Abstract
The study of coronary artery anomalies would benefit from the clarification of various fundamental issues, including the definitions, classification, incidence, pathophysiologic mechanisms, and clinical relevance of each anomaly. The greatest challenge is to identify the abnormality and determine its clinical relevance so that appropriate treatment can be instituted.
Currently, the coronary anatomy is essentially defined by the features of the (conductive) epicardial coronary tree and its dependent territory. Therefore, one must consider all the possible and observed variations in anatomic features that are used to describe the coronary arteries. We propose that the left anterior descending, circumflex, and right coronary arteries be considered the essential, elementary units of coronary anatomy. We also suggest that the coronary arteries be defined not by their origin or proximal course, but by their intermediate and distal segments or dependent microvascular bed.
A strict classification system is necessary before meaningful data can be gathered about the incidence of coronary anomalies. With respect to clinical relevance, the greatest challenge is presented by anomalies that only occasionally cause critically severe clinical events and are otherwise compatible with a normal life. In such cases, it is not known whether the specific features of a given anomaly cause adverse clinical consequences, or whether additional episodic factors are required.
To correlate subclassifiable anatomic and functional features with clinical events and prognoses, a large, multicenter database, relying on prospective, coordinated protocols, is urgently needed. In the absence of established official guidelines, we present practical protocols for diagnosing and treating coronary anomalies. (Tex Heart Inst J 2002;29:271–8)
Key words: Coronary anatomy; coronary vessel anomalies/diagnosis/epidemiology; death, sudden/causes/epidemiology; heart defects, congenital; sinus of Valsalva/abnormalities
Coronary anomalies are a poorly understood topic in modern cardiology. Clinicians and members of the general public may be aware of such anomalies, chiefly because the anomalies can result in sudden death. Nevertheless, a standard definition for coronary anomalies needs to be established, and various other fundamental issues need to be clarified, including the anatomic spectrum of coronary anomalies (which is often represented by a long, incomplete list that lacks a rationale), the pathophysiologic mechanisms involved, and the clinical repercussions and prognoses of abnormal coronary anatomy. Because these issues are not well understood, treatment guidelines tend to be unsubstantiated, inconsistent, and hence unreliable. By attempting to clarify some of these issues, the author hopes to promote an improvement in the diagnosis and treatment of coronary anomalies.
Definition and Classification
The definition of the abnormal versus the normal coronary anatomy presents a complex problem that has never been completely solved. In an attempt to simplify this matter, some researchers have suggested that coronary anomalies should be classified as major or minor, depending on their pathologic consequences. Recently, most investigators have chosen to use an exclusively anatomic definition that relegates judgments about clinical relevance to a secondary clinical classification. 1
Essentially, the normal coronary arterial circulation consists of 2 components: the large, proximal, conductive arteries and the distal, microvascular, high-resistance vessels or arteriolar–capillary network. The latter vessels, which surround and nourish the myocardial fibers, cannot be precisely evaluated in vivo. A normal myocardial nuclear scintigram, for example, can prove the adequacy of the microvascular arterial bed only in the presence of normal, large, proximal coronary arteries. It is possible that human beings may be subject to a pathologic state defined by a myocardial microvascular network that is anatomically or physiologically inadequate; such a state could account for the so-called syndrome X (myocardial ischemia in the presence of normal coronary arteries). At this time, the coronary anatomy is essentially defined by the features of the (conductive) epicardial coronary tree and its terminations in the microvascular bed. Therefore, in defining abnormal coronary anatomy, one must include all the possible and observed variations in anatomic features that are used to describe the coronary arteries (Table I). With some features, normality can be defined numerically (for example, the presence of 2 or 3 coronary ostia is normal); with other features, an exact description of a continuous variable, according to a Gaussian distribution curve in a large normal population, is required. 1 The full range of normal (no coronary anomaly) versus abnormal (a coronary anomaly) could be characterized by empirical criteria: abnormal might be considered as “that which is observed in less than 1% of the normal population,” or “that which lies more than 2 standard deviations from the mean value of a Gaussian distribution curve.” In this regard, strict definitions should be issued by a representative, authoritative group of experts.
TABLE I. Variable Features of the Coronary Artery Anatomy That May Be Normal or Abnormal

Whereas it is generally assumed that the human heart has 2 coronary arteries (the right and the left), one might ask the basic question, “What are the elementary units—the essential coronary arteries?” Consider, for example, the case of an absent left main stem with separate origination of the left anterior descending (LAD) and circumflex arteries, or the case of a single coronary artery. Both of these conditions contradict the idea that there are 2 coronary arteries. Indeed, it seems more accurate to assume that the LAD, circumflex, and right coronary artery (RCA) are the elementary units of the coronary anatomy. 2 If this concept is accepted, the left main stem should be considered a mixed proximal trunk that might or might not exist, like the initial common trunk shared by the circumflex and the RCA in the case of anomalous origination of the circumflex from the RCA.
Clearly, the next challenge is to define each elementary coronary artery with respect to its essence or minimal requirements (Table II). We recently proposed 1 that the coronary arteries be defined not by their origin or proximal course but by their intermediate and distal segments or their dependent microvascular bed; for example, the RCA should probably be defined as “the artery that runs in the right atrioventricular groove and provides nutrient branches to the free wall of the right ventricle.” It is not essential that this artery originate from the right anterior sinus of Valsalva (commonly, but improperly, called the “right” or “coronary” sinus), because it is not essential that this sinus have a coronary ostium or, specifically, a right coronary ostium. In addition, it is not essential that the RCA provide nutrient branches to the posterior interventricular septum, although this pattern is common. In the presence of a dominant circumflex artery, which occurs in about 9% of the normal population, 1 the posterior descending artery usually arises from the circumflex coronary artery.
TABLE II. Proposed Definition of the Essential Features* of the 3 (Elementary) Coronary Arteries

In describing the coronary anatomy, especially in angiographic terms, a frequently overlooked feature is the originating structure: the aortic root. Although the aortic root (specifically the sinuses of Valsalva) is normal in most patients who undergo coronary angiography, about 26% of coronary anomalies involve some kind of aortic root abnormality—at least asymmetry of the aortic sinuses. 1 The classic example is the bicuspid aortic valve, which is associated with a very high incidence of coronary anomalies. Observations in human beings correlate with recent findings in Syrian hamsters that have been inbred specifically to express bicuspid aortic valve: 3 in the presence of this anomaly, the hamsters have an abnormal coronary pattern in about 48% of cases.
It is even more relevant to consider the exact anatomy of the aortic root in the presence of more substantial cardiac defects of the outflow tracts, such as transposition of the great vessels. 2 In all such outflow tract defects, it is important to refer to the coronary anatomy by clearly describing the aortic and pulmonary valves (Fig. 1).
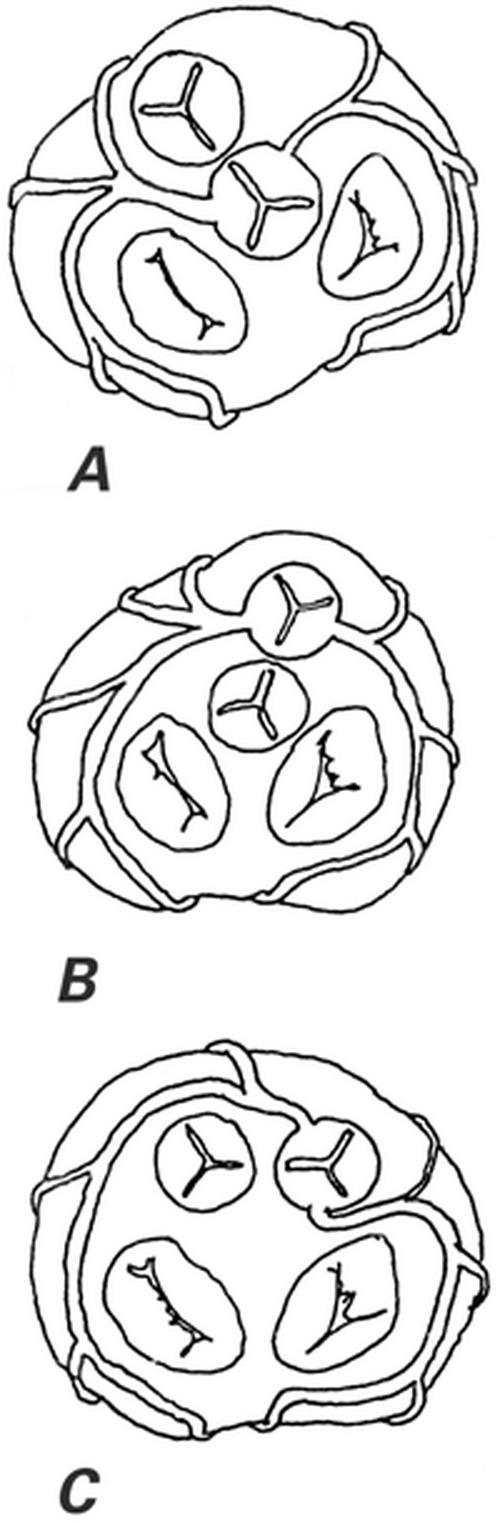
Fig. 1 The most common coronary artery anatomic patterns in 44 cases of double-outlet right ventricle. A) Normal coronary origination as seen in all 17 cases (39%) with normally related great vessels. B) Coronary artery pattern seen in 11 (73%) of the 15 patients with transposition of the great arteries (34% of the 44 cases). C) Coronary artery pattern seen in 4 (33%) of the 12 cases with side-by-side arrangement of the great arteries. Five other coronary patterns were less frequently observed.
(From Angelini P, editor. Coronary artery anomalies: a comprehensive approach. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 85. 1 [Modified from Gordillo L, Faye-Petersen O, de la Cruz MV, Soto B. Coronary arterial patterns in double-outlet right ventricle. Am J Cardiol 1993;71:1108–10.] Reprinted with permission from Lippincott Williams & Wilkins.)
Incidence
A strict system for classifying coronary anomalies is necessary in order to gather meaningful data about the incidence of these lesions. Currently, most reports regarding the incidence of coronary anomalies rely on unspecified classification criteria used in undefined populations. For example, most series involving specific anomalies, especially those series reported in the pathology literature, likely hide the existence of an unquantified referral bias, which makes it difficult to precisely determine the incidence of mortality or its associated risk. The incidence of coronary anomalies is generally reported to be about 1%. However, the incidence was found to be 5.6% in a recent prospective angiographic study of 1,950 consecutive cases, 1 which was performed according to clearly stated criteria and a strict classification scheme (Table III). Notwithstanding, even such a large series does not necessarily indicate the incidence in the general population. In our series, it is likely that some (but probably not many) patients with coronary anomalies were referred because of the known presence of the anomaly, not because of unrelated factors (as in the general population). For example, in cases involving coronary fistulas with a large flow volume, auscultation or echocardiography can clearly identify these lesions before angiography is performed. In other words, although the literature frequently implies that angiographic populations include coronary anomalies only by chance, this is not true.
TABLE III. Incidence of Coronary Anomalies and Patterns, as Observed in a Consecutive Series of 1,950 Angiograms 1

One of the most troubling questions concerns the true incidence of anomalous origination of a coronary artery from the opposite sinus (ACAOS). In the above-mentioned prospective study, 1 this incidence was 1.07%, which included a 0.92% rate of ectopic RCA originating from the left sinus and a 0.15% rate of ectopic left coronary artery (LCA) arising from the right sinus. These data are not consistent with frequently quoted autopsy reports, 4 which suggest a 57% mortality rate for ACAOS involving an ectopic LCA and a 25% mortality rate for ACAOS involving the RCA. In fact, if we assume that the ratio of ectopic LCA versus ectopic RCA in the general population is 6:1, 1 ACAOS would entail an average mortality rate of 30%. Moreover, if the 1.07% incidence were applied to the general U.S. population of 285 million persons, there would be more than 3 million carriers of this anomaly, and more than 1 million of those carriers would be expected to succumb to ACAOS-related cardiac death. Such a phenomenon has never been confirmed by any significant study of unselected populations. Interestingly, however, in autopsy series of young athletes who have died during exercise, the ratio of “LCA originating from the right sinus” to “RCA originating from the left sinus” has been 5:1, 5 which is similar to the ratio found in our angiographic series (6:1). 1
Clinical Relevance
After establishing the definition, classification, and incidence of coronary anomalies, the next task would be to evaluate the clinical relevance of each anomaly. In only a few cases is the artery's function of providing vital blood flow to the dependent myocardium impaired at baseline. For example, depending on individual variations, resting ischemia occurs to different degrees in anomalous origination of the left coronary artery from the pulmonary artery (ALCAPA) and in coronary ostial atresia or severe stenosis (anomalies involving obligatory ischemia). 6 In contrast, most coronary anomalies are at the innocuous end of the broad spectrum of clinical consequences, imposing no limitations on resting or maximal blood flow but merely requiring the clinician to be alert and knowledgeable (anomalies involving absent ischemia). Failure to recognize unusual coronary anatomy may lead to incorrect diagnosis or treatment, both in the catheterization laboratory and in the surgical suite. The greatest challenge is presented by the ill-defined group of coronary anomalies that only occasionally cause critically severe clinical events (episodes of ischemia); these anomalies involving exceptional ischemia are otherwise compatible with leading a normal life, even including athletic training.
For many years, researchers have related angina, dyspnea, syncope, acute myocardial infarction, and sudden death to coronary anomalies, mostly on the basis of necropsy series. In most of these cases, the exact pathophysiologic mechanisms that cause ischemia have not been determined: muscular bridges, coronary fistulas, and ectopic origination have not consistently been shown to cause ischemia during clinical stress testing. The recurrent fundamental question in this regard is “Can the specific features of a given coronary anomaly cause, per se, unusual clinical consequences, or are additional episodic factors (such as spasm, compression, or clotting) required in order to produce critically unfavorable conditions?”
In this context, ectopic origination of a coronary artery from the opposite sinus (ACAOS) is particularly relevant. We have recently used new techniques to study patients who were found to be carriers of ACAOS and presented with severe clinical manifestations, such as syncope or aborted sudden death.* In the catheterization laboratory, we used pressure wires in order to calculate the fractional flow reserve (FFR); during adenosine provocation testing, these patients had results within normal limits (FFR >0.9). These findings are consistent with the fact that, in similar patients, the results of nuclear stress testing with a submaximal treadmill protocol are usually negative for reversible ischemia. 7 In addition, our group at the Texas Heart Institute has recently started a new series of investigations, the basis of which is the use of intravascular ultrasound (IVUS) imaging. Using this technique, we have observed features consistent with tangential proximal coursing of such ectopic arteries, which exit the aortic lumen by passing into the aortic wall and undergoing intussusception for a variable distance (Figs. 2–4). The intramural segment is characterized by exceptionally thin inner and outer aortic-wall layers and by lateral luminal compression, which is fixed during the cardiac cycle but undergoes phasic worsening during systole (Fig. 4B). In our initial series of 10 patients, neither an ostial ridge nor atherosclerotic build-up 4 was found to contribute to a possibly obstructive mechanism. The section of aortic root that is penetrated by the intramural coronary segment becomes a localized weak spot and seems to yield more than the rest of the aortic wall during the ejection period; this leads to worsening stenosis. To simulate what probably happens during exercise, we used an experimental protocol in which the stroke volume and heart rate were increased by the rapid infusion of a saline bolus, atropine, and dobutamine. The initial results suggested that individual variations in the degree of systolic lateral compression do, indeed, occur and that they might explain the different individual behaviors and prognoses. Before any definitive statements can be made in this regard, though, a large population should be studied with similar protocols—the ultimate aim being to determine which patients can be safely treated medically and which ones might require intervention. Factors that influence aortic-wall distensibility, such as mediocystic necrosis (Marfan syndrome) or ectasia of the ascending aorta (frequently associated with bicuspid aortic valve), may further affect the prognosis. The normal thickening and stiffening of the aortic wall that occurs with aging may account for the relatively benign behavior of ACAOS in older patients. 8 Coordinated multicenter studies designed to correlate IVUS data with patients' clinical histories and prognoses are needed to reach evidence-based conclusions.
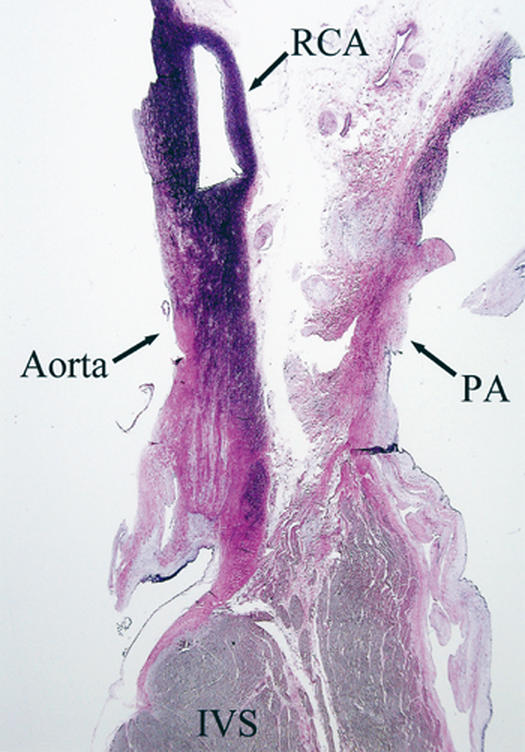
Fig. 2 Histologic longitudinal cross-section at the right ventricular outflow tract and the aortic root (closest point) obtained at necropsy from a 17-year-old basketball player who died suddenly during a game. The patient had reported no symptoms and had undergone several medical examinations that were negative for cardiovascular disease. Note that the right coronary artery (RCA) is intussuscepted into the aortic wall, which has a thickness that is 50% of normal inside the RCA, and 25% of normal outside the RCA. Also note the wide space between the aorta and the pulmonary artery (PA) at the closest site, which makes scissors-like compression of the RCA unlikely.
IVS = interventricular septum
(Photo courtesy of Dwayne A. Wolf, MD, PhD; Office of the Medical Examiner of Harris County, Texas.)
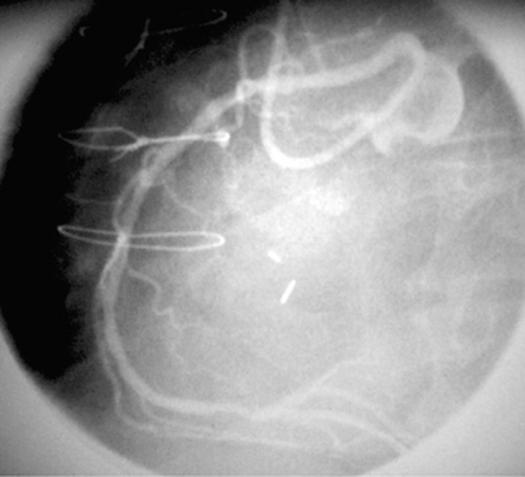
Fig. 3 Angiographic appearance of an ectopic right coronary artery (RCA) originating at the left sinus of Valsalva, next to the left coronary artery (left anterior oblique projection).
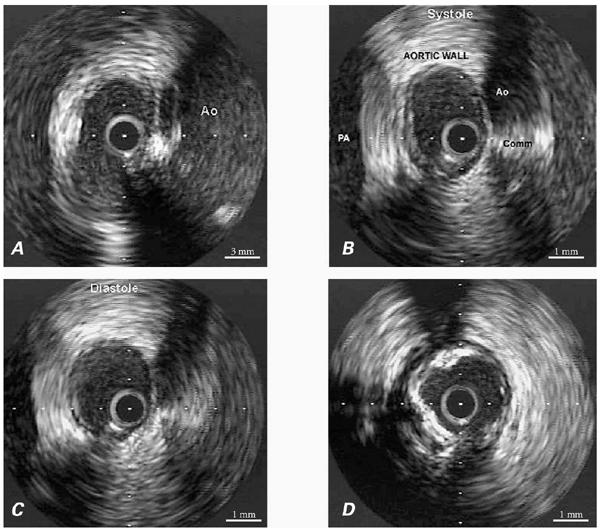
Fig. 4 A) Intravascular ultrasonographic (IVUS) view of the RCA ostium. The wall of the aorta (Ao) contains the RCA, and the inner wall of the aorta is interrupted (at the ostium), showing the tangential origination of the ectopic vessel. B) Proximal RCA, at the level of the intramural segment, as shown by IVUS during systole. The aortic lumen is on the right and the pulmonary artery (PA) on the left. The aortic wall has a total thickness of about 4.3 mm, including an inner layer of <0.02 mm and an outer layer of 1.3 mm. In this intramural segment, no intimal thickening is present. However, distal to this intussusception, the intima is quite thick and is accompanied by diffuse calcification. C) End-diastolic appearance of the RCA at the same site as shown in view B, which was during systole. In this IVUS image, the transverse diameter is wider and the luminal area rounder than in view B, proving that lateral compression is worse during systole. The luminal area was indeed 30% and 50% narrower during diastole and systole, respectively, in the intramural segment than in the distal reference vessel. D) Intravenous ultrasonographic image at the RCA, just distal to the intramural segment. Atherosclerotic intimal thickening and calcifications are clearly visible: note that they are absent at the intramural segment (see A–C).
Comm = commissure; RCA = right coronary artery
A similar critical understanding of the other forms of coronary anomalies should also be sought, so that sound criteria can be established for subclassifying these forms and for indicating intervention when necessary. Especially important in this regard are coronary artery fistulas, which are fairly common (having a 0.67% incidence in our continuous “normal series” of patients studied with angiography 1) and have individual features that present a wide spectrum of severity and clinical implications. Originally, intervention for such fistulas was generally undertaken simply because the lesions were detected and surgical treatment was available. Eventually, the following possible mechanisms for clinical manifestation were identified: left-to-right shunting, myocardial ischemia secondary to coronary steal or side-branch obstruction (acquired), mural thrombosis at sites of coronary ectasia, rupture (aneurysmal wall degeneration), endocarditis, and aortic valve disruption (secondary to an aneurysmal proximal coronary artery) with insufficiency. One concern—the amount of fistulous flow that should be considered prognostically relevant—is obviously quantifiable. Small fistulas (especially those that drain into the main pulmonary artery and the left ventricle) are much more common than large ones and are usually considered benign. The current aggressive (but inconsistent and perhaps generally unjustifiable) pursuit of intervention for these lesions is related to the recent introduction of simple, effective, catheter occlusion devices and to the frequent observation of small fistulas that coexist with coronary obstructive disease. However, the basic criterion for intervention should be the pulmonary–systemic flow ratio: if it exceeds 1.5:1, the fistula should probably be treated. Another reason for intervention might be aneurysmal degeneration, which can lead to mural thrombosis, rupture, or side-branch obstruction.
The steal phenomenon associated with coronary artery fistulas is one of the most confusing, inadequately discussed issues in the medical literature. Two types of steal phenomena can theoretically occur. One type is the persistent steal caused by the existence of large fistulous tracts, which also feed nutrient branches or receive collateral vessels that originate in the opposite coronary vessels. The nutrient flow can be compromised, especially in the presence of a relatively restrictive proximal fistulous artery. The other type of steal phenomenon, episodic steal, is caused by physiologic factors that increase shunting flow into the fistula at the expense of nutrient flow. In a worst-case situation, persistent steal can lead to ongoing ischemia at rest or to a hibernating state that entails myocardial dysfunction (possibly reversible), resting angina, or both. In cases of episodic steal, exercise and stress testing with vasodilators are likely to yield negative results, since they increase flow more to the nutrient branches than to the fistulous tract (which has no significant vasodilatory capacity). In clinical practice, nuclear stress test results are usually negative for reversible ischemia and often leave some doubt about the presence of scar tissue. Scarring can be suggested by the mere presence of the large coronary network, which can displace the myocardium. In the future, it will be important to take advantage of newer diagnostic techniques such as IVUS and pressure-wire studies. The IVUS can be used to evaluate intimal integrity, mural clots, vessel size, and localized aneurysms; and pressure-wire studies can be used to evaluate the pressure loss along fistulous arteries.
Screening and Treatment Guidelines
With most coronary anomalies, the 1st and greatest challenge is to identify the abnormality and to determine its clinical severity. The relationship between the anomaly and the presenting symptoms is frequently unclear. Ultimately, the goal is to ascertain the prognosis in order to begin appropriate treatment. Because of the great variability in the clinical relevance of the different anomalies, cost efficiency is a major concern. Although most coronary anomalies can be detected precisely with angiography, the cost and invasive nature of this method limits its usefulness for screening purposes. Moreover, cardiologists are primarily interested in identifying anomalies that might cause significant clinical consequences, not necessarily every anomaly that may be present. Unfortunately, 55% to 93% of patients who die suddenly of a coronary anomaly have no forewarning manifestations, 7,9 and fewer than 10% are known to have had a premortem cardiologic evaluation for symptoms related to the anomaly. 7,8 The usefulness of routine submaximal treadmill evaluation of coronary function is largely negated by the consistently high incidence of false-positive and false-negative results. 7,9 Echocardiography, whether transthoracic or transesophageal, evaluates the anatomy—not the functional status—and it cannot suggest clinical indications or correlations. Nevertheless, echocardiography has the potential to identify most coronary anomalies of clinical significance, especially in young patients. 10
In the absence of established official guidelines, our group has resorted to empirical protocols such as that shown in Figure 5. Patients of any age who lack symptoms or clinical signs do not generally require specific testing to rule out a coronary anomaly. When symptoms are present, one should be particularly aware of those that are likely to lead to sudden death: dyspnea (even more than just chest pains), syncope, and a history of aborted sudden death (rather than just premature ventricular beats). In such patients, particularly in male athletes, clinical evaluation should probably include heart catheterization, even if the echocardiogram and treadmill test yield negative results. In the documented presence of an anomaly and major symptoms (recurrent chest pain, dyspnea, syncope, aborted sudden death, or any combination thereof), angiography may not be adequate to document the need for intervention. A nuclear stress test that is positive for reversible ischemia in the dependent territory may confirm the need for intervention, but such a result is a rare event in our current experience.
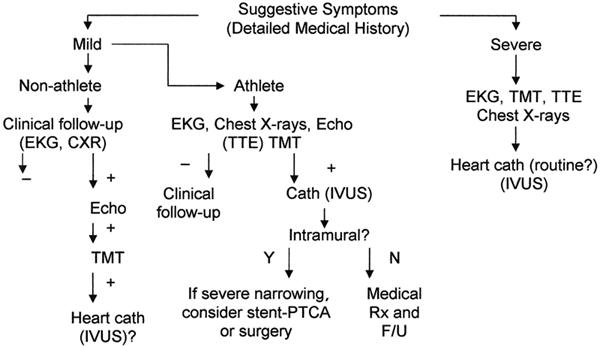
Fig. 5 Proposed diagnostic protocol for adult patients who are at risk for coronary artery anomalies.
− = negative test result; + = positive test result; CXR = chest x-ray; echo = echocardiogram; EKG = electrocardiogram; F/U = follow-up; IVUS = intravascular ultrasound; N = no; PTCA = percutaneous transluminal coronary angioplasty; Rx = treatment; TMT = treadmill test; TTE = transthoracic echocardiogram; Y = yes
As discussed in the previous section, we are in the process of validating new protocols for the evaluation of coronary anomalies with IVUS techniques, and new stress-testing protocols that could eventually be adapted for use in the catheterization laboratory.
Conclusion
Before evidence-based recommendations concerning interventions for coronary anomalies can be issued, cardiologists need to have a larger database that can correlate subclassifiable anatomic and functional features with clinical events and prognoses. For such a database to be realized, the work of isolated experts will have to coalesce into a collaborative effort among multiple specialized centers, all of which are relying on prospective coordinated protocols. 11 This would seem to be an excellent project for centers that specialize in the treatment of congenital heart defects in adults.
Footnotes
*Unpublished observations; 2000–2002.
Address for reprints: Paolo Angelini, MD, P.O. Box 20206, Houston, TX 77225-0206
This paper has its basis in a presentation made at the symposium Coronary Artery Anomalies: Morphogenesis, Morphology, Pathophysiology, and Clinical Correlations, held on 28 Feb.–1 March 2002, at the Texas Heart® Institute, Houston, Texas.
References
- 1.Angelini P, Villason S, Chan AV Jr, Diez JG. Normal and anomalous coronary arteries in humans. In: Angelini P, editor. Coronary artery anomalies: a comprehensive approach. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 27–150.
- 2.Angelini P, de la Cruz MV, Valencia AM, Sanchez-Gomez C, Kearney DL, Sadowinski S, Real GR. Coronary arteries in transposition of the great arteries. Am J Cardiol 1994;74:1037–41. [DOI] [PubMed]
- 3.Sans-Coma V, Duran AC, Fernandez B, Fernandez MC, Lopez D, Arque JM. Coronary artery anomalies and bicuspid aortic valve. In: Angelini P, editor. Coronary artery anomalies: a comprehensive approach. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 17–25.
- 4.Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol 1992;20:640–7. [DOI] [PubMed]
- 5.Virmani R, Burke AP, Farb A. The pathology of sudden cardiac death in athletes. In: Williams RA, editor. The athlete and heart disease: diagnosis, evaluation and management. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 249–72.
- 6.Shivalkar B, Borgers M, Daenen W, Gewillig M, Flameng W. ALCAPA syndrome: an example of chronic myocardial hypoperfusion? J Am Coll Cardiol 1994;23:772–8. [DOI] [PubMed]
- 7.Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol 2000;35:1493–501. [DOI] [PubMed]
- 8.Cheitlin MD. Coronary anomalies as a cause of sudden death in the athlete. In: Estes NA III, Salem DN, Wang PJ, editors. Sudden cardiac death in the athlete. Armonk (NY): Futura; 1998. p. 379–91.
- 9.Waller BF. Exercise-related sudden death in young (age less than or equal to 30 years) and old (age greater than 30 years) conditioned subjects. Cardiovasc Clin 1985;15:9–73. [PubMed]
- 10.Davis JA, Cecchin F, Jones TK, Portman MA. Major coronary artery anomalies in a pediatric population: incidence and clinical importance. J Am Coll Cardiol 2001;37:593–7. [DOI] [PubMed]
- 11.Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation 2002;105:2449–54. [DOI] [PubMed]


