Abstract
An alkaliphilic actinomycete, Nocardiopsis prasina OPC-131, secretes chitinases, ChiA, ChiB, and ChiBΔ, in the presence of chitin. The genes encoding ChiA and ChiB were cloned and sequenced. The open reading frame (ORF) of chiA encoded a protein of 336 amino acids with a calculated molecular mass of 35,257 Da. ChiA consisted of only a catalytic domain and showed a significant homology with family 18 chitinases. The chiB ORF encoded a protein of 296 amino acids with a calculated molecular mass of 31,500 Da. ChiB is a modular enzyme consisting of a chitin-binding domain type 3 (ChtBD type 3) and a catalytic domain. The catalytic domain of ChiB showed significant similarity to Streptomyces family 19 chitinases. ChiBΔ was the truncated form of ChiB lacking ChtBD type 3. Expression plasmids coding for ChiA, ChiB, and ChiBΔ were constructed to investigate the biochemical properties of these recombinant proteins. These enzymes showed pHs and temperature optima similar to those of native enzymes. ChiB showed more efficient hydrolysis of chitin and stronger antifungal activity than ChiBΔ, indicating that the ChtBD type 3 of ChiB plays an important role in the efficient hydrolysis of chitin and in antifungal activity. Furthermore, the finding of family 19 chitinase in N. prasina OPC-131 suggests that family 19 chitinases are distributed widely in actinomycetes other than the genus Streptomyces.
Chitin, an insoluble linear β-1,4-linked polymer of N-acetylglucosamine (GlcNAc), is the second most abundant polymer in nature. This polysaccharide is found in the cell walls of fungi and in the exoskeletons of insects and crustaceans. Chitinases (EC 3.2.1.14) are produced by many organisms, such as viruses, bacteria, higher plants, and animals, and play important physiological and ecological roles (7). Chitinases hydrolyze the β-1,4 linkages in chitin, yielding predominately N-N′-diacetylchitobiose, which is further degraded by N-acetylglucosaminidases to the GlcNAc monomer.
Actinomycetes are gram-positive mycelial soil bacteria with high G+C contents. In addition to having the ability to synthesize a wide variety of antibiotics and biologically active compounds, they produce extracellular hydrolytic enzymes to obtain nutrients and energy by solubilizing polymeric compounds in soil. These enzymes include proteases, nucleases, lipases, and a variety of enzymes that hydrolyze different types of polysaccharides, such as chitin and cellulose (14). Among actinomycetes, Streptomyces species make up one group regarded as well-known decomposers of chitin. Chitinases have been purified and characterized from various Streptomyces species, and the corresponding genes have been cloned and sequenced. The multiple genes for chitinases were cloned from Streptomyces plicatus (22, 23), Streptomyces lividans 66 (6, 18, 19), Streptomyces thermoviolaceus OPC-520 (33-35), and Streptomyces coelicolor A3 (2) (24). However, no work has been done on the molecular cloning and analysis of chitinase genes from the nonstreptomycete group. It has already been reported that an alkaliphilic actinomycete, Nocardiopsis prasina OPC-131, produces two types of chitinases (ChiA and ChiB) (32). The most characteristic properties of ChiA and ChiB indicated that the enzyme activities remained well within the alkaline pH range compared with those of Streptomyces species. In particular, even at pH 9.0, ChiA and ChiB showed ∼50% of the activity at the optimum pH (32). In this paper, we describe the cloning and sequencing of the chiA and chiB genes. Furthermore, we have expressed these enzymes in Escherichia coli and investigated the characterization of the cloned enzymes.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
N. prasina OPC-131, which was isolated from soil, was used as the source of chromosomal DNA (32). The strain was grown at 27°C for 6 days in a medium containing (in grams per liter) colloidal chitin, 5.0; yeast extract, 2.5; K2HPO4, 1.0; MgSO4 · 7H2O, 0.2; Na2CO3, 10.0 (pH 10.0). E. coli JM109 and TOP10 (Invitrogen Co., Carlsbad, Calif.) were grown at 37°C in Luria-Bertani (LB) broth. For agar medium, Luria-Bertani broth was solidified with 1.5% (wt/vol) agar (Nacalai Tesque, Kyoto, Japan).
Purification of chitinases.
Chitinases were purified from the culture filtrate by successive chromatographies of DEAE-Toyopearl 650 M, Sephadex G-100, and UnoQ as described previously (32).
N-terminal amino acid sequence, SDS-PAGE, and protein assay.
The amino acid sequence was analyzed with a Procise 491 HT protein sequencer (Applied Biosystems) that was connected to an online phenylthiohydantoin derivative analyzer. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done by the method of Laemmli (11). After electrophoresis, activity staining of chitinases in polyacrylamide gel was carried out by the method of Wolfgang et al. (42). Protein was assayed by the method of Bradford with bovine serum albumin as a standard (2).
Enzyme assay.
Chitinase activity was measured, as described previously, with ethylene glycol chitin (Seikagaku Co., Tokyo, Japan) or colloidal chitin (crab shell; Nacalai Tesque) as a substrate (32, 34, 36). One unit of chitinase was defined as the amount of enzyme that liberated reducing sugar corresponding to 1 μmol of GlcNAc.
Cloning of chiA and chiB genes.
General recombinant DNA techniques were performed as described by Sambrook and Russel (25). Two sets of primers (A-1, 5′-GCTACACGCACAACTTCGAT-3′, and A-2, 5′-AGATCTATGTCGACGCCGTC-3′; and B-1, 5′-GGCCCAGTTCAACCAGATGT-3′, and B-2, 5′-AGTACCACAGGCCGGTCTTC-3′) were synthesized based on the N-terminal amino acid sequences of ChiA and ChiB and the conserved sequences of family 18 and 19 chitinases. PCR amplification was performed by KOD-Plus-DNA polymerase (Toyobo, Osaka, Japan) for 30 cycles consisting of 97°C for 15 s, 51°C for 30 s, and 68°C for 1 min. The amplified fragments were phosphorylated by T4 DNA polynucleotide kinase. Each of the resulting fragments (pCHIA1 and pCHIB1) was cloned into the dephosphorylated SmaI site of pUC19, sequenced, and used as a probe. Chromosomal DNA was digested with BamHI and SalI or BamHI alone and electrophoresed on a 0.6% agarose gel. The fragments in the range of 4.0 to 5.0 kb were excised from the gel, purified with a GenElute gel extraction kit (Sigma), and then ligated into the corresponding sites of pUC19. The recombinant plasmids were inserted into competent E. coli JM109. Each of the libraries was screened by colony hybridization with alkaline phosphatase-labeled pCHIA1 or pCHIB1 as a probe (AlkPhos DIRECT; Amersham Bioscience). Hybridization and washing were performed according to the supplier's instructions.
DNA sequencing.
Nucleotide sequencing was carried out by the dideoxy chain termination method by using the DYEnamic ET terminator cycle-sequencing premix kit (Amersham Bioscience) on a DNA sequencer (Prism 310 genetic analyzer; Applied Biosystems) (26). Sequence data were analyzed by using the GENETYX-WIN program (Software Development Co., Ltd.).
Construction of expression plasmids and purification of recombinant proteins.
The expression plasmids pThioHis-ChiA, pThioHis-ChiB, and pThioHis-ChiBΔ, coding for ChiA, ChiB, and the truncated form of ChiB, respectively, were constructed as follows. The primers for ChiA (5′-CCCGTCATGACCCAGCAGACCAGCC-3′ and 5′-GCGGGCTCGAGGGGTTACTGCAGTC-3′), ChiB (5′-CTCGTTACCCTCCCCATGGCCGCCG-3′ and 5′-TGCCTCACTGCAGCCGGGGTTCAGC-3′), and ChiBΔ (5′-GACCAGGTACCCTGCGGCGACGGC-3′ and 5′-TGCCTCACTGCAGCCGGGGTTCAGC-3′), which were modified to contain BspHI and XhoI (pThioHis-ChiA), NcoI and PstI (pThioHis-ChiB), and KpnI and PstI (pThioHis-ChiBΔ) recognition sites to facilitate cloning in frame into the thioredoxin fusion protein expression vector pThioHisB (Invitrogen Co.), were synthesized. PCR amplification was performed with the full-length chiA or chiB gene as a template for 30 cycles consisting of 97°C for 15 s, 51°C for 30 s, and 68°C for 1 min. The amplified DNAs were digested by BspHI and XhoI, NcoI and PstI, and KpnI and PstI, respectively, and the resulting fragments were inserted into the corresponding sites of pThioHisB. The nucleotide sequences of pThioHis-ChiA, pThioHis-ChiB, and pThioHis-ChiBΔ were confirmed by DNA sequencing.
E. coli TOP10 cells harboring pThioHis-ChiA, pThioHis-ChiB, and pThioHis-ChiBΔ were induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at the mid-exponential growth phase and further incubated for 3 h at 27°C. The cells were harvested by centrifugation, washed, and resuspended with 20 mM phosphate buffer (pH 7.8) containing 0.5 M NaCl. The cells were disrupted by sonication, and the lysates were centrifuged at 10,000 × g for 10 min. The fusion proteins were purified from the supernatant by affinity chromatography with a nickel-charged Sepharose resin (ProBond resin; Invitrogen Co.). The purified fusion proteins were treated with enterokinase (Invitrogen Co.) for 16 h at 37°C to obtain ChiA, ChiB, and ChiBΔ. The N-terminal amino acid sequences of these proteins were confirmed by protein sequencing.
Binding study and antifungal activity.
Chitin-binding assays were carried out by adding 1.5 μg of purified ChiB or ChiBΔ to 5 mg each of α-chitin, β-chitin, and crystalline cellulose (Avicel) in 0.1 ml of 20 mM HEPES-KOH buffer (pH 7.0) in 1.5-ml microcentrifuge tubes. Samples were permitted to stand for 20 min on ice and then centrifuged at 24,650 × g for 5 min. The chitinase in the supernatant was measured using colloidal chitin as a substrate, and the activity lost from the supernatant was assumed to be the activity bound. Antifungal activity was estimated using the hyphal extension-inhibition assay as described previously (35). The mycelium of Trichoderma reesei was directly inoculated onto the center of a dish containing 10 ml of potato dextrose agar (Eiken Chemical Co., Ltd., Tokyo, Japan). After incubation at 27°C for 24 h, paper disks were placed around the edge of the T. reesei culture, and 30 μl of solution containing 80 μg of ChiA, ChiB, or ChiBΔ was put onto the disks. The plate was incubated at 27°C for 30 h, and the inhibition of hyphal extension around the disks was examined.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper have been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession numbers AB086831 (chiA) and AB086832 (chiB).
RESULTS
Purification of chitinases.
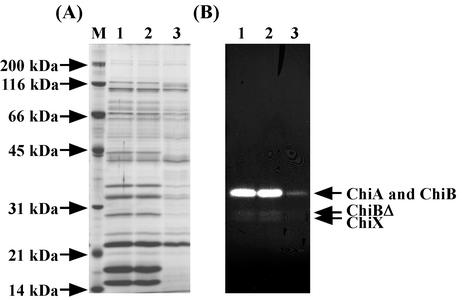
Before we began the purification of chitinases from N. prasina OPC-131, zymogram analysis was performed to investigate the multiplicity of chitinases. Zymogram analysis indicated that strain OPC-131 secreted four chitinases in the presence of chitin (Fig. 1). The molecular masses of ChiA and ChiB were 32 kDa but were overestimated as 35 kDa in a previous paper (32). The two additional chitinases, ChiBΔ and ChiX, corresponding to proteins of 28 and 27 kDa, respectively, were detected. These enzymes and several unidentified proteins were induced in the presence of chitin; however, the production of these proteins was not repressed in the presence of glucose. Among these enzymes, ChiA, ChiB, and ChiBΔ were purified, and their N-terminal amino acid sequences were determined. However, the production of ChiX was so low that we could not purify and characterize the enzyme. The BLAST search program revealed that the N-terminal amino acid sequence of ChiA (QTSQWLTGYWHNFDNGSTV) showed high homology with family 18 chitinases (8, 9), such as Bacillus circulans ChiD (39), S. thermoviolaceus Chi30 (34), Streptomyces griseus ChiI (10), and S. lividans ChiA (18). On the other hand, ChiB (ATACATAWSSSSVYTGGGQVSFEG) and ChiBΔ (GDGGGGEQPGPNDFVVSEAQF) showed high homology with family 19 chitinases (8, 9), such as S. griseus ChiC (21) and S. thermoviolaceus Chi35 (35). These results suggest that ChiA belongs to family 18 chitinases and that ChiB and ChiBΔ belong to family 19 chitinases.
FIG. 1.
SDS-PAGE and zymogram analyses of proteins in the culture supernatant of N. prasina OPC-131. (A) SDS-PAGE stained with Coomassie brilliant blue R-250. (B) Zymogram of chitinase activity. N. prasina OPC-131 was grown in medium supplemented with 0.5% chitin, 0.5% chitin plus 1% glucose, or 1% glucose. Samples were taken at 80 h for these experiments. The same volume of each culture supernatant was concentrated to 1/30 of its original volume by ultrafiltration with NanoSpin Plus. The concentrated samples were applied to SDS-PAGE. Lanes: M, marker proteins; 1, 0.5% chitin; 2, 0.5% chitin plus 1% glucose; 3, 1% glucose.
Cloning and sequencing of chiA and chiB.
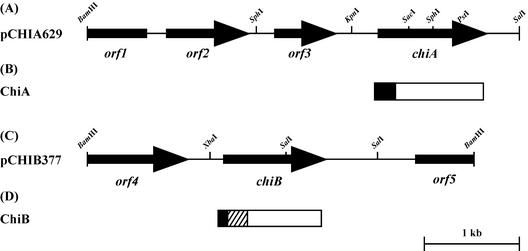
To isolate chiA and chiB genes from a genomic library of N. prasina OPC-131, PCR probes were synthesized on the basis of the N-terminal amino acid sequences of ChiA and ChiB and conserved sequences of family 18 and 19 chitinases. Two probes for the cloning of chiA (0.34 kb) and chiB (0.40 kb) were specifically amplified. Southern hybridization using these probes against total DNA digested with various restriction endonucleases showed that the probes for chiA and chiB hybridized strongly with a 4.6-kb BamHI-SalI fragment and a 4.1-kb BamHI-BamHI fragment, respectively (data not shown). Thus, for library construction, the DNA fragments with sizes between 4.0 and 5.0 kb were ligated to the corresponding sites of pUC19. Positive clones (pCHIA629 and pCHIB377) which hybridized to the probe for chiA or chiB were isolated from the libraries by colony hybridization. Analysis by restriction endonuclease digestion showed that pCHIA629 and pCHIB377 contain 4.6- and 4.1-kb insert DNAs, respectively (Fig. 2).
FIG. 2.
Restriction maps of pCHIA629 and pCHIB377 and domain structures of ChiA and ChiB. (A and C) Restriction maps of PCHIA629 (A) and pCHIB377 (B). The arrows indicate the ORFs and the direction of transcription. (B and D). Domain structures of ChiA (B) and ChiB (D). Solid, signal peptide; hatched, ChtBD; open, catalytic domain.
The nucleotide sequences of 4.6- and 4.1-kb insert DNAs were determined. An open reading frame (ORF) of chiA was identified in a 1.8-kb KpnI-SalI fragment of pCHIA629, and that of chiB was identified in the central region of pCHIB377. The ORF of chiA consists of 1,011 nucleotides encoding a protein of 336 amino acids with a predicted molecular mass of 35,257 Da. The initiation codon (ATG) was preceded at a distance of 9 bp by a possible ribosome-binding site (AGGA), which was homologous to the consensus Shine-Dalgarno sequence (27). The possible promoter sequences TTGACT and TAAAGT were identified upstream of the initiation codon. On the other hand, the 891-bp chiB ORF encoded a protein of 296 amino acids with a calculated molecular mass of 31,500 Da. The putative initiation codon, GTG, was preceded at a distance of 9 bp by a potential ribosome-binding site (AGGAG). The possible promoter sequences TGGTCT and TAGACC were identified upstream of the initiation codon. We examined whether chiA and chiB possess a pair of 12-bp direct-repeat sequences that are commonly found in the promoter regions of Streptomyces chitinase genes (5, 20). However, we could not find similar direct-repeat sequences in chiA and chiB. The deduced amino acid sequences of orf1, orf2, orf3, orf4, and orf5 showed homology with a hypothetical protein (accession number CAB88920), a putative oxide reductase (CAB88921), an Na+-H+ antiporter (AAF06676), a transpeptidase (T36689), and an ABC transporter (CAB76002) from S. coelicolor, respectively.
Structural features of ChiA and ChiB.
BLAST search analysis of the deduced amino acid sequence of ChiA revealed that the enzyme is composed of a signal sequence and a catalytic domain. The N-terminal amino acid sequence of the purified ChiA from N. prasina OPC-131 coincided precisely with the sequence starting from the Gln45 residue of the deduced amino acid sequence encoded by the chiA gene. Thus, cleavage of the signal sequence must occur between Gln44 and Gln45, which is compatible with the −3-−1 rule of von Heijne (38). The catalytic domain of ChiA showed sequence homology with chitinases classified in glycosyl hydrolase family 18, subfamily B, such as Chi30 from S. thermoviolaceus (47% identity) (34), ChiA from S. lividans (43% identity) (18), ChiA from S. coelicolor (43% identity) (24), and ChiD from B. circulans (28% identity) (39). As shown in Fig. 3, family 18 chitinases contain the consensus sequences (SXGG and DXXDXDXE) (37). The sequences SXGG and DXXDXDXE indicate substrate-binding and active sites, respectively. These consensus sequences were also perfectly conserved in the catalytic domain of ChiA. These results suggest that ChiA is classified among family 18 chitinases and that Glu165 of ChiA is involved as the proton donor in the catalytic double-displacement mechanism during hydrolysis (9, 37). On the other hand, ChiB is a modular enzyme consisting of three domains: the signal sequence (29 amino acids), the chitin-binding domain type 3 (ChtBD type 3) (41 amino acids), and the catalytic domain (203 amino acids). Between the ChtBD type 3 and the catalytic domain there was a small Gly- and Pro-rich sequence that seems to be a linker region. The N-terminal amino acid sequence of the purified ChiB coincided precisely with the sequence starting from the Ala30 residue of the deduced amino acid sequence encoded by the chiB gene. The domain from Ala36 to Gln76 showed sequence homology with ChtBD type 3, as classified by the National Center for Biotechnology Information conserved-domain database. ChtBD of ChiB had similarities to ChiC from S. griseus (85% identity) (21), a family 19 chitinase from Aeromonas sp. strain 10S-24 (65% identity) (28), and ChiA from Arthrobacter sp. strain TAD20 (55% identity) (13). The catalytic domain of ChiB showed high sequence homology to family 19 chitinases, such as ChiC (76% identity) from S. griseus (21), ChiF (73% identity) and ChiG (71% identity) from S. coelicolor (24), Chi35 (70% identity) from S. thermoviolaceus (70% identity) (35), Chi25 (37% identity) from Brassicanapus (7a), and Chi26 (36% identity) from Hordiumvulgare (12). Furthermore, the Streptomyces family 19 chitinases so far reported have deletions of several amino acid residues at two positions compared with those of class I and II chitinases (21, 35). Thus, Streptomyces family 19 chitinases are classified among class IV chitinases. As shown in Fig. 3, the catalytic domain of ChiB also has deletions similar to those of Streptomyces family 19 chitinases. These results indicate that ChiB belongs to the family of class IV chitinases. The amino acid sequence of ChiBΔ purified from N. prasina OPC-131 did not match any region near the N terminus of the deduced polypeptide. However, it coincided precisely with the sequence starting from Gly80 of the deduced amino acid sequence. These results appear to indicate that ChiBΔ is generated by the action of a protease(s) present in the culture supernatant after the initial gene product is secreted into the culture medium.
FIG. 3.
Comparison of amino acid sequences of catalytic domains of ChiA and ChiB with those of other proteins. (A) Amino acid sequence of the active-site region of ChiA compared with those of family 18 chitinases. The SXGG and DXXDXDXE motifs are boxed. A Glu residue identified as a proton donor is marked with an asterisk. The residue number of the first amino acid in each line is shown on the left. Residues that are identical are indicated by boldface letters. ChiA, N. prasina chitinase A; StChi30, S. thermoviolaceus chitinase 30; SlChiA, S. lividans chitinase A; ScChiA, S. coelicolor chitinase A; BcChiD, B. circulans chitinase D. (B) Amino acid sequence of the catalytic domain of ChiB compared with those of family 19 chitinases. ChiB, N. prasina chitinase B; SgChiC, S. griseus chitinase C; ScChiG, S. coelicolor chitinase G; ScChiF, S. coelicolor chitinase F; StChi35, S. thermoviolaceus chitinase 35; BnChi25, B. napus class I chitinase 25; HvChi26, H. vulgare class II chitinase 26. The residue number of the first amino acid in each line is shown on the left. Residues that are identical are indicated by boldface letters.
Expression and purification of recombinant proteins.
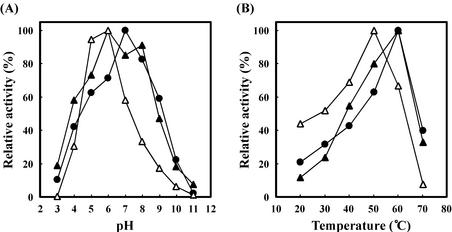
We constructed the expression plasmid coding for ChiA, ChiB, or ChiBΔ by using the thioredoxin fusion protein expression vector pThioHisB. When cell pellets from cultures grown under protein expression conditions were disrupted and centrifuged, the majority of the fusion proteins were found in the supernatant fraction. Each of the fusion proteins was digested with enterokinase, and then the recombinant ChiA, ChiB, and ChiBΔ were purified by affinity chromatography. The final enzyme preparations were shown to be homogeneous by SDS-PAGE (Fig. 4). The molecular mass of ChiA (32 kDa) calculated from the amino acid sequence without a signal peptide was in reasonable agreement with that estimated by SDS-PAGE. However, the molecular masses of ChiB (32 kDa) and ChiBΔ (28 kDa) estimated by SDS-PAGE were higher than their theoretical values (ChiB, 29 kDa; ChiBΔ, 23 kDa) despite the confirmation of their nucleotide sequences and N-terminal amino acid sequences. The purified recombinant enzymes were characterized by using colloidal chitin as the substrate (Fig. 5). The optimum temperatures of ChiA, ChiB, and ChiBΔ were 60, 60, and 50°C, respectively. The optimum pHs of ChiA, ChiB, and ChiBΔ were 7.0, 6.0, and 6.0, respectively. Even at pH 9.0, ChiA and ChiB showed ∼50% of the activities at their optimum pHs. However, ChiBΔ showed significantly lower activity, in the range of pH 7.0 to 10.0, than ChiA and ChiB. The native ChiA, ChiB, and ChiBΔ showed pH and temperature profiles similar to those of the recombinant enzymes (data not shown).
FIG. 4.
SDS-PAGE of recombinant chitinases. Lanes: M, marker proteins; 1, ChiA; 2, ChiB; 3, ChiBΔ.
FIG. 5.
Effects of pH and temperature on chitinase activities. (A) The following buffer systems were used: McIlvaine buffer (pH 3.0 to 6.0), 50 mM Tris-HCl buffer (pH 7.0 to 8.0), and 50 mM glycine-NaOH buffer (pH 9.0 to 11.0). The reaction mixtures were incubated at the optimum temperature for each enzyme (ChiA, 60°C; ChiB, 60°C; ChiBΔ, 50°C) for 10 min. The amount of each enzyme was 20 mU. (B) The reaction was carried out at various temperatures for 10 min at the optimum pH of each enzyme. The buffers used were 50 mM Tris-HCl buffer (pH 7.0) for ChiA and McIlvaine buffer (pH 6.0) for ChiB and ChiBΔ. The amount of each enzyme was 20 mU. •, ChiA; ▴, ChiB; ▵, ChiBΔ.
Binding study and antifungal activity.
To evaluate the role of ChtBD type 3 of ChiB, binding assays were carried out by adding the recombinant ChiB and ChiBΔ to α-chitin, β-chitin, and Avicel. As shown in Fig. 6, ChiB showed strong binding activity to α-chitin and β-chitin but weak affinity for Avicel; however, ChiBΔ bound to these polysaccharides much more weakly than ChiB. These results indicate that ChtBD type 3 of ChiB functions as a chitin-binding domain. When glycol chitin was used as a substrate, the specific activities of ChiB and ChiBΔ were 58.0 and 23.4 U/nmol, respectively. On the other hand, when colloidal chitin was used, ChiB had a 3.9-fold hydrolytic activity against the insoluble substrate compared with ChiBΔ.
FIG. 6.
Binding assays and hydrolytic activities of ChiB and ChiBΔ. (A) Binding assay mixtures contained 1.5 μg of ChiB (open bars) or ChiBΔ (solid bars) and 5 mg each of insoluble polysaccharides in 20 mM HEPES-KOH (pH 7.0). The mixtures were permitted to stand for 20 min on ice. The chitinase in the supernatant was measured, and the activity lost from the supernatant was assumed to be the activity bound. (B) Hydrolytic activities of ChiB (open bars) were measured at 60°C for 10 min using McIlvaine buffer (pH 6.0), and those of ChiBΔ (solid bars) were measured at 50°C for 10 min using the same buffer. Colloidal chitin or glycol chitin was used as a substrate. The error bars represent standard deviations.
The recombinant ChiB and ChiBΔ were tested for antifungal activity as indicated by the ability to inhibit the hyphal extension of T. reesei. As shown in Fig. 7, growth of T. reesei was clearly inhibited by 80 μg of ChiB, whereas ChiBΔ showed reduced inhibitory activity at the same concentration. ChiA showed no effect on the hyphal extension of T. reesei. These results indicate that ChtBD type 3 of ChiB plays an important role both in the efficient hydrolysis of chitin and in antifungal activity.
FIG. 7.
Antifungal activities of ChiA, ChiB, and ChiBΔ. The mycelium of T. reesei was directly inoculated onto the center of a potato dextrose agar plate. After 24 h at 27°C, paper disks were placed around the T. reesei colony, and samples were put onto the disks. 1, blank disk; 2, 80 μg of ChiA; 3, 80 μg of ChiB; 4, 80 μg of ChiBΔ.
DISCUSSION
In this paper, we describe for the first time the cloning, sequencing, and characterization of two chitinase genes from an alkaliphilic actinomycete, N. prasina OPC-131. Originally, members of the genus Nocardiopsis were isolated from mildewed grain (3), but the natural habitat of Nocardiopsis is soil, and many of the Nocardiopsis species grow best under moderately alkaline conditions (1, 15, 16, 43). On the other hand, the addition of chitin or cell walls of fungi to acidic soil increases the total number of Streptomyces species and leads to suppression of the growth of root-pathogenic fungi (30, 41). The suppression of root-pathogenic fungi is considered to be due to the action of family 19 chitinases produced by Streptomyces (21, 35). However, whether the amendment of alkaline soil with chitin also leads to an increase in the abundance of Streptomyces species remains to be elucidated. Thus, we isolated a number of alkaliphilic actinomycetes from soil samples using an alkaline medium containing colloidal chitin (32). One of the interesting alkaliphilic actinomycetes, N. prasina OPC-131, which produced two types of chitinases in the presence of chitin (32), was chosen for further investigation.
The study of chitinases produced by actinomycetes has been energetically pursued in Streptomyces species; however, the genus Nocardiopsis has not been considered a potential source of chitinolytic enzymes. The chitinases so far reported can be classified in two families, named 18 and 19, based on amino acid sequence similarity (8, 9). Family 18 contains chitinases from virus, bacteria, fungi, and animals and class III and V chitinases from plants. On the other hand, family 19 chitinases, consisting of classes I, II, and IV, have been identified mostly in plants. Recently, it was demonstrated that family 19 chitinases classified in class IV are distributed widely in Streptomyces species (40). However, it has not been clarified whether actinomycetes other than the genus Streptomyces produce family 19 chitinases. We have demonstrated that N. prasina OPC-131, which is phylogenetically distant from the genus Streptomyces, produces a family 19 chitinase (ChiB) classified in class IV, together with a family 18 chitinase (ChiA), in the presence of chitin. Furthermore, the strain secretes an 18-kDa chitin-binding protein in the presence of chitin (unpublished data). Recently, it was reported that the alkaliphilic Nocardiopsis sp. strain TOA-1 also produces alkaline hydrolytic enzymes, such as chitinase and protease (17). These results allow us to assume that family 19 chitinases are widely distributed not only in the genus Streptomyces but also in the non-Streptomyces group of actinomycetes.
Chitinolytic bacteria usually possess multiple chitinase genes, although the contribution of individual enzymes to chitin degradation has not yet been elucidated in detail. N. prasina OPC-131 possesses at least two types of chitinase genes, encoding ChiA and ChiB. ChiA is a family 18 chitinase consisting of only a catalytic domain. On the other hand, ChiB is a family 19 chitinase consisting of ChtBD type 3 and a catalytic domain. ChtBDs of chitinases have usually been grouped according to the classification of cellulose-binding domains (CBDs) postulated by Tomme et al. (31). Recently, ChtBDs have been seen as independent from CBDs and have been classified into types 1, 2, and 3 in the National Center for Biotechnology Information conserved-domain database (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). Types 1,2, and 3 consist of ChtBDs from fungi and plants, viruses and insects, and bacteria, respectively. Among them, ChtBD type 3 possesses a consensus sequence (AKWWTQ) that is well conserved in bacterial ChtBDs. The consensus sequence was also found in the ChtBD of ChiB, which is 41 amino acid residues long and shows homology with ChtBDs classified in type 3. The crystal structure of endoglucanase Z showed that two Trp residues of the CBD belonging to ChtBD type 3 are involved in stacking against the pyranose rings of glucose in cellulose (4, 29). By analogy with the CBD of endoglucanase Z, two similar Trp residues in the AKWWTQ sequence of the ChtBD of ChiB might participate in stacking against the pyranose rings of GlcNAc in chitin. Thus, to investigate the effects of ChtBD of ChiB on hydrolytic and antifungal activities, the activities of ChiB and ChiBΔ were compared. Removal of the ChtBD from ChiB significantly decreased the enzyme activity of ChiB toward both colloidal chitin and glycol chitin. These results suggest that the ChtBD of ChiB, unlike other ChtBDs, is necessary for the efficient hydrolysis of soluble, as well as insoluble, chitin. Furthermore, the ChtBD of ChiB was also essential for efficient antifungal activity.
In Streptomyces species, chitinase production is induced in the presence of chitin and repressed by the addition of glucose to the medium containing chitin. These phenomena are regulated at the transcriptional level. Delic et al. and Ni and Westpheling demonstrated that family 18 chitinase genes of Streptomyces contain similar pairs of direct-repeat sequences in the promoter regions, which have been suggested to be involved in both chitin induction and glucose repression (5, 20). Recently, it was reported that there are similar direct-repeat sequences in the upstream regions of several family 19 chitinase genes, such as chi35 and chi25 of S. thermoviolaceus OPC-520, chiC of S. griseus HUT6037, and chiF of S. coelicolor A3 (2, 34). These results suggest that family 18 and 19 chitinase-encoding genes of Streptomyces might be regulated by common regulatory factors or mechanisms. However, we could not find similar direct-repeat sequences in chiA and chiB, and these genes were not subjected to catabolite repression in the presence of glucose. These results indicate that the expression of these chitinase genes is regulated by mechanisms which are different from the regulatory mechanisms of Streptomyces chitinase genes. We are examining the distribution of family 19 chitinase genes in a non-Streptomyces group and the physiological role of actinomycete family 19 chitinases.
REFERENCES
- 1.Al-Tai, A. M., and J.-S. Ruan. 1994. Nocardiopsis halophila sp. nov., a new halophilic actinomycete isolated from soil. Int. J. Syst. Bacteriol. 44:474-478. [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Brocq-Rousseau, D. 1904. Sur un Streptothrix. Ref. Gen. Bot. 16:20-29. [Google Scholar]
- 4.Brun, E., F. Moriaud, P. Gans, M. J. Blackledge, F. Barras, and D. Marion. 1997. Solution structure of the cellulose-binding domain of the endoglucanase Z secreted by Erwinia chrysanthemi. Biochemistry 36:16074-16086. [DOI] [PubMed] [Google Scholar]
- 5.Delic, I., P. Robbins, and J. Westpheling. 1992. Direct repeat sequences are implicated in the regulation of two Streptomyces chitinase promoters that are subject to carbon catabolite control. Proc. Natl. Acad. Sci. USA 89:1885-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujii, T., and K. Miyashita. 1993. Multiple domain structure in a chitinase gene (chiC) of Streptomyces lividans. J. Gen. Microbiol. 139:677-686. [DOI] [PubMed] [Google Scholar]
- 7.Gooday, G. W. 1990. The ecology of chitin decomposition. Adv. Microb. Ecol. 11:387-430. [Google Scholar]
- 7a.Hamel, F., and G. Bellemare. 1993. Nucleotide sequence of a Brassica napus endochitinase gene. Plant Physiol. 101:1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrissat, B., and A. Bairoch. 1996. Updating the sequence-based classification of glycosyl hydrolases. Biochem. J. 316:695-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henrissat, B. 1999. Classification of chitinase modules. EXS 87:137-156. [DOI] [PubMed] [Google Scholar]
- 10.Kawase, T., R. Kanai, T. Ohno, T. Tanabe, N. Nikaidou, K. Miyashita, M. Mitutomi, and T. Watanabe. 2001. Identification of three family 18 chitinase genes of Streptomyces griseus HUT6037. Chitin Chitosan Res. 7:241-251. [Google Scholar]
- 11.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 12.Leah, R., H. Tommerup, I. Svendsen, and J. Mundy. 1991. Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J. Biol. Chem. 266:1564-1573. [PubMed] [Google Scholar]
- 13.Lonhienne, T., K. Mavromatis, C. E. Vorgias, L. Buchon, C. Gerday, and V. Bouriotis. 2001. Cloning, sequences, and characterization of two chitinase genes from the Antarctic Arthrobacter sp. strain TAD20: isolation and partial characterization of the enzymes. J. Bacteriol. 183:1773-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarthy, A. J., and S. T. Williams. 1992. Actinomycetes as agents of biodegradation in the environment—a review. Gene 115:189-192. [DOI] [PubMed] [Google Scholar]
- 15.Mikami, Y., K. Miyashita, and T. Arai. 1982. Diaminopimeric acid profiles of alkalophilic and alkaline-resistant strains of actinomycetes. J. Gen. Microbiol. 128:1709-1712. [DOI] [PubMed] [Google Scholar]
- 16.Mishra, S. K., J. E. Keller, J. R. Miller, R. M. Heisey, M. G. Nair, and A. R. Putnam. 1987. Insecticidal and nemacidal properties of microbial metabolites. J. Ind. Microbiol. 2:267-276. [Google Scholar]
- 17.Mitsuki, S., M. Sakai, Y. Moriyama, M. Goto, and K. Furukawa. 2002. Purification and some properties of a keratinolytic enzyme from an alkaliphilic Nocardiopsis sp. TOA-1. Biosci. Biotechnol. Biochem. 66:164-167. [DOI] [PubMed] [Google Scholar]
- 18.Miyashita, K., and T. Fujii. 1993. Nucleotide sequence and analysis of a gene (chiA) for a chitinase from Streptomyces lividans 66. Biosci. Biotechnol. Biochem. 57:1691-1698. [DOI] [PubMed] [Google Scholar]
- 19.Miyashita, K., T. Fujii, A. Watanabe, and H. Ueno. 1997. Nucleotide sequence and expression of a gene (chiB) for a chitinase from Streptomyces lividans. J. Ferment. Bioeng. 83:26-31. [Google Scholar]
- 20.Ni, X., and J. Westpheling. 1997. Direct repeat sequences in the Streptomyces chitinase-63 promoter direct both glucose repression and chitin induction. Proc. Natl. Acad. Sci. USA 94:13116-13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohno, T., S. Armand, T. Hata, N. Nikaidou, B. Henrissat, M. Mitsutomi, and T. Watanabe. 1996.. A modular family 19 chitinase found in the prokaryotic organism Streptomyces griseus HUT 6037. J. Bacteriol. 178:5065-5070. [DOI] [PMC free article] [PubMed]
- 22.Robbins, P. W., C. Albright, and B. Benfield. 1988. Cloning and expression of a Streptomyces plicatus chitinase (chitinase-63) in Escherichia coli. J. Biol. Chem. 263:443-447. [PubMed] [Google Scholar]
- 23.Robbins, P. W., K. Overbye, C. Albright, B. Benfield, and J. Pero. 1992. Cloning and high-level expression of chitinase-encoding gene of Streptomyces plicatus. Gene 111:69-76. [DOI] [PubMed] [Google Scholar]
- 24.Saito, A., T. Fujii, T. Yoneyama, M. Redenbach, T. Ohno, T. Watanabe, and K. Miyashita. 1999. High-multiplicity of chitinase genes in Streptomyces coelicolor A3(2). Biosci. Biotechnol. Biochem. 63:710-718. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 26.Sanger, F., S. Nicklen, and A. R. Coulson. 1997. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shine, J., and L. Dalgarno. 1975. Determinant of cistron specificity in bacterial ribosomes. Nature 254:34-38. [DOI] [PubMed] [Google Scholar]
- 28.Shiro, M., M. Ueda, T. Kawaguchi, and M. Arai. 1996. Cloning of a cluster of chitinase genes from Aeromonas sp. no. 10S-24. Biochim. Biophys. Acta 1305:44-48. [DOI] [PubMed] [Google Scholar]
- 29.Simpson, H. D., and F. Barras. 1999. Functional analysis of the carbohydrate-binding domains of Erwinia chrysanthemi Cel5 (endoglucanase Z) and an Escherichia coli putative chitinase. J. Bacteriol. 181:4611-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sneh, B., J. Katan, and Y. Henis. 1971. Mode of inhibition of Rhizoctonia solani in chitin amended soil. Phytopathology 61:1113-1117. [Google Scholar]
- 31.Tomme, P., R. A. J. Warren, R. C. Miller, Jr., D. G. Kilburn, and N. R. Gilkes. 1995. Cellulose-binding domains—classification and properties, p. 141-161. In J. M. Saddler and M. Penner (ed.), The enzymatic degradation of insoluble polysaccharides. American Chemical Society, Washington, D.C.
- 32.Tsujibo, H., Y. Yoshida, K. Miyamoto, T. Hasegawa, and Y. Inamori. 1992. Purification and properties of two types of chitinases produced by alkalophilic actinomycetes. Biosci. Biotechnol. Biochem. 56:1304-1305. [Google Scholar]
- 33.Tsujibo, H., H. Endo, K. Minoura, K. Miyamoto, and Y. Inamori. 1993. Cloning and sequence analysis of the gene encoding a thermostable chitinase from Streptomyces thermoviolaceus OPC-520. Gene 134:113-117. [DOI] [PubMed] [Google Scholar]
- 34.Tsujibo, H., N. Hatano, H. Endo, K. Miyamoto, and Y. Inamori. 2000. Purification and characterization of a thermostable chitinase from Streptomyces thermoviolaceus OPC-520 and cloning of the encoding gene. Biosci. Biotechnol. Biochem. 64:96-102. [DOI] [PubMed] [Google Scholar]
- 35.Tsujibo, H., T. Okamoto, N. Hatano, K. Miyamoto, T. Watanabe, M. Mitsutomi, and Y. Inamori. 2000. Family 19 chitinases from Streptomyces thermoviolaceus OPC-520: molecular cloning and characterization. Biosci. Biotechnol. Biochem. 64:2445-2453. [DOI] [PubMed] [Google Scholar]
- 36.Tsujibo, H., H. Orikoshi, N. Baba, M. Miyahara, K. Miyamoto, M. Yasuda, and Y. Inamori. 2002. Identification and characterization of the gene cluster involved in chitin degradation in a marine bacterium, Alteromonas sp. strain O-7. Appl. Environ. Microbiol. 68:263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Aalten, D. M. F., B. Synstad, M. B. Brurberg, E. Hough, B. W. Riise, V. G. H. Eijsink, and R. K. Wierenga. 2000. Structure of a two-domain chitotriosidase from Serratia marcescens at 1.9-Å resolution. Proc. Natl. Acad. Sci. USA 97:5842-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Heijne, G. 1983. Patterns of amino acids near signal sequence cleavage sites. Eur. J. Biochem. 133:17-21. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe, T., W. Oyanagi, K. Suzuki, K. Ohnishi, and H. Tanaka. 1992. Structure of the gene encoding chitinase D of Bacillus circulans WL-12 and possible homology of the enzyme to other prokaryotic chitinases and class III plant chitinases. J. Bacteriol. 174:408-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe, T., R. Kanai, T. Kawase, T. Tanabe, M. Mitsutomi, M. Sakuda, and K. Miyashita. 1999. Family 19 chitinases of Streptomyces species: characterization and distribution. Microbiology 145:3353-3363. [DOI] [PubMed] [Google Scholar]
- 41.Williams, S. T., and C. S. Robinson. 1981. The role of streptomycetes in decomposition of chitin in acidic soils. J. Gen. Microbiol. 127:55-63. [Google Scholar]
- 42.Wolfgang, H. S., K. Bronnenmeier, F. Grabnitz, and W. L. Staudenbauer. 1987. Activity staining of cellulase in polyacrylamide gels containing β-glucans. Anal. Biochem. 164:72-77. [DOI] [PubMed] [Google Scholar]
- 43.Yassin, A. F., E. A. Galinski, A. Wohlfarth, A. Jahnke, K.-D. Jahnke, K. P. Schaal, and H. G. Truper. 1993. A new actinomycete species. Nocardiopsis lucentensis sp. nov. Int. J. Syst. Bacteriol. 43:266-271. [Google Scholar]