Abstract
Type 1 T-cell responses against intracellular pathogens play a crucial role in mediating protection. We examined whether the induction of a strong type 1 T-cell response during a chronic bacterial infection influences responses to superantigens capable of inducing acute shock. Intravenous infection of mice with Mycobacterium bovis BCG appeared to induce a progressive anergy towards staphylococcal enterotoxin B (SEB) and towards antigen preparation of BCG (BCG-Ag) itself, based on diminished gamma interferon (IFN-γ) production by SEB- and BCG-Ag-stimulated splenocytes from infected mice. In contrast to these in vitro results, injection of SEB into BCG-infected mice led to a dramatic increase in the serum IFN-γ levels and the death of infected but not of control mice. In vitro hyporesponsiveness towards SEB and BCG-Ag occurred only with unfractionated splenocyte cultures, as purified T cells from infected mice produced higher levels of IFN-γ. Hyporesponsiveness towards SEB and BCG-Ag in unfractionated splenocyte cultures was not due to suppressive antigen-presenting cells (APCs), as APCs from infected mice stimulated higher levels of IFN-γ from purified T cells. The diminished IFN-γ levels observed with bulk splenocytes appear to be due to changes in the T-cell-to-APC ratio that result in a decreased proportion of T cells, coupled to reduced proliferative responses and an increased susceptibility of effector T cells to activation-induced cell death in vitro. Our results indicate that the reported phenomena of T-cell anergy during mycobacterial infection may be an in vitro consequence of the development of a strong type 1 response in vivo.
CD4+- and CD8+-T-cell activation is important for protection against infectious diseases (23). Under extreme polarizing conditions (such as chronic infections), CD4+ T cells differentiate into type 1 (producing interleukin 2 [IL-2] and gamma interferon [IFN-γ]) or type 2 (producing IL-4, IL-5, and IL-13) effectors and, once generated, mediate differential protection depending on the type of pathogen and the strain of mouse used (23). CD8+ T cells, on the other hand, are primarily involved in protection against intracellular pathogens, particularly those that replicate in the cytoplasm of the host cell (38).
Mycobacterium bovis BCG is a facultative intracellular bacterium that causes chronic infections in susceptible hosts. In mice, infection with BCG results in the development of splenomegaly in the first 4 weeks of disease, with increased accumulation of splenocytes during that time (25). Infection also leads to the development of a BCG-specific IFN-γ response at both the CD4+- and CD8+-T-cell levels (32). As with other pathogenic mycobacteria, BCG infection has been reported to induce T-cell anergy, particularly at higher bacterial burdens (25).
Superantigens hyperstimulate T cells by acting as a bridge between superantigen-restricted Vβ-containing T-cell receptors (TCRs) and major histocompatibility complex (MHC) class II molecules, resulting in a high responder frequency and massive inflammatory cytokine release (9). Gram-positive respiratory pathogens frequently produce superantigens as virulence factors during upper respiratory tract infections (9). Superantigens are secreted proteins that aid in immune evasion and cause severe pathophysiological changes in the host, often leading to shock. As superantigen-activated T cells are deleted by apoptosis or become nonresponsive (anergic) to restimulation, superantigens are often used to study inducible tolerance (33).
Since a host can potentially be challenged with multiple antigens or pathogens at any given time, understanding how an ongoing immune response to an infection alters responses to unrelated antigens is important. As BCG is a respiratory pathogen (9) and as superantigens are often produced during upper respiratory tract infections by gram-positive bacteria (9), we examined how BCG infection affected responses to the superantigen staphylococcal enterotoxin B (SEB). Our results reveal an apparent dichotomy between in vivo and in vitro responses to SEB in mice intravenously (i.v.) infected with a high dose (HD) of BCG. In addition, there was a further incongruity between results obtained in vitro with unfractionated splenocytes and those seen with fractionated T-cell and antigen-presenting cell (APC) populations. Our results question the notion that chronic mycobacterial infections induce T-cell anergy per se and suggest that this phenomenon may be an in vitro consequence of the development of a strong type 1 response in vivo.
MATERIALS AND METHODS
Bacteria and BCG-Ag preparation.
BCG (Pasteur) was kindly provided by R. North (Trudeau Institute, Saranac Lake, N.Y.) and cultured in 7H9 medium containing glycerol (0.2%), Tween 80 (0.05%), and albumin-dextrose supplement (10%; Difco Laboratories, Detroit, Mich.). At mid-log phase (optical density at 600 nm = 1.0), bacteria were harvested and frozen at −70°C (in 20% glycerol). Numbers of CFU were determined by plating serial dilutions in phosphate-buffered saline-Tween (PBS-T) (0.025% Tween 80) on Middlebrook 7H10 solid medium containing glycerol (0.5%) and oleic acid-albumin-dextrose supplement (10%; Difco Laboratories). For antigen preparation of BCG (BCG-Ag), the bacteria were grown in liquid culture (200 ml) as described earlier, harvested by centrifugation (3,000 × g for 30 min), and washed and resuspended in 2.5 ml of ice-cold PBS. The cell suspension was disrupted by sonication on ice with a Sonifier, Cell Disruptor 350 (Branson Sonic Power; SmithKline, Danbury, Conn.). This material was centrifuged at 1,900 × g for 10 min and filtered, and aliquots were stored at −80°C. The protein concentration was determined with a Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.) using bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.) as a protein standard.
Mice and in vivo challenges with BCG and SEB.
Female BALB/c mice, 6 to 8 weeks of age, were obtained from Charles River Laboratories (St. Constant, Quebec, Canada). Mice were maintained in the animal facility at the Institute for Biological Sciences (National Research Council, Ottawa, Ontario, Canada). For immunizations, BCG aliquots were thawed, washed once in PBS-T, and resuspended at 5 × 106 CFU/ml and at 5 × 104 CFU/ml. For most experiments, mice were inoculated with 106 (HD) CFU of BCG suspended in 200 μl of PBS-T via the lateral tail vein (i.v.). In some experiments, mice were injected with 104 CFU i.v. (low dose [LD]). Age-matched control mice were inoculated with 200 μl of PBS-T only (PBS mice).
For in vivo challenge with SEB (Sigma Chemical Co.), HD-infected mice immunized 30 days previously or uninfected controls were injected with 100 μg i.v. Eight hours post-SEB injection, mice were tail bled (along with noninjected controls) and serum IFN-γ was measured by enzyme-linked immunosorbent assay (ELISA). Mice were continually monitored but were allowed to progress to terminal shock. In some experiments, uninfected mice that survived SEB-induced toxic shock were rested for 1 week, prior to analysis of their splenic responses. In some experiments, SEB was heat inactivated (80°C for 1 h) prior to injection into mice. All SEB batches used in vivo were tested for lipopolysaccharide (LPS) contamination (Associates of Cape Cod Inc., Falmouth, Mass.) and were found to have <0.06 EU/100 μg of protein.
Cell culture and flow cytometry analysis of unfractionated splenocytes.
At various time points after infection, BCG-immunized mice and their age-matched controls were killed by CO2 intoxication and their spleens were removed aseptically. In some experiments SEB-exposed mice (1 week postinjection) and PBS-injected controls were used instead. Single-cell suspensions were prepared by tweezing the spleens between the frosted ends of two sterile glass slides in RPMI 1640 (GIBCO-BRL, Burlington, Ontario, Canada). Cells were subsequently passed through Falcon 2360 cell strainers (Becton Dickinson Labware, Franklin Lake, N.J.). For some experiments, erythrocytes were lysed in Tris-buffered ammonium chloride. Cells that excluded trypan blue were counted and resuspended at 107/ml in medium. Culture medium consisted of RPMI 1640 + 10% fetal bovine serum + 50 μg of gentamicin (GIBCO-BRL)/ml + 10 μg each of isonicotinic acid, rifampin, and pyrazinamide (R10A; all three from Sigma Chemical Co.) per ml.
Unfractionated splenocytes (5 × 105 cells/well; 200-μl volume in R10A) from infected (HD and LD), SEB-exposed mice or control mice were cultured in the presence of the indicated concentrations of SEB and SEA (Sigma). In some experiments, cultures were stimulated with plate-bound anti-TCR Vβ8 monoclonal antibody (MAb) (F23.1; PharMingen, San Diego, Calif.) or various concentrations of BCG-Ag. Cultures were incubated at 37°C in 8% CO2. For most experiments, supernatants were removed at 24 h for IL-2 and at 72 h for IL-4 and IFN-γ measurements, using cytokine-specific ELISAs (28). In some experiments, IFN-γ measurements were made on 24-h supernatants as well. After 72 h, 0.5 μCi (in a 50-μl volume) of tritiated thymidine was added per well. Cells were harvested 18 h after thymidine pulse, and radioactive incorporation was determined by liquid scintillation. The proliferation index is the ratio of the individual stimulus-induced incorporation to that induced by medium alone. Experiments were performed at least twice.
For flow cytometry analysis, splenocytes (5 × 105) were stained with all or some of the following MAbs (all at 1:100 dilutions in R10A): fluorescein isothiocyanate (FITC)-conjugated mouse anti-mouse Vβ8 TCR (F23.1), FITC-conjugated rat anti-mouse CD25 (IL-2 Rα; 7D4), FITC-conjugated rat anti-mouse CD122 (IL-2 Rβ; TM-β1), FITC-conjugated rat anti-mouse I-Ad/I-Ed (2G9) (all from PharMingen), or FITC-conjugated rat anti-mouse CD44 (IM7.8.1; Cedarlane Laboratories, Hornby, Canada). Cells were also stained with phycoerythrin (PE)-conjugated rat anti-mouse CD4 (YTS 191.1), PE-conjugated rat anti-mouse CD8α (YTS 169.4), or PE-conjugated rat anti-mouse CD11b/Mac1 (M170.15) (all from Cedarlane). In some experiments, splenocytes that were cultured for 24 or 72 h with SEB or BCG antigen were stained with FITC-conjugated rat anti-mouse CD4 (YTS 191.1) and rat anti-mouse CD8α (IM7.8.1; both from Cedarlane) and counterstained with propidium iodide (PI) (10 μg/ml; Sigma) to enumerate viable cells. Incubations lasted for 30 min on ice, following which the cells were washed in R10A and fixed in 1% formaldehyde in PBS. No fixation was done when cells were stained with PI. Cells (10,000 to 40,000 events) were acquired on an EPICS XL flow cytometer (Beckman Coulter, Hialeah, Fla.), and analysis was done using EXPO software (Beckman Coulter). Flow cytometry staining experiments were repeated at least three times. When warranted, statistical analysis on pooled experiments was done using a one-way analysis of variance test on a three-group comparison (PBS, LD, and HD). Results were considered significant when P was < 0.05.
T-cell purifications and culture conditions.
In order to purify CD4+ T cells by positive selection, 0.5 × 108 to 1 × 108 cells were pelleted and resuspended in 1 ml of R10A. Dynabeads mouse CD4 (Dynal Inc., Lake Success, N.Y.) was added to the resuspended cell pellet at a ratio of 4 beads/cell and was incubated at 4°C for 30 min in a rotating platform, following which the CD4+ cells were removed using the Dynal MPC-1 magnet according to the manufacturer's instructions. Three or four rounds of washing and magnetic separation were performed, with fresh R10A being added after each round. Detachment of Dynabeads from CD4+ T cells was accomplished using Detachabead mouse CD4 (Dynal Inc.) as per manufacturer's instructions: 1 U of Detachabead was added per 106 target cells, and the suspension was incubated for 60 min at ambient temperature in a rotating platform. Purified CD4+ T cells were then washed or magnetically separated from the detached Dynabeads as done before. The resulting purified T cells were greater than 90% pure (<1% CD8+ T cells) as determined by follow-up analysis with PE-conjugated rat anti-mouse CD4 (YTS 191.1). For some experiments, Dynabead-purified CD4+ T cells were subsequently stained with either FITC- or PE-labeled rat-anti-mouse CD44 (IM7.8.1) and were sorted into CD44low and CD44hi populations in an EPICS Elite ESP (Beckman Coulter).
In some experiments, the above CD4-depleted splenocytes were pelleted, resuspended in 0.5 to 1 ml of R10A, and used to purify CD8+ T cells. CELLection Biotin Binder Dynabeads precoated as per manufacturer's instructions (Dynal Inc.) with biotin-conjugated rat anti-mouse CD8β.2 MAb (53.5.8; PharMingen) were added to the resuspended cell pellet at a ratio of 5 beads/cell and were incubated for 15 to 20 min at 4°C in a rotating platform. Magnetic isolation of the CD8β+ T cells was done as described above for CD4+ cells. Dynabead detachment was done using the CELLection Biotin Binder kit Releasing Buffer (DNase; 188 U/108 Dynabeads) in a 37°C shaker for 30 to 60 min, followed by two or three rounds of washing and magnetic separation. This protocol resulted in 85 to 95% pure CD8+ cells as determined by follow-up analysis with PE-conjugated rat anti-mouse CD8α (YTS 169.4).
Purified CD4+ T cells or CD8+ T cells (105 cells/well, in a Falcon round-bottomed 96-well plate) were cocultured with various APC populations (see below) in the presence or absence of various concentrations of SEB or BCG-Ag. In some experiments, the keyhole limpet hemocyanin (KLH)-specific type 1 clone HDK1 (kindly provided by Tim Mosmann, University of Rochester, Rochester, N.Y.) was cocultured with various populations of unfractionated splenocytes (see below) in the presence or absence of KLH (10 μg/ml; Sigma). As was the case for unfractionated splenocytes, supernatants from the purified T-cell cultures were removed at 24 h for IL-2 and at 72 h for IL-4 and IFN-γ measurement, while the tritiated thymidine pulse was done after 72 h. The proliferation index is the ratio of the SEB-induced incorporation to that induced by medium alone. Experiments were performed at least twice.
Accessory cell preparation.
For some experiments, bone marrow-derived dendritic cells were used as accessory cells. Briefly, bone marrows were flushed from the femurs and tibias of one to three normal mice, and single-cell suspensions were made by passing them through Falcon 2360 cell strainers (Becton Dickinson Labware) using a sterile 1-ml syringe plunger. Cells were subsequently counted and resuspended at 106 cells/ml in R10 (including 50 μg of gentamicin/ml). Medium was supplemented with 5 ng of recombinant murine granulocyte-macrophage colony-stimulating factor (ID Labs, London, Ontario, Canada)/ml, and cells were placed in a Falcon 353111 tissue culture flask (Becton Dickinson) and cultured for 6 to 8 days at 37°C in 8% CO2. Nonadherent cells were removed at days 2 and 4 of culture, and fresh R10 + granulocyte-macrophage colony-stimulating factor was added. On the day of the experiment, nonadherent cells were harvested (>80% CD11c+), washed in R10, counted, and irradiated (2,500 rads) prior to being placed in culture with purified CD4+ or CD8+ T cells at 5 × 104 cells/well.
For other experiments, adherent splenocyte fractions from PBS-treated or LD- or HD-infected mice were obtained by incubating CD4-depleted spleens from the above-described animals in a Falcon 3003 tissue culture dish (Becton Dickinson Labware) for 2 h at 37°C in 8% CO2. Nonadherent cells were discarded, and the adherent cell fraction was harvested by incubating cells with cold PBS + 1 mM EDTA for 10 min at 4°C. Detached cells were harvested, counted, and used at 5 × 105 cells/well in the presence of the indicated concentrations of SEB or BCG-Ag.
For some experiments involving CD44low and CD44hi CD4+ T cells, irradiated B-lymphoma cells (M12.4.1) were used as APCs (at 5 × 104 cells/well) with or without BCG-Ag (10 μg/ml). For experiments involving HDK1 T cells, unfractionated splenocytes from PBS-treated or LD- or HD-infected mice were irradiated (2,500 rads) and used as APCs at the indicated concentrations in the presence or absence of KLH (10 μg/ml; Sigma).
RESULTS
Splenic hyporesponsiveness to SEB in BCG-infected mice.
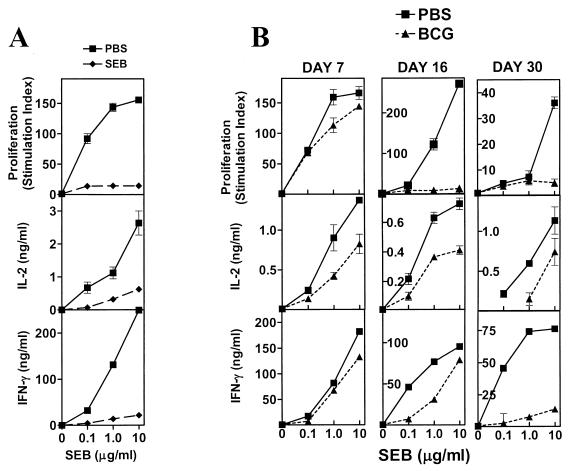
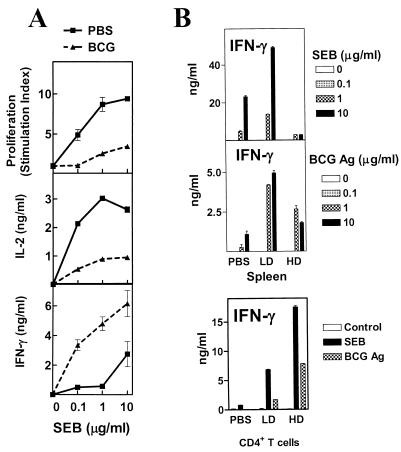
BCG induces a strong type 1 response in mice, characterized by the production of high levels of IFN-γ and little IL-4 in response to Ag (14). Since mice can encounter multiple infections that could potentially be cross-regulatory, we examined responses to SEB (a shock-inducing exotoxin produced by Staphylococcus aureus), which stimulates TCR Vβ8-bearing T cells in an MHC class II-dependent but unrestricted fashion (12). Injection of mice with SEB induces a state of splenic hyporesponsiveness to restimulation with the superantigen 1 week later (12). Reduced proliferation and reduced IL-2 and IFN-γ production characterize this in vitro splenic hyporesponsiveness seen in SEB-exposed mice, relative to what is found in unexposed controls (Fig. 1A). We observed that BCG infection appeared to have a similar impact on splenic responses to SEB (Fig. 1B), in spite of infected animals not having been experimentally exposed to the superantigen. At different time intervals after BCG infection (days 7, 16, and 30), unfractionated splenocytes from afflicted mice or age-matched PBS-injected mice were stimulated with different concentrations of SEB for 72 h. Analysis of the results depicted in Fig. 1B indicates that, as early as 7 days after infection, there was decreased production of IL-2 and IFN-γ, as well as decreased proliferation in response to higher concentrations of SEB (1 and 10 μg/ml). This difference is exacerbated as the infection progresses (day 7 < day 15 < day 30), particularly for IFN-γ, which shows the greatest difference between controls and infected mice on day 30. IL-4, when detected, was seen only in splenocyte cultures from control animals (not shown). These results indicate that BCG infection in mice induces splenic hyporesponsiveness to an in vitro stimulation with SEB and mimics the effect of an in vivo exposure to the superantigen in animals not experimentally introduced to SEB. We observed similar results when BCG-infected mice were stimulated with SEA, a staphylococcal superantigen that targets Vβ3+ T cells (20), and with a MAb specific for the Vβ8+ TCR (data not shown), indicating that BCG infection induces a splenic hyporesponsiveness to in vitro stimulations with mitogens.
FIG. 1.
BCG infection induces splenic hyporesponsiveness to SEB and mimics in vivo exposure to the superantigen. Female BALB/c mice were either injected with SEB (A), infected with BCG (Pasteur) i.v. (B), or injected with PBS (details in Materials and Methods). On day 7 post-SEB injection or day 7, 16, or 30 post-BCG infection, two or three mice were killed per group and single-cell suspensions were prepared from their spleens. Unfractionated splenocytes were stimulated with various concentrations of SEB (0, 0.1, 1.0, and 10 μg/ml) at 5 × 105 cells/well. Culture conditions, as well as cytokine levels and proliferation (stimulation index) determination, were done as described in Materials and Methods. Mean ± standard deviation of triplicate wells is shown.
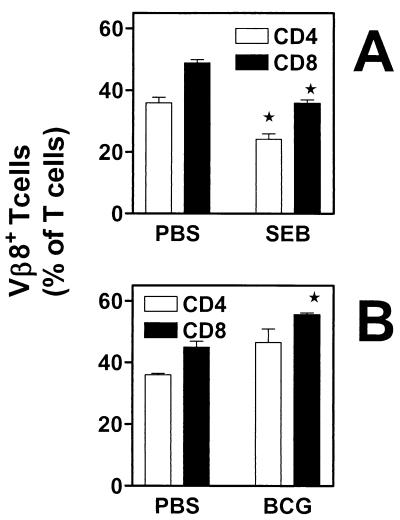
Decreased splenic responsiveness to SEB is not due to the deletion of Vβ8+ CD4+ T cells during BCG infection.
Decreased responsiveness to superantigens is usually seen only in mice that are exposed (in vivo or in vitro) to the individual superantigen (Fig. 1A), due to mechanisms involving deletion and/or anergy of T cells (2). In order to determine whether BCG infection induces deletion of SEB-responsive (Vβ8+) T cells, we examined the percentages of CD4+ and CD8+ Vβ8+ T cells in the spleens of PBS-injected or BCG-infected mice. Flow cytometry analysis of these individual T-cell populations reveals that, unlike what is seen in SEB-injected mice for Vβ8+ T cells (Fig. 2A), the percentages of splenic Vβ8+ T cells are not decreased during BCG infection (Fig. 2B) for the time point examined (2 weeks postinfection). On the contrary, there is an increase in the numbers of CD4+ and CD8+ T cells expressing Vβ3 (data not shown) and Vβ8 TCRs in the spleen of infected mice. Similar results were obtained at days 7 and 30 postinfection (data not shown). Therefore, the splenic hyporesponsiveness that is observed with staphylococcal enterotoxins in vitro due to BCG infection cannot be accounted for by an infection-induced deletion of SEB-responsive T cells.
FIG. 2.
Infection with BCG results in accumulation and not deletion of superantigen-responsive CD4+ or CD8+ T cells. Unfractionated splenocytes (5 × 105 cells/tube) from SEB-exposed mice (7 days postinjection) (A) and PBS-injected controls or day-16-infected mice and PBS-injected controls (B) were stained with FITC-labeled anti-mouse Vβ8 TCR (A and B) and either PE-labeled anti-mouse CD4 or anti-mouse CD8. The percentages of Vβ8+ CD4+ T cells (white bars) or Vβ8+ CD8+ T cells (black bars) in the spleen were determined by flow cytometry. Means ± standard errors of the means of three experiments are shown. ∗, P < 0.05.
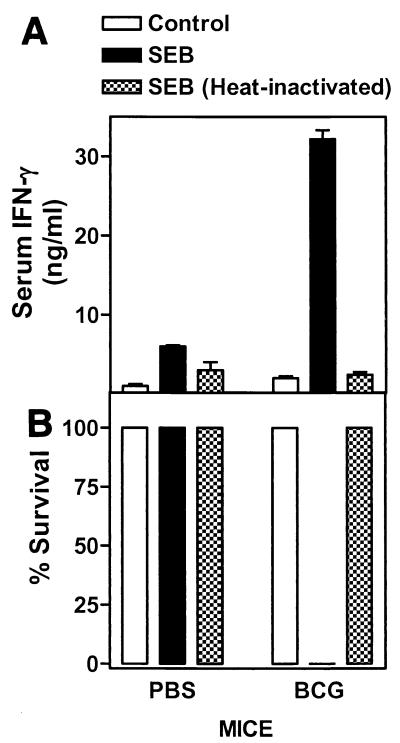
BCG-infected mice are more susceptible to SEB-induced toxic shock.
In previous work, when in vitro splenic hyporesponsiveness to SEB was present in unexposed mice, there was a concurrent increase in resistance to SEB-induced toxic shock in vivo (21). In order to ascertain whether BCG infection resulted in similarly increased resistance to toxic shock (in addition to the observed in vitro anergy), mice infected on day 30 and uninfected controls were injected i.v. with SEB. The mice were bled 8 h postinjection to determine IFN-γ levels in the serum and were monitored for symptoms of toxic shock. In direct contrast to the in vitro results with SEB, BCG-infected animals had fourfold-higher IFN-γ levels in the serum than did noninfected but SEB-injected mice (Fig. 3A). In fact, all BCG-infected mice succumbed to lethal toxic shock within 36 h of SEB injection (Fig. 3B). Uninfected mice also produced IFN-γ, albeit at lower levels, after injection with SEB, yet they survived. To determine whether the effects were mediated by SEB and were not due to endotoxin contamination in the preparation, we injected PBS control and BCG-infected mice with heat-inactivated SEB (which disrupts SEB but not endotoxin activity). Injection of mice with heat-inactivated SEB failed to induce significant IFN-γ production, and none of the mice in either experimental groups died. Furthermore, a second injection of SEB after their recovery was unable to kill the uninfected mice (data not shown). These results suggest that chronic BCG infection enhances the susceptibility of infected mice to SEB-induced toxic shock and that the apparent in vitro hyporesponsiveness seen with splenocytes has no correlate in vivo.
FIG. 3.
BCG-infected mice have increased levels of IFN-γ in their serum after SEB injection and are more susceptible to SEB-mediated toxic shock. PBS controls and BCG-infected mice were injected with heat-inactivated or normal SEB (100 μg/mouse, three mice/group) i.v. 30 days postinfection. Injected mice, along with uninjected controls, were bled 8 h postinjection, and serum IFN-γ levels were determined by ELISA (A). Animals were monitored but were allowed to progress to terminal shock (B). Experiments were performed twice.
Purified splenic T cells from infected mice are superior IFN-γ producers.
To determine whether splenic hyporesponsiveness to SEB in vitro was due to an inherent impairment in T-cell function, we purified CD4+ T cells from the spleens of mice infected for 2 weeks or PBS-injected controls. CD4+ T cells were stimulated with SEB in the presence of dendritic cells, which express high levels of class II and costimulatory molecules (1). BCG infection resulted in impaired IL-2 production and proliferation to SEB in the CD4+-T-cell population (Fig. 4A). IFN-γ production, on the contrary, was enhanced in CD4+ T cells from BCG-infected mice. Similarly, CD8+ T cells from BCG-infected mice showed reduced IL-2 production and proliferation but superior IFN-γ production (data not shown). These results suggest that BCG infection, while stimulating the development of strong IFN-γ producers, impairs the in vitro proliferative function of CD4+ and CD8+ T cells. Furthermore, the superior production of IFN-γ by these T cells mirrors the cytokine levels in the sera of BCG-infected mice that were injected with SEB and thus has an in vivo correlate.
FIG. 4.
Purified CD4+ T cells from infected mice are potent IFN-γ producers. (A) Purified CD4+ (>90% pure) from spleens of mice infected at week 2 or of PBS-injected controls were cocultured at 105 cells/well with irradiated bone marrow-derived dendritic cells (5 × 104 cells/well) with various concentrations of SEB. IL-2, IFN-γ, and proliferation (stimulation indexes) were determined as described in Materials and Methods. Mean ± standard deviation of triplicate wells is shown. (B) Unfractionated splenocytes from mice infected for 2 weeks with BCG HD (106 CFU/mouse) or LD (104 CFU/mouse) or from PBS-injected controls were stimulated with various concentrations of SEB or BCG-Ag at 5 × 105 cells/well. Purified CD4+ T cells from each type of spleen were cocultured at 105 cells/well with irradiated bone marrow-derived dendritic cells (5 × 104 cells/well) in the presence of SEB (10 μg/ml) or BCG-Ag (10 μg/ml). IFN-γ levels were determined as described in Materials and Methods. Mean ± standard deviation of triplicate wells is shown.
Infectious dose is one of the factors reported to influence the effectiveness of BCG immunization (26) with lower in vivo doses inducing stronger type 1 responses when assayed in vitro. We examined whether a 100-fold-lower infectious dose of BCG, which results in a minor splenomegaly, still induces splenic hyporesponsiveness to SEB. We found that splenocytes from LD (104 CFU/mouse)-infected mice were, as IFN-γ producers, superior to both HD (106 CFU/mouse)-infected mice's splenocytes and uninfected controls in response to SEB (Fig. 4B). Next, we examined responses to BCG-Ag in those same spleens in order to determine whether the phenomenon extended to antigen-specific responses for the HD-infected mice. In accordance with published data (26) and similar to the situation with SEB, HD splenocytes produced significantly less IFN-γ than did their LD counterparts (Fig. 4B). We also examined responses to SEB and BCG-Ag from purified CD4+ T cells of PBS-treated and LD- and HD-injected mice. Purified CD4+ T cells from HD-infected mice produced higher levels of IFN-γ to both stimuli than did either LD or control CD4+ T cells (Fig. 4B), although they had reduced IL-2 and proliferative responses (data not shown).
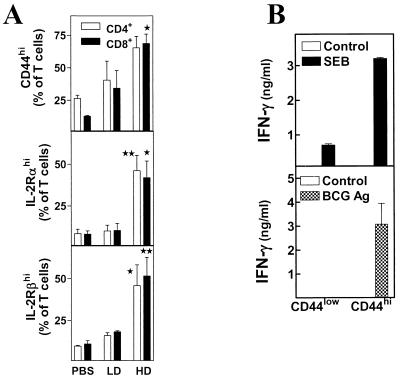
The increased proportion of CD44hi (memory/effector) cells in BCG HD-infected mice correlates with superior IFN-γ production by purified T cells responding to SEB or BCG-Ag.
To look for potential differences between the various populations of T cells, we performed flow cytometry analysis on unfractionated splenocytes from the same time point, examining the surface expression of several T-cell activation markers: CD44, CD25/IL-2Rα, and CD122/IL-2Rβ. Results indicate that T cells in HD spleens (both CD4+ and CD8+) have higher expression levels of all three of these activation markers (Fig. 5A) than do T cells in either LD or PBS splenocytes. There are also more numbers of these activated cells in each HD spleen (data not shown), largely because of the splenomegaly. These results are consistent with the superior capacity of the HD CD4+ T cells to produce IFN-γ in vitro responding to either SEB or BCG-Ag. They are also in line with the increased levels of the cytokine in the sera of HD-infected mice after SEB injection in vivo.
FIG. 5.
BCG infectious dose correlates with increased expression of the T-cell activation markers CD44, IL-2Rα, and IL-2Rβ. Unfractionated splenocytes (5 × 105 cells/tube) from each type of spleen were stained with FITC-labeled anti-mouse CD44, anti-mouse CD25/IL-2Rα, or anti-mouse CD122/IL-2Rβ and PE-labeled anti-mouse CD4 or anti-mouse CD8α. The percentages of CD4+ CD44hi, IL-2Rαhi, or IL-2Rβhi CD4+ (white bars) or CD8+ cells (black bars) in the spleen were determined by flow cytometry. Means ± standard errors of the means of three experiments are shown. ∗, P < 0.05; ∗∗, P < 0.01. Purified CD4+ T cells from the spleens of BCG HD-infected mice (day 16) were stained with FITC anti-mouse CD44 and were sorted in an EPICS Elite ESP (Beckman Coulter) into CD44low (naïve) and CD44hi (memory/effector) populations. Sorted cells were cocultured at 105 cells/well either with bone marrow-derived dendritic cells (5 × 104 cells/well) in the presence or absence of SEB (10 μg/ml) or with M12 B-lymphoma cells (5 × 104 cells/well) in the presence or absence of BCG-Ag (10 μg/ml). IFN-γ levels were determined as described in Materials and Methods. Mean ± standard deviation of triplicate wells is shown.
We were interested in determining whether this increased IFN-γ production by splenic CD4+ T cells from BCG HD-infected mice was a characteristic of the whole CD4+-T-cell population or just a subset of CD4+ T cells. To address this issue, purified CD4+ T cells from BCG HD-infected mice (2 weeks postinfection) were sorted into CD44low (naïve) and CD44hi (memory/effector) subsets and were stimulated with either SEB in the presence of dendritic cells or BCG-Ag in the presence of M12 lymphoma cells (Fig. 5B) as APCs. The results indicate that the CD44hi (memory/effector) CD4+ T cells are the primary IFN-γ producers in response to either SEB or BCG-Ag (Fig. 5B). This subset also produced relatively little IL-2 and proliferated poorly in reaction to SEB (data not shown). No measurable IL-2 was detected in response to BCG-Ag, but anti-IL-2 MAb reduced IFN-γ levels to below detection (data not shown). This result suggests that IL-2 is produced by CD44hi CD4+ T cells but is used for IFN-γ production rather than for proliferation. The CD44low CD4+ T cells, on the other hand, produced little IFN-γ in response to either SEB or BCG-Ag (Fig. 5B). They were able to proliferate better and produce higher levels of IL-2 than the CD44hi CD4+ T cells, particularly in response to SEB (data not shown). Neither subset produced any detectable IL-4 (data not shown), indicating that these cells are either phenotypically type 1 or are differentiating in that direction. Hence, the CD44hi CD4+ T cells generated during BCG HD infection are the primary IFN-γ producers in response to superantigen or bacterial antigens.
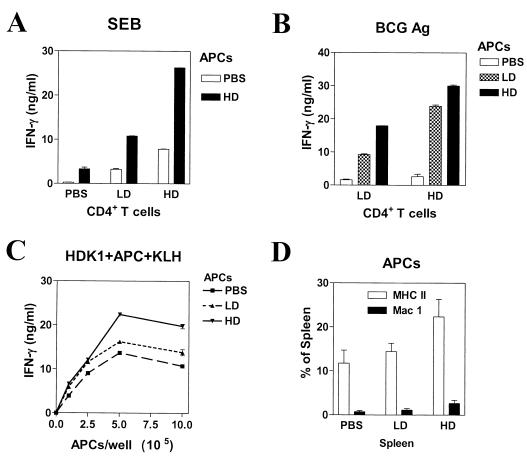
BCG HD infection induces potent APCs that stimulate rather than inhibit IFN-γ production to SEB and BCG-Ag.
While the results with purified T-cell populations correlated well with the in vivo SEB challenge, the results with bulk splenocyte cultures appeared paradoxical. Another possibility was the presence of an inhibitory APC population that would dampen IFN-γ production from those potent T cells upon in vitro stimulation with either superantigens or BCG-Ag. To test this hypothesis, purified CD4+ T cells and adherent splenocyte fractions (largely macrophages and dendritic cells) were cultured in different combinations to observe the effects on IFN-γ response to SEB or BCG-Ag. Adherent splenocytes from HD-infected mice failed to inhibit IFN-γ production of PBS, LD, or HD CD4+ T cells in response to SEB (Fig. 6A) or to BCG-Ag (Fig. 6B). Unexpectedly, HD adherent splenocytes actually stimulated higher levels of IFN-γ production from the various T-cell populations than did either the PBS adherent cells or the LD adherent splenocytes (Fig. 6A and B). To rule out the possibility that some inhibitory APCs were lost during fractionation, we used T-cell-depleted splenocytes from various groups as APCs and obtained similar results (data not shown). Lastly, unfractionated splenocytes from the various groups were used as APCs. We used CD4+ Th1 clone HDK1 cells as responders due to their high KLH-specific IFN-γ production and their unrelatedness to BCG infection. The results obtained (Fig. 6C) mirror those seen using the adherent splenocyte fractions: HD splenocytes, regardless of their numbers in culture, failed to inhibit IFN-γ production by HDK1 cells in response to KLH. In fact, HD spleen cells were actually superior APCs, particularly when cocultured at higher cell numbers. Flow cytometry analysis indicates that MHC class II+ (APCs) and CD11b/Mac1+ (present in some macrophage and dendritic cell populations) expression is increased in HD spleens (Fig. 6D). This may contribute to the superior APC function of splenocytes from infected mice. Overall, these results show that APCs from infected mice are not intrinsically inhibitory to T-cell function and could not account for decreased IFN-γ production seen in HD bulk splenocyte cultures in response to SEB or BCG-Ag.
FIG. 6.
BCG infection induces potent APCs that stimulate rather than inhibit IFN-γ production to SEB and BCG-Ag by purified CD4+ T cells. Sixteen days after injection of mice with PBS or BCG LD or BCG HD, adherent cells were obtained from bulk splenocyte suspensions by panning as described in Materials and Methods. Adherent cell populations (5 × 105 cells/well) were cocultured with purified CD4+ T cells (105 cells/well) from the same mice in the presence of either SEB (10 μg/ml) (A) or BCG-Ag (10 μg/ml) (B). (C) KLH-specific HDK1 type 1 clone was cocultured at 105 cells/well with different numbers of unfractionated splenocytes from PBS control mice or BCG LD- or HD-infected mice in the presence of KLH (10 μg/ml). IFN-γ levels were determined as described in the Fig. 1 legend. Mean ± standard deviation of triplicate wells is shown. (D) BCG-infected spleens have more MHC class II+ and Mac1+ cells. Unfractionated splenocytes (5 × 105 cells/tube) from mice that were infected for 2 weeks with BCG LD- or BCG HD-infected mice or PBS-injected controls were stained with FITC-labeled anti-mouse I-Ad/I-Ed/MHC class II and PE-labeled anti-mouse Mac1/CD11b. The percentages of MHC class II+ (white bars) and Mac1+ (black bars) APCs in the spleen were determined by flow cytometry. Means ± standard errors of the means of three experiments are shown.
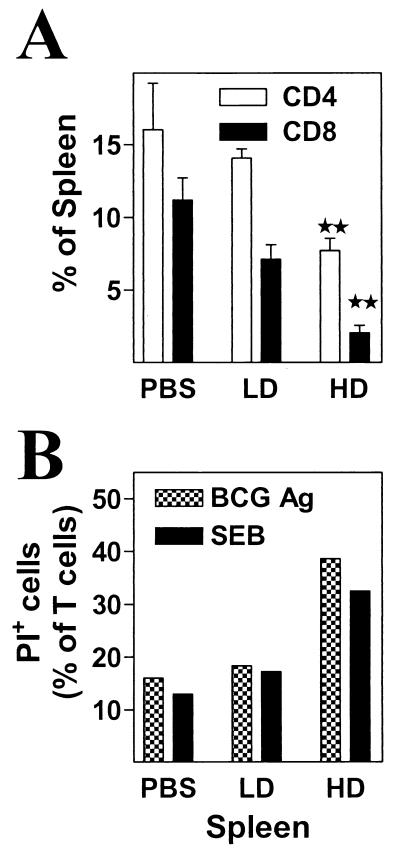
Multiple factors are involved in reducing IFN-γ production in bulk splenocyte cultures.
As both T-cell and APC functions are improved in spleens of infected mice, we examined the possibility that the differences in the relative percentages of T cells in control and infected spleens were an important factor contributing to the apparent in vitro anergy. Disproportionate proliferation of various cellular populations in the spleens of BCG HD-infected mice results in typically half the percentage of CD4+ and CD8+ T cells compared to that found in LD or PBS spleens (Fig. 7A). Another factor could be an increased propensity of type 1 effectors to undergo cell death (already a greater proportion of the T-cell population in these spleens) upon in vitro stimulation, a phenomenon that was described previously (27). To study the possible role of these factors during the in vitro stimulation of bulk splenocytes, we monitored T-cell viability and ensuing IFN-γ levels in 24- and 72-h cultures stimulated with either BCG-Ag or SEB. The percentage of dead T cells is always higher for HD cultures at the 24-h time point (Fig. 7B). Similar results are obtained at the 72-h culture period (data not shown). Lastly, HD splenocytes show higher IFN-γ production within the first 24 h of culture, but this changes by 72 h for both SEB and BCG-Ag (Fig. 8A and B). The total IFN-γ levels increase between 24 and 72 h for each type of spleen; however, the level of accumulation is higher for PBS and LD splenocytes than for their HD counterparts (Fig. 8). Thus, differential accumulation of accessory cells and T cells in control versus HD-infected mice, together with differential proliferation and decreased viability, results in a complex interplay of factors leading in turn to decreased accumulation of cytokine in the supernatants of HD cultures and therefore to an apparent defect.
FIG. 7.
HD splenocytes have a decreased percentage of T cells and show reduced viability early after culture with BCG-Ag and SEB. (A) Unfractionated splenocytes (5 × 105 cells/tube) from mice that were infected for 2 or 3 weeks with BCG LD or BCG HD or from PBS-injected controls were stained with FITC-labeled anti-mouse CD4 and PE-labeled anti-mouse CD8α. The percentages of CD4+ (white bars) or CD8+ cells (black bars) in the spleen were determined by flow cytometry. Means ± standard errors of the means of three experiments are shown. ∗, P < 0.05; ∗∗, P < 0.01. (B) Unfractionated splenocytes were subsequently stimulated with BCG-Ag (10 μg/ml) or SEB (10 μg/ml) at 5 × 105 cells/well in parallel cultures. At 24 h, cells from triplicate wells were pooled and stained with both FITC-labeled anti-mouse CD4 and anti-mouse CD8α and were counterstained with PI (10 μg/ml). The total percentage of nonviable (PI+) T cells was determined by flow cytometry.
FIG. 8.
The deficit in IFN-γ production is not apparent early in the culture period. Shown are levels of IFN-γ in 24- and 72-h supernatants from PBS control or BCG LD or BCG HD splenocyte cultures stimulated with SEB or BCG-Ag (both at 10 μg/ml). IFN-γ levels were determined as described in Materials and Methods. Mean ± standard deviation of cytokine in triplicate wells is shown.
DISCUSSION
One of the characteristics of the adaptive immune system is the ability to generate independent but appropriate immune responses to two or more unrelated insults, regardless of their temporal relationship to each other. Hence, we addressed the question whether a chronic infection that induces a potent type 1 T-cell response affects responsiveness towards superantigens. i.v. infection with BCG in mice results in the development of splenomegaly (25). Infection also induces a BCG-specific type 1 CD4+-T-cell response (this study) (14). Our study describes the apparent capacity of a BCG HD infection to induce splenic hyporesponsiveness to SEB in mice never overtly exposed to this potent staphylococcal exotoxin. This in vitro splenic hyporesponsiveness stands in sharp contrast to the increased susceptibility of BCG-infected mice to SEB-mediated toxic shock, an indication of hyperresponsiveness. The situation with BCG-infected mice is unlike that in CD28 knockout mice, which show both in vitro splenic hyporesponsiveness to SEB in unexposed mice and increased resistance to SEB-induced toxic shock in vivo (21). Lethal synergy between LPS-like molecules and superantigens has been reported (3, 4, 10, 16, 18); therefore, it is likely that BCG-infected mice suffer a similar lethal outcome upon SEB injection. Contamination of SEB with low levels of LPS is unlikely to have been the cause of the lethal shock, as the SEB batch used in vivo was tested for LPS contamination and found to have <0.06 EU/100 μg of protein. In addition only the infected mice died, indicating that, whatever the trace levels of LPS, they are not sufficient to lethally sensitize normal mice. Furthermore, heat-inactivated SEB (which disrupts SEB but not LPS activity) did not have any effect on mice in vivo or IFN-γ production in vitro (data not shown).
We also evaluated the discrepancy between in vitro splenocyte response and T-cell-mediated protection in a tumor challenge model (data not shown). Mice infected with a LD (104 CFU) of recombinant BCG-Ova (expressing ovalbumin) were partially protected after a challenge with Ova-expressing tumor cells (B16Ova), whereas mice infected with a HD (106 CFU) of BCG-Ova exhibited strong protection. These results further support the notion that chronic mycobacterial infection does not lead to functional in vivo anergy.
The decreased in vitro responsiveness to superantigens, on the other hand, resembles the reported phenomenon of anergy seen in mycobacterial infections in response to mitogens like phytohemagglutinin (24), concanavalin A (6), and anti-CD3 MAb (15). This anergy is characterized by a proliferative block and decreased IL-2 secretion and production (29) and has been attributed to both suppressor T cells and/or inhibitory macrophages via the production of inhibitory mediators like IL-6 (34), transforming growth factor beta (13), and NO (36). A similar phenomenon is seen with some preparations of mycobacterial antigens, like purified protein derivative, particularly at high infection burdens in both mice and humans (reviewed in reference 7).
An increase in CD44 expression correlates with the acquisition of memory/effector function in T cells (11). We have shown that CD44hi T cells accumulate during BCG infection, particularly at higher bacterial burdens, and that these cells are the major in vitro producers of IFN-γ in response to SEB. This is in contrast with the hyporesponsiveness of effector CD4+ T cells anergized by previous exposure (either in vivo or in vitro) to the superantigen (17). It seems likely that the CD44hi T cells are the source of the high IFN-γ levels seen in the sera of BCG-infected mice shortly after injection with SEB. The superior production of IFN-γ by purified splenic T cells from BCG HD-infected mice may be similarly linked to the increased proportion of CD44hi T cells seen in these mice.
It is still unclear whether type 1 effectors have a decreased ability to produce IL-2 compared to naïve T cells (22), although there is some evidence to indicate that this may be the case (30). We observe that the time-dependent loss in IL-2 production and proliferation by bulk splenocytes or purified T cells in response to SEB correlates with splenomegaly and resulting accumulation of CD44hi T cells. It is thus possible that the observed loss of in vitro IL-2 production and proliferation (“anergy”) could simply be the natural consequence of the in vivo immune response. This would occur during any strong chronic type 1 response and could account for the loss of responsiveness (proliferation) to mitogens or bacterial antigens at high infectious loads. The greater the antigenic burden, the higher is the number of type 1 effectors generated that respond to subsequent stimuli by exerting their effector function rapidly without proliferating.
Nevertheless, we have observed that CD44hi type 1 cells produce some IL-2, as neutralization of this cytokine early in culture results in loss of IFN-γ production (J. A. Pedras-Vasconcelos and S. Sad, unpublished data). IL-2 appears to be used not for proliferation but for IFN-γ production and perhaps other effector functions. If either IFN-γ is neutralized in or IL-4 is added to either unfractionated splenocyte or purified T-cell cultures, proliferative responses increase (Pedras-Vasconcelos and Sad, unpublished). In vitro at least, IFN-γ is having a feedback regulatory role, preventing the excessive expansion of CD44hi type 1 effector cells. According to a recent report, a similar situation may be found in vivo (8). BCG infection in IFN-γ knockout mice resulted in a longer-lasting splenomegaly, due to decreased attrition (by apoptosis) of CD44hi CD4+ T cells (8). We would predict that CD44hi CD4+ T cells from infected IFN-γ knockout mice will not be proliferatively impaired upon in vitro stimulation with SEB, at least in part due to their decreased propensity for apoptosis.
In normal mice infected with BCG, despite the reduced proliferative responses seen in vitro, splenomegaly still takes place. This would suggest that other cytokines (e.g., IL-4, IL-7, IL-9, and IL-15) might be involved in the expansion and maintenance of effector T cells in vivo (19), compensating for the decreased IL-2 levels. Another possibility is that there is a continuous turnover of effector T cells during BCG infection, with effector T-cell attrition, as well as naïve T-cell differentiation to effectors occurring on a continual basis (31). The observed decrease in proliferation for both unfractionated splenocytes and purified T cells from BCG-infected mice could in part be due to increased activation-induced cell death (AICD). Type 1 cells are also more susceptible to AICD than are Th0 and -2 cells (27, 37). Previous work with BCG-infected mice showed purified T cells to have diminished anti-CD3 and concanavalin A-triggered proliferative responses due to increased AICD, although no impact on IL-2 and IFN-γ was reported (15). The increased prevalence of nonviable T cells in unfractionated HD splenocyte cultures after stimulation with SEB or BCG-Ag is consistent with what is found in this work. However, while with splenocyte cultures there is decreased IFN-γ, the same is not seen with purified T cells, in spite of impaired proliferative responses. This apparent discrepancy could be explained in part by considering the difference in T-cell numbers in the bulk splenocyte cultures. HD splenocyte cultures typically have half the T-cell numbers of their LD and PBS counterparts. In the purified T-cell cultures, T-cell and APC numbers are equalized, but there is a higher proportion of CD44hi T cells in HD cultures. The differences in IFN-γ levels range between 2- and 10-fold for the splenocyte cultures, and this is also seen for the purified T-cell cultures, except the trend is reversed in favor of HD T cells. For the unfractionated splenocytes, the system is highly complex, and thus the relationship is not a linear one between T-cell numbers and IFN-γ levels but rather a chaotic one. In complex systems, where cooperativity is involved between multiple cell types, small changes in initial conditions (T-cell numbers at the start of the culture period) can have significant impacts in the outcome (the measurable IFN-γ levels at 72 h) (5).
The absence of a defect in IFN-γ production in purified T-cell populations from HD spleens leads us to examine the possible existence of inhibitory APCs in these spleens, as shown in other cases (8, 35). We were unable to inhibit IFN-γ production from purified T cells using adherent cell populations, T-cell-depleted splenocytes (Pedras-Vasconcelos and Sad, unpublished), or unfractionated splenocytes. For every T-cell population examined, HD APCs were superior in their ability to present and/or costimulate IFN-γ production, for SEB, BCG-Ag, and KLH. This would indicate that APCs from infected mice are not intrinsically inhibitory to T-cell function and could not alone account for the reduced cytokine responses seen in unfractionated splenocyte cultures.
The in vitro hyporesponsiveness to SEB and BCG-Ag is observable only with unfractionated splenocytes and not with purified T cells or fractionated APCs. When cytokine levels are examined earlier in the culture period, there is no defect, as HD splenocyte cultures have IFN-γ levels similar to or higher than those for their BCG LD or PBS counterparts. This is all the more remarkable, considering that there is always a smaller proportion of T cells in HD cultures than in the other two kinds of culture. Several factors may contribute to the decreased accumulation of IFN-γ in HD splenocyte cultures: the presence of fewer (if more potent) T cells and of superior APCs (in numbers and function), the inability of the effector T cells to proliferate, and their increased propensity to undergo AICD. Our study illustrates the hazard of drawing excessively on in vitro observations to extrapolate to and explain in vivo conditions. Thus, our study suggests a reevaluation of the concept that chronic mycobacterial infections truly induce T-cell anergy to mitogens and mycobacterial antigens. The degree at which the T-cell proliferative responses are impaired in vitro appears to reflect the magnitude of the type 1 T memory/effector response, which in turn correlates with the mycobacterial burden. Thus, what is termed “anergy” may simply be a natural consequence of the development of a potent type 1 T-cell response during mycobacterial infection.
Acknowledgments
We thank Margaret-Anne Gidney for cell sorting, Edward Pearce for helpful discussions and critical reading of the manuscript, and Peter Bretscher and Robert North for insightful comments.
Editor: R. N. Moore
Footnotes
This is publication no. 42455 from the National Research Council of Canada.
REFERENCES
- 1.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 2.Baschieri, S., R. K. Lees, A. R. Lussow, and H. R. MacDonald. 1993. Clonal anergy to staphylococcal enterotoxin B in vivo: selective effects on T cell subsets and lymphokines. Eur. J. Immunol. 23:2661-2666. [DOI] [PubMed] [Google Scholar]
- 3.Beno, D. W., M. R. Uhing, M. Goto, Y. Chen, V. A. Jiyamapa-Serna, and R. E. Kimura. 2001. Staphylococcal enterotoxin B potentiates LPS-induced hepatic dysfunction in chronically catheterized rats. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G866-G872. [DOI] [PubMed] [Google Scholar]
- 4.Blank, C., A. Luz, S. Bendigs, A. Erdmann, H. Wagner, and K. Heeg. 1997. Superantigen and endotoxin synergize in the induction of lethal shock. Eur. J. Immunol. 27:825-833. [DOI] [PubMed] [Google Scholar]
- 5.Callard, R., A. J. George, and J. Stark. 1999. Cytokines, chaos, and complexity. Immunity 11:507-513. [DOI] [PubMed] [Google Scholar]
- 6.Colizzi, V. 1984. In vivo and in vitro administration of interleukin 2-containing preparation reverses T-cell unresponsiveness in Mycobacterium bovis BCG-infected mice. Infect. Immun. 45:25-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins, F. M. 1993. Tuberculosis: the return of an old enemy. Crit. Rev. Microbiol. 19:1-16. [DOI] [PubMed] [Google Scholar]
- 8.Dalton, D. K., L. Haynes, C. Q. Chu, S. L. Swain, and S. Wittmer. 2000. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J. Exp. Med. 192:117-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinges, M. M., and P. M. Schlievert. 2001. Role of T cells and gamma interferon during induction of hypersensitivity to lipopolysaccharide by toxic shock syndrome toxin 1 in mice. Infect. Immun. 69:1256-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutton, R. W., L. M. Bradley, and S. L. Swain. 1998. T cell memory. Annu. Rev. Immunol. 16:201-223. [DOI] [PubMed] [Google Scholar]
- 12.Fleischer, B., and H. Schrezenmeier. 1988. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J. Exp. Med. 167:1697-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch, C. S., J. J. Ellner, R. Blinkhorn, and Z. Toossi. 1997. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc. Natl. Acad. Sci. USA 94:3926-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramnik, I., D. Radzioch, and E. Skamene. 1994. T-helper 1-like subset selection in Mycobacterium bovis bacillus Calmette-Guerin-infected resistant and susceptible mice. Immunology 81:618-625. [PMC free article] [PubMed] [Google Scholar]
- 15.Kremer, L., J. Estaquier, I. Wolowczuk, F. Biet, J. C. Ameisen, and C. Locht. 2000. Ineffective cellular immune response associated with T-cell apoptosis in susceptible Mycobacterium bovis BCG-infected mice. Infect. Immun. 68:4264-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeClaire, R. D., R. E. Hunt, S. Bavari, J. E. Estep, G. O. Nelson, and C. L. Wilhelmsen. 1996. Potentiation of inhaled staphylococcal enterotoxin B-induced toxicity by lipopolysaccharide in mice. Toxicol. Pathol. 24:619-626. [DOI] [PubMed] [Google Scholar]
- 17.Lee, W. T., and E. S. Vitetta. 1992. Memory T cells are anergic to the superantigen staphylococcal enterotoxin B. J. Exp. Med. 176:575-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luhm, J., H. Kirchner, and L. Rink. 1997. One-way synergistic effect of low superantigen concentrations on lipopolysaccharide-induced cytokine production. J. Interferon Cytokine Res. 17:229-238. [DOI] [PubMed] [Google Scholar]
- 19.Marrack, P., J. Bender, D. Hildeman, M. Jordan, T. Mitchell, M. Murakami, A. Sakamoto, B. C. Schaefer, B. Swanson, and J. Kappler. 2000. Homeostasis of alpha beta TCR+ T cells. Nat. Immunol. 1:107-111. [DOI] [PubMed] [Google Scholar]
- 20.Miller, C., J. A. Ragheb, and R. H. Schwartz. 1999. Anergy and cytokine-mediated suppression as distinct superantigen-induced tolerance mechanisms in vivo. J. Exp. Med. 190:53-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mittrucker, H. W., A. Shahinian, D. Bouchard, T. M. Kundig, and T. W. Mak. 1996. Induction of unresponsiveness and impaired T cell expansion by staphylococcal enterotoxin B in CD28-deficient mice. J. Exp. Med. 183:2481-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 23.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 24.Orme, I. M., M. J. Ratcliffe, and F. M. Collins. 1984. Acquired immunity to heavy infection with Mycobacterium bovis bacillus Calmette-Guerin and its relationship to the development of nonspecific unresponsiveness in vitro. Cell. Immunol. 88:285-296. [DOI] [PubMed] [Google Scholar]
- 25.Pelletier, M., A. Forget, D. Bourassa, P. Gros, and E. Skamene. 1982. Immunopathology of BCG infection in genetically resistant and susceptible mouse strains. J. Immunol. 129:2179-2185. [PubMed] [Google Scholar]
- 26.Power, C. A., G. Wei, and P. A. Bretscher. 1998. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect. Immun. 66:5743-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsdell, F., M. S. Seaman, R. E. Miller, K. S. Picha, M. K. Kennedy, and D. H. Lynch. 1994. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Int. Immunol. 6:1545-1553. [DOI] [PubMed] [Google Scholar]
- 28.Sad, S., R. Marcotte, and T. R. Mosmann. 1995. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity 2:271-279. [DOI] [PubMed] [Google Scholar]
- 29.Saha, B., G. Das, H. Vohra, N. K. Ganguly, and G. C. Mishra. 1994. Macrophage-T cell interaction in experimental mycobacterial infection. Selective regulation of co-stimulatory molecules on Mycobacterium-infected macrophages and its implication in the suppression of cell-mediated immune response. Eur. J. Immunol. 24:2618-2624. [DOI] [PubMed] [Google Scholar]
- 30.Seder, R. A., and W. E. Paul. 1994. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 12:635-673. [DOI] [PubMed] [Google Scholar]
- 31.Sprent, J., and D. F. Tough. 2001. T cell death and memory. Science 293:245-248. [DOI] [PubMed] [Google Scholar]
- 32.Szalay, G., and S. H. Kaufmann. 1996. Functional T cell subsets in mycobacterial and listerial infections: lessons from other intracellular pathogens. Curr. Top. Microbiol. Immunol. 215:283-302. [DOI] [PubMed] [Google Scholar]
- 33.Ulrich, R. G. 2000. Evolving superantigens of Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 27:1-7. [DOI] [PubMed] [Google Scholar]
- 34.VanHeyningen, T. K., H. L. Collins, and D. G. Russell. 1997. IL-6 produced by macrophages infected with Mycobacterium species suppresses T cell responses. J. Immunol. 158:330-337. [PubMed] [Google Scholar]
- 35.Welsh, R. M. 2001. Assessing CD8 T cell number and dysfunction in the presence of antigen. J. Exp. Med. 193:F19-F22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang, J., I. Kawamura, and M. Mitsuyama. 1997. Involvement of inflammatory cytokines and nitric oxide in the expression of non-specific resistance to Listeria monocytogenes in mice induced by viable but not killed Mycobacterium bovis BCG. Microb. Pathog. 22:79-88. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, X., T. Brunner, L. Carter, R. W. Dutton, P. Rogers, L. Bradley, T. Sato, J. C. Reed, D. Green, and S. L. Swain. 1997. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J. Exp. Med. 185:1837-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zinkernagel, R. M. 1996. Immunology taught by viruses. Science 271:173-178. [DOI] [PubMed] [Google Scholar]