Abstract
The melanoma inhibitory activity (MIA) protein is a clinically valuable marker in patients with malignant melanoma as enhanced values diagnose metastatic melanoma stages III and IV. Here, we report the backbone dynamics of human MIA studied by 15N NMR relaxation experiments. The folded core of human MIA is found to be rigid, but several loops connecting β-sheets, such as the RT-loop for example, display increased mobility on picosecond to nanosecond time scales. One of the most important dynamic features is the pronounced flexibility of the distal loop, comprising residues Asp 68 to Ala 75, where motions on time scales up to milliseconds occur. Further, significant exchange contributions are observed for residues of the canonical binding site of SH3 domains including the RT-loop, the n-Src loop, for the loop comprising residues 13 to 19, which we refer to as the“disulfide loop”, in part for the distal loop, and the carboxyl terminus of human MIA. The functional importance of this dynamic behavior is discussed with respect to the biological activity of several point mutations of human MIA. The results of this study suggest that the MIA protein and the recently identified highly homologous fibrocyte-derived protein (FDP)/MIA-like (MIAL) constitute a new family of secreted proteins that adopt an SH3 domain-like fold in solution with expanded ligand interactions.
Keywords: Backbone dynamics, binding to SH3 domains
Melanoma progression and tumor growth are regulated by a complex network of paracrine and autocrine positive and negative growth factors (Bogdahn et al. 1989). MIA provides a clinically useful parameter in patients with metastatic melanoma stages III and IV (Blesch et al. 1994;Bosserhoff et al. 1997,1998;Deichmann et al. 1999;Dréau et al. 1999). MIA mRNA was independently identified by differential display approaches comparing melanoma cell lines and cartilage cells in vitro. Therefore, MIA has also been referred to as the cartilage-derived retinoic acid-sensitive protein (CD-RAP) (Dietz and Sandell 1996). Subsequent studies of murine embryos and murine adult tissues have demonstrated specific mRNA expression patterns in cartilage, but not in any other non-neoplastic tissue. MIA thus might also be a potential serum marker for rheumatoid arthritis and cartilage damage (Müller-Ladner et al. 1999). MIA was described to possess antitumor activity by inhibiting proliferation of melanoma cell lines in vitro (Bogdahn et al. 1989;Blesch et al. 1994). However, further studies have revealed expression patterns inconsistent with a tumor suppressor. Expression of the wild-type MIA protein gene was not detected in normal skin and melanocytes but was associated with progression of melanocytic tumors (Van Groningen et al. 1995;Bosserhoff et al. 1997;Bosserhoff and Buettner 2002).
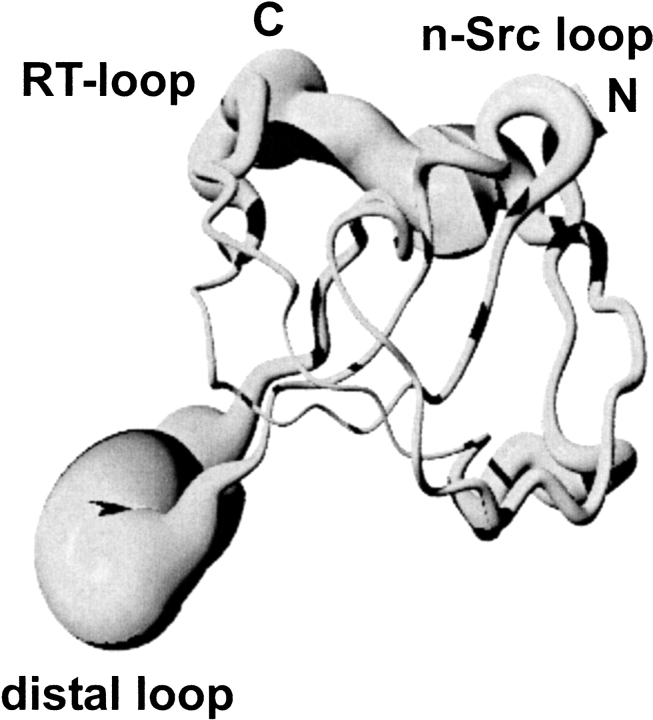
The core structure of human MIA resembles an Src homology 3 (SH3) domain (Stoll et al. 2000,2001;Lougheed et al. 2001,2002) (Fig. 1 ▶). The relative mean square deviation (rmsd) of the core of the NMR structure of human MIA and the SH3 domain of the ABL tyrosine kinase is 1.4 Å (Stoll et al. 2001). SH3 domains are small (55–70 amino acids), noncatalytic protein modules that are found in many intracellular signaling proteins (Koch et al. 1991;Yu et al. 1992,1994). SH3 domains mediate protein-protein interactions by binding to Pro-rich peptide sequences (Dalgarno et al. 1997). More than 50 SH3 domains are known, and these SH3 domains are widely distributed, having been identified in kinases, lipases, GTPases, adapter proteins, structural proteins, and viral regulatory proteins, for example (Musacchio et al. 1994;Dalgarno et al. 1997). Human MIA is, however, the first extracellular protein adopting an SH3 domain-like fold. Human MIA shares the common fold of SH3 domains consisting of two perpendicular, antiparallel, three-stranded β-sheets. Based on the first description of the SH3 structure, the strands of the β-sandwich in the core of human MIA are termed βa, βb, βb′, βc, βd, βe, and βirr (Yu et al. 1992;Dalgarno et al. 1997). These β-strands form two β-sheets, βI and βII. The smaller βI is formed by a part from βb, βa, and βe. The strand βII contains the remainder of βb′, βc, and βd, as well as βnt/βct. The portions of the βb/b′-strand participating in both βI and βII are delineated by a kink that changes the direction of the polypeptide. The β-strands pack against each other at approximately right angles to form a β-sandwich (Fig. 1 ▶). Two characteristic features of SH3 domains, the RT-loop and the n-Src loop, can also be identified in human MIA (Fig.1 ▶) (Yu et al. 1992;Dalgarno et al. 1997). The RT-loop contains an irregular antiparallel structure. In human MIA, a short antiparallel β-sheet, βirr, could be detected for Asp 30–Met 32 and Thr 40–His 42, found in only a few SH3 domains (Martínez and Serrano 1999;Riddle et al. 1999). βc and βd form the distal hairpin, the most regular element of secondary structure in the SH3 domain (Riddle et al. 1999). βc and βd are connected by a tight type-I β-turn in most SH3 domains (Musacchio and Serrano 1994;Martínez and Serrano 1999;Riddle et al. 1999). In human MIA, this distal loop is enlarged and comprises the residues Tyr 69 to Ala 75.
Figure 1.
NMR ensemble of human MIA shown as a spline with variable radius, representing the RMS of the deviation from the mean structure (Stoll et al. 2001). The N and C termini are indicated by N and by C, respectively. This figure was generated with MOLMOL and POV-Ray (http://www.povray.org) (Koradi et al. 1996).
More recently, it was shown that the MIA protein specifically inhibits attachment of melanoma cells to, at least, fibronectin (FN) type-III repeats 6, 10, and 14, thereby masking the binding site of integrins to these extracellular matrix (ECM) components and promoting invasion and metastasis in vivo (Bosserhoff et al. 1998,1999;Stoll et al. 2001;Bosserhoff and Buettner 2002). These proteins had not been described as SH3 ligands before. In this paper, we report the backbone dynamics of MIA in solution based on 15N-R1, 15N-R2, 15N{1H}-NOE, and 15N(dipole-CSA) cross-correlation rate experiments. Further, we discuss their implications for interaction between SH3 domains and their ligands with respect to the biological activity of several point mutations of human MIA.
Results
Experimental Relaxation Rates
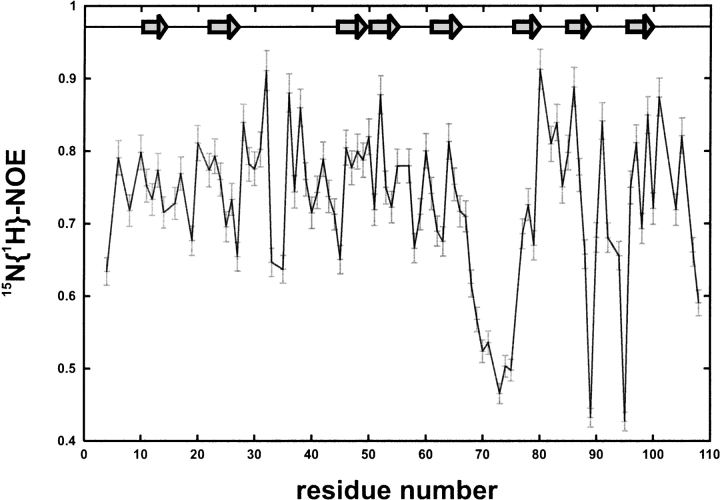
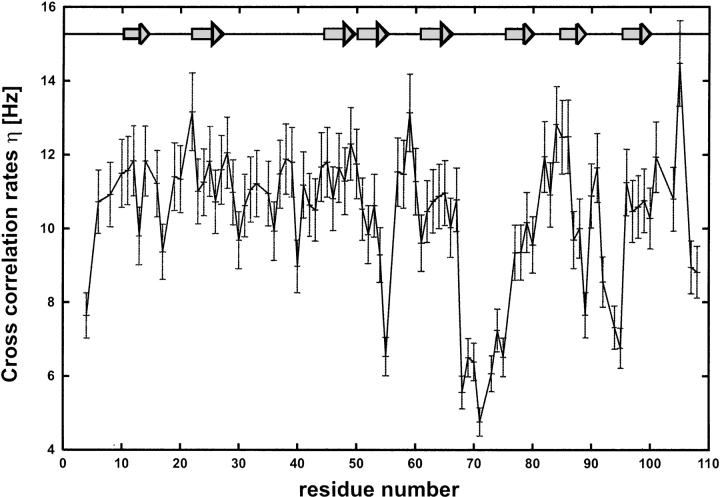
15N-R1, 15N-R2, 15N{1H}-NOE, and 15N(dipole-CSA) crosscorrelation rates at 300 K were determined at 600 MHz proton frequency. The steady-state heteronuclear 15N{1H}-NOE for the backbone amides of human MIA indicates that most of the 108 residues of human MIA are part of a compact fold. Resonances for residues Gly 2, Leu 7, and Asp 9 were not observed in the NMR spectra, therefore relaxation data could not be extracted. Apart from Gln 108, the carboxyl terminus does not exhibit any pronounced flexibility, though it is poorly defined in the NMR ensemble because of a limited number of NOEs found for these residues (Figs. 1, 2 ▶ ▶). This is in contrast to residues Tyr 69–Ala 75, which have 15N{1H} Nuclear Overhauser Effect (NOE) values <0.6, clearly suggesting an increased flexibility for this region of human MIA in agreement with decreased 15N-R2 rates for residues Asp 68 to Ala 75 (Fig. 3 ▶). Residues Ala 25, Gly 62, Leu 77, Thr 90, and Val 98 display slightly, but distinctly decreased values for the 15N{1H}-NOE also indicating a higher degree of flexibility (Fig. 2 ▶). Residues Met 4, Ser 19, Ala 27, Ala 33, and Asp 35 of the RT-loop; Gln 45 and Arg 58 of the n-Src loop; Gly 63, Asp 68, Tyr 79, Asp 88, Gln 89, Thr 90, Lys 92, Gly 94, Lys 95, Cys 107; and Gln 108 with values for the 15N{1H}-NOE <0.68 are clearly less rigid than the residues of the core SH3 structure. Significantly increased R1 rates were observed for residues Gly 55 and Gly 71–Leu 73 indicating flexibility (Fig. 3A ▶). Decreased R2 values could be extracted for residues Met4, Glu 17, Asp 68–Ala 75, Gln 89, Lys 92, Gly 94, Lys 95, and Gln 108, also pointing to a region of lower rigidity in human MIA (Fig. 3B ▶). The rest of the molecule shows R1 and R2 values typical for a rigid molecule (Fig. 3 ▶). Apart from the termini of human MIA (Met 4, Cys 107, and Gln 108), the 15N(dipole-CSA) cross-correlation rates η for residues Cys 13, Glu 17, Asp 30, Cys 36, Thr 40, Lys 54, Gly 55, Trp 61, Asp 68–Phe 80, Glu 87–Gln 89, Lys 92, Gly 94, and Lys 95 are significantly decreased (Fig. 4 ▶). The 15N(dipole-CSA) cross-correlation rates η do not only confirm the existence of fast motions on the picosecond (ps) to nanosecond (ns) time scales for the distal loop of human MIA but also indicate that small amounts of exchange broadening are present for these residues (Figs. 4, 5B ▶ ▶). The contribution of chemical exchange and the intrinsic transverse relaxation rate for the distal loop can be assessed by comparing R2 values with the cross correlation rate for human MIA (Figs. 3B, 4, 5B ▶ ▶ ▶). Apart from variations in the 15N chemical shift anisotropy tensor, which are usually small in proteins, η × T2 values are influenced by slow chemical exchange (Renner and Holak 2000). In our case, markedly decreased η × T2 values are observed for residues Asp 68–Ala 75 of the distal loop consistent with slow motions in this region. These data suggest a slow change of conformation, implying that slow conformational rearrangements occur for the distal loop in human MIA.
Figure 2.
Steady-state heteronuclear 15N{1H}-NOE for the backbone amides of human MIA. Residues for which no results are shown correspond either to prolines or to residues where relaxation data could not be extracted. For calculation of experimental errors, refer to materials and methods. The arrows indicate β-strands as described previously (Stoll et al. 2001).
Figure 3.

Plots of 15N longitudinal relaxation rates R1 (A) and transverse relaxation rates R2 (B) of MIA as a function of the residue number at 300 K. For details, refer to text.
Figure 4.
Cross correlation rates η for human MIA. For calculation of experimental errors, refer to Materials and Methods.
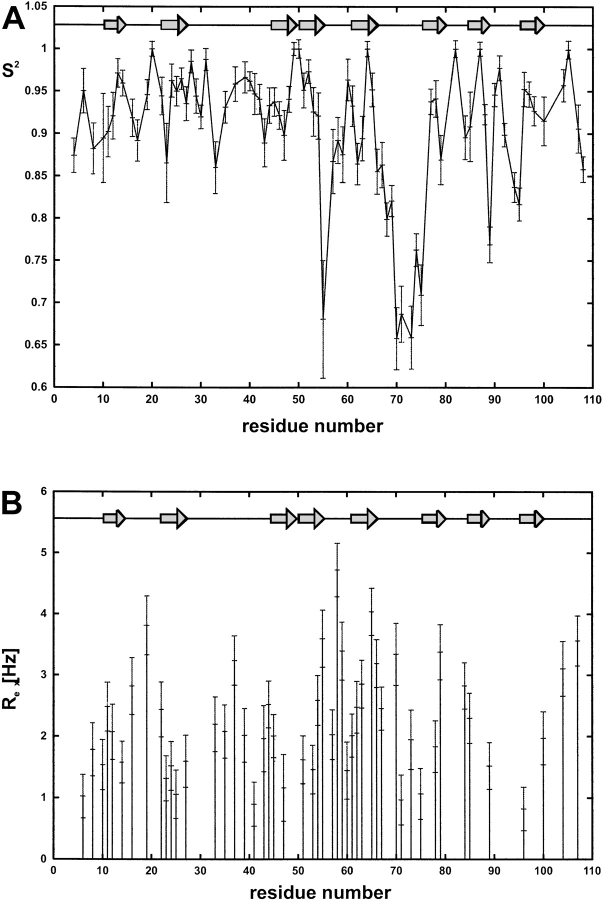
Figure 5.
Microscopic parameters of motion of S2 (A) and Rex (B) for MIA. For details, refer to text.
Lipari-Szabo analyses
To extract microscopic parameters of motion from the relaxation rates 15N-R1, 15N-R2, and 15N{1H}-NOE, a Lipari-Szabo analysis was performed with the software TENSOR2 (Dosset et al 2000). First, the overall rotation was tested for anisotropy. The disc-like shape of the MIA molecule suggests a similarity to an oblate symmetrical ellipsoid. Indeed, the fitting procedure implemented in TENSOR2 found the best agreement of experimental data and rotational model for a fully asymmetrical diffusion tensor. The statistical F-test indicates an improvement in the fitting using this most complex model (data not shown). However, the determined anisotropy is very small (Dz / Dx < 1.1) and the principal axes of the diffusion tensor are ill-defined using the Monte-Carlo–type error assessment of TENSOR2 (Dosset et al 2000). To decide whether or not to include anisotropy in the analysis, we analyzed five structures from the lowest-energy structure family of MIA trying to select structures with as different conformations as possible for less defined regions such as the distal loop, for example. For each structure, the fully anisotropic diffusion model was used and internal parameters of motion S2 and, when necessary, ti, kex, and S2 were determined. The comparison of the five data sets with one another and with a data set obtained with an isotropic diffusion tensor revealed very similar results for all cases. This led us to the conclusion that for the Lipari-Szabo analysis, the small amount of anisotropy present in the experimental data is insignificant and can be ignored. An explanation for the smaller anisotropy obtained from the experimental R1 and R2 compared to the anisotropy expected from a single structure could be that more flexible parts exhibit different conformations in different structures of the calculated structure family. Such differences have been observed before (Tjandra et al. 1995;Renner and Holak 2000).
For the final fitting, an overall isotropic tumbling (total correlation) time tc = 9.9 ns (±0.1 ns) was used. The order parameter S2, which represents the local rigidity of the structure, is depicted in Figure 6A ▶. The order parameter S2 is close to the maximum of 1.0 for most of the core residues of human MIA, suggesting a rigid structure and a well-folded protein (Figs. 5A, 6A ▶ ▶). However, S2 values for residues Gly 55, Tyr 70–Ala 75, Gln 89, and, to a lesser extent, Asp 68, Tyr 69, Gly 94, and Lys 95 are significantly <1.0 (Fig. 5A ▶). For the distal loop, Gly 55 of the n-Src loop, and other solvent-exposed residues, such as Gln 89 and Lys 95 for example, reduced S2 values show flexibility on fast time scales (ps–ns). This mobility is most prominent for the distal loop with S2 almost as low as 0.65. Only for all of the residues of the distal loop significant internal correlation times ti around 1.3 ns were obtained, whereas for the other residues large errors affect the ti as a result of the small amplitudes for internal motions. The large internal correlation time observed for the distal loop probably results from a concerted motion of the whole loop, as independent thermal fluctuations usually have correlation times <100 ps (Farrow et al 1994;Tjandra et al 1995). The carboxyl terminus is attached to the main body of the protein by a disulfide bridge, thus preventing pronounced flexibility. Finally, a significant chemical exchange contribution >2.8 Hz was observed for residues Gln 16, Ser 19, Arg 37, Gly 55, Arg 58, Leu 59, Gly 63, Val 65, Gln 66, Tyr 70, Tyr 79, Ile 84, Asp 104, and Cys 107 (Figs. 5B, 6B ▶ ▶).
Figure 6.
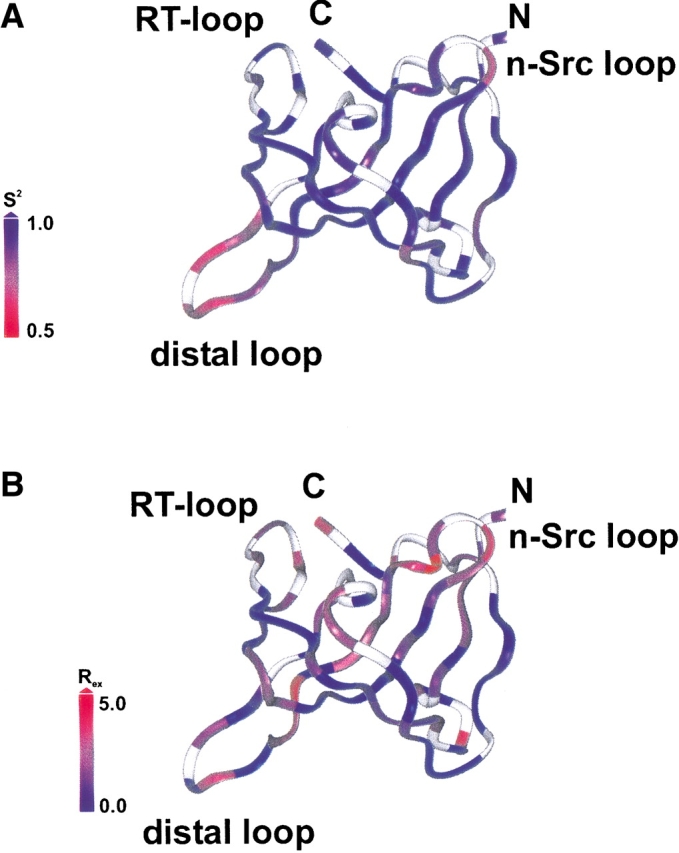
Ribbon of a NMR structure of human MIA representative for the ensemble. The N and C termini are indicated by N and by C, respectively. The color shading is based on the values of the order parameter S2 (A) and for the exchange rates Rex (B). This figure was generated with Insight II (www.accelrys.com).
Point mutations of human MIA
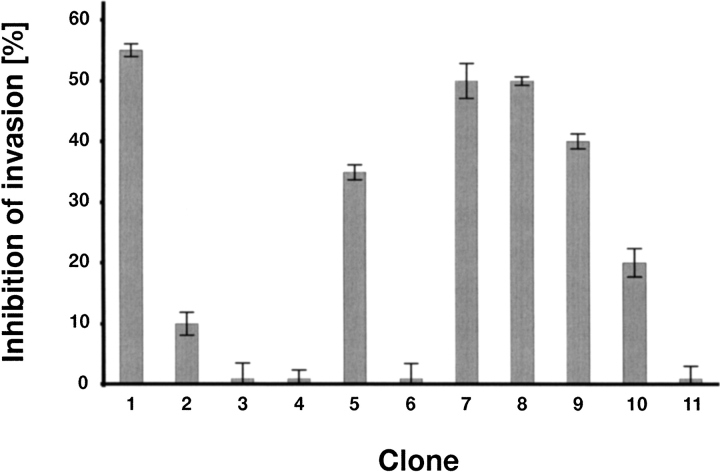
The effect of the point mutations has been evaluated by the effect on the inhibition of melanoma cell attachment in Boyden Chamber assays as described previously (Bosserhoff et al. 1998;Stoll et al. 2001). The potential of the mutation T90P in human MIA to inhibit attachment is 50% and almost as high as found for the wild type (Fig. 7 ▶). However, the V49I/R56S, the L53Q, and the G62R mutations show a dramatically decreased inhibition of attachment when compared to the wild type of human MIA (Fig. 7 ▶). Most of the residues are either part of or next to the canonical binding site of SH3 domains. The two double-point mutations E17V/D72A and E17G/A74P affecting the "disulfide loop" and the distal loop of human MIA either inhibit attachment by only 35% or do not inhibit attachment almost (Fig. 7 ▶). Finally, a series of carboxy-terminal deletion mutants show that deleting the carboxy-terminal residues starting from Ser 83 does almost not impair the function to inhibit melanoma cell attachment (Fig. 7 ▶). However, mutants of human MIA only extending either to residue Phe 80 or Ala 74 only inhibit attachment by 40% and 20%, respectively (Fig. 7 ▶). A truncated human MIA protein extending only to residue Gly 67 finally does not significantly inhibit attachment at all (Fig. 7 ▶).
Figure 7.
Inhibition of attachment of melanoma cells by different mutants of human MIA. The following point mutations have been used in the inhibition bioassay: (1) Wild-type MIA, (2) V49I/R56S, (3) L53Q, (4) G62R, (5) E17V/D72A, (6) E17G/A74P, (7) T90P, (8) dC-term 82, (9) dC-term 80, (10) dC-term 74, (11) dC-term 67. For details, refer to Materials and Methods.
Discussion
Human MIA adopts an SH3 domain-like fold in solution and binds to fibronectin type-III modules (FNIII) (Stoll et al. 2001) (Fig. 1 ▶). Human MIA is the first extracellular protein found to have an SH3 domain-like fold. The present study describes the backbone dynamics of the refolded extracellular SH3 domain-like human MIA core and its amino- and carboxy-terminal extensions. A signal peptide of human MIA is not part of the construct used in this study. Information cannot be provided regarding the first 24 residues. Nonetheless, it is generally assumed that signal sequences do not adopt any ordered conformations in solution. In the NMR data set, residues Met 1 to Leu 7 of mature MIA do not adopt any regular structure either because of missing assignments or absent NOEs in the NOESY spectra and were therefore omitted for clarity in Figures 1 and 6 ▶ ▶. Relaxation data on the amino terminus were only extracted for residues Met 4, Lys 6, and Ala 8. Met 4 shows a slightly increased flexibility according to the 15N{1H}-NOE experiment, whereas Lys 6 and Ala 8 are more rigid (Fig. 2 ▶).
Apart from connecting loops, such as the RT-loop, the n-Src loop, and the distal loop, most of the MIA structure is rigid (Figs. 2, 3, 5 ▶ ▶ ▶). Because of the structural similarities among all known SH3 domains, it can be assumed that generally the core of SH3 domains will not exhibit any increased flexibility, in agreement with previous studies (Farrow et al. 1997;Hansson et al. 1998). The conserved residues Ala 27 at the end of the βa-strand and Gln 45 at the beginning of the βb-strand also display a high degree of flexibility according to the 15N{1H}-NOE (Fig. 2 ▶). These residues are located at the beginning and at the end of the RT-loop, respectively. This fact might account for slight variations in the orientation of the RT-loop with respect to the core of human MIA in the NMR ensemble.
Residues Ala 27, Ala 33, and Asp 35 of the RT-loop; Arg 58 of the n-Src loop; Gly 62; the conserved Gly 63; and Leu 77 and Tyr 79 of human MIA show a decreased value for the 15N{1H}-NOE. Most of these residues are in proximity to or correspond to the canonical binding site of SH3 domains, which includes the conserved residues Phe 60 and Tyr 79, for example (Figs. 2, 8 ▶ ▶). Obviously, some residues of human MIA that correspond to the canonical binding site of SH3 domains display a higher degree of flexibility than the core of the molecule (Figs. 2, 5A ▶ ▶). The carboxy-terminal residues Cys 107 and Gln 108 of human MIA are also more flexible (Fig. 2 ▶). Cys 107 is covalently attached to Cys 37 by a disulfide bond. This links the carboxyl terminus of human MIA to the RT-loop that contains the flexible residues Ala 33 and Asp 35 to form a cluster of flexible residues (Fig. 6A ▶).
Figure 8.
Sequence alignment of human MIA (1HJD), bovine MIA, mouse MIA, rat MIA, human MIA-LIKE (MIAL), mouse MIAL, human fibrocyte-derived protein (FDP), and mouse FDP including their signal peptides. The numbering of amino acids follows the sequence of human MIA as previously published (Stoll et al. 2001). Conserved residues are boxed. The color code reflects the physical and chemical properties of the amino acids and was taken from the Jalview program (http://www.ebi.ac.uk). Aromatic and hydrophilic residues are colored in light blue and blue, positively charged residues are red, negatively charged residues are magenta, hydrophilic residues are green, glycines are orange, prolines are colored in yellow, and cysteines are colored in light red. For details, refer to the discussion. This figure was generated with the Swissprot software package (http://www.expasy.ch) and Jalview (http://www.ebi.ac.uk) (Appel et al. 1994).
The analysis of the chemical exchange contribution of human MIA highlights residues corresponding to the canonical binding site of SH3 domains (Fig. 5B ▶). For the n-Src loop, an elevated contribution of chemical exchange is observed (Figs. 3B, 5B, 6B ▶ ▶ ▶). In fact, the chemical exchange contribution for Gly 55, Arg 58, and Leu 59 of the n-Src loop of human MIA is 3.6 Hz, 4.7 Hz, and 3.4 Hz. This prominent feature correlates with the fact that in canonical SH3-domains, the n-Src loop plays an important role in ligand recognition (Dalgarno et al. 1997). In addition, the conserved residue Arg 37 of the RT-loop, Gly 63, Tyr 79, Ile 84, and Cys 107 display a significant contribution of chemical exchange (Figs. 5B, 6B ▶ ▶). Most of these residues are homologous or close to residues that belong to the canonical binding site on other SH3 domains.
Residues Lys 92, Gly 94, Lys 95, and to a lesser extent Val 98, which are in proximity to the “disulfide loop”, also display a higher degree of flexibility than the core of human MIA (Fig. 2 ▶). Further, Gln 16 and Ser 19 of the solvent-exposed “disulfide loop” also display a high contribution of chemical exchange, with Ser 19 also showing a significantly decreased value for the 15N{1H}-NOE (Figs. 2, 5B ▶ ▶). Interestingly, the highly conserved Glu 17 shows a significantly decreased R2 value (Fig. 3B ▶). The functional importance of this residue and the rest of the “disulfide loop” is suggested by the high degree of sequence conservation within MIA orthologs. This sequence conservation might also indicate that similar dynamical properties of the "disulfide loop" and the neighboring residues exist in the bovine, mouse, and rat MIA protein.
Another prominent dynamical feature of human MIA, however, is the increased flexibility of the distal loop that comprises residues Tyr 69–Ala 75 (Figs. 2, 5 ▶ ▶). Interestingly, Asp 68 and Asp 72 of the distal loop are highly conserved in MIA and MIA-like (MIAL)/fibrocyte-derived protein (FDP) (Fig. 8 ▶). Also, the length of the distal loop in these proteins is conserved, except for Gly 71, which is deleted in MIAL/FDP. R2 and CSA suggest slow conformational changes of the distal loop on microsecond (μsec) to millisecond (msec) time scales (Figs. 3B, 4, 5B ▶ ▶ ▶). In addition, the exchange contribution for Tyr 70 of the distal loop is >3 Hz and is further corroborating the findings for the R2 and the CSA data (Figs. 5B, 6B ▶ ▶).
Studies of the SH3 domain bound to proline-rich peptide ligands revealed a hydrophobic binding site on the surface of the protein that is lined with the side chains of conserved aromatic amino acids (Yu et al. 1992). The ligand binding site is a relatively flat surface and one end is flanked by the RT loop and the n-Src insertion, which are both of variable lengths (Mussachio et al. 1994;Dalgarno et al. 1997). The RT loop and the n-Src loop of human MIA show a higher degree of flexibility and a considerable amount of chemical exchange contributions. Moreover, neighboring residues also show a chemical exchange contribution, as they are either linked by hydrogen bonds (in cases of β-strands) or by a disulfide bridge (in the case of Cys 107) to the canonical binding site (Fig. 6B ▶). Interestingly, many residues showing chemical exchange contributions are conserved in the MIA and MIAL/FDP sequences and cluster to form a putative binding site on human MIA that resembles the canonical binding site of SH3 domains (Figs. 5B, 6B ▶ ▶). In addition, it has been shown before that flexibility in binding domains, as observed for the distal loop of human MIA, can be reflective of regions that are involved in target protein interactions and that will become more ordered upon binding (see, for example, Kay 1998;Renner and Holak 2001; and references therein). However, flexibility of certain protein segments might be necessary for the formation of biologically relevant complexes, but in itself it is probably not an indication of the presence of a binding site. In general, loops in proteins tend to be more flexible than the tightly packed hydrophobic core.
To correlate the results of the backbone dynamics of the human MIA protein, we have embarked upon performing mutagenesis studies. For this purpose, we have generated a number of point mutations affecting residues that are not only conserved in sequence but also might shed light on the functional importance of the dynamical features of the human MIA protein (Fig. 7 ▶). The point mutation T90P suggests that the slightly increased flexibility found for this region is not associated with function (Fig. 7 ▶). On the other hand, several point mutations affecting residues of or near the canonical binding site of SH3 domains, such as V49I/R56S, the L53Q, and the G62R, dramatically decrease the potential of human MIA to inhibit attachment (Fig. 7 ▶). This suggests that the residues of human MIA that correspond to the canonical binding site of SH3 domains are functionally important. Therefore, the higher degree of flexibility and a considerable amount of chemical exchange contributions of the RT loop and the n-Src loop of human MIA might be correlated with its function. Together, these data suggest a crucial role for residues of human MIA corresponding to the canonical binding site of SH3 domains including the RT loop and the n-Src loop in binding of human MIA to its target ligand(s). The two double-point mutations, E17V/072A and E17G/A74P, show that either the "disulfide loop" or the distal loop or maybe even both are important for human MIA to inhibit the attachment of melanoma cells as judged by Boyden Chamber assays (Fig. 7 ▶). Comparing the mutant E17V/D72A directly with E17G/A74P suggests a critical role for Ala 74 of the distal loop, as the latter mutant almost does not inhibit attachment (Fig. 7 ▶). However, the functional importance of the “disulphide loop” cannot be directly assessed by the results of the mutagenesis study. Nonetheless, the functional importance of residues corresponding to the canonical binding site of SH3 domains and the distal loop can be derived from a set of carboxy-terminal deletion mutants of human MIA (Fig. 7 ▶). Even more interestingly, the mutant dC-term 82 of human MIA including all residues from the amino terminus to Ser 82 is almost as capable of inhibiting attachment to the same extent as the wild type (Fig. 7 ▶). This mutant consists of the SH3 core domain (including both the “disulphide loop” and the distal loop) and the amino-terminal extension, suggesting that mainly this subdomain confers the biological activity of human MIA and that the carboxy-terminal extension to the core SH3 domain is of lesser functional importance. In addition, a mutant of human MIA that only extends from the amino terminus until Phe 80, dC-term 80, shows a slightly reduced inhibition of attachment (Fig. 7 ▶). This mutant does not contain part of the canonical binding site of SH3 domains, which comprises residues Tyr 79–Ile 84, suggesting that the highly conserved Pro 81 and/or Ser 82 are functionally important. The mutant dC-term 74, which only extents to residue Ala 74, inhibits attachment drastically less (Fig. 7 ▶). This supports the idea that not only the canonical binding-site residues of human MIA but also the highly flexible loop is important for the function of this extracellular SH3-like protein. This is not only because this mutant is missing Ala75, the last residue of this loop, but also because this loop is no longer connected to the core of the SH3 domain. In fact, this mutant is missing the β-strand βd (Leu 77–Tyr 79), that might stabilize the loop conformation. Finally, the mutant dC-term 67 does not show any significant activity (Fig. 7 ▶). However, the lack of biological activity of this mutant might also arise from improper folding as it is missing major secondary structural elements of the SH3 core domain.
These mutagenesis data strongly suggest that some residues corresponding to binding-site residues in homologous SH3 domains are functionally important in human MIA. This binding site, however, might be extended by at least the highly flexible loop of human MIA. On the other hand, this loop might be part of an additional binding site on human MIA. By scanning a wider conformational space, the flexibility of the distal loop might significantly contribute to the specificity of the human MIA in recognizing its ligand(s) and thereby enhancing the affinity for ligand(s) as recently described for IGFBP-5 (Renner and Holak 2001).
Recent studies pointed out that the canonical binding site on SH3 domains might indeed be extended. The distal loop of the stromal subunit PsaE, for instance, does interact with other components of the photo system I complex (Klukas et al. 1999;Mayer et al. 1999). Similarly, Tyr 45 and Phe 48, located in the core of the CD-loop of PsaE facing the photo system I, are involved in binding PsaE to core subunits (Klukas et al. 1999). Most interestingly, the aromatic side chains are conserved in Synechococcus sp. PCC7002 and S. elongatus (Falzone et al. 1994). Intriguingly, the distal loop of human MIA also contains two conserved aromatic residues, Tyr 69 and Tyr 70, matching the aromatic residues in the sequence of PsaE. Another example for an extended binding site on an SH3 domain is the peroxisomal membrane protein Pex13p that exhibits a novel mode of ligand interaction between the RT loop and the distal loop (Falzone et al. 1994;Barnett et al. 2000). Two suppressor mutations for Pex13p occurred on the distal-loop side of the domain (R353G and K355R) and three other in the RT loop (Barnett et al. 2000). This interaction site is distinct from the classical PXXP binding pocket of SH3 domains (Barnett et al. 2000). This finding expands the model of SH3-ligand interaction from the classical case of proline-rich ligands and non-PXXP–type SH3 ligands to ligands that interact with additional and/or other binding-site residues of SH3 domains (Dalgarno et al. 1997;Mongiovi et al. 1999;Barnett et al. 2000;Kang et al. 2000).
Very recently, an extracellular protein with 44% sequence identity and ∼80% similarity with human MIA protein has been described (Cohen-Salmon et al. 2000;Rendtdorff et al. 2001;Robertson et al. 2000) (Fig. 8 ▶). This protein, called FDP or MIAL, is expressed in the inner ear and was shown to have an in vitro effect on the early differentiation of the inner ear (Cohen-Salmon et al. 2000;Rendtdorff et al. 2001). Because of the high levels of sequence identity and similarity between MIA and FDP/MIAL, we predict that FDP/MIAL also adopts an SH3 domain-like structure. This is corroborated by the conserved, four-cysteine residues (Cys 13, Cys 18, Cys 36, and Cys 107) in all proteins, where Cys 13 and Cys 18 are flanking the highly conserved “disulphide loop” (Fig. 8 ▶). As judged from the sequence alignment, the conserved distal loop of FDP/MIAL is also enlarged when compared to other SH3 domains and might be as flexible as in human MIA (Fig. 8 ▶). FDP or MIAL and MIA thus define a new family of extracellular proteins. MIA/CD-RAP decreases the adhesive capacity of cells, thereby promoting tumoral invasion (Bosserhoff et al. 1997,1999). Similarly, extracellular matrix and cell-adhesion molecules are believed to play a prominent role in the development of complex structures of the mammalian inner ear (Bosserhoff et al. 1997;Rendtdorff et al. 2001). FPD/MIAL could, as observed for MIA/CD-RAP, regulate cell density and shape, which control for fibrocyte differentiation, thereby playing an important role in the initiation of periotic mesenchyme chondrogenesis (Bosserhoff et al. 1997,1999;Cohen-Salmon et al. 2000).
In conclusion, we have shown by 15N NMR relaxation experiments that the core of the SH3 domain-like MIA is rigid, whereas the distal loop shows a pronounced flexibility. The canonical SH3 binding site of human MIA shows a significant contribution of chemical exchange. Particularly, a chemical exchange contribution of up to 4.7 Hz for Arg 58 of the n-Src loop of human MIA could be identified. To a lower extent, chemical exchange contribution was also observed for the “disulphide loop”, the distal loop, and the carboxyl terminus. The relaxation data of human MIA, together with the results of mutagenesis studies, suggest that at least the conserved highly flexible distal loop is also involved in ligand recognition in addition to some residues of human MIA corresponding to canonical binding-site residues of SH3 domains, for which a considerable amount of exchange contribution could be identified. It appears therefore, that the observed flexibility of the distal loop is of functional importance of the human MIA protein. Due to a sequence identity of 44% and a sequence similarity of ∼80%, the human MIA protein and the recently identified highly homologous FDP/MIAL protein might constitute a new family of secreted proteins adopting an SH3 domain-like fold. Our findings thus provide support for extended interactions of these lately discovered extracellular SH3 domains.
Materials and methods
Molecular biology and protein chemistry
Expression, refolding, and purification of human MIA was performed as previously published (Stoll et al. 2000,2001). The NMR sample used for relaxation measurements contained 1 mM of protein in 100 mM potassium phosphate and 150 mM NaCl at pH 7.0, including 0.02% NaN3, protease inhibitors (Roche Molecular Biochemicals), and 10% D2O.
Generation of point mutations of human MIA
MIA cDNA optimized for expression in Escherichia coli-based systems was generated by Geneart (Regensburg, Germany). All cDNAs generated were sequenced and the mutations occurring during the chemical procedure were also characterized. cDNAs encoding mutations that are interesting for functional analysis were cloned into the pIVEx 2.3 MCS vector system as well as the wild-type MIA cDNA.
In vitro translation
In vitro translation was performed using the Rapid Translation System 500 E. coli HY Kit system (Roche Molecular Biochemicals, Roche Diagnostics) according to the manufacturer‘s instructions.
Bioassay
Boyden Chamber assays were performed to test the recombinant human MIA and mutated MIA for its biological activities (Bosserhoff et al. 1998;Stoll et al. 2001). Briefly, invasion assays were performed in Boyden Chambers containing polycarbonate filters with a pore size of 8 μm (Costar) essentially as described previously (Albini et al. 1987;Jacob et al. 1995). Filters were coated with a commercially available reconstituted basement membrane (Matrigel, diluted 1:3 in H2O; Becton Dickinson). The lower compartment was filled with fibroblast-conditioned medium as a chemoattractant. Melanoma cells were harvested by trypsinization for 2 min, resuspended in Dulbecco‘s modified Eagle‘s medium (DMEM) without fetal calf serum (FCS) at a density of 2 × 105 cells/mL, with or without recombinant MIA (50 ng/mL) or mutated MIA (50 ng/mL), respectively, and placed in the upper compartment of the chamber. After incubation at 37°C for 4 h, the filters were removed. Cells adhering to the lower surface were fixed, stained, and counted.
NMR spectroscopy
NMR experiments were carried out at 300 K on a Bruker DRX600 spectrometer. Modified versions of the experiments proposed previously were used to determine 15N R1, 15N R2, 15N{1H} NOE, and 15N(dipole-CSA) cross-correlation rates at 600 MHz proton frequency (Edison et al. 1994;Farrow et al. 1994;Tessari et al. 1997;Renner and Holak 2000). Optimal sampling strategies according to Jones et al. (1996) were used for T1 and T2. For T2, five points with relaxation periods of 16 ms + x × 32 ms with x = 0, 1, 2, 3, 4, were appropriate because of the larger spread-out of T2 values. For T1, where the expected range of values is small, only two relaxation periods differing by 880 ms were used. The experiment with longer relaxation delay was performed with four times as many scans as the experiment with shorter (essentially zero) relaxation time (Jones et al. 1996). The relaxation delays for the cross-correlation experiments were 40 ms and 80 ms. Most relaxation experiments were recorded in an interleaved manner to reduce influences from possible instabilities in experimental conditions.
Data analysis
NOE values were calculated from the ratio of the peak heights in the experiment with and without proton saturation. To obtain T2 values, the experimental data points (peak heights) were fitted to a curve A exp(−t/T2) with a simple grid search. T1 values can be calculated analytically from the two data points. Uncertainties for T2 and NOE values were determined from double recording of the whole relaxation experiment. For T1 Gaussian error propagation based on the noise level in the spectra was performed. For the cross-correlation rates η, the ratios of signal intensities (peak heights) from the cross-correlation experiment and the corresponding reference experiment follow a simple linear relation T × η (Tessari et al. 1997). The difference between the rates η obtained from the experiments with different relaxation delays served as an error estimate.
Lipari-Szabo fitting was performed with the TENSOR2 software for 90 residues (Dosset et al. 2000). Only residues in secondary structure elements were used for determination of the overall tumbling. Anisotropic and isotropic diffusion tensors resulted in very similar Lipari-Szabo parameters. Therefore, the final fitting was performed with an isotropic tumbling time tc of 9.9 ns (± 0.1 ns). Errors in tc and Lipari-Szabo parameters were determined by 100 rounds of Monte-Carlo sampling of the (assumed) distributions of the experimental values based on the experimental error. Model selection (S2; S2 and ti; S2 and Rex; S2, ti, and Rex; SS2, SF2, ti) was based on F-tests (Dosset et al. 2000). For eight residues none of the models could reproduce the experimental data satisfactorily.
Sequence comparison and analysis
Amino-acid sequences were obtained and analyzed using Swissprot (http://www.expasy.ch) and Jalview, respectively ( http://www.ebi.ac.uk) (Appel et al. 1994).
Data deposition
The coordinates of human MIA have been deposited in the PDB Data Bank under accession number 1HJD.
Acknowledgments
We are indebted to Claudia Abschlag for technical assistance. R.S. gratefully recognizes support from the Deutscher Akademischer Austauschdienst (DAAD) and is an Emmy Noether Fellow of the DFG. This work was supported by Deutsche Forschungsgemeinschaft (DFG) grants (SFB 469 and BO 1573/1–1) and the Deutsche Krebshilfe (10–1532-Bo).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
MIA, melanoma inhibitory activity
NMR, nuclear magnetic resonance
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0222603.
References
- Albini, A., Iwamoto, Y., Kleinmann, H.K., Martin, G.R., Aaronson, S.A., Kozlowski, J.M., and McEwan, R.N. 1987. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 47 3239–3245. [PubMed] [Google Scholar]
- Appel, R.D., Bairoch, A., and Hochstrasser, D.F. 1994. A new generation of information retrieval tools for biologists: The example of the ExPASy WWW server. Trends Biochem. Sci. 19 258–260. [DOI] [PubMed] [Google Scholar]
- Barnett, P., Bottger, G., Klein, A.T.J., Tabak, H.F., and Distel, B. 2000. The peroxisomal membrane protein Pex13p show a novel mode of SH3 interaction. EMBO J. 19 6382–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesch, A., Bosserhoff, A.K., Apfel, R., Behl, C., Hessdoerfer, B., Schmitt, A., Jachimczak, P., Lottspeich, F., Buettner, R., and Bogdahn, U. 1994. Cloning of a novel malignant melanoma-derived growth regulatory protein, MIA. Cancer Res 54 5695–5701. [PubMed] [Google Scholar]
- Bogdahn, U., Apfel, R., Hahn, M., Gerlach, M., Behl, C., Hoppe, J., and Martin, R. 1989. Autocrine tumor cell growth inhibiting activities from human malignant melanoma. Cancer Res. 49 5358–5363. [PubMed] [Google Scholar]
- Bosserhoff, A.K. and Buettner, R. 2002. Expression, function and clinical relevance of MIA (melanoma inhibitory activity). Histol. Histopathol. 17 289–300. [DOI] [PubMed] [Google Scholar]
- Bosserhoff, A.K., Kaufmann, M., Kaluza, B., Bartke, I., Zirngibl, H., Hein, R., Stolz, W., and Buettner, R. 1997. MIA, a novel serum marker for progression of malignant melanoma. Cancer Res. 57 3149–3153. [PubMed] [Google Scholar]
- Bosserhoff, A.K., Golob, M., Buettner, R., Landthaler, M., and Hein, R. 1998. MIA (melanoma inhibitory activity) biological functions and clinical relevance. Hautarzt 49 762–769. [DOI] [PubMed] [Google Scholar]
- Bosserhoff, A.K., Golob., M., Hein, R., and Buettner, R. 1999. Analysis of cell signalling by MIA in active detachment of melanoma cells. J. Invest. Dermatol 113 74. [Google Scholar]
- Cohen-Salmon, M., Frenz, D., Liu, W., Verpy, E., Voegeling, S., and Petit, C. 2000. Fdp, a new fibrocyte-derived protein related to MIA/CD-RAP, has an in vitro effect on the early differentiation of the inner ear mesenchyme. J. Biol. Chem 275 40036–40041. [DOI] [PubMed] [Google Scholar]
- Dalgarno, D.C., Botfield, M.C., and Rickles, R.J. 1997. SH3 domains and drug design: Ligands, structure, and biological function. Biopolymers 43 383–400. [DOI] [PubMed] [Google Scholar]
- Deichmann, M., Benner, A., Bock, M., Jackel, A., Uhl, K., Waldmann, V., and Naher, H. 1999. S100-β, melanoma inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American joint committee on cancer stage IV melanoma. J. Clin. Onc. Res 17 1891–1896. [DOI] [PubMed] [Google Scholar]
- Dietz, U. and Sandell, L.J. 1996. Cloning of a retinoic acid-sensitive mRNA expressed in cartilage and during chondrogenesis. J. Biol. Chem.271 3311–3316. [DOI] [PubMed] [Google Scholar]
- Dosset, P., Hus, J.C., Blackledge, M., and Marion, D. 2000. Efficient analysis of macromolecular rotational diffusion from heteronuclear relaxation data. J. Biomol. NMR. 16 23–28. [DOI] [PubMed] [Google Scholar]
- Dréau, D., Bosserhoff, A.K., White, R.L., Buettner, R., and Holder, W.D. 1999. Melanoma-inhibitory activity protein, a useful marker to monitor patients treated with immunotherapy. Oncol. Res 11 55–61. [PubMed] [Google Scholar]
- Edison, A.S., Abildgaard, F., Westler, W.M., Mooberry, E.S., and Markley, J.L. 1994. Practical introduction to theory and implementation of multinuclear, multidimensional nuclear magnetic resonance experiments. Methods Enzymol 239 3–79. [DOI] [PubMed] [Google Scholar]
- Falzone, C.J., Kao, Y.H., Zhao, J., Bryant, D.A., and Lecomte, J.T.J. 1994. Three-dimensional solution structure of PsaE from the cyanobacterium Synechococcus sp. Strain PCC 7002, a photosystem I protein that shows structural homology with SH3 domains. Biochemistry 33 6052–6062. [DOI] [PubMed] [Google Scholar]
- Farrow, N.A., Muhandiram, R., Singer, A.U., Pascal, S.M., Kay, C.M., Gish, G., Shoelson, S.E., Pawson, T., Forman-Kay, J.D., and Kay, L.E. 1994. Backbone dynamics of a free and a phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry 33 5984–6003. [DOI] [PubMed] [Google Scholar]
- Farrow, N.A., Zhang, O., Forman-Kay, J.D., and Kay, L.E. 1997. Characterization of the backbone dynamics of folded and denatured states of an SH3 domain. Biochemistry 36 2390–2402. [DOI] [PubMed] [Google Scholar]
- Hansson, H., Mattsson, P.T., Allard, P., Haapaniemi, P., Vihinen, M., Smith, C.I.E., and Härd, T. 1998. Solution structure of the SH3 domain from Bruton‘s tyrosine kinase. Biochemistry 37 2912–2924. [DOI] [PubMed] [Google Scholar]
- Jacob, K., Bosserhoff, A.K., Wach, F., Knuchel, R., Klein, E.C., Hein, R., and Buettner, R. 1995. Characterization of selected strongly and weakly invasive sublines of a primary human melanoma cell line and isolation of subtractive cDNA clones. Int. J. Cancer 60 668–675. [DOI] [PubMed] [Google Scholar]
- Jones, J.A., Hodgkinson, P., Barker, A.L., and Hore, P.J.1996. Optimal sampling strategies for the measurement of spin-spin relaxation times. J. Magn. Reson. B 113 25–34. [Google Scholar]
- Kang, H., Freund., C., Duke-Cohen, J.S., Musacchio, A., Wagner, G., and Rudd, C.E. 2000. SH3 domain recognition of a proline-independent tyrosine-based RKxxYxxY motif in immune cell adaptor SKAP55. EMBO J. 19 2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, L.E. 1998. Protein dynamics from NMR. Bioch. Cell. Biol. 76 145–152. [DOI] [PubMed] [Google Scholar]
- Klukas, O., Schubert, W.D., Jordan, P., Krauss, N., Fromme, P., Witt, H.T., and Saenger, W. 1999. Photosystem I, an improved model of the stromal subunits PsaC, PsaD, and PsaE. J. Biol. Chem. 274 7351–7360. [DOI] [PubMed] [Google Scholar]
- Koch, C.A., Anderson, D., Moran, M.F., Ellis, C., and Pawson, T. 1991. SH2 and SH3 domains: Elements that control interactions of cytoplasmic signaling proteins. Science 252 668–674. [DOI] [PubMed] [Google Scholar]
- Koradi, R., Billeter, M., and Wüthrich, K. 1996. MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graph.14 51–55. [DOI] [PubMed] [Google Scholar]
- Lougheed, J.C., Holton, J.M., Alber, T., Bazan, J.F., and Handel, T.M. 2001. Structure of melanoma inhibitory activity protein, a member of a recently identified family of secreted proteins. Proc. Natl. Acad. Sci. 98 5515–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lougheed, J.C., Domaille, P.J., and Handel, T.M. 2002. Solution structure and dynamics of melanoma inhibitory activity protein. J. Biomol. NMR 22 211–223. [DOI] [PubMed] [Google Scholar]
- Martínez, J.C., and Serrano, L. 1999. The folding transition state between SH3 domains is conformationally restricted and evolutionarily conserved. Nat. Struc. Biol.6 1010–1016. [DOI] [PubMed] [Google Scholar]
- Mayer, K.L., Shen, G., Bryant, D.A., Lecomte, J.T.J., and Falzone, C.J. 1999. The solution structure of photosystem I accessory protein E from the cyanobacterium Nostoc sp. Strain PCC 8009. Biochemistry 38 13736–13746. [DOI] [PubMed] [Google Scholar]
- Mongiovi, A.M., Romano, P.R., Panni, S., Mendoza, M., Wong, W.T., Musacchio, A., Cesareni, G., and Di Fiore, P.P. 1999. A novel peptide-SH3 interaction. EMBO J. 18 5300–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Ladner, U., Bosserhoff, A.K., Dreher, K., Hein, R., Neidhart, M., Gay, S., Scholmerich, J., Buettner, R., and Lang, B. 1999. MIA (melanoma inhibitory activity): A potential serum marker for rheumatoid arthritis. Rheumatology 38 148–154. [DOI] [PubMed] [Google Scholar]
- Musacchio, A., Saraste, M., and Wilmanns M. 1994. High-resolution crystal structures of tyrosine kinase SH3 domains complexed with proline-rich peptides. Nat. Struc. Biol. 1 546–551. [DOI] [PubMed] [Google Scholar]
- Rendtdorff, N.D., Frödin, M., Attié-Bitach, T., Vekemans, M., and Tommerup, N. 2001. Identification and characterization of an inner ear-expressed human melanoma inhibitory activity (MIA)-like gene (MIAL) with a frequent polymorphism that abolishes translation. Genomics 71 40–52. [DOI] [PubMed] [Google Scholar]
- Renner, C., and Holak, T.A. 2000. Separation of anisotropy and exchange broadening using 15N CSA-15N-1H dipole-dipole relaxation cross-correlation experiments. J. Magn. Reson. 145 192–200. [DOI] [PubMed] [Google Scholar]
- ———. 2001. NMR 15N relaxation of the insulin-like growth factor (IGF)-binding domain if IGF binding protein-5 (IGFBP-5) determined free in solution and in complex with IGF-II. Eur. J. Biochem. 268 1058–1065. [DOI] [PubMed] [Google Scholar]
- Riddle, D.S., Grantcharova, V.P., Santiago, J.V., Alm, E., Ruczinski, I., and Baker, D. 1999. Experiment and theory highlight role of native state topology in SH3 folding. Nat. Struc. Biol. 6 1016–1024. [DOI] [PubMed] [Google Scholar]
- Robertson, N.G., Heller, S., Lin, J.S., Resendes, B.L., Weremowicz, S., Denis, C.S., Bell, A.M., Hudspeth, A.J., and Morton, C.C. 2000. A novel conserved cochlear gene, OTOR: Identification, expression analysis, and chromosomal mapping. Genomics 66 242–248. [DOI] [PubMed] [Google Scholar]
- Stoll, R., Renner, C., Ambrosius, D., Golob, M., Voelter, W., Buettner, R., Bosserhoff, A.K., and Holak, T.A. 2000. Letter to the editor: Sequence-specific 1H, 13C, and 15N assignment of the human melanoma inhibitory activity (MIA) protein. J. Biomol. NMR 17 87–88. [DOI] [PubMed] [Google Scholar]
- Stoll, R., Renner, C., Zweckstetter, M., Bruggert, M., Ambrosius, D., Palme, S., Engh, R.A., Golob, M., Breibach, I., Buettner, R., et al. 2001. The extracellular human melanoma inhibitory activity (MIA) protein adopts an SH3 domain-like fold. EMBO J 20 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari, M.F., Mulder, A.A., Boelens, R., and Vuister, G.W. 1997. Determination of amide proton CSA in 15N-labeled proteins using 1H CSA/15N-1H dipolar and 15N CSA/15N-1H dipolar cross-correlation rates. J. Magn. Reson. 127 128–133. [Google Scholar]
- Tjandra, N., Feller, S. E., Pastor, R.W., and Bax, A. 1995. Rotational diffusion anisotropy of human ubiquitin from 15N NMR relaxation. J. Am. Chem. Soc 117 12562–12566. [Google Scholar]
- Van Groningen, J.J.M., Bloemers, H.P., and Swart, G.W. 1995. Identification of melanoma inhibitory activity and other differentially expressed messenger RNAs in human melanoma cell lines with different metastatic capacity by messenger RNA differential display. Cancer Res. 55 6237–6243. [PubMed] [Google Scholar]
- Yu, H., Rosen, M.K., Shin, T.B., Seidel-Dugan, C., Brugge, J.S., and Schreiber, S.L. 1992. Solution structure of the SH3 domain of Src and identification of its ligand binding site. Science 258 1665–1668. [DOI] [PubMed] [Google Scholar]
- Yu, H., Chen, J.K., Feng, S., Dalgarno, D.C., Brauer, A.W., and Schreiber, S.L. 1994. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell 76 933–945. [DOI] [PubMed] [Google Scholar]