Abstract
We used the cell-attached mode of patch-clamp technique to measure discrete attofarad steps in membrane capacitance (Cm), reporting area changes in the plasma membrane due to unitary exocytic and endocytic events. To investigate the role of the actin cytoskeleton in elementary exocytic and endocytic events, neuroendocrine rat melanotrophs were treated with Clostridium spiroforme toxin (CST), which specifically depolymerises F-actin. The average amplitude of exocytic events was not significantly different in control and in CST-treated cells. However, the amplitude of endocytic events was significantly smaller in CST-treated cells as compared to controls. The frequency of exocytic events increased by 2-fold in CST-treated cells relative to controls. In control cells the average frequency of exocytic events (νexo) was lower than the frequency of endocytic events (νendo) with a ratio νexo/νendo < 1. In the toxin treated cells, the predominant process was exocytosis with a ratio (νexo/νendo > 1). To study the coupling between the two processes, the slopes of regression lines relating νexo and νendo in a given patch of membrane were studied. The slopes of regression lines were similar, whereas the line intercepts with the y-axis were significantly different. The increased frequency of unitary exocytic events in CST-treated cells is consistent with the view, that the actin cytoskeleton acts as a barrier for exocytosis. While the disassembly of the actin cytoskeleton diminishes the size of unitary endocytic events, suggesting an important role of the actin cytoskeleton in determining the size of endocytic vesicles, the coupling between exocytosis and endocytosis in a given patch of membrane was independent of the state of the actin cytoskeleton.
The actin cytoskeleton plays an important role in multiple cellular events including exocytosis and endocytosis (Doussau & Augustine, 2000; Apodaca, 2001; Martin, 2001). Exocytosis is modulated by the actin cytoskeleton mainly by two mechanisms. First, cortical filamentous actin (F-actin) has been found to form a subplasmalemmal network, which is thought to prevent the translocation of secretory granules from the cytoplasm to the plasmalemma (Burgoyne & Morgan, 1993; Trifaró & Vitale, 1993). Second, in addition to this barrier role, a positive essential role of F-actin in regulated exocytosis has been suggested in other reports (Muallem et al. 1995; Norman et al. 1996). Endocytosis is a diverse set of processes used by the cell to internalise specialised regions of the plasma membrane as well as small amounts of extracellular fluid (Mukherjee et al. 1997). The classic example of endocytosis takes place at the clathrin-coated pits and involves clathrin, AP2 adaptor complexes and the dynamin GTPase (Mukherjee et al. 1997; Sever et al. 2000). Other types of endocytosis are mediated by caveolae and the clathrin-independent pathway (Parton et al. 1994; Lamaze & Schmid, 1995). Finally, some cells are capable of internalising large amounts of fluid by macropinocytosis or large amounts of particles by phagocytosis (Mukherjee et al. 1997). Therefore, several mechanisms of actin cytoskeleton modulation of endocytosis may exist (Apodaca, 2001), which may depend on the type of endocytosis. For example, actin requirement was shown in phagocytosis (Mukherjee et al. 1997) and in the internalisation of caveolae (Parton et al. 1994) but not in fluid uptake on the apical membrane of epithelial cells (Shurety et al. 1998). The sites of vesicle budding in the Golgi appear to be associated with actin (Lorra & Huttner, 1999; Fucini et al. 2000; Valderrama et al. 2000) which may be required at various stages in the formation of an endocytic vesicle, including membrane invagination, neck elongation, fission of the neck and/or its propulsion away from the plasma membrane (Apodaca, 2001; Martin, 2001).
These numerous mechanisms of modulation of exocytosis and endocytosis by the actin cytoskeleton appear to be mediated through raft-like membrane microdomains that are enriched with phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2; Martin, 2001). It was shown that the hydrolysis of PI(4,5)P2 or its sequestration by the pleckstrin homology domain decreases the cytoskeleton- plasma membrane adhesion, indicating a global role for PI(4,5)P2 in regulating cytoskeletal anchoring to the plasma membrane (Raucher et al. 2000). Thus, the control of membrane movement, vesicle fusion and vesicle fission by the actin cytoskeleton involves distinct plasma membrane microdomains.
An ideal way to learn about the microdomain physiology of the actin cytoskeleton and its interactions with membrane trafficking events would be to use a small patch of membrane. Here we studied rat neuroendocrine melanotrophs with the cell-attached patch-clamp technique (Neher & Marty, 1982; Kreft & Zorec, 1997) which allows the recording of small steps in membrane capacitance that reflect fluctuations of plasma membrane surface area due to unitary membrane trafficking events. Pituitary melanotrophs secrete a number of peptides derived from post-translational processing of pro-opiomelanocortin, including β-endorphin, α-melanocyte stimulating hormone and adrenocorticotrophin (Mains & Eipper, 1979). As is known for other neuroendocrine cells (Burgoyne & Morgan, 1993; Trifaró & Vitale, 1993), the peripheral cytoplasm of pituitary cells contains patches of subcortical actin filaments (Senda et al. 1994; Chowdhury et al. 1999). The disassembly of this peripheral cytoskeleton results in an enhanced rate of stimulated secretory activity as determined from the increased rate of membrane capacitance in CST-treated cells (Chowdhury et al. 1999). Furthermore, using the whole-cell membrane capacitance recording it was shown that in non-stimulated cells, where membrane capacitance (Cm) is slowly decreasing due to dominating endocytosis (Rupnik & Zorec, 1992, 1995), Cm was increasing after actin cytoskeleton disassembly (Chowdhury et al. 1999). Whole-cell membrane capacitance records report a net change in membrane capacitance, and preclude the unequivocal assignment of actin cytoskeleton disassembly selectively with exocytosis and/or endocytosis.
To resolve this problem one needs to selectively monitor exocytosis and endocytosis. Therefore we measured discrete steps in Cm, which report exocytosis and endocytosis selectively. Discrete increases in Cm are due to unitary exocytic events and discrete reductions in Cm are due to unitary endocytic events (Neher & Marty, 1982; Fernandez et al. 1984; Zorec et al. 1991; Zupancic et al. 1994; Kreft & Zorec, 1997; Sikdar et al. 1998; Henkel et al. 2000). The activity of unitary exocytic and endocytic events was monitored in isolated cell-attached patches in control conditions and in cells pretreated with Clostridium spiroforme toxin (CST), which specifically ADP-ribosylates cellular actin (Popoff & Boquet, 1988; Chowdhury et al. 1999).
We found that the frequency of exocytic events increased in CST-treated cells, which is consistent with the view that the subcortical actin acts as a barrier for secretory activity. On the other hand, the frequency of endocytic events was not affected by the treatment with CST. In contrast, we recorded a small but significant CST-dependent reduction in the size of endocytic vesicles, which suggests a role of the actin cytoskeleton in endocytic vesicle formation. Furthermore, correlation analysis of the frequency of exocytic and endocytic events in an isolated patch of membrane revealed that the coupling between exocytosis and endocytosis was independent of the state of the actin cytoskeleton in these non-stimulated neuroendocrine cells. These microphysiological measurements provide evidence that the mechanisms controlling exo- and endocytosis couple to the actin cytoskeleton distinctly in a membrane microdomain.
Methods
Cell culture
A cell culture of melanotrophs from the rat pars intermedia (male Wistar rats, 200-300 g) was prepared by standard methods (Rupnik & Zorec, 1992, 1995). Animals were killed by exposing them to an inflow of 100 % CO2 atmosphere followed by decapitation. This procedure was approved by the Veterinary Administration of the Slovenian Ministry for Agriculture and Forestry according to the Law for Animal Health Protection and the Instructions for Granting Permits for Animal Experimentation for Scientific Purposes. Cells plated on poly-l-lysine-covered glass coverslips were kept in an incubator at 36 °C, 95 % humidity and 5 % CO2 in cell culture medium (a mixture of: αMEM (α-minimal essential medium), DMEM (Dulbecco's modified Eagle's medium), F-12 medium, Gibco, UK) for 1-7 days before experiments. Before experimentation cell-covered coverslips were transferred to the recording chamber mounted on an inverted microscope (Nikon TMS, Japan). The recording medium in the chamber consisted of (mm): NaCl 131.8, CaCl2 1.8, KCl 5, MgCl2 2, Hepes (N-2-hydroxyethylpiperazine-N‘-2-ethanesulphonic acid)/NaOH 10, d-glucose 10, NaH2PO4 0.5, NaHCO3 5; pH 7.2. All chemicals were obtained from Sigma (St Louis, MO, USA) unless otherwise stated.
Membrane capacitance recordings
We used the cell-attached mode of the patch-clamp technique to measure membrane capacitance under an isolated patch of membrane (Neher & Marty, 1982). In the compensated mode of recording, one of the two outputs of the dual-phase lock-in amplifier signal is directly proportional to changes in Cm (Lindau & Neher, 1988; Kreft & Zorec; 1997). A two-phase lock-in amplifier was built into a patch-clamp amplifier (SWAM Cell, Celica, Slovenia (see Zorec et al. 1991)).
Signals were filtered (10 Hz, -3 dB, low pass, Bessel six-pole filter) and acquired at 100 Hz with an analog-to-digital converter (CED 1401, Cambridge, UK) using an IBM compatible PC. The acquisition software WCP was written by Dr J. Dempster (University of Strathclyde, Glasgow, UK). Membrane patches were voltage-clamped at a holding potential of 0 mV, to which a sine wave voltage (111.1 mV r.m.s.) was applied (1600 Hz). Positive steps in Cm were interpreted as single exocytic events, and negative steps as single endocytic events. Steps were resolved by progressive filtering of records. The amplitude and frequency of steps in Cm were measured as reported (Zupancic et al. 1994; Kreft & Zorec, 1997). Recordings were made at room temperature with pipette resistances between 1 and 4 MΩ. The recording pipette solution was the same as the recording medium. Unless stated otherwise, statistics are presented as means ± s.e.m. and differences between samples were tested by Student's t test, considering P < 0.02 to be statistically significant. Error bars on diagrams show s.e.m. The coefficients of both regression lines were compared with statistical method described by Pagano & Gauvreau (2000).
Binary C. spiroforme toxin purification and cell treatment
The toxin was purified and prepared according to the procedure described by Popoff et al. (1989) and Chowdhury et al. (1999). C. spiroforme toxin is a binary toxin made of two components, Sa and Sb. Cells were treated with CST by adding a bolus of the toxin stock solution (275 μg ml−1 Sa (MW 47 000) and 520 μg ml−1 Sb (MW 93 700) into the cell culture medium or into the recording bath solution. In electrophysiological experiments, cell-covered coverslips were soaked for 1 h in bathing solution containing 15 nm Sa and Sb components of the toxin, then washed with bathing solution before the electrophysiological recording. The toxin treatment affected the morphology of cells as viewed with phase-contrast microscopy (Chowdhury et al. 1999). However, patch-clamping these cells revealed that the steady-state membrane conductance was not affected significantly by this treatment (not shown), indicating that membrane was not permeabilised or rendered leaky.
Cytosolic calcium measurements
Intracellular [Ca2+] was measured after loading cells with a dual excitation calcium indicator fura-2/AM (Molecular Probes, Eugene, OR, USA; 4 μm in extracellular recording solution) at 37 °C for 30 min. The cells were rinsed three times with the extracellular recording solution before placing the coverslips into the recording chamber and measurements were made at room temperature (23 °C). Fura-2 fluorescence was excited alternately at two different wavelengths (340 and 380 nm) using a Polychrome IV light source (Till Photonics, Gräfelfing, Germany) fitted to a Zeiss Axiovert 135 inverted microscope with a LD Achroplan 40 × objective lens. Images of the emission were passed through a 410 nm dichroic mirror, filtered at 440 nm and collected by a cooled CCD camera (Imago-VGA, Till Photonics). The digital images were stored and processed by a Tillvision system (Till Photonics). The 340:380 nm ratio images were converted into [Ca2+]i using the formula: [Ca2+]i = Kdβ(R - Rmin)/(Rmax - R), where Kd is the dissociation constant of fura-2 taken as 224 nm (Grynkiewicz et al. 1985), β is the 380 nm fluorescence ratio in Ca2+-free and saturating Ca2+ conditions, and Rmin and Rmax are the fluorescence ratios in Ca2+-free and saturating Ca2+ conditions, respectively. These were determined in situ at the end of the experiment by exposing the cells to 10 μm ionomycin in an external solution containing 10 mm EGTA or 10 mm Ca2+. For a set of experiments these values were pooled and used for calibration. The mean values of Rmin, Rmax and β were 0.43, 2.06 and 5.48, respectively.
Confocal microscopy
Cells were washed with phosphate-buffered saline, pH 7.4 (PBS). After that, cells were fixed in 4 % formaldehyde solution in PBS for 10 min at room temperature. Cells were again washed with PBS. We prepared a 6.6 μm phalloidin-rhodamine solution in methanol (staining solution) and placed it on coverslips for 20 min at room temperature. Cells were then washed with 1,4-diazabicyclo(2,2,2) octane (DABCO, Molecular Probes, SlowFade, Oregon, USA). Mounted coverslips were viewed with a confocal microscope (Zeiss LSM 510, Germany). The fluorescence images were acquired through a planapochromatic oil immersion objective 63 × (NA = 1.4), excited by the 543 nm He-Ne laser line and filtered with the 560 nm long pass emission filter.
Results
Using confocal microscopy we show here that the treatment of cells with the CST resulted in a significant reduction and fragmentation of the cortical actin cytoskeleton as revealed by phalloidin-rhodamine staining (Fig. 1), which is consistent with a previous report (Chowdhury et al. 1999)
Figure 1. Phalloidin-stained actin cytoskeleton in melanotroph cell.
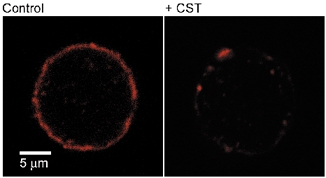
Confocal micrographs of phalloidin-stained actin cytoskeleton in a control (left) and a cell pretreated with CST.
To determine whether actin cytoskeleton disassembly modulates unitary exocytic and endocytic events we measured discrete attofarad changes in membrane capacitance (Cm) of small membrane patches (Neher & Marty, 1982; Zorec et al. 1991) in control and in CST-treated cells. Representative discrete steps in Cm are displayed in Fig. 2 recorded in control and in CST-treated cells and are similar to the steps in Cm recorded previously in rat melanotrophs (Kreft & Zorec, 1997). Cell-attached Cm recordings of at least 15 min in duration, and from 19 control and 11 CST-treated cells were analysed. We measured the amplitude and the frequency of appearance of these steps.
Figure 2. Representative recordings of unitary exocytic and endocytic events.
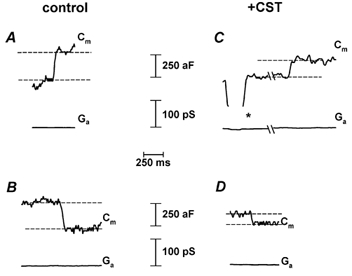
Representative recordings of exocytic (A, C) and endocytic (B, D) events in control cells (left column) and in CST-treated cells (right column). * denotes a calibration pulse used to determine the correct phase separation of the lock-in amplifier. Note that there is no projection of this pulse between the top and bottom traces in panel C. Cm stands for the imaginary part of admittance (proportional to membrane capacitance) and Ga to the real part of admittance of a cell-attached recording.
The average amplitude of unitary exocytic events in control cells was 270 ± 46 aF (n = 92), which is not significantly higher than in CST-treated cells (214 ± 17 aF, n = 121).
In contrast, the amplitude of endocytic events in CST-treated cells was 182 ± 8 aF (n = 88), which is significantly smaller (P < 0.002, see also Fig. 3) than the amplitude of steps in Cm in control cells (271 ± 27 aF, n = 118). The amplitude of these steps in Cm suggests that they are due to the interaction of constitutive vesicles with the plasma membrane, since fusion of secretory granules with the plasma membrane would result in a discrete change in Cm of at least one order of magnitude higher (Zupancic et al. 1994; Kreft & Zorec, 1997; Sikdar et al. 1998). Moreover, under cell-attached conditions the frequency of appearance of subfemtofarad steps is insensitive to the addition of ionomycin to increase cytosolic [Ca2+], indicating that the fusion of small vesicles is not sensitive to changes in cytosolic [Ca2+] in rat melanotrophs (Kreft & Zorec, 1997). Furthermore, the analysis of amplitude histograms of exocytic events revealed that the distribution of amplitudes was similar in controls and after CST treatment, indicating that the disassembly of the actin cytoskeleton did not preferentially stimulate the fusion of large hormone-containing secretory granules (not shown). This is in agreement with the report of Matter et al. (1989) where basal release of noradrenaline was not affected by the actin disassembly in PC12 cells.
Figure 3. The amplitude of exocytic and endocytic events.
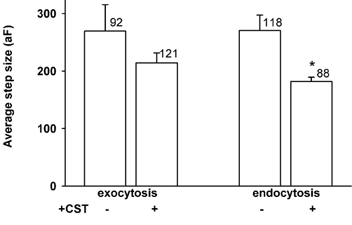
The average amplitude of exocytic (left) and endocytic (right) events in control and CST-treated cells. Numbers adjacent to columns represent a number of events. * Statistically significant (P < 0.002). Bars show s.e.m.
The frequency of unitary exocytic events in CST-treated cells was significantly higher (0.54 ± 0.1 min−1, n = 19 patches, P < 0.01) than in control cells (0.23 ± 0.04 min−1, n = 11 patches, Fig. 4A). In contrast, there was no significant difference in the frequency of endocytic events between control (0.29 ± 0.05 min−1, n = 19 patches) and CST-treated cells 0.37 ± 0.1 min−1 (n = 11 patches). If the area of the membrane is to remain constant over a longer period of time, exocytosis should be balanced by endocytosis. In Fig. 4B we show that the ratio between frequencies of exocytic and endocytic events in control cells is close to one, as expected. However, in CST-treated cells the ratio was almost two due to increased frequency of exocytic steps (Fig. 4A). To test whether the frequencies of exo- and endocytic steps in CST-treated cells are indeed coupled by a linear factor of two, as indicated by Fig. 4B, we performed regression analysis. We found that in CST-treated cells, the frequency of exocytic steps is increased independently of endocytic steps, which is indicated by the parallel shift of the regression line (Fig. 5). The intercepts of lines are significantly different (P < 0.01) while the slopes of regression lines are similar (Fig. 5). The increased frequency of exocytic events can be due to a direct effect of removal of the actin cytoskeleton by the CST pretreatment. On the other hand the pretreatment of the cells by the CST may also affect exocytosis indirectly via [Ca2+]i. Although intracellular Ca2+ measurements have been made in intact single rat melanotrophs in culture (Nemeth et al. 1990; Sikdar et al. 1998), to date there are no reports to suggest whether CST affects [Ca2+]i. To resolve the question of whether CST acts via an increase in [Ca2+]i, single melanotrophs were treated with CST, while [Ca2+]i was monitored using the Ca2+ indicator fura-2/AM. Figure 6 shows images of resting cells where [Ca2+]i was measured before the addition of the CST (Fig. 6A), 1 h after the addition of the CST (Fig. 6B) and after the addition of ionomycin (Fig. 6C). [Ca2+]i before CST treatment was 109 ± 15 nm, and after 1 h of CST treatment it was 160 ± 13 nm (n = 16), statistically different (P = 0.027), but both values equal to resting [Ca2+]i in melanotrophs (Nemeth et al. 1990; Sikdar et al. 1998). Upon the addition of ionomycin, [Ca2+]i rapidly increased to values higher than 1 to 2 μm. The threshold of [Ca2+]i that is required to activate the high-Ca2+ affinity exocytosis in melanotrophs is in the order of around 3 to 5 μm (Rupnik et al. 2000; Poberaj et al. 2002). Therefore, it is likely that the pretreatment of cells with the CST is not affecting the frequency of exocytic events indirectly via [Ca2+]i. While it is true that the small 51 nm rise in [Ca2+]i should be insufficient to trigger exocytosis, such a small sustained rise in [Ca2+]i might affect other signalling processes that could contribute to the modulation of exocytosis seen in the presence of CST.
Figure 4. The frequency of exocytic and endocytic events.
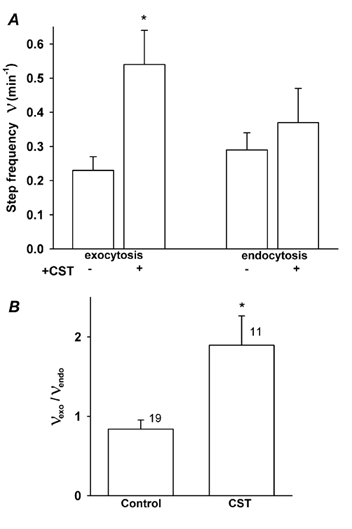
A, the average frequency of exocytic events (left) and endocytic events (right) in control and CST-treated cells. B, the ratio between frequencies of exocytic and endocytic events in control and CST-treated cells. Numbers adjacent to columns represent numbers of cells examined. * Statistically significant difference (P < 0.02).
Figure 5. Relationship between the frequencies of exocytic and endocytic events.
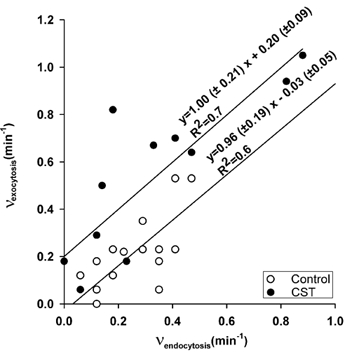
The relationship between the frequency of exocytic and the frequency of endocytic events in control (○) and in CST-treated (•) cells. Regression lines (obtained using SPSS SigmaPlot software) were drawn according to the equations depicted on the figure. Note that the intercepts, but not the slopes of lines, are significantly different (P < 0.01). Moreover, the slope of the CST-treated cells is significantly different from the slope coefficient vaalue 2 (P < 0.01).
Figure 6. Images of cytosolic [Ca2+] in control and CST-treated melanotrophs.
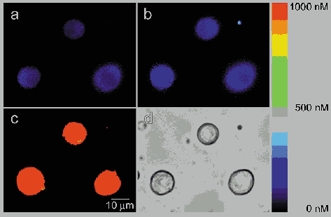
A, representative ratio fura-2 image of cells showing cytosolic [Ca2+]i in control conditions, and B, 1 h after the addition of the CST. Note that the average [Ca2+]i has not changed significantly after the CST treatment in comparison to a rise in ionomycin-induced rise in [Ca2+]i (C). Panel on the right indicates the colour-coded concentration of [Ca2+].
Discussion
The aim of this work was to investigate whether the actin cytoskeleton modulates the appearance of unitary exocytic and endocytic events. Using confocal microscopy we have confirmed that CST pretreatment significantly reduced the cortical actin cytoskeleton (Fig. 1) (Chowdhury et al. 1999). In our previous study we used the whole-cell patch-clamp technique to monitor changes in the Cm of whole-cell membrane and have shown that actin cytoskeleton depolymerisation with CST affects Cm of rat melanotrophs (Chowdhury et al. 1999). However, the whole-cell membrane capacitance measurements preclude the unequivocal determination of whether actin disassembly affects specifically exocytosis and/or endocytosis. Therefore we employed the cell-attached patch-clamp technique that allows us to observe discrete attofarad changes in Cm. Positive steps in Cm were interpreted as exocytic events and negative steps as endocytic events (Neher & Marty, 1982). We measured the frequency and amplitude of these events in control and CST-treated cells. The effects of CST treatment that we observed on these physiological parameters are most probably due to a direct effect of actin cytoskeleton disassembly, since calcium homeostasis was not affected by the CST pretreatment (Fig. 6).
While the absence of effect of CST treatment on the amplitude of exocytic events indicates that the status of the actin cytoskeleton does not affect the size of vesicles in the exocytic pathway, the reduced amplitude of endocytic vesicles (Fig. 2 and Fig. 3) is best explained by an essential role of the actin cytoskeleton in the formation of endocytic vesicles (see Apodaca, 2001). These results are consistent with reports where the sites of vesicle budding in the Golgi apparatus appear to be associated with actin (Lorra & Huttner, 1999; Fucini et al. 2000; Valderrama et al. 2000), which may be required at various stages in the formation of an endocytic vesicle including membrane invagination, neck elongation, fission of the neck and/or its propulsion away from the plasma membrane (Apodaca, 2001; Martin, 2001). The actin cytoskeleton may affect the formation of endocytic vesicles via an interaction through dynamin, which is thought to play a role in the final pinching off of endocytic vesicles from the plasma membrane (Sever et al. 2000).
From the CST-mediated reduction of the endocytic event amplitude one could predict that in experiments where changes in Cm are monitored in a whole cell, the CST-treatment should result in a net increase in Cm. Indeed, in non-stimulated cells it was reported that whole-cell Cm increases with an average rate of 0.03 % s−1 (Chowdhury et al. 1999). With an average resting Cm of these cells of 4 pF, the rate of 0.03 %−1 increase in Cm equals to 1.2 fF s−1. In a previous study it was shown that such an average rate in Cm increase is equal to a vesicle fusion rate of around 0.6 s−1 (Zupancic et al. 1994). In this work the frequency of exocytic events in a small patch of membrane was 3 × 10−3 vesicles s−1 in control conditions and 8 × 10−3 s−1 after CST treatment (Fig. 4). If one takes into account that the area of a patch is approximately 0.5 % of the total plasma membrane (Sakmann & Neher, 1983; Kreft & Zorec, 1997), then the number of fusion events per whole cell is 0.6 s−1 in controls and 1.6 s−1 after CST treatment. Although the amplitudes of unitary events in a cell-attached patch are smaller than those recorded in the whole-cell configuration, the rate of fusion events recorded in the cell-attached configuration agrees well with previous measurements in resting whole cells (0.6 vesicles s−1, see Fig. 6 in Sikdar et al. 1998).
The increased frequency of exocytic events in CST-treated cells supports the view that actin cytoskeleton disassembly reduces the barrier for vesicles entering exocytosis (Burgoyne & Morgan, 1993; Chowdhury et al. 1999). Similarly, it was proposed that actin mesh might act as a molecular fence for the formation of endocytic vesicles (Fujimoto et al. 2000). If such a mechanism operated in endocytic pathways of rat melanotrophs one would also expect an effect of actin cytoskeleton disassembly on the frequency of endocytic events. However, our results do not support this hypothesis, since there was no change in the frequency of endocytic events after the disassembly of the actin cytoskeleton. More probably, the actin cytoskeleton affects the pathway of endocytosis in rat melanotrophs at a stage of vesicle formation, since the amplitude of endocytic events was reduced after the CST treatment (Fig. 2 and Fig. 3). It is not known which pathway of endocytosis is represented by the discrete off-steps in Cm recorded in this study. However, it is probably not associated with the fluid uptake, since it was shown that in epithelial cells, fluid uptake on the apical membrane does not require intact actin cytoskeleton (Shurety et al. 1998).
Figure 3 shows that endocytic and exocytic vesicles are approximately the same size in control conditions, which is consistent with previous findings (Zorec et al. 1991; Zupancic et al. 1994; Kreft & Zorec; 1997). Similar amplitudes of exocytic and endocytic events may reflect a common mechanism determinant for the vesicle size in both processes (see Zorec et al. 1991), supporting the proposed fusion pore model for exocytosis (Zimmerberg et al. 1987) where a transiently fusing exocytic vesicle turns into an endocytic vesicle after the fusion pore closes. This form of coupling between exocytic and endocytic vesicles is supported by physiological experiments where membrane capacitance changes are stimulated by photolysis of caged calcium and show that the amount of increased surface area is typically retrieved (see Kasai et al. 1996). Although proteins that affect regulated exocytosis and endocytosis are distinct, there is some overlap. For example synaptotagmin I is thought to be the calcium sensor in regulated exocytosis (Geppert et al. 1994) and it may also interact with adaptor protein AP-2 in endocytosis (Zhang et al. 1994). Thus we investigated whether in our study the appearance of unitary exocytic and unitary endocytic events was correlated.
Under control conditions, without any stimulation of secretion, we found that membrane added by small exocytic steps is balanced by membrane retrieval along similar endocytic membrane capacitance steps (Fig. 3 and Fig. 5). In Fig. 4B, where the frequency of exocytic steps was divided by the frequency of endocytic steps in a particular membrane patch, CST treatment resulted in a ≈2-fold increase in the frequency of exocytic events in relation to the frequency of endocytic events. This could indicate at least two mechanisms of coupling between exocytosis and endocytosis (see Fig. 7).
Figure 7. Two models of coupling between exocytosis and endocytosis.
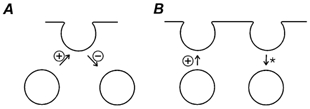
A, the same membrane added to the plasma membrane by exocytosis is retrieved by endocytosis (‘kiss-and-run’). B, exocytosed membrane appears to be loosely coupled to endocytosis that occurs in a different membrane microdomain. * denotes an inhibitory action of CST on the size of endocytic vesicles, whereas + denotes an increase and - a decrease in the frequency of events by the CST-treatment.
First, one may consider that after exocytosis the same membrane added to the plasma membrane is retrieved by endocytosis (Fig. 7A) (Zimmerberg et al. 1987). In this case the exocytic event is separated in time from the ensuing endocytic event by the fusion pore duration. To explain the CST-mediated increase in the ratio between the frequency of exocytic and endocytic events using this model, also termed ‘kiss-and-run’, one would have to consider that the fusion pore dwell time is affected in such a way that CST treatment would prolong or prevent fusion pore closure. Hence, linear regression analysis as shown on Fig. 5 should result in a line with a slope equal to the increased ratio of frequencies of exocytic and endocytic events (i.e. ≈2, Fig. 4B) and with an intercept not significantly different from zero. Moreover, if CST inhibits the closure of the fusion pore, then the frequency of endocytic events should decrease, which was not the case (Fig. 4A).
Second, if one considers that the exocytosed membrane is loosely balanced by endocytosis from a different membrane microdomain (Fig. 7B), then the CST-mediated increase in the frequency of exocytic events may not be characterised by a slope of ≈2 (see Fig. 5). Indeed, this was the case in our experiments. After CST-treatment the slope of the regression between the frequencies of exocytic and endocytic events was not significantly different from the slope coefficient of control experiments (value 1) (Fig. 5), but was significantly different from the slope coefficient value 2. Moreover, the line intercept with the y-axis was significantly higher than 0 (Fig. 5), indicating that if there was no endocytic activity in a membrane patch, there was a significant number of exocytic steps. Our results can be explained by a CST-mediated increase in the frequency of new exocytic events (see Fig. 7B). This suggests that the disassembly of the actin cytoskeleton distinctly affects exocytic and endocytic mechanisms, and that mechanisms that regulate the balance of exocytic and endocytic events for the small vesicles are very probably distinct (i.e. in biochemical terms and/or that these mechanisms are clustered in different membrane microdomains).
In summary, our microphysiological study of constitutive membrane trafficking in a small area of the plasma membrane in resting melanotrophs provided evidence that unitary exocytic and endocytic events are differentially regulated by the actin cytoskeleton. This is consistent with the view that the control of vesicle fusion and vesicle fission by the actin cytoskeleton involves distinct plasma membrane microdomains involving specific proteins and cytoskeleton assembly events.
Acknowledgments
We thank Sonja Grilc for preparing cell cultures. This work was supported by Ministry of Sciences and Technology of The Republic of Slovenia grants (nos P3 521 381 and J3 2344-7421-00) awarded to R. Z. and M.K.
References
- Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Regulated exocytosis. Biochemical Journal. 1993;293:305–316. doi: 10.1042/bj2930305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury HH, Popoff MR, Zorec R. Actin cytoskeleton depolymerization with Clostridium spiroforme toxin enhances the secretory activity of rat melanotrophs. Journal of Physiology. 1999;521:389–395. doi: 10.1111/j.1469-7793.1999.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doussau F, Augustine GJ. The actin cytoskeleton and neurotransmitter release: an overview. Biochimie. 2000;82:353–363. doi: 10.1016/s0300-9084(00)00217-0. [DOI] [PubMed] [Google Scholar]
- Fernandez JM, Neher E, Gomperts BD. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature. 1984;312:453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Fucini RV, Navarrete A, Vadakkan C, Lacomis L, Erdjument-Bromage H, Tempst P, Stamnes M. Activated ADP-ribosylation factor assembles distinct pools of actin on golgi membranes. Journal of Biological Chemistry. 2000;275:18824–18829. doi: 10.1074/jbc.M000024200. [DOI] [PubMed] [Google Scholar]
- Fujimoto LM, Roth R, Heuser JE, Schmid SL. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic. 2000;1:161–171. doi: 10.1034/j.1600-0854.2000.010208.x. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Henkel AW, Meiri H, Horstmann H, Lindau M, Almers W. Rhythmic opening and closing of vesicles during constitutive exo- and endocytosis in chromaffin cells. EMBO Journal. 2000;19:84–93. doi: 10.1093/emboj/19.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Takagi H, Ninomiya Y, Kishimoto T, Ito K, Yoshida A, Yoshioka T, Miyashita Y. Two components of exocytosis and endocytosis in phaeochromocytoma cells studied using caged Ca2+ compounds. Journal of Physiology. 1996;494:53–65. doi: 10.1113/jphysiol.1996.sp021475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft M, Zorec R. Cell-attached measurements of attofarad capacitance steps in rat melanotrophs. Pflügers Archiv. 1997;434:212–214. doi: 10.1007/s004240050387. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Schmid SL. The emergence of clathrin-independent pinocytic pathways. Current Opinion in Cell Biology. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflügers Archiv. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Lorra C, Huttner W. The mesh hypothesis of Golgi dynamics. Nature Cell Biology. 1999;1:E113–115. doi: 10.1038/12939. [DOI] [PubMed] [Google Scholar]
- Mains RE, Eipper BA. Synthesis and secretion of corticotropins, melanotropins, and endorphins by rat intermediate pituitary cells. Journal of Biological Chemistry. 1979;254:7885–7894. [PubMed] [Google Scholar]
- Martin TFJ. PI(4,5)P2 regulation of surface membrane traffic. Current Opinion in Cell Biology. 2001;13:493–499. doi: 10.1016/s0955-0674(00)00241-6. [DOI] [PubMed] [Google Scholar]
- Matter K, Dreyer F, Aktories K. Actin involvement in exocytosis from PC12 cells: Studies on the influence of botulinum C2 toxin on stimulated noradrenaline release. Journal of Neurochemistry. 1989;52:370–376. doi: 10.1111/j.1471-4159.1989.tb09131.x. [DOI] [PubMed] [Google Scholar]
- Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. Journal of Cell Biology. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiological Reviews. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proceedings of the National Academy of Sciences of the USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth EF, Taraskevich PS, Douglas WW. Cytosolic Ca2+ in melanotrophs: pharmacological insights into regulatory influences of electrical activity and ion channels. Endocrinology. 1990;126:754–758. doi: 10.1210/endo-126-2-754. [DOI] [PubMed] [Google Scholar]
- Norman JC, Price LS, Ridley AJ, Koffer A. The small GTP-binding proteins, Rac and Rho, regulate cytoskeletal organization and exocytosis in mast cells by parallel pathways. Molecular Biology of the Cell. 1996;7:1429–1442. doi: 10.1091/mbc.7.9.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M, Gauvreau K. Principles of Biostatistics. 2. Pacific Grove, USA: Duxbury Press; 2000. pp. 415–448. [Google Scholar]
- Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. Journal of Cell Biology. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poberaj I, Rupnik M, Kreft M, Sikdar SK, Zorec R. Modeling excess retrieval in rat melanotroph membrane capacitance records. Biophysical Journal. 2002;82:226–232. doi: 10.1016/S0006-3495(02)75389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff MR, Boquet P. Clostridium spiroforme toxin is a binary toxin which ADP-ribosylates cellular actin. Biochemical and Biophysical Research Communications. 1988;152:1361–1368. doi: 10.1016/s0006-291x(88)80435-2. [DOI] [PubMed] [Google Scholar]
- Popoff MR, Milward FW, Bancillon B, Boquet P. Purification of the Clostridium spiroforme binary toxin and activity of the toxin on HEp-2 cells. Infection and Immunity. 1989;57:2462–2469. doi: 10.1128/iai.57.8.2462-2469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, Sheetz MP, Meyer T. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100:221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- Rupnik M, Kreft M, Sikdar SK, Grilc S, Romih R, Zupancic G, Martin TF, Zorec R. Rapid regulated dense-core vesicle exocytosis requires the CAPS protein. Proceedings of the National Academy of Sciences of the USA. 2000;97:5627–5632. doi: 10.1073/pnas.090359097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnik M, Zorec R. Cytosolic chloride ions stimulate Ca2+-induced exocytosis in melanotrophs. FEBS Letters. 1992;303:221–223. doi: 10.1016/0014-5793(92)80524-k. [DOI] [PubMed] [Google Scholar]
- Rupnik M, Zorec R. Intracellular Cl− modulates Ca2+-induced exocytosis from rat melanotrophs through GTP-binding proteins. Pflügers Archiv. 1995;431:76–83. doi: 10.1007/BF00374379. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Neher E. Geometric parameters of pipettes and membrane patches. In: Sakmann B, Neher E, editors. Single Channel Recordings. New York: Plenum Press; 1983. pp. 37–51. [Google Scholar]
- Senda T, Okabe T, Matsuda M, Fujita H. Quick-freeze, deep-etch visualization of exocytosis in anterior pituitary secretory cells: localization and possible roles of actin and annexin II. Cell and Tissue Research. 1994;277:51–60. doi: 10.1007/BF00303080. [DOI] [PubMed] [Google Scholar]
- Sever S, Damke H, Schmid SL. Garrotes, springs, ratchets and whips: putting dynamin models to the test. Traffic. 2000;1:385–392. doi: 10.1034/j.1600-0854.2000.010503.x. [DOI] [PubMed] [Google Scholar]
- Shurety W, Stewart NL, Stow JL. Fluid-phase markers in the basolateral endocytic pathway accumulate in response to the actin assembly-promoting drug Jasplakinolide. Molecular Biology of the Cell. 1998;9:957–975. doi: 10.1091/mbc.9.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikdar SK, Kreft M, Zorec R. Modulation of the unitary exocytic event amplitude by cAMP in rat melanotrophs. Journal of Physiology. 1998;511:851–859. doi: 10.1111/j.1469-7793.1998.851bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifaró JM, Vitale ML. Cytoskeleton dynamics during neurotransmitter release. Trends in Neurosciences. 1993;16:466–472. doi: 10.1016/0166-2236(93)90079-2. [DOI] [PubMed] [Google Scholar]
- Valderrama F, Luna A, Babia T, Martinez-Menarguez JA, Ballestra J, Barth H, Charponnier C, Renau-Piqueras J, Egea G. The golgi-associated COPI-coated buds and vesicles contain beta/gamma -actin. Proceedings of the National Academy of Sciences of the USA. 2000;97:1560–1565. doi: 10.1073/pnas.97.4.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Davletov BA, Sudhof TC, Anderson RG. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Curran M, Cohen FS, Brodwick M. Simultaneous electrical and optical measurements show that membrane fusion precedes secretory granule swelling during exocytosis of beige mouse mast cells. Proceedings of the National Academy of Sciences of the USA. 1987;84:1585–1589. doi: 10.1073/pnas.84.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorec R, Henigman F, Mason WT, Kordas M. Electrophysiological study of hormone secretion by single adrenohypophysal cells. Methods in Neurosciences. 1991;4:194–210. [Google Scholar]
- Zupancic G, Kocmur L, Veranic P, Grilc S, Kordas M, Zorec R. The separation of exocytosis from endocytosis in rat melanotroph membrane capacitance records. Journal of Physiology. 1994;480:539–552. doi: 10.1113/jphysiol.1994.sp020382. [DOI] [PMC free article] [PubMed] [Google Scholar]


