Abstract
Substance P (SP) is an important modulator of neuroimmunoregulation. We have demonstrated that human T lymphocytes express SP and neurokinin-1 receptor (NK-1R), a primary SP receptor. In the present study, we investigated whether SP stimulates synthesis of macrophage inflammatory protein-1β (MIP-1β) in human T lymphocytes. SP significantly enhanced MIP-1β expression at both the mRNA and protein level in a human T cell line (Jurkat) containing the SP receptor gene (J-SPR) as determined by real-time PCR and ELISA assays. SP-induced MIP-1β expression is abrogated by the specific NK-1R antagonist (CP-96,345). The supernatants from SP-stimulated J-SPR T cell cultures enhanced T lymphocyte chemotaxis in vitro, indicating functional activity of SP-induced MIP-1β. In addition, SP augmented secretion of MIP-1β from primary cultures of peripheral blood lymphocytes (PBL) isolated from some of the donors. This donor variability was due to differential expression of the primary SP receptor (NK-1R) on PBL from different donors. PBL from two of seven donors that did not respond to SP stimulation had undetectable NK-1R expression. Our mechanistic studies showed that SP activated NF-κB promoter-directed luciferase activity, which may be responsible for its effect on MIP-1β expression in human T cells. Our data provide a potential mechanism by which SP selectively influences cellular immune responses such as β-chemokine expression in human T lymphocytes through NK-1R, which may have an important in vivo implication in inflammatory diseases.
Keywords: Substance P, Real-time PCR, MIP-1β, Lymphocytes
1. Introduction
There are bi-directional interactions between the nervous and immune systems. The neuropeptide substance P (SP), which has been extensively characterized as a modulator of neuroimmunoregulation, may play an important role in this interaction. SP is involved in immune responses and inflammation within the central and peripheral nervous systems. SP, initially described as a peptide of neuronal origin, has been identified in non-neuronal cell types. We have recently demonstrated that human fetal brain-isolated microglia (Lai et al., 2000), stem cells (Li et al., 2000), human peripheral blood mononuclear phagocytes (Ho et al., 1997), and lymphocytes (Lai et al., 1998) express SP and its receptor (NK-1R). SP, at nanomolar concentrations, effectively activates NF-κB, a transcription factor involved in the control of cytokine expression (Lieb et al., 1997; Marriott et al., 2000). SP stimulates the immune cells to produce inflammatory cytokines including interleukin-1 (IL-1), IL-6, IL-12 and tumor necrosis factor alpha (TNF-α) (Lotz et al., 1988; Laurenzi et al., 1990; Kincy-Cain and Bost, 1997). We have also demonstrated that SP enhances TNF-α and IL-10 production by monocytes and macrophages isolated from both adult peripheral and placental cord blood (Ho et al., 1996b, 1998). Increased numbers of SP receptors are found in blood vessels at sites of peripheral inflammatory lesion (Mantyh et al., 1988). SP also plays an important role in viral infections, such as respiratory syncytial virus infection(Tripp et al., 2000) and HIV infection (Ho et al., 1996a; Lai et al., 2001).
There is little information on the role of SP in the modulation of chemokine expression. Chemokines and their receptors play a crucial role in immune and inflammatory reaction. Chemokines are chemotactic cytokines that have two main classes: CXC (α) chemokines, which act primarily on neutrophils, and C-C (β) chemokines, which act on several leukocyte populations (monocytes, lymphocytes, basophils, eosinophils and natural killer cells) (Rollins, 1997). β-Chemokines, including macrophage inflammatory protein-1α (MIP-1α), macrophage inflammatory protein-1β (MIP-1β) and RANTES, directly participate in the activation and directional migration of immune cells (such as lymphocytes and monocytes) to sites of inflammation. The involvement of β-chemokines in the interaction between the immune system and CNS is currently under investigation. SP stimulates chemotaxis of T cells, monocytes, dendritic cells and B cell signaling by binding to NK-1R, a G-protein-coupled receptor for SP. However, the mechanism(s) by which SP promotes chemotactic activity of immune cells is still unknown. In the present study, we investigated the effects of SP on MIP-1β production by human T lymphocytes. We demonstrated that SP effectively up-regulates MIP-1β expression in these immune cells.
2. Materials and methods
2.1. Cell culture
Peripheral blood was obtained from healthy adult donors. Heparinized blood samples were identified as HIV antibody negative by anonymous testing with the ELISA method (Coulter Immunology, Hialeah, FL). Peripheral blood lymphocytes (PBL) were obtained according to our previously described techniques after the removal of monocytes (Hassan et al., 1986). In brief, heparinized blood obtained from healthy adult donors was separated by centrifugation over Lymphocyte Separation Medium (Organon Teknika, Durham, NC) at 400–500×g for 45 min. The mononuclear layer was collected and incubated with DMEM (Life Technologies, Grand Island, NY) in a 2% gelatin-coated flask for 45 min at 37 °C, followed by removal of the nonadherent cells with DMEM. Monocytes adhered to the bottom of gelatin-coated flask. Nonadherent cells enriched with T lymphocytes were removed and resuspended in RPMI1640 medium, which contains 10% fetal calf serum (FCS). Following the initial purification, > 95% of the cells were T lymphocytes as determined by FACS analysis using mAb against CD3. Freshly isolated T lymphocytes were prestimulated with PHA for 72 h. Cell viability was monitored by trypan blue exclusion. The cells were then washed three times and re-plated at a density of 106 cells/ml in RPMI1640 culture medium supplemented with 10% FCS. The human T-lymphoblastoid cell line (Jurkat) transfected with a cDNA encoding the NK-1R gene (J-SPR) was kindly provided by Dr. Payan (Rigel, South San Francisco, CA) (Sudduth-Klinger et al., 1992). J-SPR T cells express functional NK-1R (approximately 50,000 receptors/cell). J-SPR T cells were maintained in the presence of G418 (800 μg/ml) containing RPMI1640 culture medium supplemented with 10% FCS, 2 mM glutamine and penicillin (100 U/ml) and streptomycin (100 μg/ml).
2.2. Reagents
SP was purchased from Sigma (St. Louis, MO). Stock solution of SP (10−3 M) was stored at −80 °C as frozen aliquots in FPLC grade water. NK-1R antagonist, CP-96,345 was generously provided by Pfizer Diagnostics. Stock solution of CP-96,345 (2×10−3 M in FPLC grade water) was stored at −80 °C. SP and CP-96,345 were diluted to the appropriate concentrations in culture medium when used.
2.3. SP and CP-96,345 treatment of human T lymphocytes
For MIP-1β induction, J-SPR T cells or PBL were plated in triplicate at a density of 106 cells/well in 24-well culture plate and then incubated with or without SP for the indicated times (see figure legends). When CP-96,345 was used together with SP in these experiments, cells were first treated with CP-96,345 (10−6 M) for 30 min and then incubated with SP at different concentrations. For mRNA expression, total RNA was extracted from 2×106 cells. For MIP-1β protein production (see figure legend), cell-free supernatants were collected and stored at −70 °C for ELISA.
2.4. ELISA for MIP-1 β
ELISA kits for MIP-1β were purchased from Endogen (Cambridge, MA). The assay was performed as instructed in the protocol provided by the manufacturer. In brief, 50 Al of supernatant was added to antibody-coated wells and incubated for 1 h at room temperature. The plate was washed with the provided buffer solution and incubated with 100 μl of Biotinylated Antibody Reagent for 1 h at room temperature. The plate was washed again, treated with 100 μl of prepared Streptavidino-HRP solution, and incubated for 30 min at room temperature. After an additional wash, 100 μl of TMB substrate solution was added to each well, and color was allowed to develop at room temperature for 30 min. The reaction was stopped by the addition of 100 μl of stop solution to each well. The plate was read on a microplate reader (ELX800, Bio-Tek Instruments, Winooski, VT).
2.5. RNA isolation
Total cellular RNA was isolated from J-SPR T cells using Tri-Reagent (Molecular Research Center, Cincinnati, OH). In brief, the total RNA was extracted by a single step, guanidium thiocyanate–phenol–chloroform extraction. After centrifugation at 13,000×g for 15 min at 4 °C, the RNA-containing aqueous phase was precipitated in isopropanol. RNA precipitates were washed once in 75% ethanol and resuspended in 30 μl of RNase-free water.
2.6. Reverses transcription (RT)
Total RNA (1 μg) was subjected to reverse transcription using the reverse transcription system (Promega, Madison, WI) with specific primers (antisense) for the MIP-1β gene or the NK-1R gene (as described below) for 1 h at 42 °C. The reaction was terminated by incubating the reaction mixture at 99 °C for 5 min and then kept at 4 °C. The resulting cDNA was used as a template for PCR amplification.
2.7. Polymerase chain reaction (PCR) and nested-PCR
PCR amplification of MIP-1β cDNA was performed with one tenth of the cDNA for 35 cycles with AmpliTaq Gold (Perkin-Elmer-Cetus, Foster City, CA) in a GeneAmp PCR System 2400 (Perkin-Elmer-Cetus). The PCR reaction mixture contained 0.2 mM of dNTPs, 20 pM of each of two primers and 1.5 units of AmpliTaq Gold in 1×reaction buffer (Perkin-Elmer-Cetus). Each PCR amplification consisted of heat activation of AmpliTaq Gold for 9 min at 94 °C, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s and further elongation at 72 °C for 7 min. The specific oligonucleotide primers are listed as follows: MIP-1β gene primers: 5′-CCAAACCAAAAGAAGCAAGC-3′ (sense) and 5′-AGAAACAGTGACAGT GGACC-3′(antisense). β-Actin gene primers: 5′-ATGTGGCACCACACCTTCTACAATGAGCTGCG-3′ (sense) and 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′ (antisense). The oligonucleotide primers were synthesized by Integrated DNA Technologies (Coralville, IA).
Nested-PCR was used for amplifying the NK-1R gene from peripheral blood lymphocytes (PBL) (Lai et al., 1998). PCR amplification was performed with the one tenth of the cDNA for 45 cycles with AmpliTaq Polymerase (Perkin-Elmer, Branchburgh, NJ) in a GeneAmp PCR System 2400 (Perkin-Elmer-Cetus). The NK-1R primer pair (sense: 5′-AGGACAGTGACG-AACTATTTTC-TGG-3′ and antisense: 5′-CTGCTGGATAAACTTCTTCAGGTAG-3′) corresponds to + 190 and + 831 on mRNA NK-1R sequences with a predicted amplification size of 640 bp based on the published cDNA sequence for human NK-1R. In order to overcome nonspecific amplification, we used RT-nested-PCR for NK-1R mRNA detection. Thus, an inner PCR primer pair (5′-AACTTCTTCCCCATCGCC-3′ and 5′-ACTTGCTCGTGGTAGCGG-3′) was designed to detect NK-1R expression in human lymphocytes, resulting in the amplification of 395 bp products. In brief, 2 μl of the PCR products amplified by the outer primer pair was subjected to the second run of PCR (nested) using the inner NK-1R primer pair. The cycle condition was designed as follows: 95 °C 9 min followed by 40 cycles of 94 °C 30 s, 50 °C 30 s, 72 °C 30 s and elongation at 72 °C for 7 min. After PCR amplification, the samples were electrophoresed on a 3% NuSieve 3:1 agarose gel (FMC Bio-products, Rockland, ME).
2.8. Real-time PCR
Real-time PCR was performed with one tenth of cDNA using ABI Prism 7700 Sequence Detection System (Perkin-Elmer). For MIP-1β gene expression, the reaction mixture contained 0.25 mM of dNTPs, AmpliTaq Gold (1.5 U), 5 mM MgCl2, 50 pM of each of the two primers: 5′-GCTGCTCAGAGACAGGAAGTCTT-3′ (sense), 5′-ACAGGAACTGCGGAGAGGAGT-3′ (antisense), 20 pM of the molecular beacon probe (5′-GCGAGCCCCGGATGCTTCTCCATG-AGACACAGCTCGC-3′) labeled with FAM (a fluorophore) at its 5′ end and DABCYL (a quencher) at the 3′ end. The cycle conditions were 95 °C for 10 min followed by 45 cycles of 95 °C for 15 s and 60 °C for 1 min. The known amount of plasmid containing MIP-1β cDNA was used as a standard control. All controls and samples were run in duplicates in the same plate. The Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) mRNA levels analyzed by real-time PCR in the same plate were used as a control to normalize the mRNA contents among the samples tested.
2.9. Chemotaxis assay
Chemotaxis was performed using 96-well microplate ChemoTX system (Neuroprobe, Cabin John, MD), 3.25 mm diameter, 5 μm pore polycarbonate membranes were placed over the wells of a corresponding 96-well tissue culture plate. The bottom wells of the chamber were filled with 30 μl of supernatants from J-SPR T cell cultures, which were nonstimulated or induced with SP (10−7 M) for 6 h. Lymphocytes (3×106 cells/ml) in 50 μl of RPMI1640, 10%FCS, 2 mmol l-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin (all obtained from Cell Center) were added to the upper side of the membrane and the plate was incubated at 37 °C for 3 h. The cells which migrated to the lower wells were enumerated by light microscopy.
2.10. NF-κB activation assay
The pNF-κB-luc multimer-directed Luciferase construct was generated by Petrak et al. (1994). Two copies of the mouse κ light chain enhancer (Pierce et al., 1988) were cloned into pBLCAT3 vector (Luckow and Schutz, 1987), and then the construct was modified by replacing the CAT reporter with the luciferase gene obtained from pGEM-luc(Petrak et al., 1994). Plasmid DNA was prepared by the Miniprep technique, according to the manufacturer’s instructions (Wizard plus Minipreps, Promega) and was used in transfection experiments.
J-SPR T cells were cultured in RPMI 1640 media containing 10% FCS. For each transfection experiment, the cells were seeded in a 6-well tissue culture plate at a density of 2×106 cells/well. The cells were transfected with the pNF-κB-luc using Fugene 6 Transfection Reagent (Fugene 6) (Roche Molecular Biochemical, Indianapolis, IN) with a ratio of Fugene 6/plasmid 6:1 (μl/μg). Twenty-four hours after the transient transfection, the cells were replated and treated with SP (10−9 to 10−7 M) and/or NK-1R antagonist (CP-96,345, 10−6 M) for 6 h. If SP and CP-96,345 were used in one experiment, CP-96,345 was added 30 min before SP treatment. At the termination of the experiments, cells were harvested and washed twice with PBS by centrifugation at 3300×g for 3 min at room temperature. The cell pellets were lysed by adding 0.25 ml of 1× Reporter Lysis Buffer (Promega) and a cycle of freezing and thawing in dry ice. Cell-free lysate was obtained by centrifugation at 10,000×g for 30 s at room temperature. The effects of SP on the activation of the NF-κB in these transient transfected cells were determined by the NF-κB-directed luciferase activity. Luciferase activity in cell lysate (25 μl/sample) was quantitated using a Luciferase assay system (Promega) and a Luminometer. The results were presented as relative light units (RLU).
2.11. Statistical analysis
Where appropriate, data are expressed as mean ± S.D. Data in Fig. 4 was subjected to a one-way analysis of variance with post hoc comparisons (Dunnett’s procedure) to determine significant differences between groups.
Fig. 4.

Culture supernatants from SP-stimulated J-SPR T cells induce lymphocyte chemotaxis. Culture supernatants from SP stimulated J-SPR T cell cultures caused a significant increase of T lymphocyte migration compared with unstimulated controls. The culture supernatants from SP and/or CP-96,345-treated J-SPR T cell cultures had no effect on migration of T lymphocytes compared with that of unstimulated J-SPR T cell culture supernatants. The data shown are mean ± S.D. of three independent experiment (*P < 0.0001, SP-treated vs. untreated cells).
3. Results
3.1. SP induces MIP-1β expression in J-SPR T cells
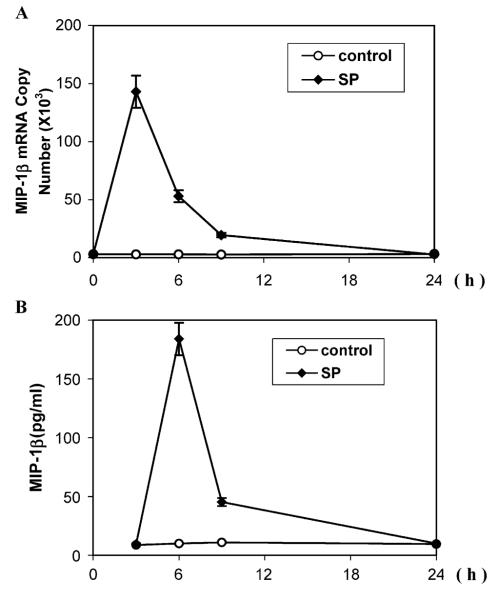
We first investigated whether MIP-1β gene expression in J-SPR T cells is affected by SP stimulation. Total RNA extracted from the cells incubated with or without SP was analyzed by RT-PCR. SP stimulated MIP-1β mRNA expression in a concentration-dependent fashion (Fig. 1A insert). In order to quantitatively determine the effects of SP on MIP-1β mRNA expression, we performed the realtime PCR assay. As demonstrated in Fig. 1A, SP (10−11, 10−9 and 10−7 M) significantly enhanced MIP-1β gene expression at 2-, 4- and 40-fold, respectively. We also examined whether SP affects MIP-1β protein synthesis in J-SPR cells. When added to J-SPR cells, SP significantly increased (2-, 5- and 20-fold at the concentrations of 10−11, 10−9 and 10−7 M, respectively) MIP-1β production in J-SPR T cells as determined by ELISA (Fig. 1B). Since SP (10−7 M) induced the highest MIP-1β expression (Fig. 1), we used SP at this concentration to determine the effects of SP on MIP-1β expression at different time points during the course of treatment. A peak expression of MIP-1β mRNA was observed at 3 h post-treatment with SP (Fig. 2A), whereas the highest level of MIP-1β protein production appeared at 6 h post-treatment with SP (Fig. 2B).
Fig. 1.
Effect of SP on the MIP-1β expression in J-SPR T cells. (A) J-SPR T cells were incubated with SP (10−11 to 10−7 M) for 3 h and total RNA was isolated and subjected to RT-PCR, electrophoresis (insert), and real-time PCR to quantify MIP-1β mRNA. The data are expressed as mean ± S.D. of triplicate cultures of MIP-1β mRNA copy number per 106 copies of GAPDH mRNA. (B) Effect of SP treatment on MIP-1β production in J-SPR T cells. MIP-1β protein was assayed using ELISA. The data shown are presented as mean ± S.D. of triplicate cultures and are representative of three independent experiments.
Fig. 2.
Time-course of SP on the MIP-1β expression in J-SPR T cells. J-SPR T cells were incubated with (+) or without (−) SP (10−7 M), and total RNA was isolated from the cells after the indicated amount of time (h). (A) Real-time PCR: MIP-1β mRNA levels were quantified by real-time PCR using the same RNA samples as indicated, and the data are expressed as mean ± S.D. of MIP-1β mRNA copy number per 106 copies of GAPDH mRNA. (B) MIP-1β protein was assayed using ELISA. The data shown are presented as mean ± S.D. of triplicate cultures and are representative of three independent experiments.
3.2. CP-96,345 blocks SP-induced MIP-1β expression in J-SPR T cells
Since SP mediates its effects by binding to specific NK-1R expressed on target cells, we hypothesized that SP stimulation of MIP-1β expression is through NK-1R expressed on J-SPR T cells. In order to identify whether the specific SP-NK-1R interaction is responsible for the SP-induced MIP-1β expression, we used a SP receptor antagonist (CP-96,345) to block the effect of SP. SP-induced MIP-1β mRNA expression in J-SPRTcells was abrogated by pre-treatment with CP-96,345 (Fig. 3A). CP-96,345 also blocked SP-induced MIP-1β protein production by J-SPR T cells (Fig. 3B). These data demonstrate specific SP-NK-1R interaction-mediated effects on MIP-1β gene expression and protein production in J-SPR T cells.
Fig. 3.
Effect of SP antagonist, CP-96,345, on SP-induced MIP-1β expression in J-SPR T cells. (A) J-SPR T cells were incubated with SP (10−7 M), SP (10−7 M) plus CP-96,345 (10−6 M), CP–96,345 (10−6 M) and without compounds (as control) for 3 h. Cellular RNA was isolated and subjected to RT-PCR, electrophoresis (insert), and real-time RT-PCR to quantify MIP-1β mRNA. The data are expressed as mean ± S.D. of triplicate cultures and are representative of three independent experiments of MIP-1β mRNA copy number per 106 copies of GAPDH mRNA. (B) MIP-1β protein was assayed using ELISA. The data shown are presented as mean ± S.D. of triplicate cultures and are representative of three independent experiments.
3.3. Supernatants from SP-stimulated J-SPR T cells promote lymphocyte chemotaxis
In order to determine whether SP-induced MIP-1β in the supernatants from J-SPR T cells have functional activity, we examined the in vitro migration of human T lymphocytes in response to the stimulation with culture supernatants collected from SP-treated J-SPR T cell cultures. SP-treated J-SPR T cell culture supernatants significantly enhanced (2-fold) T lymphocyte migration compared to the control T cells (Fig. 4). Culture supernatants from SP plus CP-96,345-treated or CP-96,345-treated J-SPR T cells had little effect on the lymphocyte migration (Fig. 4).
3.4. SP induces MIP-1β expression in peripheral blood lymphocytes
We have recently documented that human blood-isolated T lymphocytes express SP and NK-1R (Lai et al., 1998). In order to determine whether SP modulates MIP-1β secretion in primary cultures of PBL, PBL isolated from healthy adult blood were prestimulated with PHA for 72 h, and then incubated with or without SP (10−7 M) for 12 h, after which the supernatants were harvested for ELISA. Increased levels of MIP-1β protein were observed in the supernatants from SP-treated primary lymphocyte cultures in comparison with untreated control cultures from the same donors (Fig. 5). PBL isolated from two of seven donors, however, did not respond to SP stimulation. We therefore investigated whether the lack of NK-1R expression on PBL from these two donors affected SP’s effect on MIP-1β expression. We showed that the PBL from these two donors (donors 3 and 7) did not respond to SP stimulation (Fig. 5).
Fig. 5.

Effect of SP on the MIP-1β production in PBL. PBL isolated from seven healthy donors (D1–D7) were pre-stimulated with PHA for 72 h. The cells were incubated with or without SP (10−7 M) for 12 h. Total RNA was extracted from PBL isolated from different donors and nested-RT-PCR was performed to amplify NK-1R mRNA (395 bp) (insert). Cell-free supernatants were collected and MIP-1β protein was assayed using ELISA. The data shown are mean ± S.D. of MIP-1β in triplicate cultures. Plus (+) and minus (−) signs indicate presence or absence of SP.
3.5. SP activates NF-κB in J-SPR T cells
Since NF-κB is involved in the control of cytokine expression and the NF-κB binding site is also found on the MIP-1β gene (Rezzonico et al., 2001), we hypothesized that SP-NK-1R interaction participates in NF-κB activation, thus promoting MIP-1β expression in human PBL. To prove this hypothesis, J-SPR T cells were transfected with the NF-κB promoter containing plasmid (pNF-κB-luc) and then incubated with SP and/or CP-96,345. In comparison to control cells, SP stimulation showed an increase in NF-κB promoter-directed luciferase activity (Fig. 6). CP-96,345 completely blocked SP stimulation effect (Fig. 6), while CP-96,345 alone had little effect on NF-κB promoter-directed luciferase activity (Fig. 6).
Fig. 6.

Effect of SP on the activation of NF-κB in J-SPR T cells. J-SPR T cells were transfected with pNF-κB-luc using Fugene 6 Transfection Reagent (Fugene 6). The ratio of Fugene 6/plasmid was 6:1 (μl/μg). Twenty-four hours after transient transfection, the cells were treated with SP and/or CP-96,345 at different concentrations as indicated for 6 h. The effect of SP on activation of NF-κB in these transfected cells was determined by NF-κB-driven luciferase activity. Luciferase activity in the cell-free lysate was quantitated using a luciferase assay system (Promega) and a luminometor. CP: CP-96,345 (10−6 M), SP + CP: SP(10−7 M) plus CP-96,345 (10−6 M). The data are presented as relative light unit (RLU). The data shown are mean ± S.D. of three independent experiments.
4. Discussion
The nervous system has a dynamic in the regulation of adaptive immunity. Soluble products including neuropeptides and cytokines interacting with specific receptors present on both immune and neuroendocrine cells mediate neuroimmunoregulation. The neuropeptide SP is an example of one important mediator that interact with both the immune and nervous systems (Maggi, 1997). SP and its receptor (NK-1R) play an important role in immunoregulation, inflammatory response and the pathogenesis of some immunological diseases. Inflammatory responses in knockout mice devoid of the SP receptor are greatly reduced, providing compelling evidence for the importance of SP and its receptor in inflammatory events (Bozic et al., 1996). In addition, SP concentrations are elevated at local sites of inflammation (Tripp et al., 2000).
Our studies showing that human immune cells express SP and its receptor (Ho et al., 1997; Lai et al., 1998) led us to investigate the role of SP in the regulation of function of immune cells (Lai et al., 2001; Ho et al., 2002). We demonstrated that SP stimulates macrophages to produce TNF-α and IL-10 (Ho et al., 1996b, 1998). SP augments mitogen-induced IL-2 production and proliferation in T lymphocytes (Rameshwar et al., 1993). These effects of SP on lymphocytes are also observed after administration of SP in vivo (Scicchitano et al., 1988). Pharmacological antagonist of the NK-1R inhibits the immunomodulatory effects of SP on immune cells. We speculate that SP activates T lymphocytes to increase their MIP-1β production, these leading to the attraction of more monocytes to the activated T cells. These recruited monocytes in turn may augment the further activation of T lymphocytes by secreting cytokines including IL-1β and TNF-α. By this mechanism, cytokines/chemokines released by activated monocytes and lymphocytes may further amplify the inflammatory reaction.
In this communication, we demonstrate that SP up-regulates human T lymphocytes to produce MIP-1β at both protein and mRNA levels (Figs. 1, 2 and 5). The effect of SP on MIP-1β expression is specific, since the SP receptor antagonist (CP-96,345) blocked the SP effect (Fig. 3). In addition, the supernatants from SP-stimulated J-SPR T cell cultures enhanced human T lymphocyte chemotaxis (Fig. 4), suggesting SP-induced MIP-1β possesses biological activity. SP is chemotactic for a number of cell types, including dendritic cells (Dunzendorfer et al., 2001), neutrophils and monocytes (Ruff et al., 1985; Carolan and Casale, 1993). SP stimulates immune cell chemotaxis signaling through NK-1R (Wiedermann et al., 1989, 1991). A recent study (Jarpe et al., 1998) suggested that an SP derivative acts as an agonist toward neuropeptide and chemokine receptors. SP stimulates human corneal epithelial cells to produce IL-8, a powerful chemotactic agent(Tran et al., 2000). Our data demonstrating that SP induced MIP-1β expression indicate an additional mechanism by which SP functions as a chemotactic agent. Chemokines act as pro-inflammatory agents by selectively promoting the adhesion, chemotaxis, and activation of specific leukocyte effector’s population. These chemokines have been linked to the neuropathology of several diseases (e.g., multiple sclerosis, AIDS, Alzheimer’s disease). Thus, our demonstration that SP-induced MIP-1β expression by T lymphocytes may have in vivo implications in inflammatory diseases.
The intracellular pathway by which SP enhances MIP-1β expression in T lymphocytes is not known. SP specifically activates NF-κB (Lieb et al., 1997), a transcription factor involved in the control of expression of cytokines and chemokines. The functional NF-κB binding site is located in the promoter of MIP-1β gene (Rezzonico et al., 2001), through which cytokines such as TNF-α enhance MIP-1β expression. Thus, we examined the interaction of SP with NF-κB promoter in J-SPR T cells. We have observed that SP has the ability to activate NF-κB promoter in J-SPR T cells and furthermore, the SP effect on the activation of NF-κB promoter was abrogated by CP-96,345, a non-peptide SP receptor antagonist (Fig. 6). SP-induced the NF-κB activation may be responsible for its effect on MIP-1β production in human T lymphocytes.
Although the role of SP in the regulation of the expression of MIP-1β and other chemokines in human immune cells needs further investigation, our data conclusively indicate that SP indeed plays an important role in neuroimmunoregulation. The effect of SP on MIP-1β production provides additional evidence demonstrating that neuropeptide SP selectively influences cellular recruitment and consequences of inflammatory diseases by control of chemokine expression in human T cells.
Acknowledgements
This investigation was supported by National Institutes of Health Grants DA 12815 (to W.Z.H), MH 49981 and AA 13547 (to S.D.D).
References
- Bozic CR, Lu B, Hopken UE, Gerard C, Gerard NP. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- Carolan EJ, Casale TB. Effects of neuropeptides on neutrophil migration through noncellular and endothelial barriers. J. Allergy Clin. Immunol. 1993;92:589–598. doi: 10.1016/0091-6749(93)90083-r. [DOI] [PubMed] [Google Scholar]
- Dunzendorfer S, Kaser A, Meierhofer C, Tilg H, Wiedermann CJ. Cutting edge: peripheral neuropeptides attract immature and arrest mature blood-derived dendritic cells. J. Immunol. 2001;166:2167–2172. doi: 10.4049/jimmunol.166.4.2167. [DOI] [PubMed] [Google Scholar]
- Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J. Immunol. Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Cnaan A, Li YH, Zhao H, Lee HR, Song L, Douglas SD. Substance P modulates human immunodeficiency virus replication in human peripheral blood monocyte-derived macrophages. AIDS Res. Hum. Retrovir. 1996a;12:195–198. doi: 10.1089/aid.1996.12.195. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Kaufman D, Uvaydova M, Douglas SD. Substance P augments interleukin-10 and tumor necrosis factor-alpha release by human cord blood monocytes and macrophages. J. Neuroimmunol. 1996b;71:73–80. doi: 10.1016/s0165-5728(96)00132-4. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- Ho WZ, Stavropoulos G, Lai JP, Hu BF, Magafa V, Anagnostides S, Douglas SD. Substance P C-terminal octapeptide analogues augment tumor necrosis factor-alpha release by human blood monocytes and macrophages. J. Neuroimmunol. 1998;82:126–132. doi: 10.1016/s0165-5728(97)00175-6. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Lai JP, Li Y, Douglas SD. HIV enhances substance P expression in human immune cells. FASEB J. 2002;16:616–618. doi: 10.1096/fj.01-0655fje. [DOI] [PubMed] [Google Scholar]
- Jarpe MB, Knall C, Mitchell FM, Buhl AM, Duzic E, Johnson GL. [d-Arg1,d-Phe5,d-Trp7,9,Leu11]Substance P acts as a biased agonist toward neuropeptide and chemokine receptors. J. Biol. Chem. 1998;273:3097–3104. doi: 10.1074/jbc.273.5.3097. [DOI] [PubMed] [Google Scholar]
- Kincy-Cain T, Bost KL. Substance P-induced IL-12 production by murine macrophages. J. Immunol. 1997;158:2334–2339. [PubMed] [Google Scholar]
- Lai JP, Douglas SD, Ho WZ. Human lymphocytes express substance P and its receptor. J. Neuroimmunol. 1998;86:80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- Lai JP, Zhan GX, Campbell DE, Douglas SD, Ho WZ. Detection of substance P and its receptor in human fetal microglia. Neuroscience. 2000;101:1137–1144. doi: 10.1016/s0306-4522(00)00398-5. [DOI] [PubMed] [Google Scholar]
- Lai JP, Ho WZ, Zhan GX, Yi Y, Collman RG, Douglas SD. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3970–3975. doi: 10.1073/pnas.071052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenzi MA, Persson MA, Dalsgaard CJ, Haegerstrand A. The neuropeptide substance P stimulates production of interleukin 1 in human blood monocytes: activated cells are preferentially influenced by the neuropeptide. Scand. J. Immunol. 1990;31:529–533. doi: 10.1111/j.1365-3083.1990.tb02801.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Douglas SD, Ho W. Human stem cells express substance P gene and its receptor. J. Hemat. Stem Cell Res. 2000;9:445–452. doi: 10.1089/152581600419107. [DOI] [PubMed] [Google Scholar]
- Lieb K, Fiebich BL, Berger M, Bauer J, Schulze-Osthoff K. The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J. Immunol. 1997;159:4952–4958. [PubMed] [Google Scholar]
- Lotz M, Vaughan JH, Carson DA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- Luckow B, Schutz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987;15:5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA. The effects of tachykinins on inflammatory and immune cells. Regul. Pept. 1997;70:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- Mantyh CR, Gates TS, Zimmerman RP, Welton ML, Passaro EP, Jr., Vigna SR, Maggio JE, Kruger L, Mantyh PW. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc. Natl. Acad. Sci. U. S. A. 1988;85:3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott I, Mason MJ, Elhofy A, Bost KL. Substance P activates NF-kappa B independent of elevations in intracellular calcium in murine macrophages and dendritic cells. J. Neuroimmunol. 2000;102:163–171. doi: 10.1016/s0165-5728(99)00182-4. [DOI] [PubMed] [Google Scholar]
- Petrak D, Memon SA, Birrer MJ, Ashwell JD, Zacharchuk CM. Dominant negative mutant of c-Jun inhibits NF-AT transcriptional activity and prevents IL-2 gene transcription. J. Immunol. 1994;153:2046–2051. [PubMed] [Google Scholar]
- Pierce JW, Lenardo M, Baltimore D. Oligonucleotide that binds nuclear factor NF-kappa B acts as a lymphoid-specific and inducible enhancer element. Proc. Natl. Acad. Sci. U. S. A. 1988;85:1482–1486. doi: 10.1073/pnas.85.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshwar P, Gascon P, Ganea D. Stimulation of IL-2 production in murine lymphocytes by substance P and related tachykinins. J. Immunol. 1993;151:2484–2496. [PubMed] [Google Scholar]
- Rezzonico R, Imbert V, Chicheportiche R, Dayer JM. Ligation of CD11b and CD11c beta(2) integrins by antibodies or soluble CD23 induces macrophage inflammatory protein 1 alpha (MIP-1 alpha) and MIP-1 beta production in primary human monocytes through a pathway dependent on nuclear factor-kappa B. Blood. 2001;97:2932–2940. doi: 10.1182/blood.v97.10.2932. [DOI] [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Ruff MR, Wahl SM, Pert CB. Substance P receptor-mediated chemotaxis of human monocytes. Peptides. 1985;6:107–111. doi: 10.1016/0196-9781(85)90142-1. [DOI] [PubMed] [Google Scholar]
- Scicchitano R, Biennenstock J, Stanisz AM. In vivo immunomodulation by the neuropeptide substance P. Immunology. 1988;63:733–735. [PMC free article] [PubMed] [Google Scholar]
- Sudduth-Klinger J, Schumann M, Gardner P, Payan DG. Functional and immunological responses of Jurkat lymphocytes transfected with the substance P receptor. Cell. Mol. Neurobiol. 1992;12:379–395. doi: 10.1007/BF00711540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran MT, Lausch RN, Oakes JE. Substance P differentially stimulates IL-8 synthesis in human corneal epithelial cells. Invest. Ophthalmol. Visual Sci. 2000;41:3871–3877. [PubMed] [Google Scholar]
- Tripp RA, Moore D, Winter J, Anderson LJ. Respiratory syncytial virus infection and G and/or SH protein expression contribute to substance P, which mediates inflammation and enhanced pulmonary disease in BALB/c mice. J. Virol. 2000;74:1614–1622. doi: 10.1128/jvi.74.4.1614-1622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedermann CJ, Wiedermann FJ, Apperl A, Kieselbach G, Konwalinka G, Braunsteiner H. In vitro human polymorphonuclear leukocyte chemokinesis and human monocyte chemotaxis are different activities of aminoterminal and carboxyterminal substance P. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1989;340:185–190. doi: 10.1007/BF00168967. [DOI] [PubMed] [Google Scholar]
- Wiedermann CJ, Niedermuhlbichler M, Zilian U, Geissler D, Lindley I, Braunsteiner H. Priming of normal human neutrophils by tachykinins: tuftsin-like inhibition of in vitro chemotaxis stimulated by formylpeptide or interleukin-8. Regul. Pept. 1991;36:359–368. doi: 10.1016/0167-0115(91)90069-s. [DOI] [PubMed] [Google Scholar]