Abstract
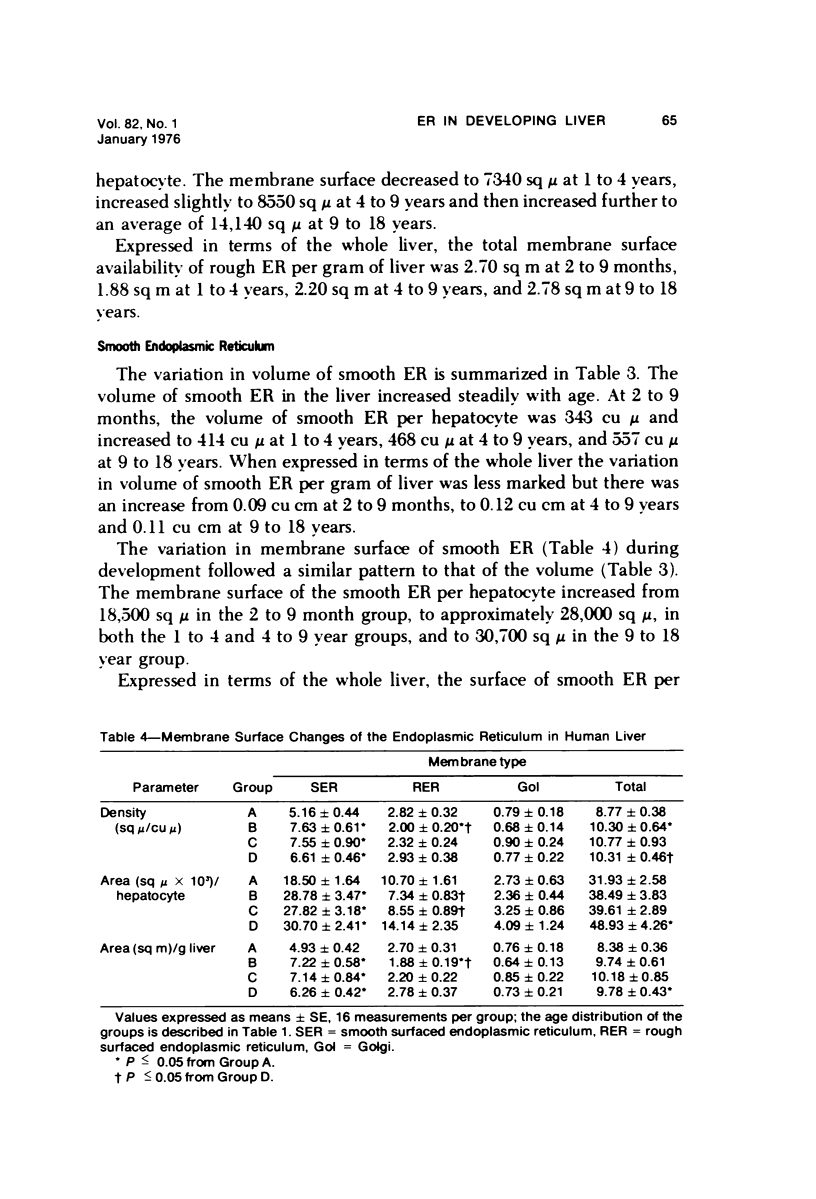
Quantitative electron microscopic aspects of the liver have not been explored in detail, and the numerical characterization of tissue changes may contribute to the understanding of basic cellular mechanisms in disease processes. Sixteen liver biopsies from children 2 months to 18 years old were analyzed by stereology to study the composition and relative distribution of endoplasmic reticulum membranes within hepatocytes. The histologic aspects of the liver as well as the clinical laboratory data of these patients revealed no abnormalities when being observed for suspected hepatic ailment. Morphometric analysis of four tissue blocks per biopsy was undertaken by means of combined light and electron microscopy, using standard stereologic formulae. The results showed less endoplasmic reticulum in liver cells from children 2 to 9 months old. These low levels were accounted for by reduced surface of smooth membranes. There was a first-order relationship in the growth of smooth endoplasmic reticulum between 2 months and 4 years at a rate of 17.1 sq cm/hr, similar to the membrane accumulation rate in experimental animals. Membrane dimensions from Golgi apparatus and rough endoplasmic reticulum were cell-size dependent, and these organelles matured within 2 months of postnatal life. The significance of these findings resides in the low amounts of smooth endoplasmic reticulum membranes at an early age. This lack of membrane surface agrees with findings in developing liver of various species. Experimental studies showed reduced membrane population and low microsome-bound enzyme activities which, under normal circumstances, allow the hepatocyte to undertake detoxification and drug metabolizing processes. Thus, the reduced membrane availability of the liver in infants may account for their inability to metabolize foreign compounds.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann E., Rane A., Ericsson J. L. The liver microsomal monooxygenase system in the human fetus: distribution in different centrifugal fractions. Clin Pharmacol Ther. 1972 Sep-Oct;13(5):652–662. doi: 10.1002/cpt1972135part1652. [DOI] [PubMed] [Google Scholar]
- Arcasoy M., Smuckler E. A., Benditt E. P. Acute effects of 3'-methyl-4-dimethylaminoazobenzene intoxication on rat liver. Structural and functional changes in the endoplasmic reticulum and NADPH-related electron transport. Am J Pathol. 1968 Apr;52(4):841–867. [PMC free article] [PubMed] [Google Scholar]
- Basu T. K., Dickerson J. W., Parke D. V. Effect of development on the activity of microsomal drug-metabolizing enzymes in rat liver. Biochem J. 1971 Aug;124(1):19–24. doi: 10.1042/bj1240019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolender R. P., Weibel E. R. A morphometric study of the removal of phenobarbital-induced membranes from hepatocytes after cessation of threatment. J Cell Biol. 1973 Mar;56(3):746–761. doi: 10.1083/jcb.56.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. I. Structural and chemical differentiation in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):73–96. doi: 10.1083/jcb.30.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. II. Synthesis of constitutive microsomal enzymes in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):97–117. doi: 10.1083/jcb.30.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorchik B. H., Stenger B. G., Quattropani S. L. Fetal hepatic drug metabolism in the nonhuman primate, Macaca arctoides. Drug Metab Dispos. 1974 Nov-Dec;2(6):539–544. [PubMed] [Google Scholar]
- FOUTS J. R., ADAMSON R. H. Drug metabolism in the newborn rabbit. Science. 1959 Apr 3;129(3353):897–898. doi: 10.1126/science.129.3353.897. [DOI] [PubMed] [Google Scholar]
- Gillette J. R., Stripp B. Pre- and postnatal enzyme capacity for drug metabolite production. Fed Proc. 1975 Feb;34(2):172–178. [PubMed] [Google Scholar]
- Jézéquel A. M., Koch M., Orlandi F. A morphometric study of the endoplasmic reticulum in human hepatocytes. Correlation between morphological and biochemical data in subjects under treatment with certain drugs. Gut. 1974 Sep;15(9):737–747. doi: 10.1136/gut.15.9.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardish R., Feuer G. Relationship between maternal progesterones and the delayed drug metabolism in the neonate. Biol Neonate. 1972;20(1):58–67. doi: 10.1159/000240446. [DOI] [PubMed] [Google Scholar]
- MacLeod S. M., Renton K. W., Eade N. R. Development of hepatic microsomal drug-oxidizing enzymes in immature male and female rats. J Pharmacol Exp Ther. 1972 Dec;183(3):489–498. [PubMed] [Google Scholar]
- Rohr H. P., Wirz A., Henning L. C., Riede U. N., Bianchi L. Morphometric analysis of the rat liver cell in the perinatal period. Lab Invest. 1971 Feb;24(2):128–139. [PubMed] [Google Scholar]
- Smuckler E. A., Arcasoy M. Structural and functional changes of the endoplasmic reticulum of hepatic parenchymal cells. Int Rev Exp Pathol. 1969;7:305–418. [PubMed] [Google Scholar]
- Sturgess J. M., De la Iglesia F. A. Morphometry of the golgi apparatus in developing liver. J Cell Biol. 1972 Nov;55(2):524–530. doi: 10.1083/jcb.55.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS C. F., GLAZKO A. J., WESTON J. K. Chloramphenicol in the newborn infant. A physiologic explanation of its toxicity when given in excessive doses. N Engl J Med. 1960 Apr 21;262:787–794. doi: 10.1056/NEJM196004212621601. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. J., Rabin B. R. Distruption by carcinogens of the hormone dependent association of membranes with polysomes. Nature. 1971 Jul 9;232(5306):102–105. doi: 10.1038/232102a0. [DOI] [PubMed] [Google Scholar]
- Yaffe S. J., Rane A., Sjöqvist F., Boréus L. O., Orrenius S. The presence of a monooxygenase system in human fetal liver microsomes. Life Sci II. 1970 Oct 22;9(20):1189–1200. doi: 10.1016/0024-3205(70)90038-x. [DOI] [PubMed] [Google Scholar]


