIzol (Life Technologies; Grand Island, NY). RT-PCR reactions were performed using kits from Life Technologies. The primer sets used were the following: for mouse PS-R' (GenBank Accession no. AF304118) forward CTC ACG ATG AAC CAC AAG AGC, reverse GGA CCA GCC CTC TTG TGC ATT; for mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH, GenBank Accession no. M32599) forward GGT CGG TGT GAA CGG ATT TGG, reverse ATG AGG TCC ACC ACC CTG TTG. The expected PCR reaction product sizes are 245 bp for PS-R' and 968 bp for GAPDH. PCR products were analyzed on a 2% agarose gel and stained with ethidium bromide. The identity of the target products was confirmed by sequencing.
Flow cytometric analysis
Apoptosis was measured by simultaneous annexin V and propidium iodide staining (Apoptosis Detection Kit; R & D Systems; Minneapolis, MN) according to the manufacturer's protocol. Cells were analyzed without fixation by flow cytometry within 1 h of staining. Staining of surface receptors and flow cytometry were performed as previously described in detail (33) using a FACSCAN cytometer (Becton Dickinson; Mountain View, CA) running Cell Quest software on a PowerPC microcomputer (Apple; Cupertino, CA) for data collection and analysis. A minimum of 10,000 viable cells was analyzed.
Phagocytosis assay
Phagocytosis of apoptotic thymocytes in vitro was assayed by coincubation of 0.5-2.0 × 105 adherent Mø with 2.0 × 106 apoptotic thymocytes for 90 min at 37° C in 5% CO2 as previously described (10). Results are expressed as percentage of Mø containing at least one ingested thymocyte (percent phagocytosis), and as phagocytic index, which was generated by multiplying the percentage of phagocytosis by the mean number of ingested cells per Mø. Cell-permeable PKC inhibitors were added either 30 min (rottlerin) or 18 h (Ro-32-0432 and PMA) before addition of apoptotic thymocytes. Myristylated PKC peptides from the carboxy terminus of the V5 region of PKC α (myr-PQFVHPILQSAV-amide)g, PKC βI (myr-DQNEFAGFSYTNPEFVINV-amide) or PKC βII (myr-SFVNSEFLKPEVKS-amide) were added to a final concentration of 100 μM 30 min before addition of apoptotic thymocytes. The myristate moieties coupled to the amino terminus of these peptides allow membrane permeability, permitting their use in primary cells (34). All inhibitors were nontoxic at the times and concentrations utilized, as determined by the LDH cytotoxicity detection kit4.
Adhesion assay
Adherence of apoptotic thymocytes to Mø in vitro was assayed in the same fashion as phagocytosis, except that 2 × 107 apoptotic thymocytes suspended in 400 μl of complete medium were added to each well, yielding a 100:1 ratio of thymocytes to Mø. The slides were incubated for various times at 37°C, and then washed in a standardized fashion, by dipping individual slides 5 times in each of two Wheaton jars filled with ice-cold PBS. Slides were then stained using hematoxylin-eosin Y (H & E) (Richard-Allan; Kalamazoo, MI), and coverslipped. Adhesion was evaluated by counting 200 to 300 Mø per well at 1000 × magnification under oil immersion, and scoring for bound thymocytes. Results are expressed as percentage of Mø binding at least one thymocyte (percent adhesive Mø), and as adhesion index, which was generated by multiplying the percentage of adherence positive Mø by the mean number of adherent thymocytes per Mø. In blocking experiments, mAbs were used at concentrations previously determined to be saturating by flow cytometry, and culture supernatant containing mAb 217 was concentrated 10-fold using Centriprep tubes (Amicon; Bedford, MA).
Statistical analysis
Data are expressed as mean ± SEM. Statistical calculations were performed using Statview on a Macintosh Power PC G3 computer. Continuous ratio scale data were evaluated by unpaired Student t-test (for two samples) or ANOVA (for multiple comparisons) with post hoc analysis by the Tukey-Kramer test or by the two-tailed Dunnett test, which specifically compares treatment groups to a control group (35). Use of these parametric statistics was deemed appropriate, as phagocytosis of apoptotic thymocytes by PMø has been shown to follow a Gaussian distribution (36). Significant differences were defined as p<0.05.
RESULTS
AMø express lower amounts of most PKC isoforms than do PMø
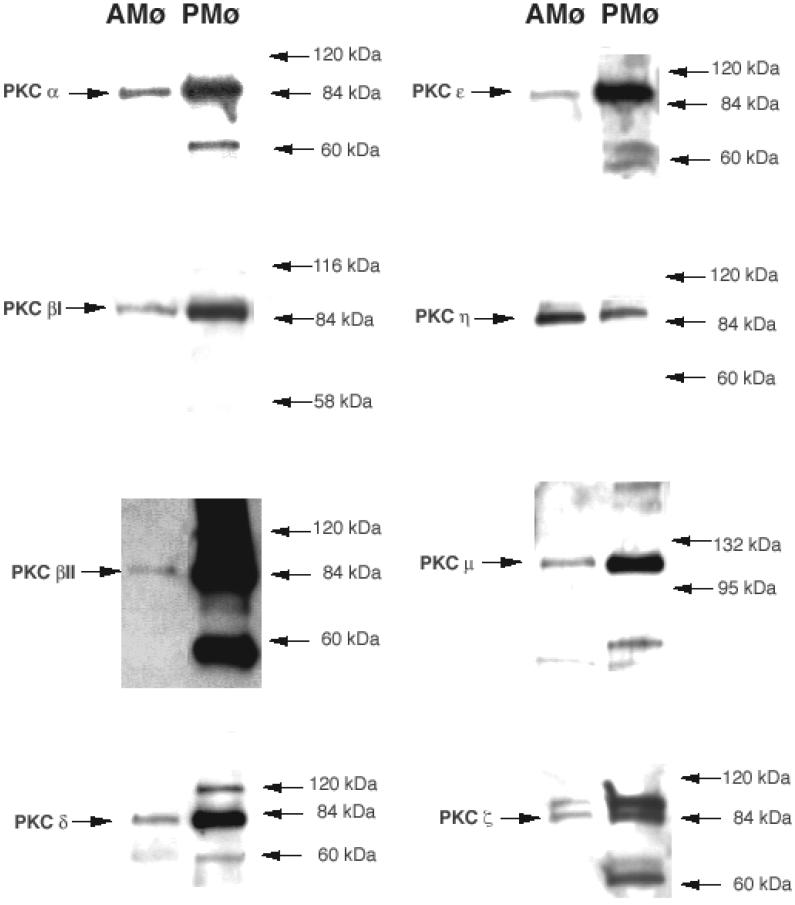
Western blot analysis using PKC isoform-specific antibodies demonstrated markedly lower expression of PKC α, βI, βII, δ, ε, μ, and ζ in resident murine AMø in comparison with resident murine PMø (Fig. 1). By contrast, AMø had slightly higher expression of PKC η. Staining did not detect expression of PKC γ or PKC λ in either murine AMø or PMø4, in agreement with previous analyses of human tissue Mø (24,37), and we did not test expression of the lymphocyte-specific isoform PKC θ. Thus, the lower expression of several PKC isoforms by AMø is a potential explanation for the previously observed decreased phagocytosis of apoptotic cells by this cell type.
Figure 1. Resident murine AMø and PMø differ in expression of multiple PKC isoforms.
Whole cell lysates of AMø and PMø of normal C57BL/6 mice cultured as adherent monolayers for 2 hours were analyzed for expression of PKC isoforms by Western blotting using isoform-specific antibodies.
Effect of overnight PMA treatment on Mø phagocytosis of apoptotic thymocytes
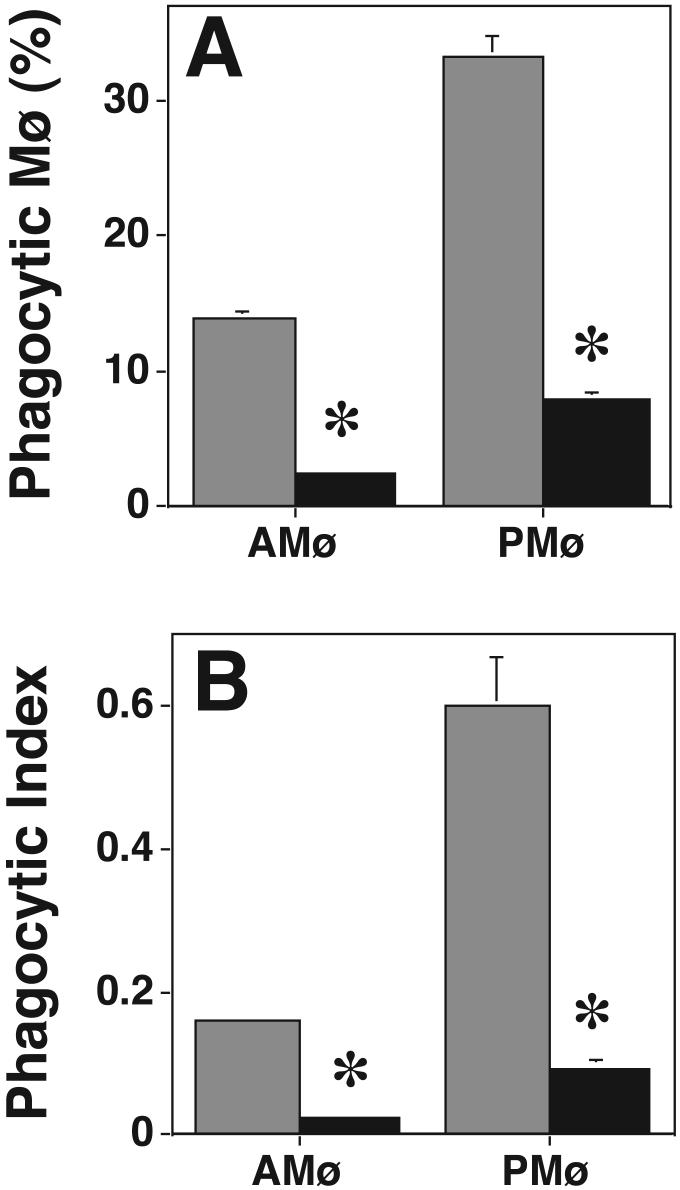
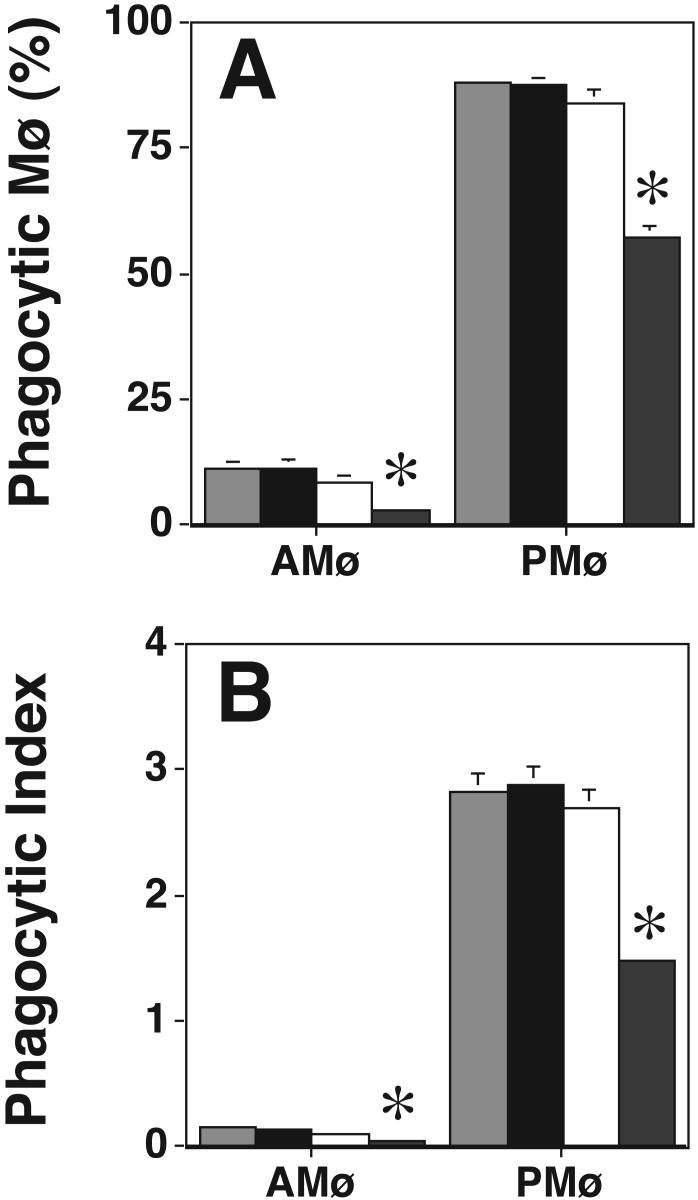
Chronic (18 h) PMA treatment depletes cPKCs and nPKCs but not aPKCs, with the degree to which individual isoforms are affected depending on the cell type and the PMA concentration (27,37-39). In preliminary experiments, the concentration and time of PMA addition were optimized to achieve maximal inhibition of apoptotic cell phagocytosis. We determined that the conditions used were nontoxic as indicated by assay of LDH release4. Western blot analysis on AMø and PMø after overnight treatment using 8.1 μM PMA confirmed that PKC α, βI, βII, and δ were significantly depleted (p<0.05, unpaired t test) by PMA treatment in either of the two types of Mø (Fig. 2). As anticipated, PKC ε, η, μ, and ζ were not depleted by overnight PMA treatment in either Mø type. In the functional assay, the same overnight PMA treatment significantly decreased phagocytosis by AMø and PMø (p<0.05, unpaired Student t-test) (Fig. 3). Control experiments showed that overnight PMA treatment did not influence adhesion of thymocytes to PMø (percent adhesive Mø, control 86.1 ± 1.7% versus PMA-treated 82.0 ± 2.0%, p = 0.16; adhesion index, control 2.6 ± 0.2 versus PMA-treated 2.7 ± 0.1; mean ± SEM, n = 4, p = 0.47, both comparisons made by unpaired t-test) and actually slightly increased adhesion to AMø (adhesive Mø, control 60.5 ± 1.5% versus PMA-treated 71.5 ± 2.2%; mean SEM, n = 4; p < 0.001; adhesion index, control 1.2 ± 0.1 versus PMA-treated 1.7 ± 0.0; p < 0.001, unpaired t-test). Collectively, these data indicate that the nPKC isoforms ε and η, the aPKC isoform ζ, and PKC μ/PKD are not essential for Mø phagocytosis of apoptotic cells, but leave open the question of which cPKC or other nPKC isoforms are required.
Figure 2. Overnight PMA treatment decreases expression of cPKC and nPKC isoforms by AMø and PMø.
Resident AMø and PMø were incubated overnight at 37° C and 5% CO2 with either PMA (8.1 μM final concentration) or the appropriate amount of ethanol as the control, and then analyzed for expression of PKC isoforms by Western blotting using isoform-specific antibodies.
Figure 3. Depletion of cPKCs and nPKCs by overnight PMA treatment reduces Mø phagocytosis of apoptotic thymocytes.
Phagocytosis of apoptotic thymocytes by resident AMø and PMø incubated overnight in medium containing a final concentration of 8.1 μM PMA (black bars) or the appropriate amount of ethanol as a control (gray bars) was determined by examining H & E stained slides under oil immersion. (A) percentage of phagocytic Mø; (B) phagocytic index. Data are mean ± SEM of 4 replicates in two experiments. *, p<0.05, unpaired Student t-test.
Rottlerin and Ro-32-0432 decrease phagocytosis of apoptotic thymocytes at concentrations at which they inhibit cPKCs
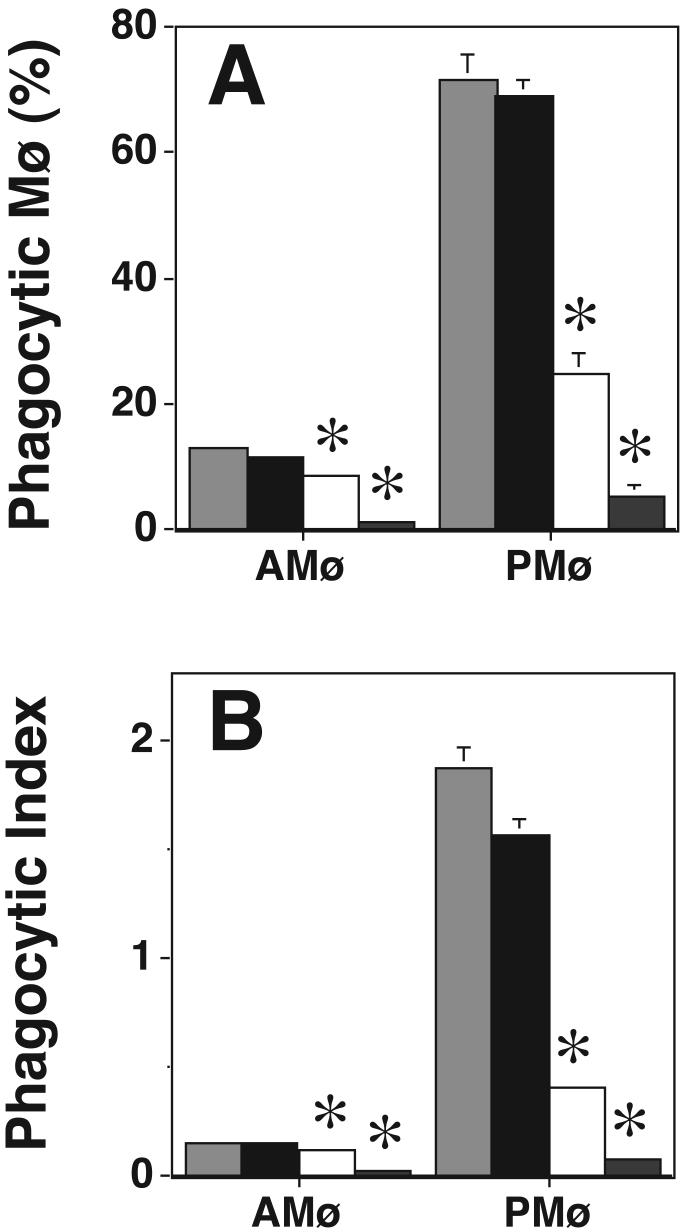
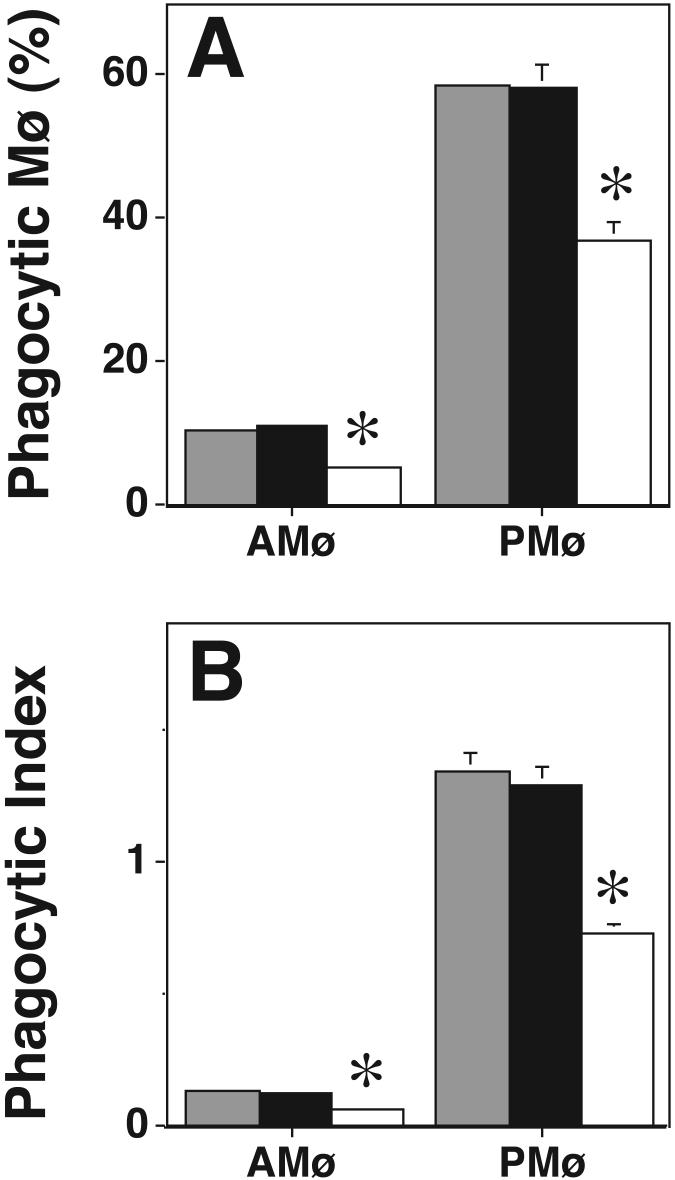
To further define which PKC isoforms are essential for phagocytosis, we used two cell-permeable PKC inhibitors, rottlerin and Ro-32-0432, which show a degree of isoform specificity. Rottlerin specifically inhibits PKC δ at relatively low concentration (IC50 = 3-6 μM) and other PKC isoforms only at higher concentrations (IC50 for PKC α and β = 30-42 μM; IC50 for PKC ε, η, and ζ is 80-100 μM) (40). We found that rottlerin did not inhibit AMø or PMø phagocytosis of apoptotic thymocytes at 10 μM (a concentration at which PKC δ should be inhibited (40)), whereas there was significantly decreased phagocytosis at 40 μM (a concentration at which cPKCs should be inhibited (40)) (Fig. 4). Ro-32-0432 had no effect on phagocytosis of apoptotic thymocytes at 9 nM (a concentration at which PKC α should be inhibited (41)), but did inhibit phagocytosis of apoptotic thymocyte at 28 nM (a concentration at which PKC β, but not PKC ε should be inhibited (41)) (Fig. 5). These data argue against a requirement for PKC δ or PKC ε for phagocytosis of apoptotic cells, and provide additional support that a cPKC, especially PKC βI or βII, is required. However, rottlerin can inhibit casein kinase II and cAMP-dependent protein kinase (PKA) with IC50 values of 30 μM and 78 μM, respectively, and can also inhibit calmodulin kinase III with IC50 of 5.3 μM (32). Moreover, interpretation of these experiments is complicated by the uncertainty in the degree to which the reported IC50s for these inhibitors can be extrapolated from the in vitro assays with which they were developed to intact cells. Therefore, we looked for an additional method to verify a requirement of PKC β during phagocytosis.
Figure 4. Rottlerin inhibits Mø phagocytosis of apoptotic thymocytes in a dose-dependent fashion.
Phagocytosis of apoptotic thymocytes by resident AMø and PMø was determined after 30 minutes incubation under the following conditions: control medium (light gray bars), 10 μM rottlerin (black bars), 40 μM rottlerin (white bars), and 100 μM rottlerin (dark gray bars). (A) percentage of phagocytic Mø; (B) phagocytic index. Data are mean ± SEM of 3 replicates and are representative of results of two experiments. *, p<0.05, ANOVA with post-hoc Dunnett's testing.
Figure 5. Ro-32-0432 inhibits Mø phagocytosis of apoptotic thymocytes in a dose-dependent fashion.
Phagocytosis of apoptotic thymocytes by resident AMø and PMø was determined after 30 minutes incubation under the following conditions: control medium (gray bars), 9 μM Ro-32-0432 (black bars), 28 μM Ro-32-043 (white bars). (A) percentage of phagocytic Mø; (B) phagocytic index. Control, gray bars;, black bars; 2, white bars. Data are mean ± SEM of 4 replicates in two experiments. *, p<0.05, ANOVA with post-hoc Dunnett's testing.
Myristylated PKC βII blocking peptide inhibits phagocytosis of apoptotic thymocytes by AMø and PMø
Because all data thus far implicated a cPKC as a necessary signaling component during phagocytosis of apoptotic thymocytes, we tested the effect of cell-permeable myristylated peptides derived from the carboxy terminus of the V5 region of PKC α, βI, or βII, on phagocytosis of apoptotic thymocytes. Peptides from this region were selected based on the following rationale. First, this region (specifically the last 13 amino acids) has been suggested to contribute to phosphatidylglycerol-induced activation of PKC βII (29), and via interaction with the C2 region, in calcium-and PS-mediated activation of PKC βII (42). Second, the V5 region of PKC α, specifically the QSAV sequence, has been shown to interact with PICK1, a PKC-binding protein that appears to target PKC α to appropriate intracellular sites for transduction of isozyme-specific signals (43). Third, the PKC βII peptide used in this study has previously been shown to inhibit the binding of a maltose-binding protein-PKC βII fusion protein to RACK 1 (34), confirming its functional activity. Fourth, the V5 region is the only site at which PKC βI and PKC βII amino acid sequences differ. Finally, these βI and βII peptides comprise part of the antigenic sequences used for development of the isozyme-specific antibodies used in this study.
We found that the myristylated PKC βII peptide significantly decreased phagocytosis of apoptotic thymocytes by both AMø and PMø, whereas the myristylated PKC α and PKC βI peptides had no effect (Fig. 6). These results provide direct evidence that PKC βII is required for Mø phagocytosis of apoptotic thymocytes.
Figure 6. Myristylated PKC βII blocking peptide specifically inhibits Mø phagocytosis of apoptotic thymocytes.
Phagocytosis of apoptotic thymocytes by resident AMø and PMø was determined after 30 minutes incubation in control medium (light gray bars) or in medium containing a final concentration of 100 μM in water of one of the following myristylated blocking peptides: PKC α peptide (black bars), PKC βI (white bars), PKC βII peptide (dark gray bars). (A) percentage of phagocytic Mø; (B) phagocytic index. Data are mean ± SEM of three replicates and are representative of two experiments.
PS liposomes stimulate translocation of PKC isoforms βI, βII, δ, ε, μ, and ζ to membrane and cytoskeleton fractions
Translocation from cytosol to membrane or cytoskeleton fractions upon activation is a major mechanism of PKC regulation (26,44), leading us to investigate translocation of individual PKC isoforms during the phagocytic process. However, preliminary experiments showed that the abundant expression of multiple PKC isoforms in viable murine thymocytes was retained upon induction of apoptosis 4, making use of apoptotic thymocytes for these studies infeasible. Instead, we substituted PS liposomes, which have been used previously as models for apoptotic cells (45,46). Liposomes clearance by AMø and PMø has been shown to require phagocytosis and not to result from membrane fusion (47,48), further supporting use of PS liposomes for these studies. Based on the practical consideration that our previous experiment showed that AMø express much lower amounts of multiple PKC isoforms (Fig. 1), we performed these experiments using only PMø. Time-points were selected based on our previous study of the kinetics of phagocytosis (10).
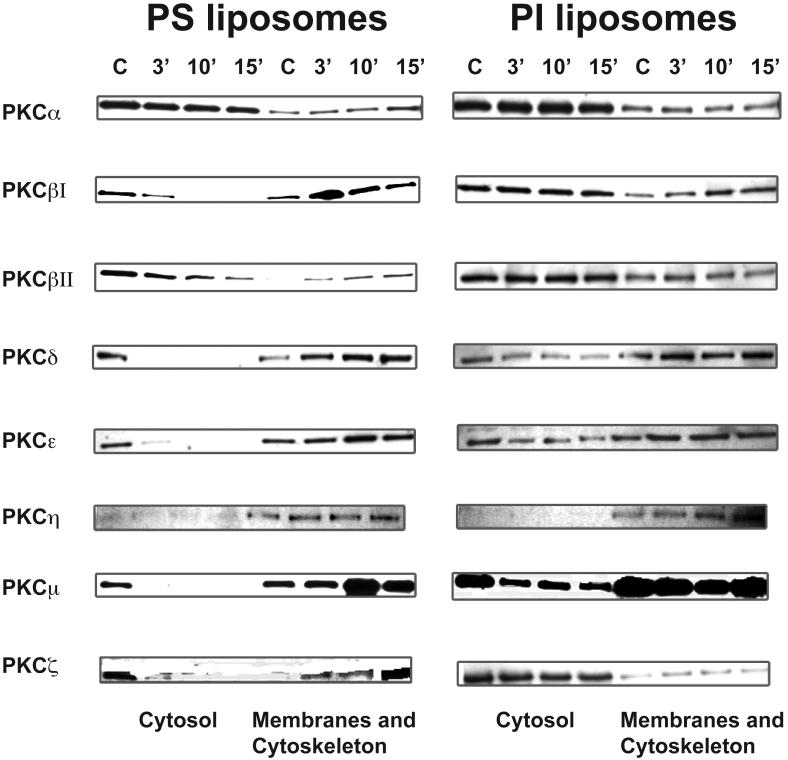
We found that PS liposomes strongly stimulated translocation of PKC βI, βII, δ, ε, μ, and ζ from the cytosol to the membrane and cytoskeleton fraction of PMø by 10 min, whereas control PI liposomes had no effect (Fig. 7). PS liposomes specifically stimulated slight translocation of PKC α, and PI liposomes also slightly stimulated translocation of PKC δ and ε, whereas neither type of liposome had significant effect on the localization of PKC η. Thus, several members of all three groups of PKC isoforms are specifically translocated in response to PS exposure.
Figure 7. PS liposomes induce translocation of multiple PKC isoforms into the membrane and cytoskeletal fractions.
Resident murine PMø were incubated for various times with liposomes consisting of L-α-PS (left-hand column) or control L-α-PI (right-hand column), and then expression of PKC isoforms in the cytosolic fraction and in the membrane & cytoskeletal fraction was analyzed by Western blotting. C, control (no liposomes).
Resident murine Mø express the stereo-specific PS-R', which mediates PKC translocation in response to PS liposomes
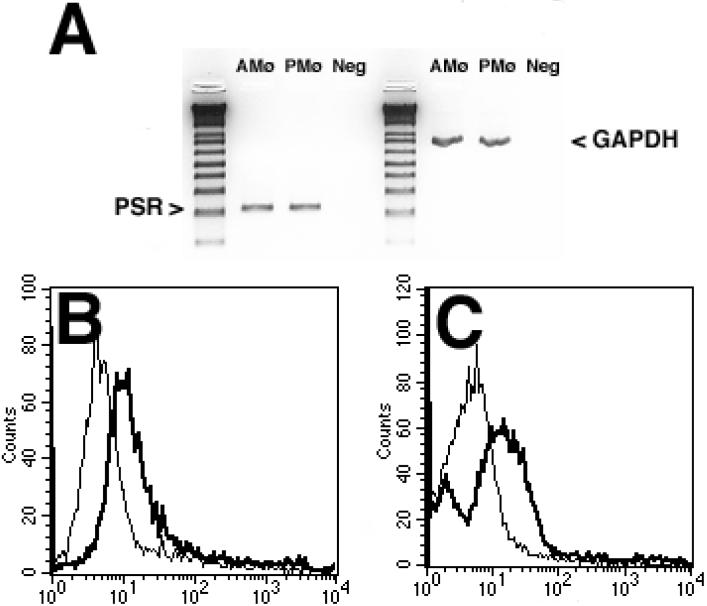
These data raised the question of whether the stereo-specific PS-R' identified by Fadok and associates could contribute directly to PKC activation during Mø recognition of apoptotic cells. Expression of PS-R' was detected by multiple methods in both types of Mø. RT-PCR demonstrated equivalent PS-R' mRNA in AMø and PMø (Fig. 8A). Flow cytometry showed distinct surface expression of PS-R' by both types of Mø (Fig. 8B & 8C). Although specific staining and background staining of both Mø types varied slightly between experiments, mean channel fluorescence of PMø was generally somewhat higher than that of AMø (e.g., in the experiment shown in Fig. 8, mean channel fluorescence: 484.7 for PMø versus 161.4 for AMø). Hence, absence of PS-R' expression by AMø did not appear to explain our previous finding of decreased phagocytosis of apoptotic cells (10).
Figure 8. Resident murine AMø & PMø express PS-R'.
A. RT-PCR analysis of mRNA. Equally loaded PCR reactions were normalized to achieve the same expression of the housekeeping gene GAPDH (right side of the figure), by adjusting the volume of the cDNA product used in each reaction. The black and white image was inverted after scanning. Neg is a negative control where no RT reaction was performed and aliquots of RNAs from both resident Mø were mixed. The reference ladder is 1 kb Plus. Results are representative of two experiments performed with identical outcomes. B, C. Flow cytometric analysis of surface protein expression. AMø & PMø from normal C57BL/6 mice were stained in suspension with mAb 217 culture-supernatant or with control IgM from a murine myeloma, with biotinylated goat antimouse IgM F(ab'2) and with streptavidin-phycoerythrin, and then analyzed by flow cytometry, counting 10,000 cells per condition. B, AMø; C, PMø. The narrow line depicts staining with isotype control and the dark line depicts staining with mAb 217. Representative histograms are shown; similar results were obtained in four independent experiments.
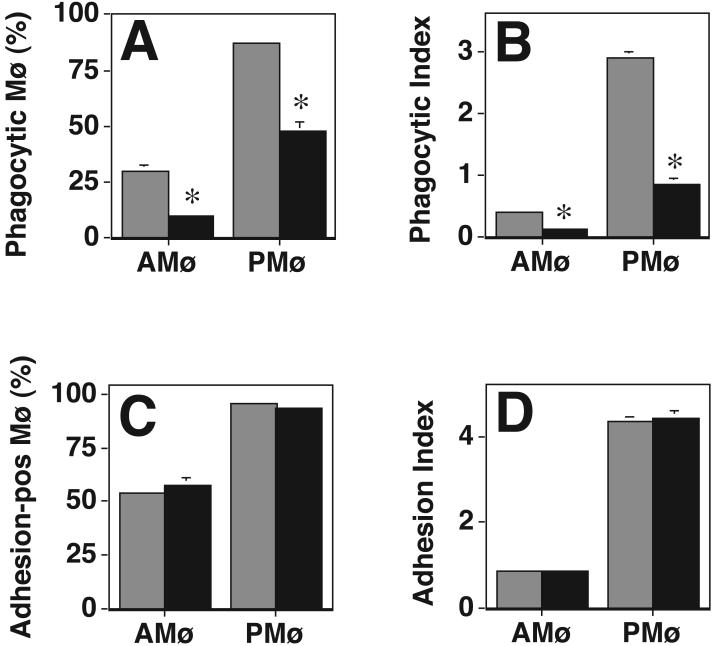
To determine whether PS-R' was functional on both types of Mø, we performed phagocytosis and adhesion assays in the presence of the blocking mAb 217. Blockade of PS-R' specifically and profoundly inhibited phagocytosis of apoptotic thymocytes (Fig. 9A & 9B), in agreement with previous data using this mAb on other cell types (12). However, the same concentration of mAb 217 had no effect on adhesion of apoptotic thymocytes to either type of Mø (Fig. 9C & 9D). These data confirm that both AMø and PMø express functional PS-R', but that this receptor does not mediate apoptotic cell adhesion.
Figure 9. mAb against PS-R' blocks Mø phagocytosis but not adhesion of apoptotic thymocytes.
Resident AMø and PMø from normal C57BL/6 mice were pre-treated with saturating amounts of control antibody (gray bars) or anti-PS-R' mAb 217 (black bars) and then phagocytosis or adhesion of apoptotic thymocytes was determined by examining H-E stained slides under oil immersion. A, B. Phagocytosis. Data are mean ± SEM of 3-5 replicates per condition in a single experiment; *, p<0.05, unpaired t-test. C, D. Adhesion. Data are mean ± SEM of 3-5 replicates per condition in a single experiment. Similar results were found in an additional experiment.
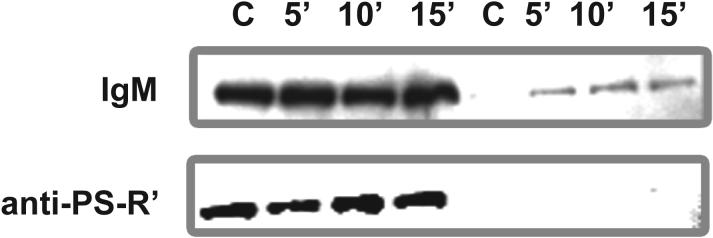
To determine whether stimulation via PS-R' induced PKC activation, we tested whether mAb 217 could inhibit PS liposome-induced translocation of PKC βII to particulate fractions. In the presence of control IgM, PS liposomes induced translocation of PKC βII from cytosolic fractions to membrane and cytoskeletal fractions (Fig. 10, top row), as anticipated from our previous results (Fig. 7). Such induced translocation was inhibited when PS-R' was blocked using mAb 217 (Fig. 10, bottom row). These results indicate that the induction of PKC βII translocation by PS liposomes, and by implication, by apoptotic cells, is mediated through PS-R'. Because mAb 217 itself has been shown to have agonist activity as indicated by its ability to stimulate TGF-β expression (12), in separate experiments, we tested whether this mAb in the absence of PS liposomes could induce PKC translocation. No induction of PKC translocation was observed for PKC βII or any of the other seven isoforms tested 4. Thus, the effects shown in Figure 10 result from inhibition of PS-induced translocation mediated via PS-R', and not from agonist activity of mAb 217 itself.
Figure 10. Antibody against PS-R' inhibits PKC βII translocation induced by PS liposomes.
Resident PMø were incubated with saturating amounts of control rabbit IgM (top row) or anti-PS-R' mAb 217 (bottom row), exposed to PS-liposomes to a final concentration of 0.11 mM for up to 15 min at 37° C and 5% CO2 , and then PKC βII expression in cytosolic versus membrane and cytoskeletal fractions was assayed by Western analysis. C, control (no liposomes).
DISCUSSION
The principal findings of this study indicate that PKC βII is required for phagocytosis of apoptotic cells by murine tissue Mø, and that the activation of this PKC isozyme in response to PS liposomes is mediated by PS-R'. These conclusions are based on a consistent body of evidence, including the effects on phagocytosis of PKC depletion by overnight PMA treatment, dose-specific inhibition using rottlerin and Ro-32-0432, and a specific myristylated blocking peptide corresponding to the carboxy-terminus of PKC βII, as well as by inhibition of PS-induced translocation of PKC βII when PS-R' was blocked. Because adhesion of apoptotic thymocytes to Mø was not decreased by the PKC inhibitor staurosporine (11) or by overnight PMA treatment, the inhibition of phagocytosis seen in this study appears to result from recognition events following binding. Our results also showed that exposure to PS liposomes induced translocation of several other PKC isoforms (βI, δ, ε, μ, and ζ, and to a lesser degree α) to membrane and cytoskeletal fractions. However, based on results of the inhibitor and PMA studies, translocation of these latter isoforms appears to be unnecessary for phagocytosis. Furthermore, our results indicated that the stereo-specific PS-R' did not mediate adhesion of apoptotic cells, consistent with its predicted short extracellular domain. Finally, Western blot analysis showed that murine AMø have markedly lower expression of PKC βII and of multiple other PKC isoforms (α, βI, δ, ε, μ, and ζ), providing a partial explanation for the previously demonstrated relative deficit in apoptotic cell phagocytosis by that cell type (10). These novel findings advance the understanding of signal transduction during Mø recognition and ingestion of apoptotic cells.
The finding that PKC βII is necessary for Mø phagocytosis of apoptotic cells is important because it differs from previously defined requisites for Mø phagocytosis of other types of particles. Allen and Aderem found that during ingestion of zymosan by LPS-stimulated PMø, PKC α and myristylated, alanine-rich C-kinase substrate (MARCKS) co-localized with F-actin and talin adjacent to nascent phagocytic cups (49). During FcλR-mediated phagocytosis, PKC α (50), β (51), λ (52), δ (53), and ε (50) have all been shown to localize to the phagosome membrane, with the specific PKC isoform recruited dependent on the state of Mø differentiation and the exact FcgR involved (52). However, because PKC co-localization may be a consequence rather than a necessary process, other groups have investigated blockade of PKC function. Overexpression of a dominant-negative mutant of PKC α in the murine Mø cell line RAW 264.7 reduced phagocytosis of IgG-opsonized sheep red blood cells (54), but not phagocytosis of Leishmania donovani promastigotes (55). Although the former finding was interpreted to indicate a requirement for PKC a in FcλR-mediated phagocytosis, such observations must be interpreted with caution, because overexpression of one PKC isozyme can alter the levels of other PKC isoforms (56). By contrast, using a combination of confocal microscopy, various inhibitors, and biochemical evidence of PKC translocation in RAW 264.7 cells, Larsen and coworkers found that PKC α was needed for FcλR-stimulated respiratory burst, but that only PKC δ and ε were necessary for FcgR-mediated phagocytosis itself (50). PKC βII and its anchoring protein RACK1 have been found to be up-regulated in rat AMø upon maturation of functional responses such as TNF-α or hydrogen peroxide production, and lysozyme release (57). Significantly, however, none of these previous studies have confirmed a role for PKC βII in Mø phagocytosis of other particles.
PKC βI and βII are products of alternative mRNA splicing of a single gene; they are identical for the first 621 amino acids and differ only in their carboxyl-terminal 50-52 amino acids. The myristylated blocking peptides we used correspond to this area of difference, and therefore were specific. The absence of effect of myristylatedβIpeptide on phagocytosis agrees with previous reports that these isoforms have unique functions (28). Early studies found that although both isoforms translocate to membranes in response to short term PMA exposure, only PKC βII is an actin-binding protein that translocates to the actin-based cytoskeleton (58). However, recent studies have reached conflicting conclusions, showing either that PKC βII does not co-distribute with actin-based cytoskeleton upon PMA treatment (59), or that PKCβIas well as other PKC isoforms bind to and are activated by F-actin (60,61).
That phagocytosis of apoptotic targets might involve unique signal transduction elements should not be surprising. In contrast to the pro-inflammatory responses activated by phagocytosis via other pathways (1), phagocytosis of apoptotic cells is continuous during development, and anti-phlogistic during resolving inflammation (62-65). The finding that murine thioglycollate-elicited PMø secreted MIP-2/CXCL2 on ingestion of apoptotic T cells has been interpreted as a contradiction to this principle (66), but an alternative view would be that production of this chemokine in the absence of other pro-inflammatory signals simply recruits Mø as part of the beneficial clean-up process. Ingestion of apoptotic T cells has been found to compromise Mø host defense functions in vivo (67). Hence, we speculate that the relatively deficient phagocytosis of apoptotic leukocytes by AMø may be a beneficial evolutionary adaptation. Even moderate suppression of chemokine and monokine secretion in the lungs might tip the balance in favor of virulent pathogens such as enteric gram negative bacteria and S. aureus, or may be sufficient to permit evasion of innate immunity by less virulent pathogens such as anaerobes.
Our current data showing that both murine AMø and PMø respond to PMA agrees with the preponderance of results in other systems. Heale and Speert found that PMA can reverse the inability of murine AMø to ingest P. aeruginosa(68). Although Peters-Golden and coworkers initially reported that PMA caused release of arachidonic acid in a PKC-dependent manner in rat PMø but not AMø (69), a more recent paper from that group showed that the augmentation of FcλR-mediated phagocytosis in rat AMø by leukotrienes is PKC-dependent, indicating that AMø can respond to PKC stimulatory molecules (70).
Our analyses of PS-R' advance the understanding of this receptor's role in the process of apoptotic cell clearance. The gene for this receptor has been conserved with exceedingly high fidelity across greater than 600 million years of evolution between organisms as disparate as C. elegans, D. melanogaster, and mammals (12). This conservation and the fact that it was not previously identified by genetic analysis of C. elegans mutants (which suggests that its deletion may be lethal) speaks to its fundamental importance. Because we show that mAb 217 does not block adhesion, the PS-R' function must depend on initial adhesion via other receptors. Thus, it is a recognition molecule that imparts specificity to the interaction. We cannot at present exclude the possibility that only those AMø that express high amounts of surface PS-R' participate in phagocytosis. However, the fact that only roughly 40% of PMø showed significant staining, yet virtually all PMø ingest apoptotic cells in an annexin V-inhibitable fashion (10), urges caution in interpretation of flow cytometry data using mAb 217. The high background staining seen using this IgM mAb precludes accurate definition of the lower limit of expression, and even small amounts of PS-R' may be sufficient. In addition to the stereo-specific PS-R', PS can be bound by multiple receptors including class B scavenger receptors, CD14, macrosialin (CD68) and thrombospondin-dependent vitronectin receptors (1,71-73).
The demonstration that resident murine AMø express lesser amounts of multiple PKC isoforms, compared to resident Mø at another body surface, further highlights the distinctive differentiation of this cell type (74,75). AMø appear to have evolved as specialized phagocytes whose principal task is to keep the gas exchanging surface of the alveoli clear, yet not compromised by excessive inflammation. AMø have a mostly suppressive role on the induction of adaptive immune responses within the lung itself (76,77), permitting local expression of T cell effector functions while inhibiting their proliferation (78). Indeed, AMø differ from other Mø types in expression of surface receptors (79,80), in antigen-presenting capacity (81), and in eicosanoid production (82,83). Our results agree with previous studies that found markedly lower expression of multiple PKC isoforms, including PKC βII, in normal human or rat AMø compared with monocytes or other Mø (37,69). Interestingly, we have also found that, like murine AMø, the murine Mø cell lines RAW 264.7 and IC21 also have markedly reduced expression of PKC βII (but not PKC α or βI), which correlates with their greatly reduced phagocytosis (but not adhesion) of apoptotic thymocytes relative to PMø4. Significantly, the fact that Monick and coworkers found the greatest difference in PKC isoform expression between human AMø and blood monocytes to be a reduction in PKC βII in AMø (37) implies that our findings can be extrapolated to normal human AMø, which we have recently found also have relatively depressed phagocytosis of apoptotic lymphocytes4.
In summary, these data demonstrate for the first time that Mø phagocytosis of apoptotic cells requires PKC βII, an isoform not shown previously to be required for phagocytosis of other types of particles. This unique PKC isoform requirement may be an important clue to the signaling pathways that mediate suppression of Mø production of pro-inflammatory mediators on ingestion of apoptotic cells (64,65). Activation of PKC βII by PS-R' provides a partial explanation for the essential role of this receptor in apoptotic cell ingestion.
Acknowledgments
Supported by RO1 HL56309 and RO-1 HL6157 from the USPHS; and by Merit Review funding and a Research Enhancement Award Program (REAP) grant from the Department of Veterans Affairs.
We thank Dr. Lilli Petruzzelli and all the members of the Ann Arbor VAMC REAP for helpful suggestions, Dr. Valerie Fadok for the generous gift of mAb 217, and Joyce O'Brien for secretarial support.
Footnotes
Portions of this work have been presented previously at the Autumn Immunology Conference, Chicago, IL, November 17, 2001, and at the American Thoracic Society Meeting in Atlanta, GA, May 22, 2002.
Unpublished observation.
Abbreviations used are: AMø, alveolar macrophages; aPKC, atypical PKC isoform; BAL, bronchoalveolar lavage; cPKC, conventional PKC isoform; DAG, diacylglycerol; DMSO, dimethyl sulfoxide; Ig, immunoglobulin; mAb, monoclonal antibody; Mø, macrophage; nPKC, novel PKC isoform; PI, phosphatidylinositol; PKA, cAMP-dependent protein kinase; PKC, protein kinase C; PL, peritoneal lavage; PMø, peritoneal macrophages; PMA, phorbol 12-myristate 12-acetate; PS, phosphatidylserine; RACKs, receptors for activated C kinaes.
References
- 1.Aderem A, Underhill DM. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 2.Fadok VA, Chimini G. Semin Immunol. 2001;13:365–372. doi: 10.1006/smim.2001.0333. [DOI] [PubMed] [Google Scholar]
- 3.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akbar AN, Savill J, Gombert W, Bofill M, Borthwick NJ, Whitelaw F, Grundy J, Janossy G, Salmon M. J Exp Med. 1994;180:1943–1947. doi: 10.1084/jem.180.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern M, Savill J, Haslett C. Am J Pathol. 1996;149:911–921. [PMC free article] [PubMed] [Google Scholar]
- 6.Haslett C. Am J Respir Crit Care Med. 1999;160:S5–11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 7.Surh CD, Sprent J. Nature. 1994;372:100–103. [PubMed] [Google Scholar]
- 8.Milik AM, Buechner-Maxwell VA, Sonstein J, Kim S, Seitzman GD, Beals TF, Curtis JL. J Clin Invest. 1997;99:1082–1091. doi: 10.1172/JCI119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman SL, Henson JE, Henson PM. J Exp Med. 1982;156:430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu B, Sonstein J, Christensen PJ, Punturieri A, Curtis JL. J Immunol. 2000;165:2124–2133. doi: 10.4049/jimmunol.165.4.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu B, Punturieri A, Todt J, Sonstein J, Polak T, Curtis JL. J Leukoc Biol. 2002;71:881–889. [PMC free article] [PubMed] [Google Scholar]
- 12.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 13.Verhoven B, Krahling S, Schlegel RA, Williamson P. Cell Death Differ. 1999;6:262–270. doi: 10.1038/sj.cdd.4400491. [DOI] [PubMed] [Google Scholar]
- 14.Schlegel RA, Stevens M, Lumley-Sapanski K, Williamson P. Immunol Lett. 1993;36:283–288. doi: 10.1016/0165-2478(93)90101-7. [DOI] [PubMed] [Google Scholar]
- 15.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 16.Homburg CH, de Haas M, von dem Borne AE, Verhoeven AJ, Reutelingsperger CP, Roos D. Blood. 1995;85:532–540. [PubMed] [Google Scholar]
- 17.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. J Biol Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 19.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 20.Gregory CD. Curr Opin Immunol. 2000;12:27–34. doi: 10.1016/s0952-7915(99)00047-3. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platt N, da Silva RP, Gordon S. Immunol Lett. 1999;65:15–19. doi: 10.1016/s0165-2478(98)00118-7. [DOI] [PubMed] [Google Scholar]
- 23.Marguet D, Luciani MF, Moynault A, Williamson P, Chimini G. Nat Cell Biol. 1999;1:454–456. doi: 10.1038/15690. [DOI] [PubMed] [Google Scholar]
- 24.Webb BL, Hirst SJ, Giembycz MA. Br J Pharmacol. 2000;130:1433–1452. doi: 10.1038/sj.bjp.0703452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newton AC. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 26.Mochly-Rosen D, Gordon AS. FASEB J. 1998;12:35–42. [PubMed] [Google Scholar]
- 27.Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 28.Chalfant CE, Ohno S, Konno Y, Fisher AA, Bisnauth LD, Watson JE, Cooper DR. Mol Endocrinol. 1996;10:1273–1281. doi: 10.1210/mend.10.10.9121494. [DOI] [PubMed] [Google Scholar]
- 29.Gokmen-Polar Y, Fields AP. J Biol Chem. 1998;273:20261–20266. doi: 10.1074/jbc.273.32.20261. [DOI] [PubMed] [Google Scholar]
- 30.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 31.Keenan C, Goode N, Pears C. FEBS Lett. 1997;415:101–108. doi: 10.1016/s0014-5793(97)01104-6. [DOI] [PubMed] [Google Scholar]
- 32.Way KJ, Chou E, King GL. Trends Pharmacol Sci. 2000;21:181–187. doi: 10.1016/s0165-6147(00)01468-1. [DOI] [PubMed] [Google Scholar]
- 33.Curtis JL, Kim S, Scott PJ, Buechner-Maxwell VA. Am J Respir Cell Mol Biol. 1995;12:520–530. doi: 10.1165/ajrcmb.12.5.7537969. [DOI] [PubMed] [Google Scholar]
- 34.Stebbins EG, Mochly-Rosen D. J Biol Chem. 2001;276:29644–29650. doi: 10.1074/jbc.M101044200. [DOI] [PubMed] [Google Scholar]
- 35.Zar JH. Biostatistical analysis. 2nd Ed. Prentice-Hall; Englewood Cliffs: 1974. [Google Scholar]
- 36.Licht R, Jacobs CW, Tax WJ, Berden JH. J Immunol Methods. 1999;223:237–248. doi: 10.1016/s0022-1759(98)00212-9. [DOI] [PubMed] [Google Scholar]
- 37.Monick MM, Carter AB, Gudmundsson G, Geist LJ, Hunninghake GW. Am J Physiol. 1998;275:L389–397. doi: 10.1152/ajplung.1998.275.2.L389. [DOI] [PubMed] [Google Scholar]
- 38.Nixon JB, McPhail LC. J Immunol. 1999;163:4574–4582. [PubMed] [Google Scholar]
- 39.Ahnadi CE, Giguere P, Gravel S, Gagne D, Goulet AC, Fulop T, Jr., Payet MD, Dupuis G. J Leukoc Biol. 2000;68:293–300. [PubMed] [Google Scholar]
- 40.Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson SE, Parker PJ, Nixon JS. Biochem J. 1993;294:335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keranen LM, Newton AC. J Biol Chem. 1997;272:25959–25967. doi: 10.1074/jbc.272.41.25959. [DOI] [PubMed] [Google Scholar]
- 43.Staudinger J, Lu J, Olson EN. J Biol Chem. 1997;272:32019–32024. doi: 10.1074/jbc.272.51.32019. [DOI] [PubMed] [Google Scholar]
- 44.Mochly-Rosen D, Kauvar LM. Semin Immunol. 2000;12:55–61. doi: 10.1006/smim.2000.0207. [DOI] [PubMed] [Google Scholar]
- 45.Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM. J Immunol. 1992;149:4029–4035. [PubMed] [Google Scholar]
- 46.Tait JF, Smith C. J Biol Chem. 1999;274:3048–3054. doi: 10.1074/jbc.274.5.3048. [DOI] [PubMed] [Google Scholar]
- 47.Raz A, Bucana C, Fogler WE, Poste G, Fidler IJ. Cancer Res. 1981;41:487–494. [PubMed] [Google Scholar]
- 48.Perry DG, Martin WJ., 2nd J Immunol Methods. 1995;181:269–285. doi: 10.1016/0022-1759(95)00011-x. [DOI] [PubMed] [Google Scholar]
- 49.Allen LH, Aderem A. J Exp Med. 1995;182:829–840. doi: 10.1084/jem.182.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larsen EC, DiGennaro JA, Saito N, Mehta S, Loegering DJ, Mazurkiewicz JE, Lennartz MR. J Immunol. 2000;165:2809–2817. doi: 10.4049/jimmunol.165.5.2809. [DOI] [PubMed] [Google Scholar]
- 51.Dekker LV, Leitges M, Altschuler G, Mistry N, McDermott A, Roes J, Segal AW. Biochem J. 2000;347:285–289. [PMC free article] [PubMed] [Google Scholar]
- 52.Melendez AJ, Harnett MM, Allen JM. Immunology. 1999;96:457–464. doi: 10.1046/j.1365-2567.1999.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brumell JH, Howard JC, Craig K, Grinstein S, Schreiber AD, Tyers M. J Immunol. 1999;163:3388–3395. [PubMed] [Google Scholar]
- 54.Breton A, Descoteaux A. Biochem Biophys Res Commun. 2000;276:472–476. doi: 10.1006/bbrc.2000.3511. [DOI] [PubMed] [Google Scholar]
- 55.St-Denis A, Caouras V, Gervais F, Descoteaux A. J Immunol. 1999;163:5505–5511. [PubMed] [Google Scholar]
- 56.Ways DK, Kukoly CA, deVente J, Hooker JL, Bryant WO, Posekany KJ, Fletcher DJ, Cook PP, Parker PJ. J Clin Invest. 1995;95:1906–1915. doi: 10.1172/JCI117872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corsini E, Viviani B, Lucchi L, Marinovich M, Racchi M, Galli CL. Immunol Lett. 2001;76:89–93. doi: 10.1016/s0165-2478(00)00327-8. [DOI] [PubMed] [Google Scholar]
- 58.Blobe GC, Stribling DS, Fabbro D, Stabel S, Hannun YA. J Biol Chem. 1996;271:15823–15830. doi: 10.1074/jbc.271.26.15823. [DOI] [PubMed] [Google Scholar]
- 59.Edwards AS, Faux MC, Scott JD, Newton AC. J Biol Chem. 1999;274:6461–6468. doi: 10.1074/jbc.274.10.6461. [DOI] [PubMed] [Google Scholar]
- 60.Slater SJ, Milano SK, Stagliano BA, Gergich KJ, Curry JP, Taddeo FJ, Stubbs CD. Biochemistry. 2000;39:271–280. doi: 10.1021/bi9916527. [DOI] [PubMed] [Google Scholar]
- 61.Lopez-Lluch G, Bird MM, Canas B, Godovac-Zimmerman J, Ridley A, Segal AW, Dekker LV. Biochem J. 2001;357:39–47. doi: 10.1042/0264-6021:3570039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meagher LC, Savill JS, Baker A, Fuller RW, Haslett C. J Leukoc Biol. 1992;52:269–273. [PubMed] [Google Scholar]
- 63.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 64.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDonald PP, Fadok VA, Bratton D, Henson PM. J Immunol. 1999;163:6164–6172. [PubMed] [Google Scholar]
- 66.Uchimura E, Kodaira T, Kurosaka K, Yang D, Watanabe N, Kobayashi Y. Biochem Biophys Res Commun. 1997;239:799–803. doi: 10.1006/bbrc.1997.7556. [DOI] [PubMed] [Google Scholar]
- 67.Freire-de-Lima CG, Nacimento DO, Soares MBP, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 68.Heale JP, Speert DP. J Leukoc Biol. 2001;69:158–160. [PubMed] [Google Scholar]
- 69.Peters-Golden M, McNish RW, Sporn PH, Balazovich K. Am J Physiol. 1991;261:L462–471. doi: 10.1152/ajplung.1991.261.6.L462. [DOI] [PubMed] [Google Scholar]
- 70.Mancuso P, Peters-Golden M. Am J Respir Cell Mol Biol. 2000;23:727–733. doi: 10.1165/ajrcmb.23.6.4246. [DOI] [PubMed] [Google Scholar]
- 71.Savill J, Fadok V, Henson P, Haslett C. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 72.Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 73.Ramprasad MP, Fischer W, Witztum JL, Sambrano GR, Quehenberger O, Steinberg D. Proc Natl Acad Sci U S A. 1995;92:9580–9584. doi: 10.1073/pnas.92.21.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lipscomb MF, Russell SW, Lenfent C, editors. Lung macrophages and dendritic cells in health and disease. Vol. 107. Marcel Dekker; New York: 1997. Lung biology in health and disease. [Google Scholar]
- 75.Brody AR. J Lab Clin Med. 1998;131:391–392. doi: 10.1016/s0022-2143(98)90138-x. [DOI] [PubMed] [Google Scholar]
- 76.Thepen T, Van Rooijen N, Kraal G. J Exp Med. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, Thepen T. J Exp Med. 1993;177:397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Upham JW, Strickland DH, Bilyk N, Robinson BWS, Holt PG. Immunology. 1995;84:142–147. [PMC free article] [PubMed] [Google Scholar]
- 79.Hance AJ, Douches S, Winchester RJ, Ferrans VJ, Crystal RG. J Immunol. 1985;134:284–292. [PubMed] [Google Scholar]
- 80.Yoshikawa K, Suzuki Y, Kawai M, Fukada M, Yokochi T. Microbiol Immunol. 1991;35:803–807. doi: 10.1111/j.1348-0421.1991.tb01613.x. [DOI] [PubMed] [Google Scholar]
- 81.Lipscomb MF, Lyons CR, Nunez G, Ball EJ, Stanstny P, Vial W, Lem V, Weissler JC, Miller LM, Toews GB. J Immunol. 1986;136:497–504. [PubMed] [Google Scholar]
- 82.Bigby TD, Holtzman MJ. J Immunol. 1987;138:1546–1550. [PubMed] [Google Scholar]
- 83.Balter MS, Toews GB, Peters-Golden M. J Immunol. 1989;142:602–608. [PubMed] [Google Scholar]