Abstract
The validation of flow cytometry analysis of anti-live trypomastigote antibodies (FC-ALTA) to monitor cure after treatment of Chagas' disease was evaluated with serum samples from treated and nontreated chagasic patients. After optimization of the original technique, toward better sensitivity and applicability to field surveys, we design a double blind study of 94 coded samples classified into the following categories: patients not treated (NT) and patients treated but not cured (TNC), both presenting positive conventional serology and xenodiagnosis; patients treated and cured (TC), showing negative serology and xenodiagnosis; and patients treated under evaluation (TUE), who presented positive or oscillating conventional serology (CSA) but negative xenodiagnosis. Coded samples, diluted 1:256, were assayed by incubation with live cell culture trypomastigotes, which were subsequently stained with fluorescein isothiocyanate-conjugated anti-human immunoglobulin G, with prior fixation and analysis by flow cytometry. The results were expressed as the percentages of positive fluorescent parasites (PPFP) for each individual sample, establishing 20% PPFP as the cutoff between negative and positive results. Our data demonstrated that all NT and TNC presented positive results while all but one TC had a PPFP lower than 20%. Analysis of TUE demonstrated a wide degree of reactivity, with PPFP values that were negative (PPFP ≤ 20%), low positive (20% < PPFP ≤ 50%), and high positive (PPFP > 50%). As TUE with negative PPFP presented negative xenodiagnosis and positive or oscillating CSA, they were classified as dissociated according to the criteria of Krettli and Brener (J. Immunol. 128:2009-2012, 1982) and could indeed be considered cured after chemotherapy. This study demonstrates and validates the use of FC-ALTA to easily identify anti-live trypomastigote membrane-bound antibodies, offering another approach for investigating and monitoring the efficacy of specific chemotherapy in cases of human Chagas' disease.
Chagas' disease is a parasitic infection caused by the protozoan Trypanosoma cruzi, which is widespread in South and Central America, and transmitted mainly by an invertebrate vector, triatomine (20). Although autochthonous cases are rare in other localities, transmission of Chagas' disease by blood transfusion may represent an increasing public health problem in countries in which Chagas' disease is not endemic (20).
Chemotherapy with nitroheterocyclic compounds has been indicated for the treatment of acute cases (4, 6, 10). In patients with chronic disease, however, the value of chemotherapy is still controversial. So far, it has been recommended for the treatment of patients with recent chronic disease who have not yet developed clinical symptoms (11).
One of the major challenges regarding the evaluation of treatment effectiveness is the lack of truthful laboratorial approaches for use as tools for cure criteria. Two categories of tests are available, including serological and parasitological methods. Parasitological tests are based on parasite demonstration by hemoculture, xenodiagnosis, or parasitological molecular test (PCR), whereas serological methods evaluate the presence of specific antibodies by immunological methods, such as indirect hemagglutination (IHA), indirect immunofluorescence assay (IFA), and enzyme-linked immunosorbent assay (ELISA).
Using serological approaches, Krettli and Brener (15) proposed that sera from chronic chagasic patients present two types of anti-parasite antibodies with different functional activities named lytic antibodies (LA) and conventional serology antibodies (CSA). LA are associated with resistance in active ongoing infection and can be detected by complement-mediated lysis (CoML) and indirect immunofluorescence, all with live trypomastigotes (14, 15). On the other hand, CSA are neither associated with resistance nor able to bind to live parasites, but they do react to soluble or fixed epimastigote antigens and are detected by different immunological methods (16).
Considering these findings, clinical trials have demonstrated that LA gradually become negative after treatment, whereas CSA can remain positive over decades. So it has been proposed that the cure criterion for Chagas' disease should be based on either negative parasitological tests or the absence of anti-live trypomastigote antibodies (ALTA) (13).
A flow cytometric method to detect ALTA (FC-ALTA) has been previously described as a new approach for monitoring the efficacy of treatment in cases of human Chagas' disease. The performance of FC-ALTA has been demonstrated to be comparable to that of CoML, offering an alternative method for easily identifying anti-live T. cruzi membrane-bound antibodies (18).
In this study, we describe an optimization of FC-ALTA analysis and evaluate its performance for clinical studies. A double-blind study, with serum samples from treated and nontreated chagasic patients, was conducted to validate the method, reemphasizing its applicability for monitoring cure after treatment of Chagas' disease. Moreover, the data presented here offer an optimization of the original technique, making our method more sensitive and applicable to field studies.
MATERIALS AND METHODS
Patients.
The inclusion of all subjects in our investigation had the approval of the FIOCRUZ Ethical Committee (Brazilian Health Ministry). In this study, we analyzed 94 patients (53 females and 41 males) ranging in age from 6 months to 68 years assisted by one of us at the Faculdade de Medicina, Universidade Federal de Goiás, GoiÂnia, Goiás, Brazil. Chagas' disease diagnosis was established in all patients by positive xenodiagnosis and three positive serological tests, including IHA, IFA, and ELISA. All patients in the acute (n = 13) and subacute (n = 3) phases were treated with benznidazole (BZ) (60 mg/kg of body weight/day for 60 days). Patients in the chronic phase (n = 78) were treated with BZ (n = 44), allopurinol (n = 3), nifurtimox (n = 3), or BW349C59 (n = 6) or not treated (n = 22). In this study, we did not focus on the efficacy of the different therapeutic schemes used since it was not the major goal of our present investigation. After clinical, parasitological, and serological follow-up studies, ranging from 3 to 26 years, patients were classified into three different categories: not treated (NT) (n = 22), all patients with chronic disease; treated but not cured (TNC) (n = 19), 4 patients with acute- and 15 patients with chronic-phase disease; treated and cured (TC) (n = 25), 8 patients with acute-, 3 patients with subacute-, and 14 patients with chronic-phase disease; and treated under evaluation (TUE) (n = 28), 1 patient with acute- and 27 patients with chronic-phase disease (Table 1). NT and TNC patients had positive results on both serological and parasitological tests. Patients were considered TC only when both tests, serological and parasitological, were consistently and repeatedly (at least eight times) negative. Patients presenting with positive (n = 17) or oscillating (n = 11) serology with negative parasitological tests were considered TUE. Samples were coded and stored pure at −20°C or in 50% glycerol at room temperature.
TABLE 1.
Patients, conventional serology, and xenodiagnosis
| Patient group | n | No. of patients treated witha:
|
Result with:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| BZ | NF | ALLO | BW | IFA | ELISA | IHA | Xeno- diagnosis | ||
| NT | 22 | 0 | 0 | 0 | 0 | + | + | + | + |
| TNC | 19 | 14 | 1 | 3 | 1 | + | + | + | + |
| TC | 25 | 23 | 1 | 0 | 1 | − | − | − | − |
| TUE | 17 | 15 | 0 | 0 | 2 | + | + | + | − |
| 11 | 8 | 1 | 0 | 2 | OSCb | OSC | OSC | − | |
NF, nifurtimox; ALLO, allopurinol; BW, BW349C59.
OSC, oscillating results with serologic tests.
Conventional serological tests.
Three kinds of serological tests were performed as recommended by the World Health Organization (20): IHA (Imunoserum, São Paulo, Brazil), IFA (Biolab, Rio de Janeiro, Brazil), and ELISA (Abbott). At the beginning of the study, we used the complement fixation reaction, which was available at that time, to screen some patients.
Xenodiagnosis.
Xenodiagnosis was performed on all patients before and after treatment as described by Cerisola et al. (7) by using triatomine from Dipetalogaster maximus and Triatoma infestans according to availability. Forty triatomines, after a 15-day fast, were allowed to feed on each patient for 30 min. Microscopic examination of intestinal contents was carried out 30 and 60 days after feeding. The number of xenodiagnoses per patient ranged from 8 (320 triatomines) to 114 (4,500 triatomines).
Cell line and parasites.
NTCT clone 929 cells (L929, ATCC CCL 1) were maintained in our laboratory by serial passages and frozen in liquid nitrogen. For the assays, 5 × 105 L929 cells were seeded in tissue culture flasks (25 or 75 cm2, Falcon; Becton Dickinson, San Jose, Calif.) with 10 ml of RPMI medium (GIBCO, Grand Island, N.Y.) containing 10% fetal bovine serum (FBS) and incubated at 37°C in humidified air containing 5% CO2. After 2 or 3 days, the monolayer was infected with 3 × 106 to 7 × 106 trypomastigotes of T. cruzi strain CL (5). Trypomastigotes were obtained initially from infected mice and subsequently from the supernatant of another infected monolayer. Infected cells were washed daily with RPMI-5% FBS. The cultures were maintained in RPMI and 10% FBS at 33°C, 5% CO2, and 95% humidity (3). After 5 to 7 days of incubation, the trypomastigotes started being released into the supernatant. The cell culture was transferred to a 50-ml tube and centrifuged at low speed (100 × g for 10 min at room temperature) to remove cell debris and some contaminating amastigotes. Trypomastigotes recovered from the supernatant were washed three times (1,000 × g for 15 min at 4°C) and resuspended in phosphate-buffered saline (PBS) and 10% FBS at a concentration of 1 × 106 parasites/ml. A 50-μl sample was used for FC-ALTA.
FC-ALTA.
The immunofluorescence reaction was performed as described by Martins-Filho et al. (18), modified as follows. Live trypomastigotes (500,000) were incubated at 37°C for 30 min in the presence of a 1:256 final dilution of serum (whole or diluted 1:1 in glycerol), using a coded serum sample. After incubation with sera, parasites were washed once with PBS containing 10% FBS. Parasite suspensions were reincubated at 4°C for 1 h or 37°C for 30 min in the dark in the presence of fluorescein isothiocyanate (FITC)-conjugated anti-human immunoglobulin G (IgG) antibody (anti-whole molecule) (Sigma, St. Louis, Mo.) diluted 1:400 in PBS-10% FBS. The use of different temperatures and times of incubation with FITC-conjugated anti-human IgG antibody represented the major change from the original method described by Martins-Filho et al. (18), where incubation was performed only at 4°C for 60 min. Each assay included an internal control of nonspecific binding as well as positive and negative controls. To monitor unspecific binding, parasites not exposed to human serum were incubated with FITC-conjugated anti-human IgG. Positive controls included sera from NT chagasic patients, whereas samples from uninfected individuals were included as negative controls. After being stained, labeled parasites were washed with PBS-10% FBS and fixed on ice for 30 min with a fix solution (10 g of paraformaldehyde per liter, 1% sodium cacodylate, 6.65 g of sodium chloride per liter, and 0.01% sodium azide, pH 7.2). Flow cytometry analysis was performed up to 24 h after parasite fixation.
FACScan data storage and analysis.
Flow-cytometric measurements were performed on a Becton Dickinson FACScan interfaced to an Apple Quadra FACStation. CellQuest software was used for both data storage and analysis. Trypomastigotes were identified based on their specific forward (FSC) and side (SSC) laser-scattering properties. Following FSC and SSC adjustments, parasites were localized on FSC (size) by SSC (internal complexity-granularity) dot plot distribution (Fig. 1a). Parasites were then selected by gating on the FSC by SSC dot plot distribution. An average of 8,000 to 9,000 gated trypomastigotes was analyzed for relative fluorescence intensity by using single FITC histograms for each individual sample. A control marker of up to 2% of the parasites that were fluorescence positive was set up on the FITC-conjugated internal control histogram (Fig. 1b). This marker was used to determine the percentage of positive fluorescent parasites (PPFP) for each sample. Figures 1c and d show representative histograms for the negative and positive controls, respectively. Data analysis was performed by establishing 20% PPFP as the cutoff between negative and positive results as described by Martins-Filho et al. (18). Thus, samples were considered positive when the PPFP was >20% and negative when the PPFP was ≤20%. Positive results were additionally classified according to the method of Cordeiro et al. (9) as low positive (20% < PPFP ≤ 50%) and high positive (PPFP > 50%).
FIG. 1.
FC-ALTA. (a) Dot plot analysis of a representative trypomastigote distribution (R1) based on size and granularity. Single histograms represent PPFP values for FITC-conjugated anti-human IgG internal control (b), negative control (c), and positive control (d).
Statistics.
Statistical analysis was performed using one-way analysis of variance followed by Student's t test. Differences were considered significant when the P value was <0.05.
RESULTS
Optimization of original method for FC-ALTA. (i) Effect of incubation temperature on PPFP values.
In order to evaluate the effect of incubation conditions on the performance of FITC-conjugated anti-human IgG antibody binding, we performed a parallel study in which a 1-h incubation at 4°C was compared to a 30-min incubation at 37°C. Figure 2 shows representative histogram analyses demonstrating that the latter condition led to higher PPFP values for samples from one NT patient, whereas no change was detected for a sample from a TC patient (Fig. 2a and b). Using a range of samples, our data demonstrated that incubation of parasites for 30 min at 37°C increases the sensitivity of FC-ALTA (P = 0.0007 for NT patients and P = 0.0001 for TNC patients) with no effect on its ability to discriminate samples with PPFP values of ≤20% (P = 0.68) (Fig. 2c). Therefore, we have adjusted this condition in the original method in order to improve the sensitivity of FC-ALTA.
FIG. 2.
Representative histograms showing effect of incubation temperature on PPFP values. Parasites were incubated in parallel study with a 1:256 dilution of PBS-10% FBS-diluted human sera (from NT and TC patients) followed by a 1-h incubation at 4°C (a) or a 30-min incubation at 37°C (b) with FITC-conjugated anti-human IgG (whole-molecule) antibody. (c) A range of samples were tested for both protocols (−4°C for 60 min [○] and 37°C for 30 min [•]), including samples from the NT (n = 12), TNC (n = 9), and TC (n = 4) patient groups. Statistical analysis demonstrated significant differences between PPFP values obtained at 37°C for 60 min in comparison to those obtained at 4°C for 60 min for the NT and TNC patient groups but not for the TC patient group.
(ii) Comparison of PPFP values between pure and GPS.
With the purpose of fitting the method for field studies, in which storage of frozen samples (FS) may not be possible, we tested the applicability of our method to glycerol-preserved samples (GPS) that can easily be maintained at room temperature. Parallel studies which used 70 pairs of FS and GPS samples were carried out, and the PPFP values for each individual sample are presented in Fig. 3. Data analysis was performed by grouping the samples based on the PPFP values obtained with FS as negative (PPFP ≤ 20%), low positive (20% < PPFP ≤ 50%), or high positive (PPFP > 50%) followed by comparison with the PPFP values obtained with GPS. One out of 29 samples with negative results (PPFP = 20.0%) became low positive (PPFP = 25.0%) when tested as GPS. Two out of 11 samples with low-positive results showed a distinct result when tested as GPS. One became negative (PPFP = 26.0 to 17.0%) and another one became high positive (PPFP = 50.0 to 66.0%). No changes were observed in the performance of FC-ALTA for all 40 samples that showed high-positive results. Statistical analysis did not show any differences between the mean PPFP values obtained from GPS and FS (P = 0.89 for results with PPFP ≤ 20%, P = 0.66 for results with 20% < PPFP ≤ 50%, and P = 0.68 for results with PPFP > 50%).
FIG. 3.
Comparison of PPFP values between pure and GPS. Parallel studies with pairs of FS (•) and GPS (○) samples were carried out, and the results were presented as PPFP values. Samples were grouped based on the PPFP values obtained with FS followed by comparison with the PPFP values obtained with GPS. No significant differences were observed between mean PPFP values obtained from FS and GPS.
Performance of FC-ALTA with treated and nontreated patient samples. (i) PPFP values from NT and TNC patients.
PPFP values from chagasic patients with positive serology and xenodiagnosis are presented in Fig. 4. Our data demonstrated that all NT patients showed positive PPFP values. Moreover, it was interesting that NT samples were always high positive (PPFP > 50%) (Fig. 4a). The TNC patients were subdivided into two groups as they received treatment during the acute or chronic phase of the disease (Fig. 4b). In the same manner, all TNC patients presented PPFP values greater than the 20% cutoff, and then they did not differ from NT patients. However, in this group we found two low-positive samples with PPFP values of >20% and ≤50% (samples 38 and 76), suggesting that the therapy was able to decrease parasitemia and also lower antibody titers but not enough to make them free of infection (Fig. 4b).
FIG. 4.
Analysis of PPFP values from patients with positive xenodiagnosis and positive conventional serology. The performance of FC-ALTA with samples from the NT (a) and TNC (b) patient groups was investigated by using FS, and data analysis was performed by establishing a 20% PPFP value as the cutoff between negative and positive results, as described by Martins-Filho et al. (18). Positive results were additionally classified according to the method of Cordeiro et al. (9) as low positive (20% < PPFP ≤ 50%) and high positive (PPFP > 50%). The TNC patients were subdivided into two groups, as they received treatment during the acute (A) or chronic (C) phase of the disease.
(ii) PPFP values from TC individuals.
PPFP results for TC individuals are presented in Fig. 5. The patients were subdivided into three groups as they received treatment during the acute, subacute, or chronic phase of the disease. All but one (sample 17) TC individual had PPFP values lower than 20%. With the aim of further investigating this unexpected result, after decoding the patient identification number, we retested this sample from 1997 along with two other previous samples from 1981 and 1989, taken before treatment and early after treatment, respectively. The results of this analysis confirm the high reactivity of this patient before and at different times after treatment (PPFP = 95.5, 94.3, and 95.1%, respectively).
FIG. 5.
Analysis of PPFP values from patients with negative xenodiagnosis and negative conventional serology. The performance of FC-ALTA for samples from the TC patient group was investigated by using FS, and data analysis was performed by establishing a 20% PPFP value as the cutoff between negative and positive results, as described by Martins-Filho et al. (18). Positive results were additionally classified according to the method of Cordeiro et al. (9) as low positive (20% < PPFP ≤ 50%) and high positive (PPFP > 50%). The TC patients were subdivided into three groups, as they received treatment during the acute (A), subacute (SA), or chronic (C) phase of the disease.
(iii) PPFP values from TUE patients.
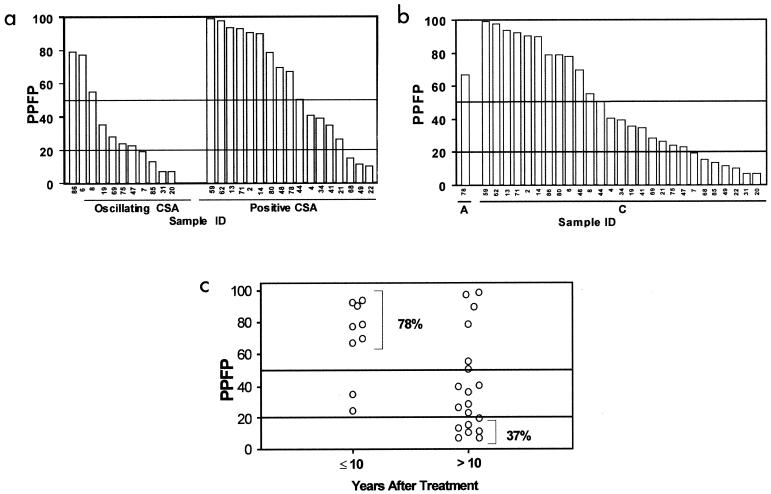
PPFP results for TUE individuals are presented in Fig. 6. The data demonstrated a wide degree of reactivity, including 12 samples classified as high positive (PPFP > 50%), 9 samples classified as low positive (20% < PPFP ≤ 50%), and 7 samples presenting negative results (PPFP ≤ 20%) (Fig. 6a). These seven samples with negative PPFP results are classified as dissociated according to the Krettli and Brener criteria (15). As previously described (13, 15, 18), these individuals should be considered cured since they presented negative results for xenodiagnosis and PPFP.
FIG. 6.
Analysis of PPFP values from patients with negative xenodiagnosis but positive or oscillating conventional serology. The performance of FC-ALTA for samples from the TUE patient group was investigated by using FS, and data analysis was performed by establishing a 20% PPFP value as the cutoff between negative and positive results, as described by Martins-Filho et al. (18). Positive results were additionally classified according to the method of Cordeiro et al. (9) as low positive (20% < PPFP ≤ 50%) and high positive (PPFP > 50%). The TC patients were subdivided into groups, as they received treatment during the acute (A) or chronic (C) phase of the disease (b), had positive or oscillating serology (a), and had less or more than 10 years of follow-up after treatment (c).
Additional analysis demonstrated that all patients with less than 10 years of follow-up after treatment (9 of 9) showed positive PPFP values and 78.0% of them (7 of 9) had high-positive results (PFPF > 50%) (Fig. 6c). Patients with more than 10 years of follow-up after treatment presented a wide range of results. The frequency of samples with high positive results dropped to 31.5% (6 of 19), and the frequency of low-positive and negative results increased to 31.5% (6 of 19) and 37.0% (7 of 19), respectively. Statistical analysis demonstrated that results for patients in the ≤10 years after treatment group differed significantly from those for patients in the >10 years after treatment group (P < 0.05).
To further address the differential reactivity of the TUE patient group, we subdivided the patients based on their results from CSA analysis (Fig. 6b). Then the PPFP values from patients with positive CSA and those with oscillating CSA were compared. The data demonstrated that both subgroups had negative, low-positive, and high-positive results. The percentages of samples with negative, low-positive, and high-positive PPFP values were 36.5% (4 of 11), 36.5% (4 of 11), and 27.0% (3 of 11) for patients with oscillating CSA and 18.0% (3 of 17), 29.0% (5 of 17), and 53.0% (9 of 17) for those with positive CSA, respectively. Interestingly, the frequency of high-positive results was higher within the group with positive CSA (53.0 versus 27.0%).
Together, our data support the hypothesis that high-positive results with FC-ALTA are in general associated with positive CSA, regardless of the clinical group analyzed (NT, TNC, or TUE).
DISCUSSION
Different studies performed with either human or murine models have focused on strategies to evaluate the efficacy of specific chemotherapy against Chagas' disease (2, 11, 13). Conflicting reports have arisen because of differences in the approaches applied as cure criteria (15, 16). Positive parasitological tests indicate the persistence of circulating parasites and represent a definitive marker of ongoing infection and treatment failure (8). However, lack of sensitivity is the major feature that leads to a low-negative predictive value of these methods (12, 17). On the other hand, most types of CSA tests, evaluating the presence of specific antibodies, remain positive for long periods after treatment. Therefore, despite their high sensitivity, CSA tests are not recommended as cure criteria (13). For this reason, there is an urgent need for the development of serological tests, which avoid the occurrence of positive serology after parasite elimination. One of the strategies is to search for new immunological techniques, which are more reliable, to be used in follow-up studies after treatment. The finding of two types of anti-T. cruzi antibodies (LA and CSA) enabled Krettli and Brener (14) to describe a phenomenon of serology dissociation and postulate that the absence of LA should be considered a cure marker, despite residual positive CSA tests. Considering the technical laboriousness of CoML, we have used an alternative tool (FC-ALTA) in our laboratory that was shown to be comparable to CoML for monitoring the treatment effectiveness in cases of human Chagas' disease (18). In fact, the sensitivity of FC-ALTA was much higher than that of CoML, as shown by the optimal dilutions required, 1:256 and 1:4, respectively. Furthermore, the capacity of flow cytometry to count thousands of trypomastigotes per assay improved the data confidence. Like CoML, the original FC-ALTA method allows for distinguishing sera from NT and TNC patients from both TC and noninfected individuals. However, the original FC-ALTA let two out of nine TNC patients with positive CoML results fall below the 20% cutoff level. For this reason, in the present investigation we designed an optimization of the original method in order to improve its performance and sensitivity. Our data demonstrated that incubation with FITC-conjugated anti-human IgG antibody for 30 min at 37°C led to higher PPFP values for samples from chagasic patients, with no changes for samples from cured individuals. Therefore, we have incorporated this adjustment in the original method for further studies. Another great advance achieved by this study was the perspective of fitting the method for field surveys since the PPFP values were not adversely affected when testing GPS. Parallel analysis of FS and GPS demonstrated that only 3 out of 70 samples showed discrepancies in PPFP values. From those, only two samples interchanged between negative and low-positive results within PPFP intervals of 20% ± 10%. Then we recommended that when testing GPS, the interpretation of PPFP values should take this variation into account.
In the study described here, the major goal of the research was to conduct a double-blind investigation to reinforce the use of FC-ALTA for clinical survey. For this purpose, we tested coded serum samples from four different categories of chagasic patients, including TC, TNC, TUE, and NT patients. Our data confirmed, in another study population, our previous statement that patients with ongoing infection (NT and TNC), in this case identified by positive xenodiagnosis, had positive results with FC-ALTA. It was interesting that the optimization of the original method, as described above, held all TNC patient samples with PPFP values higher than 20%, improving the performance of the original technique, in which 2 out of 9 TNC patient samples fell bellow the cutoff level. In our pioneer study, we suggested that those low PPFP values should be the result of antibody shedding from the parasite membrane and that shorter incubation times at a lower temperature should resolve this question. Here, we confirm that lower incubation time but higher temperature was the optimal condition to improve the sensitivity of FA-ALTA with no effect on its ability to discriminate negative samples. Together, the data from NT and TNC patient samples reemphasizes that the persistence of circulating parasites is associated with the presence of ALTA, which can be used as a valid immunological marker for active infection. Furthermore, it should be stressed that, in general, patients with negative CSA and negative xenodiagnosis persistently had PPFP values under 20%. Unexpectedly, we found that one cured patient had PPFP values over 50% in sequential samples and repeated tests. Interestingly, we found that this patient had been treated for tuberculosis 10 years before inclusion in this study. This patient had more than 140 negative xenodiagnoses and persistent negative CSA results over the past 10 years (data not shown). Several studies have reported the possibility of cross-reactivity in the serology for Chagas' disease in patients with tuberculosis (1, 19). We are now testing to see if samples from patients with tuberculosis may present cross-reactivity in FC-ALTA assay. Considering these observations, we suggest the association of FC-ALTA with other tests already indicated as cure criteria to evaluate therapeutic efficacy in Chagas' disease. Parasitological and CSA tests should be performed repeatedly before a single FC-ALTA. Individuals presenting negative results on conventional tests should be considered cured, without requirement of confirmation by FC-ALTA. However, the FC-ALTA should be indicated, to confirm cure, in cases of individuals with several negative parasitological tests and positive or oscillating CSA tests (TUE patients), since this approach could save several years of inconclusive diagnosis. In fact, we have found that seven samples within the TUE patient population had negative PPFP results and could be classified as dissociated according to the Krettli and Brener criteria (15). As previously described (13, 15, 18), these individuals should be considered cured since they had negative results for xenodiagnosis and PPFP. We therefore postulated that patients who have been treated and show negative parasitological tests and the absence of ALTA should be considered cured despite residual positive or oscillating CSA. Here, using a 1:256 serum dilution from a TUE patient, we have observed that a period of 10 years after treatment was required to identify negative results with FC-ALTA for patients treated during the chronic phase of the disease. Then, we hypothesized that FC-ALTA follow-up with serial dilution could identify the antibody clearance sooner by the shift of positive PPFP values to lower titers. This hypothesis is currently under investigation in our laboratory.
Finally, our study optimized and validated our method, offering another tool, less laborious than CoML and parasitological tests, for investigating and monitoring results of specific chemotherapy. Furthermore, these data reinforce the relevance of studies regarding etiological treatment of chronic patients since a considerable number of successful treatments were achieved during the chronic phase of Chagas' disease.
Acknowledgments
We thank Maria Elizabeth Pereira for technical assistance during this study.
This work was supported by the Fundação Oswaldo Cruz and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
REFERENCES
- 1.Almeida, J. O., J. L. P. Freitas, and H. Brandão. 1954. Complement fixation test with a triple antigen for syphilis, tuberculosis, leprosy or Chagas disease in blood banks. Am. J. Trop. Med. Hyg. 3:490-494. [DOI] [PubMed] [Google Scholar]
- 2.Andrade, Z. A. 1991. Pathogenesis of Chagas' disease. Res. Immunol. 142:126-129. [DOI] [PubMed] [Google Scholar]
- 3.Bertelli, M. S., R. R. Golgher, and Z. Brener. 1977. Intraspecific variation in Trypanosoma cruzi: effect of temperature on the intracellular differentiation in tissue culture. J. Parasitol. 63:434-437. [PubMed] [Google Scholar]
- 4.Brener, Z. 1984. Recent advances in the chemotherapy of Chagas' disease. Mem. Inst. Oswaldo Cruz 79(Suppl.):149-155. [Google Scholar]
- 5.Brener, Z., and E. Chiari. 1963. Variações morfológicas observadas em diferentes amostras de Trypanosoma cruzi. Rev. Inst. Med. Trop. Sao Paulo 5:220-224. [PubMed] [Google Scholar]
- 6.Cançado, J. R., A. A. Salgado, S. M. Batista, and C. A. Chiari. 1979. Specific treatment of human Chagas'disease, p. 2-7. In Congresso Internacional sobre Doença de Chagas, Rio de Janeiro. Fundação Oswaldo Cruz, Rio de Janeiro, Brazil.
- 7.Cerisola, J. A., R. W. Rohwedder, and C. E. Del Prado. 1971. Yield of xenodiagnosis in human chronic Chagas' infection using nymphs of different species of triatomid bugs. Bol. Chil. Parasitol. 26:57-58. [PubMed] [Google Scholar]
- 8.Chiari, E., J. C. P. Dias, M. Lana, and C. A. Chiari. 1989. Hemocultures for the parasitological diagnosis of human chronic Chagas' disease. Rev. Soc. Bras. Med. Trop. 22:19-23. [DOI] [PubMed] [Google Scholar]
- 9.Cordeiro, F. D., O. A. Martins-Filho, M. O. Da Costa Rocha, S. J. Adad, R. Corrêa-Oliveira, and A. J. Romanha. 2001. Anti-Trypanosoma cruzi immunoglobulin G1 can be a useful tool for diagnosis and prognosis of human Chagas' disease. Clin. Diagn. Lab. Immunol. 8:112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coura, J. R. 1996. Current prospects of specific treatment of Chagas' disease. Bol. Chil. Parasitol. 51:69-75. [PubMed] [Google Scholar]
- 11.de Andrade, A. L., F. Zicker, R. M. de Oliveira, S. Almeida Silva, A. Luquetti, L. R. Travassos, I. C. Almeida, S. S. de Andrade, J. G. de Andrade, and C. M. Martelli. 1996. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 348:1407-1413. [DOI] [PubMed] [Google Scholar]
- 12.Franco, Y. B. A., I. G. Silva, A. Rassi, A. C. R. G. Rocha, H. H. G. Silva, and G. G. Rassi. 2002. Correlação entre a positividade do xenodiagnóstico artificial e a quantidade de sangue e triatomíneos utilizados no exame, em pacientes chagásicos crônicos. Rev. Soc. Bras. Med. Trop. 35:29-33. [DOI] [PubMed] [Google Scholar]
- 13.Galvão, L. M., R. M. Nunes, J. R. Cançado, Z. Brener, and A. U. Krettli. 1993. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans. R. Soc. Trop. Med. Hyg. 87:220-223. [DOI] [PubMed] [Google Scholar]
- 14.Krettli, A. U., and Z. Brener. 1976. Protective effects of specific antibodies in Trypanosoma cruzi infections. J. Immunol. 116:755-760. [PubMed] [Google Scholar]
- 15.Krettli, A. U., and Z. Brener. 1982. Resistance against Trypanosoma cruzi associated to anti-living trypomastigote antibodies. J. Immunol. 128:2009-2012. [PubMed] [Google Scholar]
- 16.Luquetti, A. O., and A. Rassi. 2000. Diagnóstico laboratorial da infecção pelo Trypanosoma cruzi, p. 344-378. In Z. Brener, Z. Andrade, and M. Barral-Neto (ed.), Trypanosoma cruzi e doença de Chagas, 2nd ed. Guanabara Koogan, Rio de Janeiro, Brazil.
- 17.Luz, Z. M. P., M. G. Coutinho, J. R. Cançado, and A. U. Krettli. 1994. Hemocultura: técnica sensível na detecção do Trypanosoma cruzi em pacientes chagásicos na fase crônica da Doença de Chagas. Rev. Soc. Bras. Med. Trop. 27:143-148. [DOI] [PubMed] [Google Scholar]
- 18.Martins-Filho, O. A., M. E. S. Pereira, J. F. Carvalho, J. R. Cançado, and Z. Brener. 1995. Flow cytometry, a new approach to detect anti-live trypomastigote antibodies and monitor the efficacy of specific treatment in human Chagas' disease. Clin. Diagn. Lab. Immunol. 2:569-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nussenzweig, V. 1957. Reação de fixação do complemento para Leishmaniose visceral com antígeno extraído do bacilo da Tuberculose. Técnica, sensibilidade e especificidade. Hospital 51:115-126. [Google Scholar]
- 20.World Health Organization. 1991. Control of Chagas' disease. WHO Tech. Rep. Ser. 811:1-91. [PubMed] [Google Scholar]