Abstract
Cell motility and cell polarity are essential for morphogenesis, immune system function, and tissue repair. Many animal cells move by crawling, and one main driving force for movement is derived from the coordinated assembly and disassembly of actin filaments. As tissue culture cells migrate to close a scratch wound, this directional extension is accompanied by Golgi apparatus reorientation, to face the leading wound edge, giving the motile cell inherent polarity aligned relative to the wound edge and to the direction of cell migration. Cellular proteins essential for actin polymerization downstream of Rho family GTPases include the Arp2/3 complex as an actin nucleator and members of the Wiskott–Aldrich Syndrome protein (WASP) family as activators of the Arp2/3 complex. We therefore analyzed the involvement of the Arp2/3 complex and WASP-family proteins in in vitro wound healing assays using NIH 3T3 fibroblasts and astrocytes. In NIH 3T3 cells, we found that actin and Arp2/3 complex contributed to cell polarity establishment. Moreover, overexpression of N-terminal fragments of Scar2 (but not N-WASP or Scar1 or Scar3) interfere with NIH 3T3 Golgi polarization but not with cell migration. In contrast, actin, Arp2/3, and WASP-family proteins did not appear to be involved in Golgi polarization in astrocytes. Our results thus indicate that the requirement for Golgi polarity establishment is cell-type specific. Furthermore, in NIH 3T3 cells, Scar2 and the Arp2/3 complex appear to be involved in the establishment and maintenance of Golgi polarity during directed migration.
INTRODUCTION
Fibroblastic cell migration can be divided into four mechanistically separate steps: lamellipodium extension, formation of new adhesions, cell body contraction, and tail detachment. The Rho family of GTPases are important controllers of multiple steps in cell migration. In particular, Rho, Rac, and Cdc42 have been well characterized for their roles in regulation of the actin cytoskeleton (Tapon and Hall, 1997; Hall, 1998). Signals from diverse cell surface receptors through Rac and Cdc42 as well as other mediators are postulated to converge on specific Wiskott–Aldrich Syndrome protein (WASP) family members and other nucleation promoting factors that regulate actin polymerization through the Arp2/3 complex, leading to a common pathway to generate actin filaments (Machesky and Insall, 1998, 1999; Machesky and Gould, 1999).
There are five known mammalian WASP-family members, including WASP, N-WASP, and three Scar/WAVE proteins (Millard and Machesky, 2001). Scars contain from N- to C-terminus a Scar Homology domain (SHD), a basic motif (B), a polyproline-rich region (Polypro), and a WASP Homology 2/central/acidic (WCA) domain (see Figure 4). On the other hand, N-WASP contains, at the N-terminus, a WASP Homology 1 (WH1) domain rather than an SHD and a GTPase binding domain (GBD; see Figure 4). All the WASP-family members have carboxy-terminal domains that bind to actin-monomers and to the Arp2/3 complex, causing activation of the actin nucleating activity (Machesky and Insall, 1998; Machesky et al., 1999; Rohatgi et al., 1999).
Figure 4.
Golgi polarization, at the edge of the wound, of N-WASP-PWCA– and N-WASP-deltaA–expressing cells. Golgi reorientation to face the wound was evaluated when NIH 3T3 cells at the edge of the wound were microinjected with the two different N-WASP expression constructs (pEGFPC1- or pRK5-myc; see Figure 4). (A) Percentage of Golgi orientated toward the wound was determined at 3, 5, and 7 h after wounding for cells microinjected with N-WASP-PWCA and N-WASP-deltaA expression vectors. Golgi polarization of non-microinjected wound edge cells, over the time course of wound closure, is included as a control (see legend on the right side of the graph). (B) Quantification data. The first number corresponds to the number of Golgi observed and the second, in parentheses, is the number of separate experiments.
Movement of cell sheets is essential for embryonic development, defense against infections, and healing of tissue wounds (Martin, 1997). In some respects, movement of a cell sheet is more complex than single cell movement, because it may involve purse-string and/or protrusion-based crawling dependent on the cell type and model chosen: embryonic or tissue-culture wound healing models (Martin and Lewis, 1992; Fenteany et al., 2000; Jacinto and Martin, 2001; Jacinto et al., 2001). When wounds close by a purse-string mechanism, the obvious driving force is the contraction of actomyosin complexes. For wound closure by crawling, the mechanisms are less clear. In both cases, small GTPases of the Rho family are implicated. Purse-string contraction is dependent on the activity of Rho but not Rac (Brock et al., 1996). In protrusion-based crawling models, such as rat embryo fibroblasts, Rac is essential for protrusion of lamellipodia and forward movement, Rho activity is required to maintain cell substrate adhesion, and Cdc42 is required for cell polarity (Nobes and Hall, 1999). In previous and present studies, polarity was measured by the movement of the Golgi complex to the side of the cell facing the wound (Kupfer et al., 1982; Nobes and Hall, 1999). It was more recently reported that wounds induced in Madin-Darby canine kidney epithelial cell monolayers close by Rac-dependent cell crawling, with formation of lamellipodia at the margin and that Rho and Cdc42 are necessary for the regularity of the wound closure (Fenteany et al., 2000).
As a model for cell protrusion, polarity establishment, and migration, we analyze downstream targets of Rho family of GTPases in NIH 3T3 fibroblasts and astrocytes. Our data demonstrate for the first time that Arp2/3, an actin nucleator, as well as Scar2, an activator of Arp2/3, are involved in Golgi polarization in wound edge NIH 3T3 cells. We also show that interference with Scar2 does not disrupt cell migration. In comparison, we found that primary astrocytes (Etienne-Manneville and Hall, 2001) do not use the actin cystokeleton for Golgi polarization, neither Arp2/3 nor any WASP-family proteins. Thus in different cell types, polarity is likely to have different underlying mechanisms.
MATERIALS AND METHODS
Antibodies and Reagents
The monoclonal anti-myc antibody (9E10), from Alan Hall, and the anti–beta-COP antibody from ABR Affinity Bioreagents (Cambridge, UK) were used at dilutions 1:250 and 1:750, respectively, in immunofluorescence microscopy. The polyclonal anti-WASP antibody 07–066 was purchased from US Biological (Swampscott, MA). The polyclonal anti–N-WASP antibody was from Dr. P. Aspenström (Uppsala, Sweden). The polyclonal antibody directed against p34-Arc (ARPC2) was previously described (Machesky et al., 1997). A pan anti-Scar antibody was from John Scott, (Oregon), and was previously characterized (Westphal et al., 2000). Polyclonal anti-Scar1 antibody was prepared in rabbits immunized against the N-terminal region of human Scar1 (S. Launay et al., 2003; Eurogentec, Herstal, Belgium). The antibodies directed against WASP, N-WASP, p34-Arc (ARPC2), pan-Scar, and Scar1 were used at dilution 1:500 in Western blot and 1:150 in immunofluorescence experiments. The horseradish peroxidase–conjugated anti-rabbit antibodies (1:5000) used in Western blots were from Jackson ImmunoResearch Laboratories (West Grove, PA). Secondary antibodies used in immunofluorescence staining (Texas Red–conjugated anti-rabbit or anti-mouse) were from Molecular Probes (Leiden, The Netherlands) and were used at dilution 1:750. Texas Red–conjugated phalloidin and Alexa fluor 594–conjugated dextran (10,000 MW) were purchased from Molecular Probes.
Preparation of NIH 3T3 Extracts and Western Blots
Proteins of NIH 3T3 cells were precipitated in the dish with cold 5% trichloroacetic acid and kept at 4°C for 1 h. The precipitate was harvested and then centrifuged for 10 min at 7000 × g at 4°C. The pellet was dissolved in electrophoresis sample buffer as previously described (Launay et al., 1999). Cellular proteins, 12 μg per well, were run on a Laemmli-type 10% SDS-PAGE. Proteins were electroblotted onto nitrocellulose. Blocking of nitrocellulose and immunostaining was performed in a buffer containing 10 mM Tris (pH 7.4), 150 mM NaCl, 5% dry milk and 0.1% Tween-20. The membranes were stained with either the antibodies directed against WASP, N-WASP, p34-Arc (ARPC2), pan-Scar, or Scar1. Thereafter, the nitrocellulose sheets were incubated with the anti-rabbit-IgG horseradish-peroxidase conjugate. The antibody binding was detected using SuperSignal chemiluminescence substrate reagents according to the manufacturer's instructions (Pierce, Rockford, IL). The chemiluminescence was recorded and processed using a GeneGenius (Syngene/Synoptics, Cambridge, UK).
Cell Culture and Wounding
NIH 3T3 cells were grown in DMEM supplemented with 5% DCS and penicillin/streptomycin (100 iU/ml and 100 μg/ml, respectively) and incubated at 37°C and 10% CO2. For the wound healing assays, cells were seeded on coverslips and grown to confluence, and the wound was made by scraping the cell monolayer across the coverslip with a microinjection needle. Astrocyte cell culture and wounding were performed as recently described (Etienne-Manneville and Hall, 2001).
Drug Treatment
Cytochalasin D was used at final concentrations of 0.5 and 2 μM. Confluent monolayers of cells (NIH 3T3 and astrocytes) were incubated with the drug and directly wounded (performed at least in triplicate). Two hours later, NIH 3T3 cells were fixed and labeled for actin and Golgi. Astrocytes were incubated for 8 h before fixation and staining for Golgi.
DNA Constructs
Tagged expression vectors encoding the full-length Scar1, the WCA and deltaA constructs of both Scar1 and N-WASP, and the deltaA constructs of both Scar2 and Scar3 as well as deletion constructs of Scar2-deltaA were obtained as described below, and the expressed proteins are shown in Figure 4. The full-length Scar2 construct was also generated. 1) For pEGFPN1-Scar1, cDNA encoding human Scar1 (KIAA0429, Kasuza, cDNA project, Japan) was amplified by PCR (Table 1). The product was digested with BamHI and EcoRI and cloned into pEGFPN1 (Clontech, Palo Alto, CA), which had been digested with BglII and EcoRI. 2) For pEGFPC1-Scar1, the PCR amplified full-length Scar1 was digested with BamHI and EcoRI and ligated into pEGFPC1 (Clontech) digested with BglII and EcoRI. 3) pEGFPC1-Scar1-WCA was a kind gift of Bob Mark, Wyeth-Ayerst, Princeton, NJ. 4) For pEGFPC1-Scar1-deltaA, cDNA encoding amino acids 1–530 of human Scar1 was amplified by PCR (Table 1). The PCR product was digested with BamHI and EcoRI and cloned into pEGFPC1, which had been digested with BglII and EcoRI. 5) The plasmid encoding myc-tagged N-WASP-PWCA was previously described (Machesky and Insall, 1998). 6) For pEGFPC1-N-WASP-deltaA, cDNA encoding amino acids 1–484 of bovine N-WASP was amplified by PCR (Table 1). The PCR product was digested with BclI and EcoRI and cloned into pEGFPC1, which had been digested with BglII and EcoRI. 7) For pEGFPC1-Scar2-deltaA and pEGFPC1-Scar3-deltaA: cDNA encoding amino acids 1–468 of Scar2 and cDNA encoding amino acids 1–473 of Scar3 were PCR amplified (Table 1). Both PCR products were digested with BglII and KpnI and ligated into pEGFPC1 digested with the same enzymes. 8) Four pEGFPC1-Scar2-deltaA deletion constructs (Scar2-SB, Scar2-SBP, Scar2-BPWC, Scar2-PWC) and the pEGFPC1-Scar2 construct (full-length Scar2) were generated. PCR amplifications were performed using the primers listed in Table 1. All PCR products were digested with BglII and KpnI and ligated into pEGFPC1 cut with BglII and KpnI.
Table 1.
PCR primers used in this study
| Sense primer | Antisense primer | Final construct |
|---|---|---|
| 5′-cgggatccatgccgctagtgaaaagaaacatcgatcc-3′ | 5′-cgaattccctccaaccaatctacttcatc-3′ | PEGFPN1-Scar1 |
| 5′-cgggatccatgccgctagtgaaaagaaacatcgatcc-3′ | 5′-ggaattcttactccaaccaatctacttcatcaaattc-3′ | PEGFPC1-Scar1 |
| 5′-cgggatccatgccgctagtgaaaagaaacatcgatcc-3′ | 5′-ggaattcttaatcgttttcaatgcgttcatg-3′ | PEGFPC1-Scar1-deltaA |
| 5′-ccctgatcatccgtccagcagcagccg-3′ | 5′-ggaattcaagaatgaatggctttgctcctc-3′ | PEGFPN1-N-WASP-deltaA |
| 5′-ccagatctatgcctttagtgaagagg-3′ | 5′-gcggtaacctcagtcattccccacccg-3′ | PEGFPC1-Scar3-deltaA |
| 5′-ccagatctatgccgttagtaacgagg-3′ | 5′-gcggtaccctacaggtcattgcccacaac-3′ | PEGFPC1-Scar2-deltaA |
| 5′-ccagatctatgccgttagtaacgagg-3′ | 5′-gcggtacctcaatagctacttgcatccacgtt-3′ | PEGFPC1-Scar2-SB |
| 5′-ccagatctatgccgttagtaacgagg-3′ | 5′-gcggtacctcagctcacggcaggcaagga-3′ | PEGFPC1-Scar2-SBP |
| 5′-ccagatctaaggatatcatgaaagagaag-3′ | 5′-gcggtaccctacaggtcattgcccacaac-3′ | PEGFPC1-Scar2-BPWC |
| 5′-ccagatctctggggacttctgggtat-3′ | 5′-gcggtaccctacaggtcattgcccacaac-3′ | PEGFPC1-Scar2-PWC |
| 5′-ccagatctctggggacttctgggtat-3′ | 5′-gcggtaccttaatcggaccagtcgtcctc-3′ | PEGFPC1-Scar2 |
We verified that the constructs are expressed stably and at similar levels. NIH 3T3 cells were transiently transfected and followed by Western blots with anti-GFP antibodies to document the expression of the GFP-fusion constructs. Transfection was performed using Fugene 6 (Roche, Nutley, NJ), and blots were probed with an antibody recognizing GFP (obtained from Cancer Research UK).
Microinjection
Nuclear microinjection in the first row of the wound edge NIH 3T3 cells were performed about 1 h after wounding. Expression vectors were used at 100–200 μg/ml. When needed, Alexa fluor 594–conjugated dextran was used at a final concentration of 2 mg/ml as marker of microinjection in conjunction with vector or alone. Microinjection of wound edge astrocytes was performed as recently described (Etienne-Manneville and Hall, 2001).
Immunofluorescence: Golgi Polarization and Wound Closure
Cells were stained and mounted on glass slides as previously described (Machesky and Hall, 1997). In brief, cells were fixed with 4% para-formaldehyde in PBS, blocked in 50 mM NH4Cl in PBS, and permeabilized in 0.1% Triton X-100 in PBS and stained with phalloidin or the appropriate antibodies. The expressed proteins from the microinjected vectors were all GFP labeled, except for N-WASP-PWCA, which was myc tagged, so expression was visualized using anti-myc antibodies (9E10) or by coinjecting with Alexa fluor 594–conjugated dextran as a marker. For cell polarity analysis, cells were fixed 2, 4, and 6 h after microinjection and labeled for the Golgi apparatus with anti–beta-COP antibodies. The orientation of the Golgi was assessed as described previously (Nobes and Hall, 1999), and the significance of the inhibition (or lack of inhibition) of the Golgi polarization was determined by the statistical t test (p < 0.025) for all the performed experiments. In each case, we compared treated and untreated cells at the same time point to determine significance. The number of Golgi examined and the number of separately performed experiments is indicated in the RESULTS. The data were collected by two observers blinded as to the construct identity. The Golgi polarization is expressed as average ± SD for each construct at each time point after wounding. When observing the motility of microinjected cells, the cells were fixed 4 h postmicroinjection and labeled with Texas Red–conjugated phalloidin and the position of the microinjected cells were observed versus the wound edge. We scored the number of microinjected cells still present at the wound edge (migrated forward) and the number left behind the advancing cell sheet margin (did not migrate). As positive control for migration and wound closure, wound edge cells were microinjected with Alexa fluor 594–conjugated dextran. The cells were examined on a Zeiss microscope (Thornwood, NY) using oil immersion lenses. Fluorescence images were recorded and processed using Openlab software (Improvision, Lexington, MA) with Hammamatsu C4880 camera (Bridgewater, NJ).
RESULTS
Expression of Arp2/3 and WASP-family Proteins in NIH 3T3 Cells
Because not all tissues express the same WASP-family proteins, we first determined which cytoskeletal proteins were expressed in NIH 3T3 cells. We used antibodies directed against the p34-Arc (ARPC2) subunit of the Arp2/3 complex to confirm by Western blot the presence of Arp2/3 in the NIH 3T3 cell extracts (Figure 1A). NIH3T3 cells also express other subunits of the Arp2/3 complex, as assessed with anti-Arp2, anti-Arp3, and anti-p16Arc (ARPC5) antibodies (unpublished data and Machesky et al., 1997). Because antibodies specifc to WASP-family proteins are still being developed, we could only test WASP, N-WASP, and Scar1 expression. NIH 3T3 cells express Scar1 and N-WASP but not WASP (Figure 1A). The Arp2/3 complex is present in the cytoplasm of all cells and at the leading edge of the wound (Figure 1B). Anti-pan Scar antibodies stained both the cell junctions and the leading edge of the cells at the wound margin; the nonspecific nuclear labeling was previously reported (Westphal et al., 2000). Anti-Scar1 and anti–N-WASP antibodies did not significantly stain NIH 3T3 cells by immunofluorescence microscopy (unpublished data).
Figure 1.
Expression of Arp2/3 and WASP-family proteins by NIH 3T3 cells. Analyses were performed by Western blot and by immunofluorescence. (A) NIH 3T3 cell extracts were analyzed by Western blot with the following antibodies as described from left to right: Scar1, pan-Scar, WASP, N-WASP, and p34-Arc (ARPC2) of the Arp2/3 complex. Molecular weight markers (in kDa) are shown. (B) Cells were fixed and stained for pan-Scar (top: middle and right panels) and for the p34-Arc (bottom: middle and right panels), and in both cases labeled concomitantly for actin (left panels). The right panels are enlargements of a region from the middle panels as indicated.
NIH 3T3 Wound Model Analysis
We analyzed the polarity and motility of the cells present at the edge of a scratch wound made across an NIH 3T3 fibroblast monolayer. The wound width was ∼6–8 cells across (300–700 μm), and the wound was ∼300–400 cells long (∼7–8 mm). Wounding induced migration of the remaining intact cell sheet into the gap as described earlier, and cells extended lamellipodial protrusions in the direction of migration (Nobes and Hall, 1999; Etienne-Manneville and Hall, 2001; Fenteany et al., 2000). In our assays, the wound was usually closed in 10–11 h.
Cells at the edge of the wound acquired a polarized morphology that was revealed by reorientation of the Golgi apparatus in the direction of the movement (Nobes and Hall, 1999). Cells in which the Golgi (beta-COP) labeling was within the 120° sector facing the wound were scored positive. As a control, cell polarity was measured without wounding, taking a line as virtual wound. Approximately 33% of these cells showed a Golgi oriented toward the line, confirming random Golgi orientation if the monolayer is not wounded (Nobes and Hall, 1999).
Golgi polarization of the wound edge cells was determined 0.1, 3, 5, 7, 8, and 10 h after wounding. Cells exhibited a polarized Golgi quickly after wounding, and it lasted for several hours. Data for untreated control cells are shown in Figures 3 and 4.
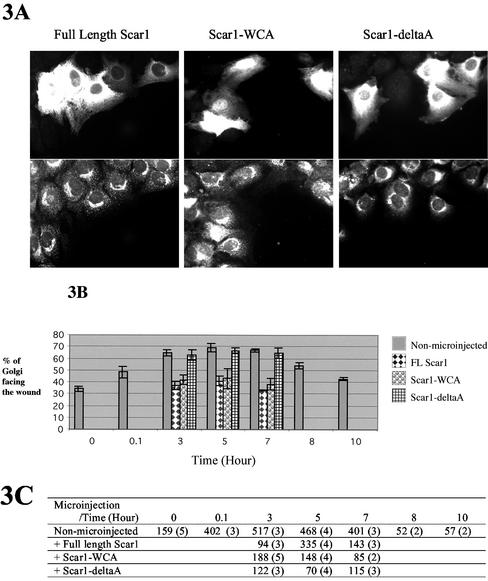
Figure 3.
Golgi polarization, at the edge of the wound, of fulllength Scar1-, Scar1-WCA–, and Scar1-deltaA–expresisng cells. Golgi reorientation to face the wound was evaluated when NIH 3T3 cells at the edge of the wound was microinjected with the three different pEGFP-Scar1 expression constructs described in Figure 2. (A) The microinjected cells expressing the GFP-fusion protein are shown (top panels): full-length Scar1 (left), Scar1-WCA (middle), and Scar1-deltaA (right). Cells were fixed and stained for the Golgi apparatus with anti-beta-COP (bottom panels.) (B) Percentage of Golgi orientated toward the wound was determined at 3, 5, and 7 h after wounding for cells microinjected with full-length Scar1, Scar1-WCA, and Scar1-deltaA expression vectors. Golgi polarization of non-microinjected wound edge cells, over the time course of wound closure, is included as a control (see legend on the right side of the graph). (C) quantification data. The first number corresponds to the number of Golgi observed and second, in parentheses, is the number of separate experiments.
Arp2/3 and Golgi Polarization in the NIH 3T3 Wound Model
To determine whether Arp2/3 localization is required for the establishment of NIH 3T3 cell polarity, Golgi apparatus reorientation to face the wound was measured in cells expressing Scar1 and various deletion constructs described in Figure 2. Overexpression of the WASP-family acidic domain constructs interferes with endogenous Arp2/3 localization (Machesky and Insall, 1998; Linder et al., 2000; Qualmann and Kelly, 2000; Alrutz et al., 2001; Hufner et al., 2002). Cells expressing GFP–full-length Scar1 exhibited a reduced percentage of Golgi polarization compared with non-microinjected control wound edge cells (Figure 3). As shown in Figure 3, between 30 and 40% of these cells had the Golgi apparatus facing the wound, which is close to random as confirmed by t test (p < 0.025). This reduction was persistent over the time course of wound healing: at 3, 5, and 7 h after wounding. We observed similar results regardless of whether the GFP tag was fused to the N- or C-termini.
Figure 2.
Scar and N-WASP constructs used in this study. Full-length Scar1 contains the following domains: Scar Homology domain (SHD), basic motif (B), a polyproline-rich region (Polypro), WASP Homology 2/central/acidic (WH2/C/A) domain also called WCA domain. N-WASP contains: WASP Homology 1 (WH1) domain, basic motif (B), GTPase binding domain (GBD), a polyproline-rich region (Polypro), WASP Homology 2/central/acidic (WH2WH2/C/A) domain. Scar1–2-3-deltaA and N-WASP-deltaA constructs are lacking the C-terminal region as shown in the Figure. Scar1-WCA and N-WASP-PWCA constructs are lacking the N-terminal region. All constructs are tagged, with the green fluorescent protein (GFP) or with the myc-tag, as indicated. The numbers at the top of the constructs give the amino acid number of the protein sequence that is at the beginning and the end of the indicated domains.
To assess whether Golgi polarization inhibition can be attributed to the interaction of Scar1 with Arp2/3, we tested Scar1-WCA, a minimal Arp2/3 and actin-binding construct and Scar1-deltaA, which does not bind to Arp2/3 complex but still interacts with actin and presumably with other targets of Scar1 (Machesky and Insall, 1999; May et al., 1999). The expression of GFP-Scar1-WCA interfered with Golgi reorientation to a similar extent to GFP–full-length Scar1, whereas GFP-Scar1-deltaA did not cause any detectable change (Figure 3).
We determined whether the acidic domain of N-WASP was also able to interfere with NIH 3T3 cell polarity as shown with Scar1 (Figure 4). However, unlike Scar1, full-length N-WASP has a folded conformation resulting in autoinhibition of its activity toward Arp2/3 (Kim et al., 2000). Similar to our findings with Scar1, the expression of N-WASP-PWCA interfered with Golgi polarization in response to wounding, whereas N-WASP-deltaA did not (Figure 4).
Thus, delocalization of the Arp2/3 complex due to overexpression of an Arp2/3 binding domain (WCA) inhibited the Golgi polarization (Figures 3 and 4; see also Figure 7B), and our experiments suggest that correct localization/activity of the Arp2/3 complex is important for the establishment of NIH 3T3 Golgi polarity during directed cell migration.
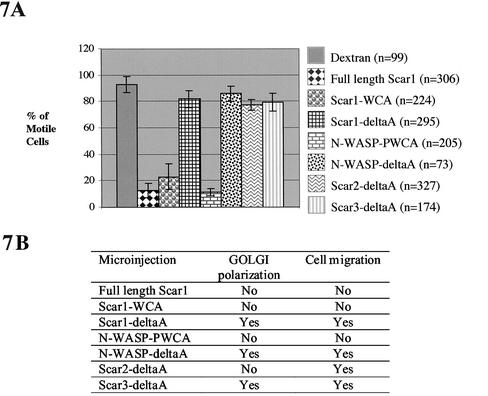
Figure 7.
NIH 3T3 motility and Golgi polarization of the wound edge cells during wound closure. Wound edge cells were microinjected with the expression vectors coding for the Scar and the N-WASP constructs (described in Figure 2 and listed in A and B). (A) Motility. Percentage of microinjected cells still able to carry out wound closure and staying at the edge of the wound as it closed. These percentages were determined from quantification of data as described in MATERIALS AND METHODS; n is the number of cells observed; quantification was performed in at least three separate experiments. (B) Cell polarity of microinjected cells was determined by Golgi reorientation to face the wound. These results were described over time as % of Golgi polarization, in Figures 3, 4, and 5. Cell motility was determined as shown in section A.
Scar2 Is Involved in Golgi Polarization in the NIH 3T3 Wound Model
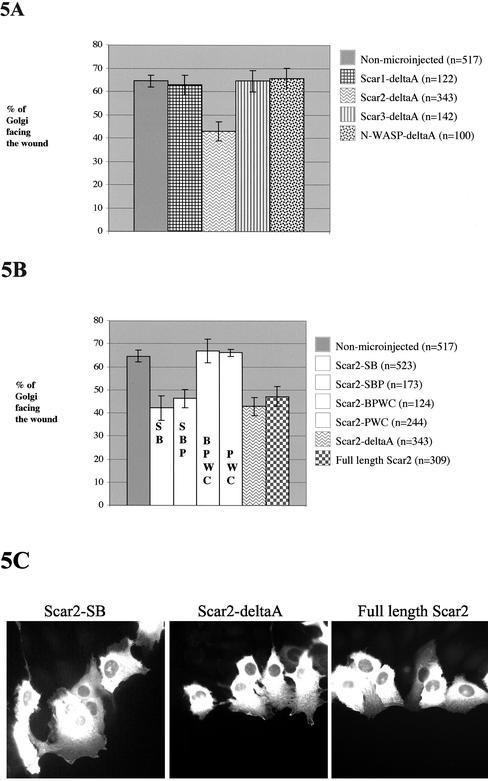
Because Arp2/3 is needed for Golgi polarization of NIH 3T3 fibroblasts, we postulated that a WASP-family protein is likely to be involved as a signal mediator between Rho family GTPases and Arp2/3. Previous studies have shown that N-terminal constructs of WASP-family proteins will specifically inhibit certain cellular processes (see DISCUSSION). We observed no effect of Scar1-deltaA or N-WASP-deltaA on cell behavior in the scratch wound assay, so we therefore tested the effect of the remaining WASP-family proteins, Scar2-deltaA and Scar3-deltaA (Figure 5A). Among the deltaA constructs of the WASP-family proteins only Scar2 interfered with Golgi polarization (Figure 5A).
Figure 5.
Scar2-deltaA inhibits Golgi polarization of NIH 3T3 fibroblasts and the SHD domain of Scar2 is necessary. Golgi polarization, at the wound edge, of cells expressing (A) the deltaA constructs of four WASP-family proteins: Scar1, Scar2, Scar3, N-WASP and (B) full-length Scar2 and Scar2-deltaA deletion constructs. Golgi reorientation to face the wound was evaluated when NIH 3T3 cells at the edge of the wound were microinjected with the appropriate pEGFPC1 expression constructs described in Figures 2 and 6. The percentage of Golgi oriented toward the wound was determined at 3 h after wounding for cells microinjected with the WASP-family protein deletion constructs. Golgi polarization of non-microinjected wound edge cells is included as a control of cell polarity (see legend on the right side of the graph; n is the number of Golgi observed; quantification was performed in at least 3 separate experiments). (C) Microinjected cells expressing the GFP-fusion proteins are shown as indicated: Scar2-SB (left), Scar2-deltaA (middle), and full-length Scar2 (right).
Several deletion constructs of Scar2-deltaA as well as the full-length Scar2 construct were generated, and their effect on Golgi polarization was tested (Figures 5B and 6). All Scar2 deletion constructs containing the S domain showed inhibition of Golgi polarization, seen with Scar2-SB, Scar2-SBP, and Scar2-deltaA. On the other hand, Scar2-BPWC– and Scar2-PWC–expressing cells exhibited Golgi polarization. Of note, full-length Scar2 overexpression inhibited Golgi polarity. Thus, the SHD domain of Scar2 is necessary for the inhibition of the Golgi polarization (Figures 5 and 6).
Figure 6.
The five different pEGFPC1-Scar2-deltaA deletion constructs used in this study. Domains are described in the Figure 2 legend.
To explore whether it was possible that the deletion constructs were blocking interaction of endogenous Scar2 SHD with its ligands, we demonstrated by RT-PCR that Scar2 is expressed in NIH 3T3 cells (unpublished data). We also verified that the constructs were expressed stably and at similar levels (unpublished data). Thus, the most likely explanation of our results is that Scar2 SHD is involved in Golgi polarity.
Scar2-deltaA Overexpressing NIH 3T3 Cells Can Drive Wound Closure
Lamellipodia formation and migration of single cells is prevented by Arp2/3 delocalization (Machesky and Insall, 1998; Weiner et al., 1999; Jones, 2000; Linder et al., 2000; Bailly et al., 2001; Hufner et al., 2002). We showed that Arp2/3 delocalization interferes with Golgi polarization in response to wounding. In addition, we confirmed that migration of wound edge NIH 3T3 cells within a sheet was also not possible with delocalized endogenous Arp2/3 (Figure 7). Thus, cells that overexpressed a construct containing a WCA domain, were nonmotile, whereas cells that overexpressed a deltaA construct could move even if the Golgi did not polarize (Figure 7).
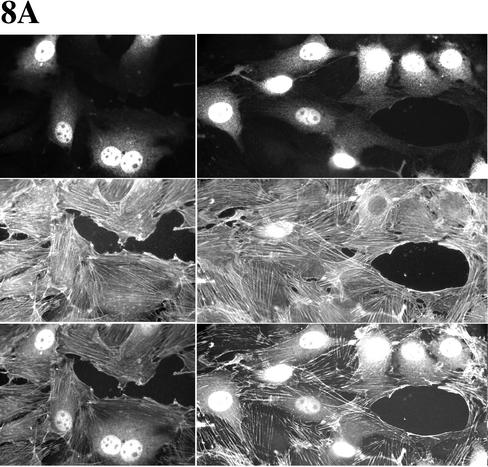
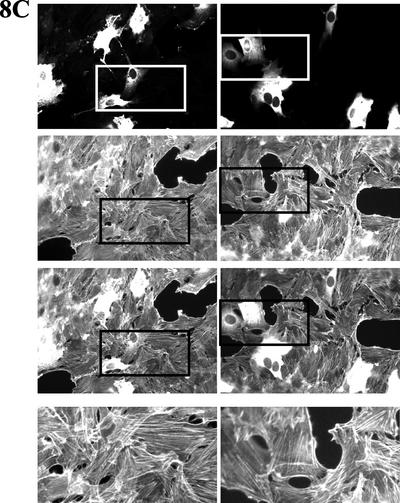
WASP-family proteins, including Scar2, are localized in protruding lamellipodia (Hahne et al., 2001; Nakagawa et al., 2001; Takenawa and Miki, 2001; Yamaguchi et al., 2002). Although WASP is not essential for migration, WASP and N-WASP proteins have been reported as regulators of migration of single cells (Haddad et al., 1999; Burns et al., 2001; Okabe et al., 2002; Yamaguchi et al., 2002). So far, there are no reports about the involvement of mammalian Scar proteins in cell migration. We found that overexpression of Scar2-deltaA inhibits NIH 3T3 Golgi polarization but not cell migration (Figure 7). So, we next tested whether Scar2-deltaA–expressing wound edge cells can drive wound closure properly. We also tested Scar1-deltaA and Scar1-WCA to determine whether Arp2/3 binding is likely to be the only requirement for the observed effects of full-length Scar1 overexpression. With the deltaA constructs, microinjected cells moved from both sides of the wound meeting each other and forming bridges to close the wound. DeltaA constructs and dextran microinjected wound edge cells behaved similarly (Figure 8, A–C). Scar1-WCA expressing cells, in contrast, were no longer located at the wound margin and never formed part of bridges between the two opposite sides of the closing wound (Figure 8D).
Figure 8.
Wound closure can be performed by Scar1-deltaA as well as Scar2-deltaA but not Scar1-WCA expressing wound edge NIH 3T3 cells. Cells were fixed 10 h after microinjection to look at the final stages of wound closure. Wound edge cells were microinjected with (A) Alexa fluor 594–conjugated dextran (as positive control of wound closure); (B) the Scar1-deltaA expression vector; (C) the Scar2-deltaA expression vector; and (D) the Scar1-WCA expression vector. The top panels show the labeled microinjected cells, middle panels are the corresponding actin labeling panels, and third bottom panels corresponding to the merge of top and middle ones. For sections B and C, the fourth bottom panels are enlargements of the actin labeling region as indicated above them in the corresponding column.
Different Mechanisms in Response to Wounding by Astrocytes and NIH 3T3 Cells
Although astrocytes show Golgi polarization in response to wounding, unlike with NIH 3T3 cells, astrocyte wounding induces an actin polymerization–independent polarization of the leading wound edge cells (Etienne-Manneville and Hall, 2001). This was characterized by formation of protrusions and by the polarization of the microtubule organizing center (Etienne-Manneville and Hall, 2001). We compared the actin dependence of astrocytes and NIH 3T3 cells for Golgi polarity in the scratch wound assay.
Cytochalasin D–treated NIH 3T3 wounded monolayers did not show Golgi polarity at the wound margin. At a cytochalasin D final concentration of 2 μM, only ∼37 ± 4% of the Golgi of wound edge cells were found facing the wound (n = 382). At that drug concentration, the actin cytoskeleton was clearly affected as well as cell polarity (unpublished data). Cytochalasin D was also used at a final concentration of 0.5 μM to treat the cells, and the effect on actin was milder; nevertheless, the Golgi polarization was ∼44 ± 6%, which was still significantly lower than for nondrug-treated wound edge cells (n = 374).
On the other hand, cytochalasin D did not significantly prevent the polarization of the Golgi in astrocytes (p < 0.025). At final drug concentration of 2 μM, ∼51 ± 7% of Golgi in wound edge cells faced the wound (n = 300). At 0.5 μM, wound edge cells exhibited 66 ± 4% of Golgi polarization (n = 300).
In addition, wound edge astrocytes were microinjected with the five Scar deletion constructs described in Figure 4. There was no significant effect of any of these fusion proteins on the Golgi reorientation of the wound edge astrocytes (Figure 9). Therefore, Golgi polarization in astrocytes appears to be independent of actin assembly, Arp2/3 complex, and WASP-family proteins (Figure 9).
Figure 9.
Astrocytes do not depend on Arp2/3 and WASP-family proteins for Golgi polarization. Polarity of the wound edge cells expressing full-length Scar1, Scar1-WCA, and the deltaA constructs of the three Scar was examined. Golgi reorientation to face the wound was evaluated when astrocytes at the edge of the wound were microinjected with the corresponding pEGFP-Scar expression constructs described in Figure 2. (A) The microinjected cells expressing the GFP-fusion proteins are shown (top panels): full-length Scar1 (left) and Scar1-WCA (right). Cells were fixed and stained for the Golgi apparatus with anti–beta-COP and Hoechst was used to stain the nucleus (bottom panels). (B) Percentage of Golgi orientated toward the wound was determined at 8 h after wounding. Golgi polarization of microinjected wound edge cells expressing the GFP and the N17Cdc42 mutated protein (Etienne-Manneville and Hall, 2001) was included as positive and negative control, respectively (see legend on the right side of the graph; n is the number of Golgi observed; quantification was performed in three separate experiments per vector).
In conclusion, Arp2/3 delocalization and overexpression of Scar2-deltaA in wound edge cells interfere with Golgi polarization when using NIH 3T3 fibroblasts but not in astrocytes. These data indicate that different cell types respond to wounding using different mechanisms: Golgi polarization is actin dependent for NIH 3T3 but actin independent for astrocytes.
DISCUSSION
Arp2/3 Complex Is Important for NIH 3T3 Golgi Polarity
We investigated the effect of disruption of Arp2/3 complex–mediated actin cytoskeletal assembly on Golgi polarity establishment using a scratch wound assay. It was recently reported that in astrocytes, the formation of cell protrusions and polarization of the microtubule-organizing center during cell migration is independent of actin assembly (Etienne-Manneville and Hall, 2001). Thus, here, we focused on the actin involvement for Golgi polarity in response to wounding in both NIH 3T3 cells and astrocytes. Cytochalasin D affects cellular organelle positioning (Hilaire et al., 1995) and Golgi repositioning during morphological development (Zmuda and Rivas, 2000). In addition, we found that wound edge NIH 3T3 cells treated with cytochalasin D did not exhibit Golgi polarization but that wound edge astrocytes treated with cytochalasin D exhibited Golgi polarization. We can conclude that Golgi polarity in NIH 3T3 cells but not in astrocytes appears to be sensitive to disruption of the actin cytoskeleton.
Because actin assembly appeared to be required for polarity of NIH 3T3 cells, we investigated a role for the Arp2/3 complex. Actin nucleation and branch formation induced in vitro by the Arp2/3 complex requires activation, which is likely mediated by a WASP-family protein directly binding to and activating the Arp2/3 complex (Machesky et al., 1999; Rohatgi et al., 1999; Yarar et al., 1999). Expressing the WCA domain of WASP-family proteins in cells interferes with Arp2/3 localization and prevents lamellipodia formation (Machesky and Insall, 1998; Alrutz et al., 2001). We found that Golgi polarization of wound edge NIH 3T3 cells was inhibited when expressing the WCA domain both from N-WASP and Scar1. These results point to an importance of Arp2/3 in Golgi polarity, but we cannot rule out the possibility that the WCA constructs also act by affecting the steady state levels of F-actin in the cells (Machesky and Insall, 1998). On the other hand, wound edge NIH 3T3 cells expressing their corresponding deltaA constructs did not show inhibition of Golgi polarity. In astrocytes, however, Golgi polarity was unaffected by the WCA and deltaA constructs. This suggests that Arp2/3 and/or F-actin assembly are involved in Golgi polarization of NIH 3T3 cells but not in Golgi polarization of astrocytes.
Scar2 or One of Its Ligands Appears to be Involved in NIH 3T3 Golgi Polarization
Expression of the N-terminal sequences of WASP-family proteins, lacking only the acidic Arp2/3 complex binding sequence have yielded much information about specific involvement of WASP-family proteins in cellular processes and in mechanisms of pathogen invasion and infection. For example, Moreau et al. (2000) found that N-WASP was specifically involved in vaccinia virus motility and Gruenheid et al. (2001) found a similar involvement of N-WASP in pedestal formation by EPEC Escherichia coli. Likewise, N-WASP-deltaA expression inhibits PIP5-kinase–mediated actin comets (Rozelle et al., 2000) but not phagocytosis via the Fc- or CR- receptors (May et al., 2000 and R.C. May, unpublished observations). Scar1, in contrast, is involved in actin assembly by cryptosporidium (Elliott et al., 2001). We therefore expressed deltaA constructs of N-WASP and Scar1–3 in NIH 3T3 to determine whether we could identify involvement of a specific WASP-family protein in establishment of Golgi polarity or migration. The deltaA constructs of Scar1, Scar3, and N-WASP had no effect on Golgi polarization or migration. However, Scar2-deltaA disrupted Golgi polarization, without affecting migration. Thus, Scar2 is the most likely candidate WASP-family protein involved in Golgi polarity in NIH 3T3 cells and is the likely intermediate needed between the Rho family of GTPases and Arp2/3. Furthermore, we found that the N-terminal SHD region of Scar2 is sufficient to inhibit the Golgi polarization. It may seem curious that Golgi polarization requires Cdc42 but not Rac (Nobes and Hall, 1999), yet we find a potential role for Scar2, a putative Rac effector, in Golgi polarity. However, because the mechanism of Golgi polarity is not yet well understood and because the connection between Rac and Scar2 is also not well established, this may simply mean that the pathways connecting these proteins are not linear and that the system for regulating polarity and actin assembly is quite complex. We think it likely that proteins such as Scar and WASP may have more than one role in cells, because they have many potential binding partners and are likely to function as a part of large signaling complexes, which may vary depending on the context.
Thus far, specific ligands of the SHDs of Scar proteins have not been identified, so the reason for specificity is not yet clear. Northern blot expression profiles suggest that Scar2 expression has a very wide cell distribution compared with Scar1 and Scar3 (Suetsugu et al., 1999). Scar1 does appear to be expressed in NIH 3T3 cells (Figure 1). In addition, we showed by RT-PCR that Scar2 is also present in NIH 3T3 cells. The more homologous regions between the 3 Scars are the C-terminal WCA and the N-terminal SHD regions. The SHD region of Scar1 is 75 and 73% identical to Scar2 and Scar3, respectively, so it is hard to predict which regions might be important for specific ligand interactions. It will be interesting to identify which ligand(s) of Scar2 might be responsible for its involvement in Golgi polarity in NIH 3T3 cells.
NIH 3T3 Golgi Polarization Is Not Essential for Cell Migration
Cells overexpressing Scar2-deltaA were still able to drive wound closure even though the Golgi did not polarize. It was shown previously that inhibition of Cdc42 completely prevents Golgi apparatus realignment but did not inhibit cell movement in response to a scratch wound and that wound closure would occur (Nobes and Hall, 1999). These reported data are therefore in accordance with our data, which showed that in tissue-wound healing repair the polarization of the Golgi is not essential for wound closure. However we cannot exclude a requirement for Golgi polarity in a genuine in vivo situation, such as during embryo development, or a change in the efficiency or coordination of migration that was not detectable in our assays.
The lack of inhibition of migration by overexpression of any truncated WASP-family protein may indicate that migration uses multiple parallel pathways involving more than one WASP-family protein and/or a collection of other cytoskeletal mediators of actin assembly. To our knowledge, thus far, no knockout cell has been reported either that lacks migration, including WASP or N-WASP (Westerberg et al., 2001), but the data are not yet available for mammalian Scar proteins.
Different Cell Types Use Different Mechanisms for Golgi Polarity Establishment
We demonstrated the involvement of Arp2/3 and Scar2 as downstream components in Golgi polarization in wound healing of NIH 3T3 monolayers but not in astrocytes. We also showed that Golgi polarization is cytochalasin D sensitive in fibroblasts and cytochalasin D resistant in astrocytes. Taken together, our data indicate that Golgi polarization is actin dependent in the case of NIH 3T3 cells and that it is actin independent for astrocytes. Thus, two different cell types, which present very distinct morphologies, use different mechanisms for cell polarity establishment. It will be interesting to explore the relative contributions of the microtubule cytoskeleton to polarity and motility in these two different systems, given the reported importance of microtubules downstream of Cdc42 in astrocytes (Etienne-Manneville and Hall, 2001).
The Molecular Mechanism for Golgi Polarization Is Not Well Understood
Cells reposition the Golgi to face in the direction of subsequent migration. This rotation is thought to occur to produce a vectorial flow of Golgi-derived vesicles to the newly formed leading edge (Abercrombie et al., 1970; Izzard and Lochner, 1980; Hay, 1981; Kupfer et al., 1982; Singer and Kupfer, 1986). Of note, in the secretory pathway, actin filaments are required to maintain the organization of the Golgi complex (Valderrama et al., 1998) and for protein transport from the Golgi to the plasma membrane and the endoplasmic reticulum (Valderrama et al., 2001).
The molecular mechanism by which the Golgi moves to face the wound is not completely understood. Cdc42 is required for this (Nobes and Hall, 1999; Fenteany et al., 2000), and we now show that in NIH 3T3 cells Arp2/3 complex and Scar2 are important, but clearly this pathway is different in astrocytes. Interestingly, Cdc42 also controls Golgi-to-ER protein transport in an N-WASP–dependent manner in Hela cells (Luna et al., 2002). So, for at least some cell types, the connection with Arp2/3 complex and Golgi polarity may be through Cdc42, which is localized on the Golgi complex.
ACKNOWLEDGMENTS
We thank Robin C. May for preparation of some of the DNA constructs used in this study. This work was funded by European Community 5th framework (J.M. and L.M.M), Medical Research Council (T.H.M. and L.M.M), EMBO long-term fellowship (S. E.-M.), and CRC (S.L. and L.M.M).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–06–0345. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–06–0345.
REFERENCES
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. I. Movements of the leading edge. Exp Cell Res. 1970;59:393–398. doi: 10.1016/0014-4827(70)90646-4. [DOI] [PubMed] [Google Scholar]
- Alrutz MA, Srivastava A, Wong KW, D'Souza-Schorey C, Tang M, Ch'Ng LE, Snapper SB, Isberg RR. Efficient uptake of Yersinia pseudotuberculosis via integrin receptors involves a Rac1-Arp 2/3 pathway that bypasses N-WASP function. Mol Microbiol. 2001;42:689–703. doi: 10.1046/j.1365-2958.2001.02676.x. [DOI] [PubMed] [Google Scholar]
- Bailly M, Ichetovkin I, Grant W, Zebda N, Machesky LM, Segall JE, Condeelis J. The F-actin side binding activity of the Arp2/3 complex is essential for actin nucleation and lamellipod extension. Curr Biol. 2001;11:620–625. doi: 10.1016/s0960-9822(01)00152-x. [DOI] [PubMed] [Google Scholar]
- Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol. 1996;135:1097–1107. doi: 10.1083/jcb.135.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 2001;98:1142–1149. doi: 10.1182/blood.v98.4.1142. [DOI] [PubMed] [Google Scholar]
- Elliott DA, Coleman DJ, Lane MA, May RC, Machesky LM, Clark DP. Cryptosporidium parvum infection requires host cell actin polymerization. Infect Immun. 2001;69:5940–5942. doi: 10.1128/IAI.69.9.5940-5942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Fenteany G, Janmey PA, Stossel TP. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr Biol. 2000;10:831–838. doi: 10.1016/s0960-9822(00)00579-0. [DOI] [PubMed] [Google Scholar]
- Gruenheid S, DeVinney R, Bladt F, Goosney D, Gelkop S, Gish GD, Pawson T, Finlay BB. E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat Cell Biol. 2001;3:856–859. doi: 10.1038/ncb0901-856. [DOI] [PubMed] [Google Scholar]
- Haddad E, et al. The thrombocytopenia of Wiskott Aldrich syndrome is not related to a defect in proplatelet formation. Blood. 1999;94:509–518. [PubMed] [Google Scholar]
- Hahne P, Sechi A, Benesch S, Small JV. Scar/WAVE is localized at the tips of protruding lamellipodia in living cells. FEBS Lett. 2001;492:215–220. doi: 10.1016/s0014-5793(01)02239-6. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hay ED. Extracellular matrix. J Cell Biol. 1981;91:205s–223s. doi: 10.1083/jcb.91.3.205s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire E, Paulsen AQ, Brown CS, Guikema JA. Effects of clinorotation and microgravity on sweet clover columella cells treated with cytochalasin D. Physiol Plant. 1995;95:267–273. [PubMed] [Google Scholar]
- Hufner K, Schell B, Aepfelbacher M, Linder S. The acidic regions of WASp and N-WASP can synergize with CDC42Hs and Rac1 to induce filopodia and lamellipodia. FEBS Lett. 2002;514:168–174. doi: 10.1016/s0014-5793(02)02358-x. [DOI] [PubMed] [Google Scholar]
- Izzard CS, Lochner LR. Formation of cell-to-substrate contacts during fibroblast motility: an interference-reflexion study. J Cell Sci. 1980;42:81–116. doi: 10.1242/jcs.42.1.81. [DOI] [PubMed] [Google Scholar]
- Jacinto A, Martin P. Morphogenesis: unraveling the cell biology of hole closure. Curr Biol. 2001;11:R705–R707. doi: 10.1016/s0960-9822(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Jacinto A, Martinez-Arias A, Martin P. Mechanisms of epithelial fusion and repair. Nat Cell Biol. 2001;3:E117–E123. doi: 10.1038/35074643. [DOI] [PubMed] [Google Scholar]
- Jones GE. Cellular signaling in macrophage migration and chemotaxis. J Leukoc Biol. 2000;68:593–602. [PubMed] [Google Scholar]
- Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- Kupfer A, Louvard D, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc Natl Acad Sci USA. 1982;79:2603–2607. doi: 10.1073/pnas.79.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay S, et al. Lineage-specific modulation of calcium pump expression during myeloid differentiation. Blood. 1999;93:4395–4405. [PubMed] [Google Scholar]
- Launay, S., Brown, G., and Machesky, L.M. (2003). Expression of WASP and Scar1/WAVE actin-associated proteins is differently modulated during differentiation of HL-60 cells. Cell Motil. Cytoskel. in press. [DOI] [PubMed]
- Linder S, Higgs H, Hufner K, Schwarz K, Pannicke U, Aepfelbacher M. The polarization defect of Wiskott-Aldrich syndrome macrophages is linked to dislocalization of the Arp2/3 complex. J Immunol. 2000;165:221–225. doi: 10.4049/jimmunol.165.1.221. [DOI] [PubMed] [Google Scholar]
- Luna A, Matas OB, Martinez-Menarguez JA, Mato E, Duran JM, Ballesta J, Way M, Egea G. Regulation of protein transport from the Golgi complex to the endoplasmic reticulum by CDC42 and N-WASP. Mol Biol Cell. 2002;13:866–879. doi: 10.1091/mbc.01-12-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Gould KL. The Arp2/3 complex: a multifunctional actin organizer. Curr Opin Cell Biol. 1999;11:117–121. doi: 10.1016/s0955-0674(99)80014-3. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J Cell Biol. 1997;138:913–926. doi: 10.1083/jcb.138.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Insall RH. Signaling to actin dynamics. J Cell Biol. 1999;146:267–272. doi: 10.1083/jcb.146.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, Hall ME, Pollard TD. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci USA. 1999;96:3739–3744. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Reeves E, Wientjes F, Mattheyse FJ, Grogan A, Totty NF, Burlingame AL, Hsuan JJ, Segal AW. Mammalian actin-related protein 2/3 complex localizes to regions of lamellipodial protrusion and is composed of evolutionarily conserved proteins. Biochem J. 1997;328:105–112. doi: 10.1042/bj3280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- May RC, Caron E, Hall A, Machesky LM. Involvement of the Arp2/3 complex in phagocytosis mediated by FcgammaR or CR3. Nat Cell Biol. 2000;2:246–248. doi: 10.1038/35008673. [DOI] [PubMed] [Google Scholar]
- May RC, Hall ME, Higgs HN, Pollard TD, Chakraborty T, Wehland J, Machesky LM, Sechi AS. The Arp2/3 complex is essential for the actin-based motility of Listeria monocytogenes. Curr Biol. 1999;9:759–762. doi: 10.1016/s0960-9822(99)80337-6. [DOI] [PubMed] [Google Scholar]
- Millard TH, Machesky LM. The Wiskott-Aldrich syndrome protein (WASP) family. Trends Biochem Sci. 2001;26:198–199. doi: 10.1016/s0968-0004(01)01788-1. [DOI] [PubMed] [Google Scholar]
- Moreau V, Frischknecht F, Reckmann I, Vincentelli R, Rabut G, Stewart D, Way M. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat Cell Biol. 2000;2:441–448. doi: 10.1038/35017080. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Miki H, Ito M, Ohashi K, Takenawa T, Miyamoto S. N-WASP, WAVE, and Mena play different roles in the organization of actin cytoskeleton in lamellipodia. J Cell Sci. 2001;114:1555–1565. doi: 10.1242/jcs.114.8.1555. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Fukuda S, Broxmeyer HE. Activation of Wiskott-Aldrich syndrome protein and its association with other proteins by stromal cell-derived factor-1alpha is associated with cell migration in a T-lymphocyte line. Exp Hematol. 2002;30:761–766. doi: 10.1016/s0301-472x(02)00823-8. [DOI] [PubMed] [Google Scholar]
- Qualmann B, Kelly RB. Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J Cell Biol. 2000;148:1047–1062. doi: 10.1083/jcb.148.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Rozelle AL, et al. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol. 2000;10:311–320. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- Singer SJ, Kupfer A. The directed migration of eukaryotic cells. Annu Rev Cell Biol. 1986;2:337–365. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Miki H, Takenawa T. Identification of two human WAVE/SCAR homologues as general actin regulatory molecules which associate with the Arp2/3 complex. Biochem Biophys Res Commun. 1999;260:296–302. doi: 10.1006/bbrc.1999.0894. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci. 2001;114:1801–1809. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Valderrama F, Babia T, Ayala I, Kok JW, Renau-Piqueras J, Egea G. Actin microfilaments are essential for the cytological positioning and morphology of the Golgi complex. Eur J Cell Biol. 1998;76:9–17. doi: 10.1016/S0171-9335(98)80012-5. [DOI] [PubMed] [Google Scholar]
- Valderrama F, Duran JM, Babia T, Barth H, Renau-Piqueras J, Egea G. Actin microfilaments facilitate the retrograde transport from the Golgi complex to the endoplasmic reticulum in mammalian cells. Traffic. 2001;2:717–726. doi: 10.1034/j.1600-0854.2001.21006.x. [DOI] [PubMed] [Google Scholar]
- Weiner OD, Servant G, Welch MD, Mitchison TJ, Sedat JW, Bourne HR. Spatial control of actin polymerization during neutrophil chemotaxis. Nat Cell Biol. 1999;1:75–81. doi: 10.1038/10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg L, Greicius G, Snapper SB, Aspenstrom P, Severinson E. Cdc42, Rac1, and the Wiskott-Aldrich syndrome protein are involved in the cytoskeletal regulation of B lymphocytes. Blood. 2001;98:1086–1094. doi: 10.1182/blood.v98.4.1086. [DOI] [PubMed] [Google Scholar]
- Westphal RS, Soderling SH, Alto NM, Langeberg LK, Scott JD. Scar/WAVE-1, a Wiskott-Aldrich syndrome protein, assembles an actin-associated multi-kinase scaffold. EMBO J. 2000;19:4589–4600. doi: 10.1093/emboj/19.17.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Miki H, Takenawa T. Neural Wiskott-Aldrich syndrome protein is involved in hepatocyte growth factor-induced migration, invasion, and tubulogenesis of epithelial cells. Cancer Res. 2002;62:2503–2509. [PubMed] [Google Scholar]
- Yarar D, To W, Abo A, Welch MD. The Wiskott-Aldrich syndrome protein directs actin-based motility by stimulating actin nucleation with the Arp2/3 complex. Curr Biol. 1999;9:555–558. doi: 10.1016/s0960-9822(99)80243-7. [DOI] [PubMed] [Google Scholar]
- Zmuda JF, Rivas RJ. Actin filament disruption blocks cerebellar granule neurons at the unipolar stage of differentiation in vitro. J Neurobiol. 2000;43:313–328. doi: 10.1002/1097-4695(20000615)43:4<313::aid-neu1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]