Abstract
In studies of the outstanding salt tolerance of the unicellular green alga Dunaliella salina, we isolated a cDNA for a salt-inducible mRNA encoding a protein homologous to plant β-ketoacyl-coenzyme A (CoA) synthases (Kcs). These microsomal enzymes catalyze the condensation of malonyl-CoA with acyl-CoA, the first and rate-limiting step in fatty acid elongation. Kcs activity, localized to a D. salina microsomal fraction, increased in cells transferred from 0.5 to 3.5 m NaCl, as did the level of the kcs mRNA. The function of the kcs gene product was directly demonstrated by the condensing activity exhibited by Escherichia coli cells expressing the kcs cDNA. The effect of salinity on kcs expression in D. salina suggested the possibility that salt adaptation entailed modifications in the fatty acid composition of algal membranes. Lipid analyses indicated that microsomes, but not plasma membranes or thylakoids, from cells grown in 3.5 m NaCl contained a considerably higher ratio of C18 (mostly unsaturated) to C16 (mostly saturated) fatty acids compared with cells grown in 0.5 m salt. Thus, the salt-inducible Kcs, jointly with fatty acid desaturases, may play a role in adapting intracellular membrane compartments to function in the high internal glycerol concentrations balancing the external osmotic pressure.
Unicellular green algae of the genus Dunaliella are exceptional in the plant kingdom in their ability to proliferate over practically the entire range of salinities. The algae grow in media with NaCl concentrations ranging from <0.1 m to near saturation, while maintaining a low intracellular ionic concentration (Avron, 1986). Its outstanding salt tolerance makes the genus Dunaliella an intriguing model to identify and characterize mechanisms underlying this capacity. Mechanisms of salt tolerance of plants (Hasegawa et al., 2000) and model systems such as yeast (Saccharomyces cerevisiae; Serrano, 1996, 1999) are now vigorously pursued. Major aspects addressed include the osmotic regulation by osmolytes, control of water, and ion fluxes and underlying signaling pathways.
Dunaliella salina, devoid of a rigid cell wall, overcomes external osmotic variations by adjusting intracellular levels of glycerol to concentrations balancing the external osmotic pressure. Therefore, the cells maintain a constant volume independent of the external salinity (Avron, 1986; Sadka et al., 1991). Mechanisms governing ionic homeostasis and other aspects of salt tolerance of D. salina still remain largely unknown.
Identification of genes/proteins of D. salina that are preferentially expressed/accumulated under high salinities have previously led us to unravel novel components contributing to salt tolerance. These include two plasma membrane proteins implicated in alleviating salt-imposed limitations on the availability of CO2 (Fisher et al., 1996) or iron (Fisher et al., 1997, 1998). Furthermore, these extracellularly exposed proteins are unique in remaining active over a very broad range of salinities in contrast to their mesophilic homologs (Fisher et al., 1996). The present study sheds light on still a different aspect of salt tolerance. A cloned cDNA for a salt-inducible gene was found to encode the microsomal enzyme β-ketoacyl-CoA synthase (Kcs) that catalyzes the condensation of acyl-CoAs with malonyl-CoA to yield β-ketoacyl-CoA and CO2. This reaction is the first and rate-limiting of four reactions leading to fatty acid elongation by sequential addition of C2 units to acyls of at least C12 (Lessire et al., 1989; Millar and Kunst, 1997). The primary substrates for elongation are the products of de novo fatty acid biosynthesis in the plastid that employs the acyl carrier protein, rather than CoA, as acyl carrier.
Cloning of plant kcs genes was initially based on directed transposon tagging in Arabidopsis (James et al., 1995) and partial amino acid sequencing of a protein from developing embryos of jojoba (Simmondsia chinensis; Lassner et al., 1996). Additional plant genes belonging to this family were identified on the basis of sequence homology or as transposon insertion sites in tagged mutants (Fourman et al., 1998; Millar et al., 1998; Yephremov et al., 1999; Priutt et al., 2000). Functionally characterized plant kcs genes include genes expressed in seeds and active in the biosynthesis of storage lipids or waxes (Lassner et al., 1996), as well as genes expressed in vegetative tissues and involved in the biosynthesis of cuticular waxes (Millar et al., 1999; Todd et al., 1999). The D. salina Kcs, identified in the context of algal salt responses, is most likely involved in membrane lipid biosynthesis. Specifically, this enzyme may play a role in salt-related changes of microsomal fatty acid composition.
RESULTS
Cloning of a Salt-Inducible Kcs from D. salina
Cells of D. salina transferred from 0.5 to 3.5 m NaCl cease to divide and suffer rapid water loss causing the cells to shrink. Subsequent rapid accumulation of glycerol, to a level osmotically balancing the external salinity, allows the cells to regain their original volume already within approximately 2 h, but cell proliferation resumes only approximately 10 h after the osmotic shock (Sadka et al., 1989; Fisher et al., 1994, 1997). Based on the expectation that genes involved in salt tolerance are preferentially expressed at this time, a cDNA library for poly(A+) mRNAs was constructed for D. salina cells 9 h after their transfer from 0.5 to 3.5 m NaCl (Fisher et al., 1996). The isolation from this library of a cDNA for a novel salt-inducible gene, cloning of its full length, as well as most of the corresponding genomic sequence are described in “Materials and Methods.”
Sequence determination indicated that the cDNA included an open reading frame for a 621-amino acid protein of Mr 69,867 with a predicted pI of 9.26 (Fig. 1; GenBank accession no. AF333040). The nucleotides flanking the assigned initiation codon agree with the core consensus sequence AGNATGNC for translation initiation in plants and animals (Lutcke et al., 1987). Furthermore, an in-frame termination codon is located upstream of the assigned initiation codon (data not shown).
Figure 1.
Sequence alignment of Kcs from D. salina and plant Kcs/Fae proteins. Protein sequences were aligned by the use of the PILEUP program (Genetics Computer Group, Madison, WI). GenBank accession numbers for the aligned proteins are: Fae from Brassica napus, U50771; Fae1 from Arabidopsis, U29142; Kcs from Simmondsia chinesis, U37088; Cut1 from Arabidopsis, AF129511 (recently, CUT1 was shown to be identical to CER6, a gene critical for Arabidopsis pollination; Fiebig et al., 2000); Kcs1 from Arabidopsis, AF053345; FDH from Arabidopsis, ATH010713; and Kcs2 from D. salina (denoted elsewhere in this paper as Kcs), AF333040. Black circles, Conserved Cys; white circles, conserved Cys with one exception.
The predicted product of the cloned cDNA belonged to the family of plant β-ketoacyl CoA synthases that act in fatty acid elongation in embryos or vegetative tissues. The sequences included in the alignment (Fig. 1) are of proteins for which Kcs activity has been demonstrated directly or inferred from phenotypes of mutant or transformed plants. The close similarity between the algal and plant proteins extends along most of their length, including the two predicted membrane-spanning domains (Millar et al., 1999), corresponding to residues 111 through 132 and 153 through 175.
However, the predicted algal protein is outstanding in its extended N-terminal sequence: 52 amino acids longer than FDH, the next in N-terminal extension length. N-terminal sequences have not been directly determined yet for any of the mature Kcs proteins. However, results of partial amino acid sequencing of peptides derived from the jojoba Kcs were in keeping with the absence of a cleavable leader peptide of meaningful length (Lassner et al., 1996). Nonetheless, as shown below, the first 46 N-terminal residues are not essential for enzymatic activity. Another difference noted is that only four of the six Cys residues conserved in other Kcs proteins (Todd et al., 1999) are present in the algal Kcs. Recently reported mutagenesis experiments indicated that only a single Cys residue (residue 223 in the Arabidopsis FAE1) was essential for activity (Ghanevati and Jaworski, 2001).
Genomic sequences overlapping most of the cDNA sequence were determined, indicating that the coding sequence is interrupted by at least nine introns (data not shown). Most plant kcs genes characterized so far lack introns. Only the FDH gene from Arabidopsis has been shown to include two introns, one of which is positioned close to an intron insertion site (codon 193) in D. salina (Yephremov et al., 1999).
Kcs Activity in D. salina
To further study the algal kcs, D. salina cells were fractionated and assayed to localize Kcs enzymatic activity. A lysate of cells grown in 3.5 m NaCl was first spun to separate chloroplasts, and the supernatant was further resolved into soluble and mixed membrane fractions (fraction 1). Centrifugation of fraction 1 through a glycerol gradient yielded purified plasma membranes (fraction 2). Analyses of fractions 1 and 2 (Table I) for the microsomal marker NADPH cytochrome c reductase (Peeler et al., 1989) and for chlorophyll, a thylakoid membrane marker, indicated that fraction 1, but not fraction 2, contained microsomal membranes and that both fractions were largely free of thylakoid membranes. Together with the detection of the D. salina plasma membrane proteins Dca (Fisher et al., 1996) and TTf (Fisher et al., 1997) in both fractions 1 and 2 (data not shown), these results led to the conclusion that fraction 1 contained both microsomes and plasma membranes, whereas fraction 2 contained microsome-free plasma membranes. Attempts to obtain a microsomal fraction free of plasma membranes in reasonable yield were unsuccessful.
Table I.
Characterization of two membrane fractions from D. salina
| NADPH Cytochrome c Reductase | Chlorophyll | Kcs Activity | |
|---|---|---|---|
| units | μg mg protein−1 | units | |
| Fraction 1 | 2.03 | 0.05 | 37.6 |
| Fraction 2 | 0.002 | nda | 3.6 |
Cell growth and isolation of fractions 1 and 2 were as described in “Materials and Methods.” Assay of antimycin A-insensitive NADPH CytC reductase and unit definition were as described (Peeler et al., 1989). Chlorophyll was determined spectroscopically. The characterization and assay of Kcs activity [with 30 μg of protein and acyl(18:1)-CoA as the acceptor substrates] are described in “Results” and “Materials and Methods.”
nd, Not detected.
Analyses for Kcs activity (Table I), using the assay detailed in “Materials and Methods” and characterized in following experiments, revealed significant Kcs activity only in fraction 1, and not in fraction 2. No activity was detected in other cell fractions (data not shown). Thus, Kcs activity was localized to the microsomal fraction, similar to its localization in higher plants.
The Kcs activity of fraction 1, first characterized for dependence on enzyme concentration and time course of the reaction (data not shown), was analyzed for its dependence on added acyl-CoA (Fig. 2A). The results clearly demonstrate the dependence of the reaction on acyl(18:1)-CoA, up to 100 μm, with only a low background noted in the absence of substrate. Thus, the added acyl-CoA, rather than an endogenous acceptor, served as the predominant substrate for condensation with malonyl-CoA. Assays of the condensing activity with several different acyl-CoAs, at 100 μm, were performed to evaluate the substrate preferences of the algal Kcs (Fig. 2B). The results, reflecting relative substrate preferences but not precise affinities, indicated that saturated acyls were preferred (in the order C14:0 < C16:0 > C18:0) over the mono-unsaturated acyls C16:1 and C18:1. As shown below (Fig. 3), the relative efficiencies of the least and maximally effective substrates tested, i.e. acyl(18:1) and acyl(16:0), respectively, were essentially retained over a broad range of enzyme concentrations.
Figure 2.
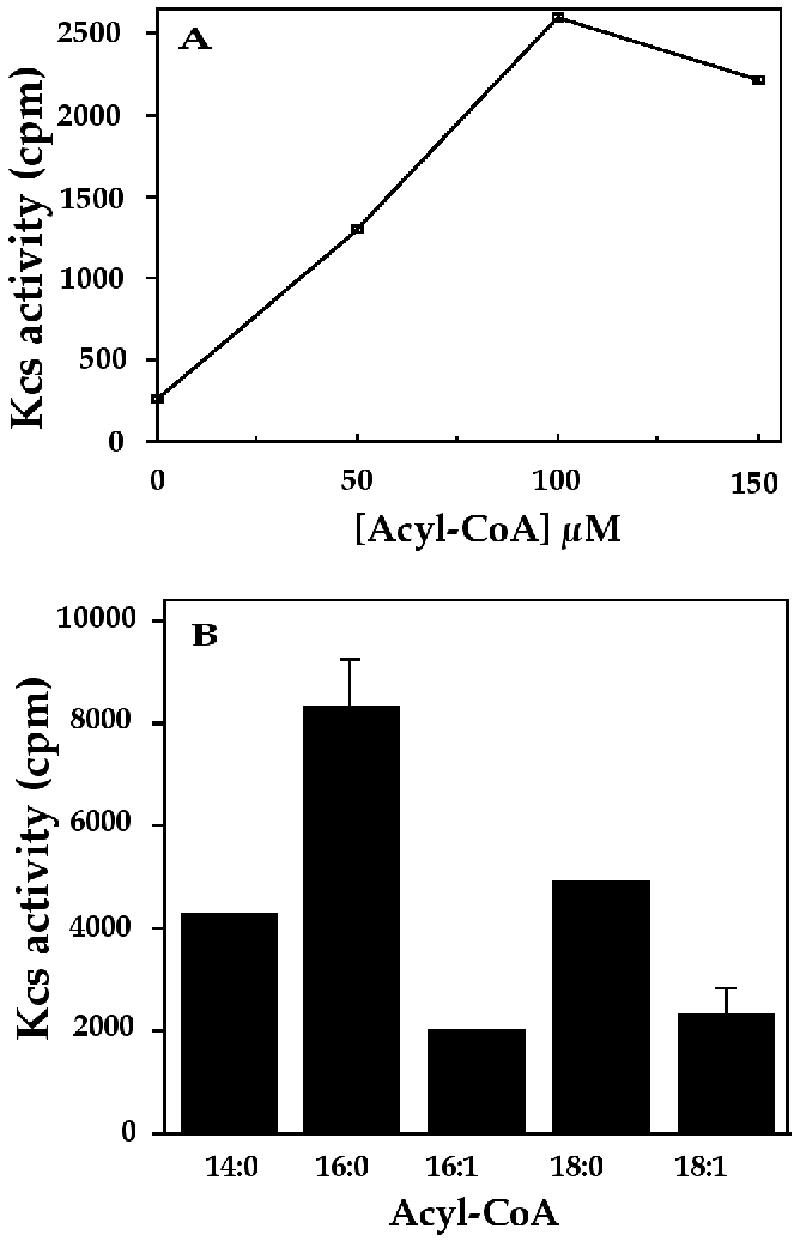
Acyl-CoA dependence of Kcs activity. A, Dependence on Acyl-CoA concentration. Assay mixtures, with 30 μg of protein of fraction 1 from cells grown in 3.5 m NaCl, contained the indicated concentrations of acyl(18:1)-CoA. Assay conditions and product analysis were as described in “Materials and Methods.” B, Kcs activity with different acyl-CoAs. Activity assays, as described in A, were performed with 100 μm of the acyl-CoAs indicated. sd values are indicated for acyl(18:1)-CoA and acyl(16:0)-CoA substrates. Kcs activity, cpm in 0.6 mL of chloroform extracts, as detailed in “Materials and Methods.”
Figure 3.
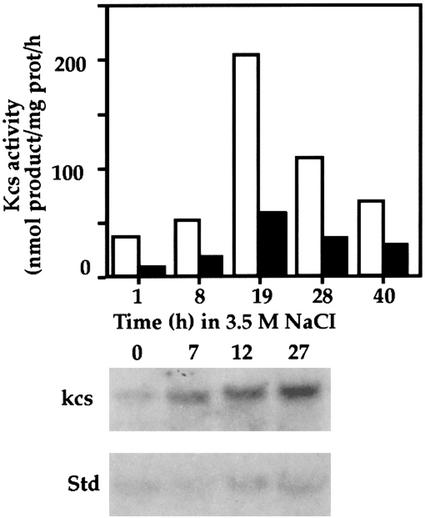
Analysis of kcs transcript and Kcs activity in D. salina cells after exposure to high salt. Cultures were transferred, in two steps, from a medium with 0.5 m to a medium with 3.5 m NaCl. Top, Assay of Kcs activity. Cell culture batches of approximately 1 L were removed at the indicated times after transfer to 3.5 m NaCl and the cells were fractionated to give fraction 1. Kcs activity was determined with 30 μg of protein of fraction 1 and 100 μm acyl(16:0)-CoA (white bars) or acyl(18:1)-CoA (black bars) as described in “Materials and Methods.” Bottom, Northern-blot analysis of kcs mRNA. Samples of approximately 200 mL were removed at the indicated times after transfer to 3.5 m NaCl and total RNA was extracted. Northern-blot hybridization with kcs cDNA (kcs) or standard (std) probe was as described in “Materials and Methods.”
Kcs activity was further characterized for the condensation products of [2-14C]malonyl-CoA, with myristyl(14:0)-CoA or palmityl(16:0)-CoA as acceptor substrates (Fig. 4). In this case, the reaction mixtures were supplemented with NADH and NADPH to allow subsequent reduction steps in the formation of the fully reduced acyl moieties. The TLC analysis of the reaction mixtures indicates the formation of condensation products comigrating with palmitic(16:0) or stearic(18:0) acids (as methyl esters), corresponding to the C2-elongated myristyl(14:0)-CoA or palmityl(16:0)-CoA substrates, respectively. The additional, faster migrating spots evident in the chromatogram must correspond to hydroxy or desaturated intermediates in the conversion of the β-ketoacyl primary condensation products into the fully reduced acyls. These results show that the algal Kcs, similar to plant counterparts, catalyzes the condensation of malonyl-CoA with the provided acyl-CoA substrates to form the expected elongated products.
Figure 4.
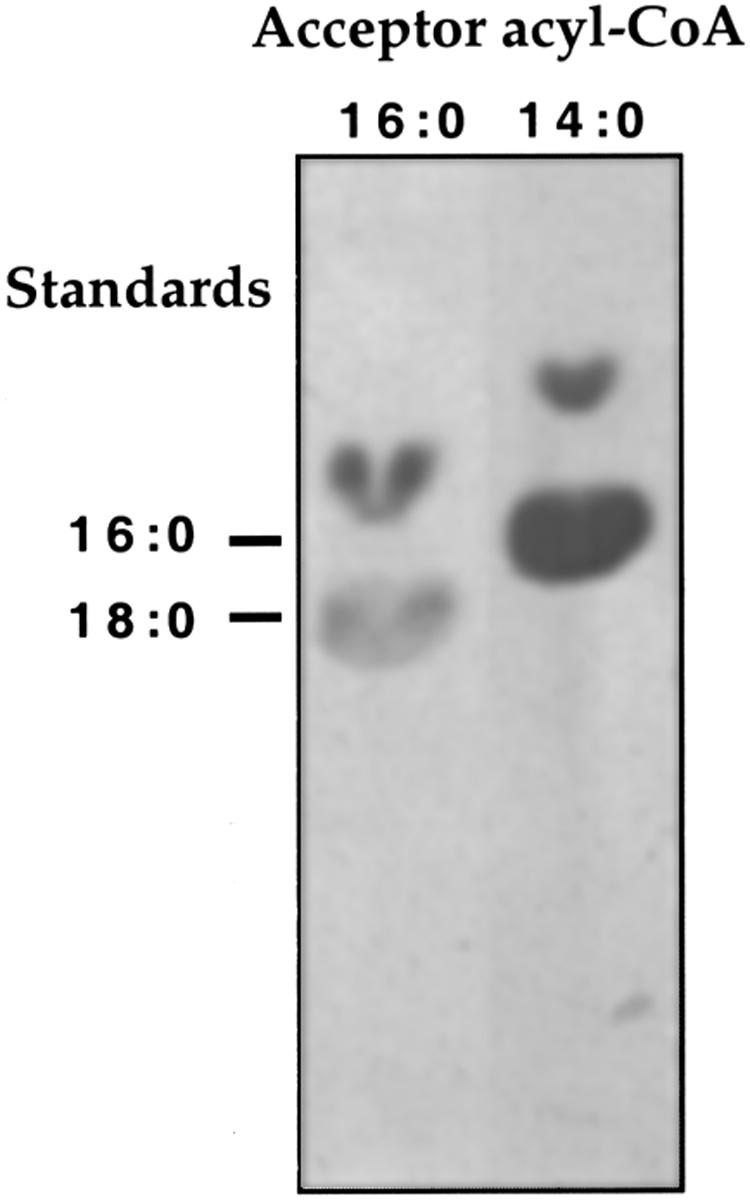
Analysis of Kcs reaction products. Kcs assay mixtures with 100 μm of the indicated Acyl-CoAs were supplemented with 0.5 mm each NADH and NADPH. Assay conditions, reaction product processing, resolution of the reaction products by thin-layer chromatography (TLC), and autoradiography were as described in “Materials and Methods,” Markers: 16:0, methyl ester of [3H] palmitic acid; 18:0, methyl ester of [14C] stearic acid.
Salt Induction of kcs mRNA Accumulation and Kcs Activity
Levels of kcs mRNA and Kcs activity were determined in D. salina cells at different times after transfer from 0.5 to 3.5 m NaCl (Fig. 3). In these cultures, cell division started approximately 10 h after transfer. Assays of Kcs activity, with acyl(16:0)-CoA or acyl(18:1)-CoA as acceptor substrates, indicated for both substrates a progressive rise in activity from 8 to 19 h after the salt shock followed by a decline at 28 h. By 40 h and onwards (data not shown) the activity reached nearly a constant level severalfold higher than the activity exhibited by cells grown in 0.5 m NaCl. A similar difference in Kcs activity was observed between cells grown continuously in 0.5 or 3.5 m NaCl. Northern-blot analysis of kcs mRNA during the course of adaptation to 3.5 m NaCl indicated an increase in the level of the transcript 7 h after the salt shock and further increases at 12 and 28 h. A reduction in the level of the kcs transcript was observed at later times (data not shown). A comparison of the course of change in Kcs activity and transcript accumulation indicates both reach a maximum within 12 to 27 h after the salt shock.
To determine whether kcs induction reflected a salt-specific or general osmotic response, the level of the kcs transcript was determined in cells subjected to osmotic upshock generated by added glycerol rather than NaCl. The results (data not shown) indicate only a low rise in both kcs transcript and Kcs activity, compared with salt-shocked cells. Thus, kcs is induced in response to salt to a far greater extent than to nonionic osmotic shock.
Functional Expression of kcs in Escherichia coli
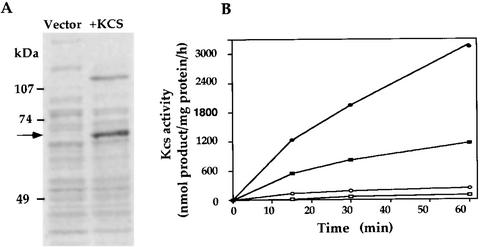
To critically establish that kcs encoded Kcs, a cDNA fragment starting at Ser-47 (Fig. 1) was cloned into pET28c, in phase with the initiator ATG and 37 additional N-terminal codons of the vector encoding His tag and T7 tag sequences. Expression of the fusion protein in E. coli was monitored by [35S]Met labeling of isopropyl-β-d-thiogalactoside (IPTG)-induced cells treated with rifampicin to enhance expression selectivity. The results (Fig. 5A) show that cells transformed with the recombinant plasmid, but not with the cloning vector alone, synthesized a protein of the expected size. Another preferentially expressed protein, of >107 kD, likely represents residual Kcs dimers that escaped dissociation by SDS. Evidence for the homodimeric structure of native plant Kcs has been presented (Lassner et al., 1996).
Figure 5.
Expression and activity of Kcs in E. coli. A, Detection of heterologously expressed Kcs. The kcs cDNA cloned in the pET28c expression vector was transformed into E. coli. Transformants with the recombinant plasmid (+) or vector alone (−) were metabolically labeled with [35S]Met and membrane fractions were analyzed by SDS-PAGE and autoradiography as described in “Materials and Methods.” The major translation product is marked by an arrow. A preferentially labeled >107-kD polypeptide is likely to represent an incompletely dissociated dimeric form of Kcs, as discussed in “Results.” B, E. Coli transformants with the kcs cDNA containing plasmid (black symbols), or the pET28c vector alone (white symbols), were treated under conditions inducing kcs expression and a membrane fraction was isolated and assayed for Kcs activity as described in “Materials and Methods.” Reaction mixtures contained 3 (rectangles) or 6 (circles) μg of protein of the E. coli fraction.
Membrane fractions from E. coli transformed with the kcs recombinant plasmid, or vector alone, were solubilized and assayed for Kcs enzymatic activity according to a similar protocol as used to assay fraction 1 from D. salina. The results (Fig. 5B) clearly show Kcs activity in fractions from transformants with the recombinant plasmid, but not with vector alone, confirming that the cloned gene encoded a functional Kcs enzyme. Furthermore, the results show that the truncation of the N-terminal 46 amino acid residues did not abolish the activity of the expressed protein. The cofractionation of the Kcs activity with a membrane fraction in E. coli suggests, but does not prove, the membrane integration of the protein in the heterologous host.
Fatty Acid Composition of Membrane Fractions from Cells Grown in Different Salinities
The salt inducibility of kcs in D. salina suggested that adaptation to salt might entail the elongation of fatty acids in membrane lipids. To test this possibility, lipids isolated from membrane fractions from low- or high-salt-grown D. salina were analyzed for fatty acids composition (Table II). The results are presented as the relative distributions of the major fatty acids in each of the fractions (C14–C22 in fraction 1 and C14–C18 in fraction 2), excluding longer fatty acids present in minute amounts.
Table II.
Fatty acid composition of fractions 1 and 2 from D. salina grown in low and high salinities
| Fatty Acid | Fraction 1 (Microsomes/PMa)
|
Fraction 2 (PM)
|
||
|---|---|---|---|---|
| 3.5 m NaCl in growth medium | 0.5 m NaCl in growth medium | 3.5 m NaCl in growth medium | 0.5 m NaCl in growth medium | |
| Fatty acid content (wt %) | ||||
| 14:0 | ndb | 0.39 ± 0.01 | nd | nd |
| 14:1 | 2.20 ± 0.1 | 2.9 ± 0.17 | 2.1 | 0.83 ± 0.06 |
| 14:2 | 2.56 ± 2.03 | 6.63 ± 0.5 | 7.8 ± 0.87 | 8.3 ± 0.61 |
| 14c | 4.76 ± 2.11 | 9.79 ± 0.44 | 9.9 ± 1.04 | 9.23 ± 0.64 |
| 16:0 | 75.30 ± 3.21 | 48.91 ± 0.33 | 41.87 ± 0.38 | 39.83 ± 0.29 |
| 16:1 | nd | nd | 0.87 ± 0.06 | 0.63 ± 0.12 |
| 16 | 75.31 ± 3.21 | 48.91 ± 0.33 | 42.73 ± 0.42 | 40.47 ± 0.25 |
| 18:0 | 1.39 ± 0.01 | 0.46 ± 0.08 | 1.43 ± 0.06 | 1.5 ± 0.35 |
| 18:1(w6 + w9) | 9.57 ± 2.46 | 17.45 ± 1.08 | ndd | ndd |
| 18:2(w6) | 1.95 ± 1.17 | 8.14 ± 0.31 | 8.47 ± 0.31 | 16.67 ± 0.12 |
| 18:3(w3 + w6) | 1.61 ± 0.6 | 2.37 ± 0.12 | 31.83 ± 1.79 | 28.9 ± 0.2 |
| 18:4(w3) | 2.34 ± 0.74 | 2.83 ± 0.31 | 5.63 ± 0.55 | 3.23 ± 0.06 |
| 18 | 16.40 ± 4.16 | 31.25 ± 1.48 | 47.37 ± 0.67 | 50.3 ± 0.5 |
| 20:0 | nd | 0.81 ± 0.27 | ||
| 20:3 | nd | 0.58 ± 0.07 | ||
| 20 | nd | 1.39 ± 0.28 | ||
| 22:0 | nd | 1.63 ± 0.04 | ||
| 22:1 | 4.45 ± 1.2 | 7.71 ± 1.14 | ||
| 22 | 4.45 ± 1.2 | 8.8 ± 2.08 | ||
D. salina cells collected from 121 cultures grown to ∼106 cells mL−1 in the indicated NaCl concentrations were used to isolate fraction 1, containing microsomes and plasma membrane, and fraction 2, containing purified plasma membrane, as detailed in “Materials and Methods” and “Results.” Lipid extraction and fatty acid analysis were performed as described in “Materials and Methods.” Wt %, wt of the indicated fatty acids relative to their total amount (100%). Fatty acids of longer chain lengths than those indicated were negligible in amount.
PM, plasma membrane.
nd, Not detected.
Bold, Total wt % fatty acids of the indicated chain length.
In some experiments 18:1(w9) was not fully resolved from 18:3(w3).
The analysis reveals that Fraction 1 from cells grown in 3.5 m NaCl differs from the corresponding fraction from cells grown in 0.5 m NaCl in containing a markedly lower proportion of C16 fatty acids, which is mainly accounted for by an increase in the proportion of C18 fatty acids, and to a lesser degree in the proportion of the minor C14 and C22 fatty acids. On the other hand, the relative proportions of C14, C16, and C18 in fraction 2 remain practically unaltered between cells grown in high and low salt, although an altered distribution of different desaturated C18 fatty acids is noticeable.
In both fractions 1 and 2, the fully saturated 16:0 palmitic acid is the predominant C16 fatty acid, whereas C18 fatty acids mostly consist of desaturated species. Hence, the salt-related shift from C16 to C18 fatty acids in fraction 1 also entails a rise in the overall proportion of desaturated fatty acids. Thus, microsomal membranes of cells grown in high salinity contain a higher proportion of C18 to C16 fatty acids and, as a consequence, a higher content of desaturated fatty acids, compared with low-salt-grown cells.
DISCUSSION
Transcriptional activation of the D. salina kcs gene and enhancement of Kcs activity were shown to follow a hyperosmotic salt shock. These responses do not constitute a transient stage in salt adaptation, but persist in cells growing continuously in high salinity.
Plant Kcs enzymes have not been obtained yet in homogeneous, pure form (Lassner et al., 1996; Todd et al., 1999). Studies of mutant or transformed plants or partially purified enzymes led to the conclusion that plant Kcs enzymes vary in their preference for different chain length acyl-CoA substrates, i.e. each enzyme typically participates in several rounds of elongation within a given range of chain lengths (Lassner et al., 1996). The D. salina enzyme does not seem to be involved in the formation of very long-chain fatty acids, which are very scarce in the algal membranes, but apparently prefers relatively shorter chain acyl-CoA substrates than enzymes such as the jojoba Kcs (Lassner et al., 1996), or the heterologously expressed Arabidopsis KCS1 (Todd et al., 1999). However, the D. salina Kcs resembles these plant enzymes in preferring saturated over mono-unsaturated acyl-CoA substrates.
The salt inducibility of the D. salina Kcs raised the possibility that salt adaptation may entail a shift toward longer chain fatty acids in algal lipids. Because D. salina is devoid of waxes and energy storage lipids (Thompson, 1996), we anticipated the shift to occur in membrane lipids. A pronounced salt-induced shift in fatty acid chain length was detected in a fraction containing both microsomes and plasma membranes. Because no shift was observed in purified plasma membranes, we concluded that fatty acid elongation, for which the analysis necessarily provides only a minimal estimate, was confined to the microsomal fraction.
Palmitic acid (C16:0) is the predominant C16 fatty acid in both plasma membranes and microsomes, whereas the bulk of C18 fatty acids is made up of unsaturated fatty acids. Therefore, it is envisaged that palmitic acid is first elongated to stearic acid (C18:0), which subsequently undergoes various degrees of desaturation. By increasing the proportion of C18:0, the elongation reaction thus provides additional substrate for desaturation. Hence, the salt-related modifications concern not only fatty acid chain elongation but also increased overall desaturation.
Modification of membrane lipid fatty acids in plants has been studied mostly in relation to chilling sensitivity. In this instance, increased desaturation of thylakoid fatty acids was causally linked to enhanced salt tolerance (Somerville, 1995). In D. salina, increased desaturation was reported in polar lipid fractions from plasma membranes of D. salina grown in high compared with low salinity (Peeler et al., 1989). Another analysis (Al-Hasan et al., 1987) detected a salt-related increase in the relative proportion of linolenic acid (18:3) in the total lipids of another D. salina strain.
The establishment of a direct functional link between the cloned kcs, fatty acid modifications, and enhancement of salt tolerance must await the development of appropriate genetic tools for D. salina. Nonetheless, taken together, our results provide support to the scheme whereby fatty acid elongation, and probably desaturation, contribute to the salt tolerance of D. salina. The understanding of this contribution is made difficult by the fact that homeostatic mechanisms maintain the intracellular ionic concentration at a rather low level, regardless of the external salinity (Pick et al., 1986). As a consequence, the endoplasmic reticulum or Golgi apparatus are not exposed to high ionic concentrations. The most pronounced biochemical change in the intracellular milieu is the accumulation of high concentrations of glycerol (in excess of 4.0 m) that osmotically balance the external high salinity. Glycerol is generally thought to be fully compatible with the stability and function of cellular components (Roberts, 2000). Yet, one cannot dismiss the possibility that some cellular components may not operate optimally in the presence of such high levels of glycerol and need to undergo adaptive modifications exemplified by the present observations.
The function(s)/structures potentially affected by glycerol in D. salina remain elusive. Yet, a plausible direction involves the secretory pathway responsible for transport of proteins and lipids between the endoplasmic reticulum and Golgi apparatus by secretory vesicles that bud from one compartment and fuse with another. Vesicle budding and fusion, as well as discrimination of cargo and targeting molecules, depend on specific protein-membrane and membrane-membrane interactions that were shown to be affected by membrane lipid composition (Rothman and Wieland, 1996; Schenkman and Orci, 1996; Weber et al., 1998; Matsuoka and Schenkman, 2000). It is not unlikely that some of these interactions are hindered in high intracellular glycerol concentrations. Hence, the activity of the salt-induced Kcs in D. salina may be required, alongside additional activities, to modify membranes of the endoplasmic reticulum and/or Golgi apparatus so as to optimize vesicular transport in cells grown in high salinity. Adaptations of this sort are not likely to be unique to D. salina because intracellular accumulation of inorganic or organic solutes is a ubiquitous salt-adaptive, osmoregulatory response in taxonomically varied organisms (Roberts, 2000).
MATERIALS AND METHODS
Algae and Growth Conditions
The source of the Dunaliella salina strain used in this study, media, and growth conditions were essentially as described (Ben Amotz and Avron, 1983; Sadka et al., 1989; Lers et al., 1990). Axenic cultures were grown continuously in media with 0.5 or 3.5 m NaCl. Cells were osmotically shocked by transferring cultures grown to 6 × 105 cells mL−1 in a medium with 0.5 m NaCl, to a medium with 3.5 m NaCl essentially as previously described (Fisher et al., 1994).
Isolation and Cloning of kcs cDNA
A cDNA library from salt-shocked D. salina was constructed as previously described (Fisher et al., 1996). Specifically, D. salina cells grown in 0.5 m NaCl were transferred in two steps to 3.5 m NaCl (Fisher et al., 1994). Total RNA was extracted from cells 9 h after transfer to 3.5 m NaCl and cDNA to poly(A+) mRNA was synthesized and cloned into the λ Uni-ZAP XR expression vector (Promega, Madison, WI) as described (Fisher et al., 1996). A phage clone initially isolated in a screen with anti-TTf antibodies (Fisher et al., 1997) was subsequently shown to include a cDNA for an unrelated, salt-inducible gene. The approximately 1.0-kb insert in the original clone, including the 3′ end of the cDNA, was used to clone the full-length cDNA by the 5′-RACE procedure (CLONTECH Laboratories, Palo Alto, CA). To obtain corresponding genomic DNA sequences, primers based on cDNA sequences were used with templates of genomic DNA, digested by Sau 3A, HaeIII, TaqI, or MspI, followed by fragment circularization by ligase, in several consecutive steps of inverted PCR amplification and subsequent cloning.
Cell Fractionation
D. salina cells, grown in 0.5 or 3.5 m NaCl to 1 × 106 cells mL−1, were collected by centrifugation at 5,000g for 10 min at 4°C. Cell pellets were washed, in accordance with the growth medium, with 0.5 or 3.5 m NaCl in medium buffer (10 mm KCl, 2 mm MgCl2, 5 mm β-mercaptoethanol, and 25 mm Tricine-NaOH, pH 7.8), followed by washing with buffers containing 0.9 or 4.6 m glycerol, respectively, in medium buffer. The cell pellets were resuspended in the same buffers to a density of 3 to 5 × 108 cells mL−1 and cells were lysed by adding 3 or 4 volumes of medium buffer to cells grown in 0.5 or 3.5 m NaCl, respectively, and gentle stirring of the suspensions for 20 min at 4°C. The lysates were centrifuged at 8,000g for 20 min at 4°C and Na-EDTA (pH 7.5) was added to the supernatants to a final concentration of 5 mm, followed by gentle stirring for 30 min at 4°C and centrifugation at 235,000g for 45 min at 4°C. The supernatants (soluble fraction) were removed and the pellets were resuspended (1 × 106 cell equivalents/100 μL) in storage buffer (1 m NaCl, 10% [w/v] glycerol, 5 mm β-mercaptoethanol, 2% [w/v] CHAPS, and 25 mm Tricine-NaOH, pH 7.8) and stored at −80°C until assayed for Kcs activity (Fraction 1). For further fractionation, the pellets were suspended in medium buffer containing 0.2 or 1.2 m glycerol for pellets from cells grown in 0.5 or 3.5 m NaCl, respectively, and the suspensions were centrifuged at 290,000g for 90 min at 4°C. The pellets were resuspended in 0.3 mL of the respective suspension buffers and loaded on top of glycerol step gradients containing 5 mL of 30% (v/v) glycerol in 30 mm KCl, 1 mm MgCl2, 5 mm β-mercaptoethanol, and 25 mm mm Tricine-NaOH (pH 7.8) layered on top of 6 mL of 60% (v/v) glycerol in the same buffer. The gradients were spun in swinging buckets at 210,000g for 2 h at 4°C. Pellets (from 1 × 106 cell equivalents) were resuspended in 50 μL of medium buffer and the suspensions were spun at 5,000g for 10 min at 4°C. The supernatants (fraction 2) were stored in liquid N2 until analyzed.
Fractions for Lipid Analysis
The procedure was essentially as described above, but with slight modifications. The medium buffer contained 10 mm Tris-MOPS (pH 7.5) instead of Tricine-NaOH, and contained no β-mercaptoethanol. In all steps after cell lysis, the buffers contained 1 mm benzamidine and 5 mm ε-aminocaproic acid. Fraction 1 pellets were suspended in 3.0 mL and fraction 2 pellets were suspended in 1.5 mL of medium buffer, containing 0.2 or 1.2 m glycerol, for pellets originating in cells grown in 0.5 or 3.5 m NaCl, respectively.
Kcs Assay
The assay of Kcs activity was essentially similar to previously described protocols (Fehling et al., 1992; Lassner et al., 1996). Specifically, 30 μL of reaction mixtures contained 25 mm Tricine-NaOH (pH 7.8), 0.38% (w/v) CHAPS, 0.3 m NaCl, 2 mm β-mercaptoethanol, 17 μm [2-14C]malonyl-CoA (specific activity: 56 mCi mmol−1, Amersham, Buckinghamshire, UK), 100 μm of the specified acyl-CoAs (Sigma, St. Louis), and fractions from D. salina or transformed Escherichia coli (as specified). The mixtures were incubated on a gyratory shaker for 1 h at 30°C. The reaction was stopped by adding 30 μL of 20% (w/v) KOH in water:methanol (9:1, v/v) and incubating at 80°C for 1 h. The mixtures were acidified with 30 μL of 5 m H2SO4 and extracted twice with 0.6 mL of chloroform. The pooled chloroform extracts were washed twice with 1 mL of water and radioactivity in 0.6-mL aliquots of the chloroform solution was determined in a liquid scintillation counter. Enzyme activity is presented as the cpm measured in such aliquots, or by units defined by nmol of condensation product/mg protein/h.
Reaction Product Analysis
The reaction conditions, with 30 μg of protein of D. salina fraction 1 as the source of enzyme, were essentially as described above but the mixtures were supplemented with 0.5 mm each of NADH and NADPH to allow the elongation to proceed beyond the condensation step. The mixtures were processed as in the assay described above and 0.6-mL aliquots of the chloroform extracts were incubated in sealed tubes with 0.5 mL of 10% (w/v) BCl3 in methanol for 30 min at 70°C. After addition of 2.5 mL of water, the fatty acid methyl esters were extracted twice with 2 mL of hexane. The combined extracts were concentrated under N2 and resolved on KC18 reverse-phase TLC plates (Whatman, Clifton, NJ) developed in acetonitrile:tetrahydrofuran (80:20, w/v) essentially as described (Evenson and Post-Beittenmiller, 1995), and exposed to BioMax MS film with BioMax transcreen (Eastman-Kodak, Rochester, NY) for 4 d at −80°C.
Heterologous Expression of kcs in E. coli
A kcs cDNA fragment, extending from the T residue at position 139 (start of the codon for Ser-47, compare with Figs. 1 and 2) to the poly(A+) tail, was isolated from the plasmid rescued from the original λZap recombinant phage (Fisher et al., 1996) by restriction with EcoRI and XhoI. The cDNA fragment was ligated into the pET28c vector (Novagen, Madison, WI), previously digested with EcoRI and XhoI, and the recombinant plasmid was transformed into and propagated in E. coli HB101. Shortly before analysis, the recombinant plasmid, or the pET28c vector, was transformed into E. coli BL21(DE3) pLysS and fresh transformant clones were grown at 26°C to an optical density at 600 nm (OD600) of 0.6 in 20 mL of 2× Luria-Bertani medium containing 30 μg mL−1 kanamycin and 34 μg mL−1 chloramphenicol. Kcs synthesis was induced by the addition of 1 mm IPTG and incubation for 3 h at 26°C. The cultures were cooled on ice, centrifuged at 5,000g for 5 min at 4°C, and the cell pellets were washed with 20 mL of sonication buffer (1 m NaCl, 5 mm β-mercaptoethanol, and 25 mm Tricine-NaOH, pH 7.8) and resuspended in 0.5 mL of the same buffer containing 1 mm phenylmethyl sulfonyl fluoride. The cells were lysed by three 20-s pulses of sonication (Ultrasonic Cell Disruptor, model XL2005, Microson, Misonix, NY), the sonicates were cleared by 1 min of centrifugation at 5,000g at 4°C, and the supernatants were spun at 235,000g for 45 min at 4°C. The pellets were resuspended in sonication buffer containing 10% (w/v) glycerol and 2% (w/v) CHAPS and stored at −80°C until assayed for Kcs activity as the D. salina fractions. To detect the recombinant Kcs, E. coli transformants grown to 0.6 OD600 in 10 mL of Luria-Bertani medium with the antibiotics indicated above were collected by centrifugation at 5,000g for 15 min at room temperature and the pellets were washed twice with 1 mL of M9 medium and resuspended in 1 mL of M9 medium containing Glc and an amino acid mixture without Met. After 1 h of incubation at 37°C, 1 mm IPTG was added and incubation was continued for 30 min at 37°C followed by the addition of 0.4 mm rifampicin (Sigma). After 20 min at 37°C, 20 μCi [35S]Met (specific activity: 1,000 Ci mmol−1, Amersham) was added and the cells were incubated for an additional 30 min at 37°C. Nonradioactive Met was added to 40 μg mL−1 and the cultures were kept in ice for 10 min and centrifuged at 5,000g for 10 min at 4°C. Cell pellets were washed with 10% (w/v) Suc and 10 mm Tris-HCl (pH 8.0), resuspended in 80 μL of 1 mm Na EDTA, 25 mm KCl, 1 mm dithiothreitol, and 50 mm Tris-HCl (pH 8.0), and the suspensions were subjected to two cycles of freezing in liquid N2 and thawing at room temperature. After adding 10 μL each of 20 μg mL−1 DNase I and 10 mm MgCl2, the suspensions were again frozen, thawed, and then clarified by 10 min of centrifugation at top speed in a microfuge at 4°C. The supernatants, 40 μL per lane, were resolved on 10% (w/v) SDS-PAGE followed by autoradiography.
Fatty Acid Analysis
Lipids were extracted as described (Bligh and Dyer, 1959) from 0.9-mL aliquots (1 × 1010 cell equivalents) of fraction 1 or 2. The chloroform extracts were concentrated under N2 to 0.1 to 0.2 mL, and mixed with 1 mL of 1.2 n NaOH in 50% (w/v) aqueous methanol. The tubes, tightly sealed with Teflon caps, were placed in a boiling water bath for 30 min, cooled to room temperature, and the solutions were brought to pH ≤ 2 with 0.5 mL of 6 n HCl. To form fatty acid methyl esters, 1 mL of 12% (w/v) FCl3 in methanol was added, the tubes were tightly sealed with Teflon caps, mixed gently, and heated in a water bath at 85°C for 20 min. After cooling to room temperature, 1 mL of hexane:diethyl ether (1:1, w/v) was added and after mixing and phase separation, the bottom phase was discarded and 3 mL of 0.3 n NaOH was added to the upper phase. After mixing and phase separation, the top phase was removed and stored at −80°C until analyzed by gas chromatography, essentially as described by Landry (1994).
Total RNA Isolation and Northern-Blot Hybridization
Total RNA, prepared from samples harvested at different times after osmotic shock, was analyzed (30 μg per lane) by northern blot-hybridization essentially as previously described (Fisher et al., 1994). The hybridization probe used was a 1.2-kb 5′ end fragment of the of kcs cDNA. The probe used as a hybridization standard was as described (Fisher et al., 1997).
ACKNOWLEDGMENT
We thank Prof. Uri Pick for helpful discussions.
Footnotes
This work was supported by Nature Beta Technologies (Eilat, Israel), by the Nikken-Sohonsha Corporation (Gifu, Japan), and by the Magnet Program, Israeli Ministry of Industry and Commerce.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.001909.
LITERATURE CITED
- Al-Hasan R, Ghannoum M, Sallal A, Abu-Elteen K, Radwan S. Correlative changes of growth, pigmentation and lipid composition of Dunaliella salina in response to halostress. J Gen Microbiol. 1987;133:2607–2616. [Google Scholar]
- Avron M. The osmotic components of halotolerant algae. Trends Biochem. 1986;11:5–6. [Google Scholar]
- Ben Amotz A, Avron M. On the factors that determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol. 1983;72:593–597. doi: 10.1104/pp.72.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Evenson KJ, Post-Beittenmiller D. Fatty acid-elongating activity in rapidly expanding leek epidermis. Plant Physiol. 1995;109:707–716. doi: 10.1104/pp.109.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehling E, Lessire R, Cassagne C, Mukherjee KD. Solubilization and partial purification of constituents of acyl-CoA elongase from Lunaria annua. Biochim Biophys Acta. 1992;1126:88–94. doi: 10.1016/0005-2760(92)90221-g. [DOI] [PubMed] [Google Scholar]
- Fiebig A, Mayfield J, Miley N, Chau S, Fischer R, Preuss D. Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems. Plant Cell. 2000;12:2001–2008. doi: 10.1105/tpc.12.10.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Gokhman I, Pick U, Zamir A. A salt-resistant plasma-membrane carbonic anhydrase is induced by salt in Dunaliella salina. J Biol Chem. 1996;271:17718–17723. doi: 10.1074/jbc.271.30.17718. [DOI] [PubMed] [Google Scholar]
- Fisher M, Gokhman I, Pick U, Zamir A. A structurally novel transferrin-like protein accumulates in the plasma membrane of the unicellular green alga Dunaliella salina grown in high salinities. J Biol Chem. 1997;272:1565–1570. doi: 10.1074/jbc.272.3.1565. [DOI] [PubMed] [Google Scholar]
- Fisher M, Pick U, Zamir A. A salt-induced 60-kilodalton plasma membrane protein plays a potential role in the extreme halotolerance of the alga Dunaliella. Plant Physiol. 1994;106:1359–1365. doi: 10.1104/pp.106.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Zamir A, Pick U. Iron uptake by the halotolerant alga Dunaliella is mediated by a plasma membrane protein. J Biol Chem. 1998;273:17553–17558. doi: 10.1074/jbc.273.28.17553. [DOI] [PubMed] [Google Scholar]
- Fourman F, Barret P, Renard M, Pelletier G, Delourme R, Brunel D. The two genes homologous to Arabidopsis FAE1 co-segregate with the two loci governing erucic acid content on Brassica napus. Theor Appl Genet. 1998;96:852–858. [Google Scholar]
- Ghanevati M, Jaworski J. Active-site residues of a plant membrane-bound fatty acid elongase beta-ketoacyl-CoA synthase. Biochim Biophys Acta. 2001;1530:77–85. doi: 10.1016/s1388-1981(00)00168-2. [DOI] [PubMed] [Google Scholar]
- Hasegawa P, Bressan R, Zhu J-K, Bohnert H. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Mol Biol. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- James DW, Lim E, Keller J, Plooy I, Ralston E, Dooner HK. Directed tagging of the Arabidopsis FATTY ACID ELONGATION (FAE1) gene with the maize transposon Activator. Plant Cell. 1995;7:309–319. doi: 10.1105/tpc.7.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry WL. Identification of vibrio vulnificus by cellular fatty acid composition using the Hewlett-Packard 5898A microbial identification system: collaborative study. J AOAC Int. 1994;77:1492–1499. [PubMed] [Google Scholar]
- Lassner MW, Lardizabal K, Metz JG. A jojoba β-ketoacyl-CoA synthase complements the canola fatty acid mutation in transgenic plants. Plant Cell. 1996;8:281–292. doi: 10.1105/tpc.8.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lers A, Biener Y, Zamir A. Photoinduction of massive β-carotene accumulation by the alga Dunaliella bardawil. Plant Physiol. 1990;93:389–395. doi: 10.1104/pp.93.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lers A, Levy H, Zamir A. Co-regulation of a gene homologous to early light-induced genes in higher plants and β-carotene biosynthesis in the alga Dunaliella bardawil. J Biol Chem. 1991;266:13698–13705. [PubMed] [Google Scholar]
- Lessire R, Bessoule JJ, Cassagne C. Involvement of a β-ketoacyl-CoA intermediate in acyl-CoA elongation by an acyl-CoA elongase purified from leek epidermal cells. Biochim Biophys Acta. 1989;1006:35–40. [Google Scholar]
- Lutcke HA, Chow KC, Mickel FS, Moss KA, Kern HF, Scheele GA. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987;6:43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Schenkman R. The use of liposomes to study COPII- and COPI-coated vesicle formation and membrane sorting. METHODS. 2000;20:417–428. doi: 10.1006/meth.2000.0955. [DOI] [PubMed] [Google Scholar]
- Millar A, Kunst L. Very-long-chain fatty acid biosynthesis is controlled through the expression and specificity of the condensing enzyme. Plant J. 1997;12:121–131. doi: 10.1046/j.1365-313x.1997.12010121.x. [DOI] [PubMed] [Google Scholar]
- Millar AA, Clemens S, Zachgo S, Giblin EM, Taylor DC. CUT1, an Arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain-fatty acid condensing enzyme. Plant Cell. 1999;11:825–838. doi: 10.1105/tpc.11.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AA, Wrischer M, Kunst L. Accumulation of very-long-chain-fatty acids in membrane glycerolipids is associated with dramatic alterations in plant morphology. Plant Cell. 1998;11:1889–1902. doi: 10.1105/tpc.10.11.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeler TC, Stephenson MB, Einspaht KJ, Thompson GA. Lipid characterization of enriched plasma membrane fraction of Dunaliella salina grown in media of varying salinity. Plant Physiol. 1989;89:970–976. doi: 10.1104/pp.89.3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U, Karni A, Avron M. Determination of ion content and ion fluxes in the halotolerant alga Dunaliella salina. Plant Physiol. 1986;81:92–96. doi: 10.1104/pp.81.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priutt RE, Vielle-Calzada J-P, Ploense SE, Grossinklaus U, Lolle SJ. Fiddlehead, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. Proc Natl Acad Sci USA. 2000;97:1311–1316. doi: 10.1073/pnas.97.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MF. Osmoadaptation and osmoregulation in Arachaea. Frontiers Biosci. 2000;5:d796–812. doi: 10.2741/roberts. [DOI] [PubMed] [Google Scholar]
- Rothman J, Wieland F. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Sadka A, Himmelhoch S, Zamir A. A 150 KDa cell surface protein is induced by salt in the halotolerant green alga Dunaliella salina. Plant Physiol. 1991;95:822–831. doi: 10.1104/pp.95.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadka A, Lers A, Zamir A, Avron M. A critical examination of the role of de novo protein synthesis in the osmotic adaptation of the halotolerant alga Dunaliella. FEBS Lett. 1989;244:93–98. [Google Scholar]
- Schenkman R, Orci L. Coat proteins and vesicle budding. Science. 1996;171:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Serrano R. Salt tolerance in plants and microorganisms: toxicity targets and defense responses. Int Rev Cytol. 1996;165:1–52. doi: 10.1016/s0074-7696(08)62219-6. [DOI] [PubMed] [Google Scholar]
- Serrano R. Genetic engineering of salt and drought tolerance with yeast regulatory genes. Sci Hortic. 1999;78:261–269. [Google Scholar]
- Somerville C. Direct tests of the role of membrane lipid composition in low-temperature-induced photoinhibition and chilling sensitivity in plants and cyanobacteria. Proc Natl Acad Sci USA. 1995;92:6215–6218. doi: 10.1073/pnas.92.14.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. Lipids and membrane function in green algae. Biochim Biophys Acta. 1996;1302:17–45. doi: 10.1016/0005-2760(96)00045-8. [DOI] [PubMed] [Google Scholar]
- Todd J, Post-Beittenmiller D, Jaworsky JG. KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana. Plant J. 1999;17:119–130. doi: 10.1046/j.1365-313x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman B, McNew J, Westermann B, Gmachi M, Parlati F, Sollner T, Rothman J. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–777. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Yephremov A, Wissman E, Hujsler P, Wellesen K, Saedler H. Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell. 1999;11:2187–2201. doi: 10.1105/tpc.11.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]