Abstract
Inhibitors of human immunodeficiency virus type 1 attachment (CD4-immunoglobulin G subclass 2), CCR5 usage (PRO 140), and fusion (T-20) were tested on diverse primary cell types that represent the major targets both for infection in vivo and for the inhibition of trans infection of target cells by virus bound to dendritic cells. Although minor cell-type-dependent differences in potency were observed, each inhibitor was active on each cell type and trans infection was similarly vulnerable to inhibition at each stage of the fusion cascade.
Human immunodeficiency virus type 1 (HIV-1) entry proceeds via the sequential steps of gp120-CD4 attachment, gp120-coreceptor interactions, and gp41-mediated fusion (9, 21). CD4-immunoglobulin G subclass 2 (CD4-IgG2) (PRO 542) is an HIV-1 attachment inhibitor that has shown promise in phase I/II testing (18, 26, 31). PRO 140 (PA14) is an anti-CCR5 monoclonal antibody (27, 37) that is entering phase I testing. The fusion inhibitor T-20 is a peptide derived from HIV-1 gp41; it targets a transient gp41 conformation formed after coreceptor binding (5). T-20 has demonstrated promising safety and antiviral effects in phase III testing (B. Clotet, A. Lazzarin, D. Cooper, J. Reynes, K. Arasteh, M. Nelson, C. Katlama, J. Chung, L. Fang, J. Delehanty, and M. Salgo, 14th Int. AIDS Conf., abstr. LbOr19A, 2002; K. Henry, J. Lalezari, M. O'Hearn, B. Trottier, J. Montaner, P. Piliero, S. Walmsley, J. Chung, L. Fang, J. Delehanty, and M. Salgo, 14th Int. AIDS Conf., abstr. LbOr19B, 2002).
Studies of entry inhibitors typically have utilized cultured cell lines or peripheral blood mononuclear cells (PBMC). However, HIV-1 replicates in additional cell types and tissues in vivo (4, 12, 33, 40) and these have important implications for therapy.
Entry inhibitors could also be used prophylactically. Dendritic cells (DC) at surface epithelia may disseminate virus to susceptible cells in draining lymphoid tissue (16, 35). Cord blood mononuclear cells (CBMC) provide an available source of cells relevant to vertical transmission.
Whereas immature DC replicate HIV-1 efficiently, mature DC are poorly infectible (14). However, mature DC can bind virus and mediate the infection of cocultured CD4+ T cells in trans. There are conflicting reports on the susceptibility of cell-bound virus to neutralizing antibodies (13, 15, 34), and other classes of entry inhibitors have not been similarly studied. Here, we explore the breadth of activity in vitro of the three main classes of HIV-1 entry inhibitors.
Direct infection of diverse primary cell types.
We first tested the inhibitors for activity on diverse primary cells. CD4-IgG2, murine PRO 140, and T-20 were prepared as described previously (1, 24, 37). RANTES (PeproTech, Rocky Hill, N.J.), a natural chemokine ligand for CCR5, was tested for comparison. The studies examined the subtype B CCR5-using (R5) primary isolates HIV-1JR-FL (25), HIV-1SF162 (7), and HIV-1Case C 1/85 (8).
PBMC and CBMC (36), macrophages (37), and mature and immature DC (11) were isolated and cultured according to published methods. The inhibition assay was performed essentially as described previously (38). Briefly, inhibitors were combined for 1 h at 37°C with virus (CD4-IgG2 and T-20) or target cells (PRO 140 and RANTES). Inhibitors, virus, and cells were then incubated for 5 to 7 days at 37°C, and the extent of viral replication was determined by p24 antigen enzyme-linked immunosorbent assay of the culture supernatants. The viral inocula in 50% tissue culture infective doses were 400 to 1,000 for PBMC and CBMC, 1,000 for macrophages, and 5,000 to 10,000 for DC.
Table 1 summarizes the concentrations required for 90% (IC90) and 50% (IC50) inhibition for the various cell types. The median IC90s for CD4-IgG2, PRO 140, RANTES, and T-20 in PBMC cultures were 13, 31, 16, and 62 nM, respectively (Table 1). These values are consistent with those from prior studies of these agents (24, 37-39).
TABLE 1.
Interisolate variation in inhibitor activity for direct and trans infection of primary cells
| Inhibitor and cell type | Virusa
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case C 1/85
|
SF162
|
JR-FL
|
Composite
|
|||||||||
| IC90 | IC50 | No. of replicates | IC90 | IC50 | No. of replicates | IC90 | IC50 | No. of replicates | IC90 | IC50 | No. of replicates | |
| CD4-IgG2 | ||||||||||||
| PBMC | 40 | 12 | 7 | 13 | 10 | 7 | 7.1 | 2.3 | 7 | 13 | 3.3 | 21 |
| Immature DC | 33 | 6.4 | 5 | 50 | 8.2 | 3 | 1.8 | 0.3 | 1 | 33 | 6.4 | 9 |
| Macrophage | 2.0 | 0.2 | 4 | 1.7 | 0.3 | 4 | 1.8 | 0.5 | 2 | 1.7 | 0.3 | 10 |
| CBMC | 2.9 | 1.4 | 2 | 5.7 | 2.1 | 2 | 5.1 | 1.0 | 4 | 2.9 | 1.3 | 8 |
| DC (in trans) | 50 | 7.1 | 10 | 8.3 | 0.7 | 1 | 0.04 | 0.04 | 1 | 37 | 3.6 | 12 |
| T-20 | ||||||||||||
| PBMC | 154 | 22 | 7 | 45 | 12 | 7 | 22 | 2.9 | 6 | 62 | 12 | 20 |
| Immature DC | 917 | 190 | 5 | 360 | 7.6 | 3 | 67 | 4.2 | 1 | 394 | 15 | 9 |
| Macrophage | 156 | 15 | 4 | 149 | 20.3 | 4 | 55 | 6.5 | 2 | 137 | 11 | 10 |
| CBMC | 145 | 40 | 2 | 40 | 17 | 2 | 146 | 26 | 4 | 89 | 31 | 8 |
| DC (in trans) | 56 | 15 | 10 | 152 | 19 | 2 | 0.4 | 0.4 | 1 | 41 | 14 | 13 |
| PRO 140 | ||||||||||||
| PBMC | 28 | 3.6 | 18 | 97 | 10 | 20 | 15 | 1.8 | 30 | 31 | 4.5 | 68 |
| Immature DC | 198 | 37 | 5 | 334 | 25 | 3 | 50 | 0.5 | 1 | 198 | 25 | 9 |
| Macrophage | 12 | 5.7 | 5 | 26 | 10 | 7 | 65 | 10 | 9 | 37 | 5.7 | 21 |
| CBMC | 16 | 5.5 | 2 | 46 | 5.6 | 2 | 10 | 1.9 | 2 | 15 | 3.4 | 6 |
| DC (in trans) | 8.9 | 1.8 | 10 | 89 | 4.4 | 2 | 6.1 | 3.8 | 1 | 10 | 3.0 | 13 |
| RANTES | ||||||||||||
| PBMC | 14 | 3.4 | 12 | 30 | 5.9 | 9 | 8.7 | 5.9 | 6 | 16 | 3.9 | 27 |
| Immature DC | 127b | 4.2 | 5 | 33 | 6.9 | 3 | 64 | 51 | 1 | 127 | 6.9 | 9 |
| Macrophage | 410b | 64 | 3 | 64b | 29 | 6 | 64b | 31 | 9 | 64b | 33 | 18 |
| CBMC | 46 | 14 | 2 | 17 | 6.6 | 2 | 20 | 4.7 | 3 | 24 | 5.6 | 7 |
| DC (in trans) | 13 | 2.4 | 10 | 74 | 39 | 2 | 0.3 | 0.04 | 1 | 15 | 3.6 | 13 |
Median IC50 and IC90 values (nM) are shown for direct infection of PBMC, immature DC, macrophages, and CMBC by CD4-IgG2, T-20, PRO 140, and RANTES. The last row for each inhibitor lists IC50s and IC90s for infection in trans of CD14-cell-depleted PBMC by mature DC-associated virus. The number of replicates of each drug-virus combination, pooled from different experiments, is given.
90% inhibition was not achieved at the indicated concentration of inhibitor.
CD4-IgG2, PRO 140, and T-20 each mediated 90% inhibition of viral replication in PBMC, CBMC, macrophages, and immature DC (Table 1). In contrast, RANTES was ineffective in macrophage cultures, as reported previously (2, 3, 10, 28-30, 32, 37). Although RANTES blocked virus replication by 50% at moderate concentrations (Table 1), higher concentrations often led to the enhancement of infection (data not shown). Inhibition studies were not conducted on mature DC, which were poorly susceptible to infection.
For comparison of cell type differences in inhibitor potency, we used log-transformed IC50s and IC90s, which more closely followed a Gaussian distribution than did the raw values, facilitating comparison of the means by two-tailed t tests. ICs observed for PBMC were compared with those for other cell types. Since the t test was performed four times for each drug, the threshold P value was adjusted from 0.05 to 0.013 in accordance with Bonferroni's correction.
For a given inhibitor, the mean ICs for the other cell types tended to cluster about the values observed for PBMC (Fig. 1). Thus CD4-IgG2, PRO 140, and T-20 are broadly active in blocking the entry of HIV-1 into PBMC, CBMC, macrophages, and immature DC. These findings are consistent with those of a similarly designed study employing RANTES, T-20, and the CCR5 antagonist SCH-C (19a).
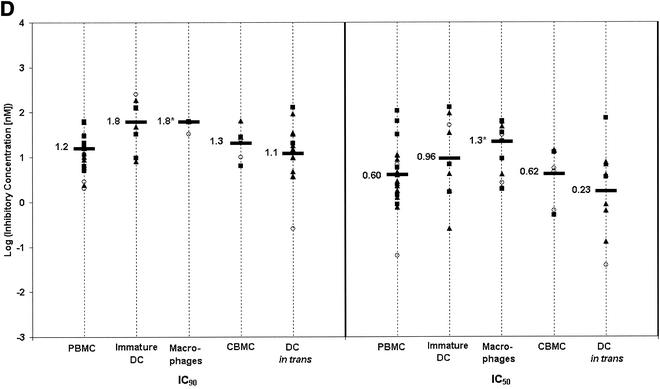
FIG. 1.
Cell-type-specific variations in inhibitor activity. IC50s and IC90s were observed for CD4-IgG2 (A), PRO 140 (B), T-20 (C), and RANTES (D) against HIV-1JR-FL (open circles), HIV-1SF162 (filled squares), and HIV-1Case C 1/85 (filled triangles) on the indicated cell types. Overall mean log IC50s and IC90s for all assays are indicated with horizontal bars. Mean log ICs that differed significantly (P < 0.013) from the corresponding value for PBMC are indicated with an asterisk. (A) CD4-IgG2. The mean log IC90s and IC50s obtained for macrophages were significantly lower than those obtained for PBMC (P = 7.9 × 10−7 for both IC90 and IC50). Mean log ICs for CBMC were also lower than those for PBMC (P = 0.0039 and 0.0045 for IC90 and IC50, respectively). None of the other values were significantly different from those for PBMC. (For IC90s, P = 0.96 for immature DC and 0.78 for DC in trans. For IC50s, P = 0.91 for immature DC and 0.82 for DC in trans.) (B) PRO 140. The mean log IC90 for immature DC was significantly higher than that for PBMC (P = 5.7 × 10−4). None of the other mean log IC90s were different from those for PBMC (P = 0.63 for macrophages, 0.19 for CBMC, and 0.38 for DC in trans). None of the IC50s differed from that for PBMC (P = 0.33 for immature DC, 0.23 for macrophages, 0.94 for CBMC, and 0.89 for DC in trans). (C) T-20. Immature DC had an IC90 higher than that for PBMC (P = 8.8 × 10−4). No other cell types had IC90s different from those for PBMC (P = 0.47 for macrophages, 0.62 for CBMC, and 0.19 for DC in trans). No IC50s were different compared to those for PBMC (P = 0.17 for immature DC, 0.34 for macrophages, 0.32 for CBMC, and 0.76 for DC in trans). (D) RANTES. Macrophages had higher mean log ICs than did PBMC (P = 4.8 × 10−7 for IC90 and 2.9 × 10−4 for IC50). The IC90s for immature DC bordered on being significantly higher than those for PBMC (P = 0.013). No other cell types had ICs different from those for PBMC. (For IC90s, P = 0.43 for CBMC and 0.63 for DC in trans. For IC50s, P = 0.31 for immature DC, 0.96 for CBMC, and 0.21 for DC in trans.) Any higher ICs for immature DC may reflect the 10-fold greater virus doses used to detect production infection in these assays.
Although the entry inhibitors were broadly active, cell type differences in potency were observed. CD4-IgG2 was ninefold (P = 7.9 × 10−7) and sixfold (P = 0.0039) more potent when assayed on macrophages and CBMC, respectively, than on PBMC. Notably, macrophages and CBMC express comparatively low levels of CD4 (6, 23). Immature DC required higher ICs, with the mean IC90s of T-20 and PRO 140 being ninefold (P = 8.8 × 10−4) and sixfold (P = 5.7 × 10−4) higher than their respective PBMC values. These findings may reflect the higher concentration of virus inoculum required to infect immature DC.
Correlation between the potencies of entry inhibitors.
We next performed linear regression analysis of the log IC90s observed for a pair of inhibitors within a given experiment, keeping the virus, cell type, and cell donor constant. Data obtained with different cell types were pooled to increase the statistical power. An r2 of >0.25 was deemed indicative of a strong correlation, and in those cases, P values were calculated by using JMP software (SAS Institute, Inc., Cary, N.C.).
Significant correlations were observed in the cases of PRO 140 and RANTES (r2 = 0.26, P = 0.0005) and PRO 140 and T-20 (r2 = 0.34, P < 0.0001). Both correlations were positive; i.e., a virus culture that was sensitive to one inhibitor was sensitive to the other. The correlation observed for PRO 140 and RANTES is perhaps unsurprising, given both are CCR5 inhibitors. The correlation between PRO 140 and T-20 may reflect the fact that these agents act at sequential, interdependent stages of fusion.
CD4-IgG2's potency did not correlate with that of any other inhibitor and thus may be influenced by an independent set of viral and host determinants. Distinct resistance determinants are desirable for therapy.
Regression analysis was used to examine other potential correlates of activity. However, for a given cell type and inhibitor, no significant correlation could be established based on the virus isolate, cell donor, or degree of viral replication or “fitness.” These variables may have complex combinatorial effects on inhibition, and further studies are needed to dissect the relative contributions.
DC-mediated infection in trans.
We next examined the inhibition of trans infection. Briefly, CD14+ and CD14− cells were isolated as described previously (11, 36). The CD14− cells were cryopreserved, while the CD14+ cells were differentiated into mature DC (11). DC were incubated overnight at 37°C with 10,000 tissue culture infective doses/ml of virus, washed five times, resuspended in PBMC culture medium (36), seeded at 2 × 105 cells/well in 96-well plates, and incubated with CD4-IgG2 or T-20 for 1 h at 37°C. CD14− cells were thawed, stimulated as described previously (36), and incubated with PRO 140 or RANTES. CD14− cells (1.4 × 105 cells/well) were then added to DC, and HIV-1 replication was determined as described above.
Each inhibitor effectively blocked trans infection. In no case did the ICs for a given inhibitor vary by more than threefold between direct and trans infection of PBMC (Table 1 and Fig. 1). Thus, during trans infection, DC-associated virus is sensitive to inhibition throughout the fusion cascade.
Thus DC-mediated trans infection is inhibited by CD4-IgG2 as well as HIV-1-neutralizing monoclonal antibodies (13, 17). In contrast, follicular DC-mediated trans infection reportedly is unaffected by neutralizing antibody (15). The divergent results could reflect differences both in how DC versus follicular DC process immune-complexed virus and in assay methodology.
Similarly, we observed that T-20 efficiently blocks DC-mediated trans infection. Our findings stand in apparent contrast to those reported for trans infection mediated by intestinal epithelial cells, where T-20 was inactive (22). However, the different outcomes may again reflect methodology differences. In the earlier study, T-20 was removed from the epithelial cells prior to the addition of CD4+ target cells (22), whereas T-20 was present in our DC-T-cell cocultures.
In the present study, DC were added in excess of T cells such that most of the T cells could be infected in trans during the first round of infection. To explore the potential importance of secondary rounds of infection, inhibitors were washed away prior to the combination of DC and T cells. CD4-IgG2 was fully active in the washout setting (data not shown), indicating that the agent blocks trans infection. These findings support the notion that the inhibitors block trans infection.
Our findings shed light on the mechanism of HIV-1 infection in trans. Notably, during this process, virus is sensitive to each of the known classes of entry inhibitors. This result is surprising for two reasons. Firstly, lentiviruses are internalized in prominent vacuoles in mature DC (11) and internalization may be critical for infection (20). Secondly, trans infection may occur at polarized interfaces between the cells (19) and internalized virus moves to this immunologic synapse within minutes of cell contact (D. McDonald, D. Unatmaz, V. KewalRamani, and T. Hope, Keystone Symp. HIV Pathog., abstr. 121, 2002). Nonetheless, our findings indicate that virus remains vulnerable to inhibition by membrane-impermeable drugs.
In summary, we report that the main classes of HIV-1 entry inhibitors block the direct and trans infection of multiple primary cell types. Although minor cell-type-specific differences in activity were observed, CD4-IgG2, PRO 140, and T-20 were effective in each setting. Our findings also offer new insights into the nature of the DC/T-cell synapse during trans infection.
Acknowledgments
M.P. is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation. J.P.M. is a Stavros S. Niarchos Scholar.
This work was funded by NIH grants AI46871, R01 AI43084, R01 AI40877, R01 AI41420, and P01 AI52048; The Rockefeller Foundation; the Elizabeth Glaser Pediatric AIDS Foundation; and Progenics Pharmaceuticals, Inc. The Department of Microbiology and Immunology at the Weill Medical College gratefully acknowledges the support of the William Randolph Hearst Foundation.
REFERENCES
- 1.Allaway, G. P., K. L. Davis-Bruno, G. A. Beaudry, E. B. Garcia, E. L. Wong, A. M. Ryder, K. W. Hasel, M.-C. Gauduin, R. A. Koup, J. S. McDougal, and P. J. Maddon. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer which neutralizes primary HIV-1 isolates. AIDS Res. Hum. Retrovir. 11:533-539. [DOI] [PubMed] [Google Scholar]
- 2.Amzazi, S., L. Ylisastigui, Y. Bakri, L. Rabehi, L. Gattegno, M. Parmentier, J. C. Gluckman, and A. Benjouad. 1998. The inhibitory effect of RANTES on the infection of primary macrophages by R5 human immunodeficiency virus type-1 depends on the macrophage activation state. Virology 252:96-105. [DOI] [PubMed] [Google Scholar]
- 3.Arenzana-Seisdedos, F., J. L. Virelizier, D. Rousset, I. Clark-Lewis, P. Loetscher, B. Moser, and M. Baggiolini. 1996. HIV blocked by chemokine antagonist. Nature 383:400.. [DOI] [PubMed] [Google Scholar]
- 4.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 5.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 6.Collman, R., B. Godfrey, J. Cutilli, A. Rhodes, N. F. Hassan, R. Sweet, S. D. Douglas, H. Friedman, N. Nathanson, and F. Gonzalez-Scarano. 1990. Macrophage-tropic strains of human immunodeficiency virus type 1 utilize the CD4 receptor. J. Virol. 64:4468-4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collman, R., N. F. Hassan, R. Walker, B. Godfrey, J. Cutilli, J. C. Hastings, H. Friedman, S. D. Douglas, and N. Nathanson. 1989. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J. Exp. Med. 170:1149-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor, R., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doms, R. W., and J. P. Moore. 2000. HIV-1 membrane fusion: targets of opportunity. J. Cell Biol. 151:F9-F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dragic, T., V. Litwin, G. P. Allaway, S. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 11.Frank, I., M. J. Piatak, H. Stoessel, N. Romani, D. Bonnyay, J. D. Lifson, and M. Pope. 2002. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J. Virol. 76:2936-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank, I., and M. Pope. 2002. The enigma of dendritic cell-immunodeficiency virus interplay. Curr. Mol. Med. 2:229-248. [DOI] [PubMed] [Google Scholar]
- 13.Frankel, S. S., R. M. Steinman, N. L. Michael, S. R. Kim, N. Bhardwaj, M. Pope, M. K. Louder, P. K. Ehrenberg, P. W. Parren, D. R. Burton, H. Katinger, T. C. VanCott, M. L. Robb, D. L. Birx, and J. R. Mascola. 1998. Neutralizing monoclonal antibodies block human immunodeficiency virus type 1 infection of dendritic cells and transmission to T cells. J. Virol. 72:9788-9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath, S. L., J. G. Tew, A. K. Szakal, and G. F. Burton. 1995. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature 377:740-744. [DOI] [PubMed] [Google Scholar]
- 16.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ignatius, R., R. M. Steinman, A. Granelli-Piperno, D. Messmer, C. Stahl-Hennig, K. Tenner-Racz, I. Frank, L. Zhong, S. Schlesinger Frankel, and M. Pope. 2001. Dendritic cells during infection with HIV-1 and SIV, p. 487-504. In M. T. Lotze and A. W. Thomson (ed.), Dendritic cells: biology and clinical applications. Academic Press, New York, N.Y.
- 18.Jacobson, J. M., I. Lowy, C. V. Fletcher, T. J. O'Neill, D. N. H. Tran, T. J. Ketas, A. Trkola, M. E. Klotman, P. J. Maddon, W. C. Olson, and R. J. Israel. 2000. Single-dose safety, pharmacology, and antiviral activity of the human immunodeficiency virus (HIV) type 1 entry inhibitor PRO 542 in HIV-infected adults. J. Infect. Dis. 182:326-329. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, D. C., and M. T. Huber. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Ketas, T., P.-J. Klasse, C. Spenlehauer, M. Nessin, I. Frank, M. Pope, J. M. Strizki, G. R. Reyes, B. M. Baroudy, and J. P. Moore. Antagonism of HIV-1 replication by the entry inhibitors SCH-C, RANTES in multiple cell types. AIDS Res. Hum. Retrovir., in press. [DOI] [PubMed]
- 20.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 21.LaBranche, C. C., G. Galasso, J. P. Moore, D. P. Bolognesi, M. S. Hirsch, and S. M. Hammer. 2001. HIV fusion and its inhibition. Antivir. Res. 50:95-115. [DOI] [PubMed] [Google Scholar]
- 22.Meng, G., X. Wei, X. Wu, M. T. Sellers, J. M. Decker, Z. Moldoveanu, J. M. Orenstein, M. F. Graham, J. C. Kappes, J. Mestecky, G. M. Shaw, and P. D. Smith. 2002. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat. Med. 8:150-156. [DOI] [PubMed] [Google Scholar]
- 23.Mo, H., S. Monard, H. Pollack, J. Ip, G. Rochford, L. Wu, J. Hoxie, W. Borkowsky, D. D. Ho, and J. P. Moore. 1998. Expression patterns of the HIV type 1 coreceptors CCR5 and CXCR4 on CD4+ T cells and monocytes from cord and adult blood. AIDS Res. Hum. Retrovir. 14:607-617. [DOI] [PubMed] [Google Scholar]
- 24.Nagashima, K. A., D. A. D. Thompson, S. I. Rosenfield, P. J. Maddon, T. Dragic, and W. C. Olson. 2001. Human immunodeficiency virus type 1 entry inhibitors PRO 542 and T-20 are potently synergistic in blocking virus-cell and cell-cell fusion. J. Infect. Dis. 183:1121-1125. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien, W. A., Y. Koyanagi, A. Namazie, J. Q. Zhao, A. Diagne, K. Idler, J. A. Zack, and I. S. Chen. 1990. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature 348:69-73. [DOI] [PubMed] [Google Scholar]
- 26.O'Hara, B. M., and W. C. Olson. 2002. HIV entry inhibitors in clinical development. Curr. Opin. Pharmacol. 2:523-528. [DOI] [PubMed] [Google Scholar]
- 27.Olson, W. C., G. E. Rabut, K. A. Nagashima, D. N. Tran, D. J. Anselma, S. P. Monard, J. P. Segal, D. A. Thompson, F. Kajumo, Y. Guo, J. P. Moore, P. J. Maddon, and T. Dragic. 1999. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J. Virol. 73:4145-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oravecz, T., M. Pall, and M. A. Norcross. 1996. Beta-chemokine inhibition of monocytotropic HIV-1 infection. Interference with a postbinding fusion step. J. Immunol. 157:1329-1332. [PubMed] [Google Scholar]
- 29.Oravecz, T., M. Pall, J. Wang, G. Roderiquez, M. Ditto, and M. A. Norcross. 1997. Regulation of anti-HIV-1 activity of RANTES by heparan sulfate proteoglycans. J. Immunol. 159:4587-4592. [PubMed] [Google Scholar]
- 30.Schmidtmayerova, H., B. Sherry, and M. Bukrinsky. 1996. Chemokines and HIV replication. Nature 382:767.. [DOI] [PubMed] [Google Scholar]
- 31.Shearer, W. T., R. J. Israel, S. Starr, C. V. Fletcher, D. Wara, M. Rathore, J. Church, J. DeVille, T. Fenton, B. Graham, P. Samson, S. Staprans, J. McNamara, J. Moye, P. J. Maddon, W. C. Olson, et al. 2000. Recombinant CD4-IgG2 in human immunodeficiency virus type 1-infected children: phase I/II study. J. Infect. Dis. 182:1774-1779. [DOI] [PubMed] [Google Scholar]
- 32.Simmons, G., P. R. Clapham, L. Picard, R. E. Offord, M. Rosenkilde, T. W. Schwartz, R. Buser, T. N. Wells, and A. E. Proudfoot. 1997. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science 276:276-279. [DOI] [PubMed] [Google Scholar]
- 33.Smith, P. D., G. Meng, M. T. Sellers, T. S. Rogers, and G. M. Shaw. 2000. Biological parameters of HIV-1 infection in primary intestinal lymphocytes and macrophages. J. Leukoc. Biol. 68:360-365. [PubMed] [Google Scholar]
- 34.Spenlehauer, C., A. Kirn, A. M. Aubertin, and C. Moog. 2001. Antibody-mediated neutralization of primary human immunodeficiency virus type 1 isolates: investigation of the mechanism of inhibition. J. Virol. 75:2235-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trkola, A., T. Ketas, V. N. KewalRamani, F. Endorf, J. M. Binley, H. Katinger, J. Robinson, D. R. Littman, and J. P. Moore. 1998. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J. Virol. 72:1876-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trkola, A., T. J. Ketas, K. A. Nagashima, L. Zhao, T. Cilliers, L. Morris, J. P. Moore, P. J. Maddon, and W. C. Olson. 2001. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol. 75:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trkola, A., W. A. Paxton, S. P. Monard, J. A. Hoxie, M. A. Siani, D. A. Thompson, L. Wu, C. R. Mackay, R. Horuk, and J. P. Moore. 1998. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J. Virol. 72:396-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trkola, A., A. P. Pomales, H. Yuan, B. Korber, P. J. Maddon, G. P. Allaway, H. Katinger, C. F. Barbas, D. R. Burton, D. D. Ho, and J. P. Moore. 1995. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG2. J. Virol. 69:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wesselingh, S. L., and K. A. Thompson. 2001. Immunopathogenesis of HIV-associated dementia. Curr. Opin. Neurol. 14:375-379. [DOI] [PubMed] [Google Scholar]