Abstract
Background: Infants requiring extracorporeal membrane oxygenation (ECMO) support represent a high risk group in terms of cerebral injury. Mild hypothermia both during and after cerebral hypoxic ischaemia appears to be a promising strategy for offering neuroprotection.
Objective: To investigate whether mild hypothermia was both feasible and safe in infants receiving ECMO as a prelude to any formal assessment of this approach in a randomised trial.
Methods: Twenty infants (body weight less than 5 kg) with severe cardiopulmonary insufficiency, referred for ECMO support at Glenfield Hospital, Leicester, were enrolled in this study. Twenty consecutive infants (compromising four groups of five) were studied. Baseline data were obtained from a control group who were run throughout their course at 37°C. The patients in the next group were managed with a core temperature of 36°C for the first 12 hours of their ECMO run, before being warmed up to 37°C. After successful completion, the next group of five were cared for at 35°C for the first 12 hours, and, there having been no previous complications, the final group were cared for at 34°C for the first 12 hours. Patients were assessed clinically and biologically. In addition to routine laboratory tests, cytokines (interleukin 6, interleukin 8, tumour necrosis factor α, and C reactive protein) were measured and coagulation tests (D-dimer, thrombin-antithrombin III complex, plasmin-α2-antiplasmin complex) were performed serially for five days.
Results: There were no significant differences among the four groups in gestational age, birth weight, age at the time of ECMO, Apgar scores at one and five minutes, pH before cannulation, oxygenation index, duration of ECMO, and survival rate to discharge from hospital. No adverse effects of mild hypothermia were found on patient management during ECMO. Laboratory data for up to five days of ECMO also showed no difference among the four groups.
Conclusion: Mild hypothermia (34°C) for the initial 12 hours of an ECMO run is feasible.
Full Text
The Full Text of this article is available as a PDF (217.3 KB).
Figure 1 .
Blood sampling schedule adopted for each patient receiving extracorporeal membrane oxygenation (ECMO).
Figure 2 .
Temperature of each group studied. Values are presented as mean (SD).
Figure 3 .

Changes in plasma concentrations of (A) thrombin-antithrombin III complex and (B) plasmin-α2-antiplasmin complex are shown for each of the groups. Values are presented as median (SD).
Figure 4 .
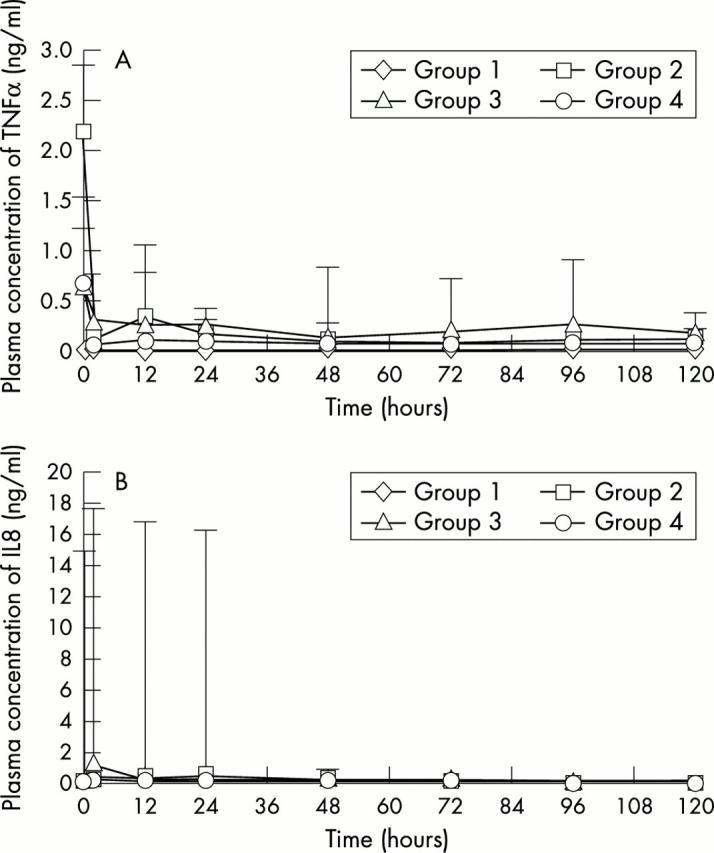
Changes in plasma concentrations of (A) tumour necrosis factor α (TNFα) and (B) interleukin 8 (IL8) for each of the groups as examples of the observed changes in cytokines. Values are presented as median (SD).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzopardi D., Robertson N. J., Cowan F. M., Rutherford M. A., Rampling M., Edwards A. D. Pilot study of treatment with whole body hypothermia for neonatal encephalopathy. Pediatrics. 2000 Oct;106(4):684–694. doi: 10.1542/peds.106.4.684. [DOI] [PubMed] [Google Scholar]
- Boldt J., Knothe C., Welters I., Dapper F. L., Hempelmann G. Normothermic versus hypothermic cardiopulmonary bypass: do changes in coagulation differ? Ann Thorac Surg. 1996 Jul;62(1):130–135. doi: 10.1016/0003-4975(96)00239-1. [DOI] [PubMed] [Google Scholar]
- Clifton G. L., Miller E. R., Choi S. C., Levin H. S., McCauley S., Smith K. R., Jr, Muizelaar J. P., Wagner F. C., Jr, Marion D. W., Luerssen T. G. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001 Feb 22;344(8):556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- Edwards A. D., Yue X., Squier M. V., Thoresen M., Cady E. B., Penrice J., Cooper C. E., Wyatt J. S., Reynolds E. O., Mehmet H. Specific inhibition of apoptosis after cerebral hypoxia-ischaemia by moderate post-insult hypothermia. Biochem Biophys Res Commun. 1995 Dec 26;217(3):1193–1199. doi: 10.1006/bbrc.1995.2895. [DOI] [PubMed] [Google Scholar]
- Fortenberry J. D., Bhardwaj V., Niemer P., Cornish J. D., Wright J. A., Bland L. Neutrophil and cytokine activation with neonatal extracorporeal membrane oxygenation. J Pediatr. 1996 May;128(5 Pt 1):670–678. doi: 10.1016/s0022-3476(96)80133-8. [DOI] [PubMed] [Google Scholar]
- Graziani L. J., Baumgart S., Desai S., Stanley C., Gringlas M., Spitzer A. R. Clinical antecedents of neurologic and audiologic abnormalities in survivors of neonatal extracorporeal membrane oxygenation. J Child Neurol. 1997 Oct;12(7):415–422. doi: 10.1177/088307389701200702. [DOI] [PubMed] [Google Scholar]
- Gunn A. J., Gluckman P. D., Gunn T. R. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics. 1998 Oct;102(4 Pt 1):885–892. doi: 10.1542/peds.102.4.885. [DOI] [PubMed] [Google Scholar]
- Hofkosh D., Thompson A. E., Nozza R. J., Kemp S. S., Bowen A., Feldman H. M. Ten years of extracorporeal membrane oxygenation: neurodevelopmental outcome. Pediatrics. 1991 Apr;87(4):549–555. [PubMed] [Google Scholar]
- Johnston T. D., Chen Y., Reed R. L., 2nd Functional equivalence of hypothermia to specific clotting factor deficiencies. J Trauma. 1994 Sep;37(3):413–417. doi: 10.1097/00005373-199409000-00014. [DOI] [PubMed] [Google Scholar]
- Lazar H. L. The treatment of hypothermia. N Engl J Med. 1997 Nov 20;337(21):1545–1547. doi: 10.1056/NEJM199711203372111. [DOI] [PubMed] [Google Scholar]
- Lehot J. J., Villard J., Piriz H., Philbin D. M., Carry P. Y., Gauquelin G., Claustrat B., Sassolas G., Galliot J., Estanove S. Hemodynamic and hormonal responses to hypothermic and normothermic cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1992 Apr;6(2):132–139. doi: 10.1016/1053-0770(92)90186-b. [DOI] [PubMed] [Google Scholar]
- Lorek A., Takei Y., Cady E. B., Wyatt J. S., Penrice J., Edwards A. D., Peebles D., Wylezinska M., Owen-Reece H., Kirkbride V. Delayed ("secondary") cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1994 Dec;36(6):699–706. doi: 10.1203/00006450-199412000-00003. [DOI] [PubMed] [Google Scholar]
- Mehmet H., Edwards A. D. Hypoxia, ischaemia, and apoptosis. Arch Dis Child Fetal Neonatal Ed. 1996 Sep;75(2):F73–F75. doi: 10.1136/fn.75.2.f73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. D., Christian T. F., Hopfenspirger M. R., Hodge D. O., Gersh B. J., Gibbons R. J. Infarct size after acute myocardial infarction measured by quantitative tomographic 99mTc sestamibi imaging predicts subsequent mortality. Circulation. 1995 Aug 1;92(3):334–341. doi: 10.1161/01.cir.92.3.334. [DOI] [PubMed] [Google Scholar]
- Plötz F. B., van Oeveren W., Bartlett R. H., Wildevuur C. R. Blood activation during neonatal extracorporeal life support. J Thorac Cardiovasc Surg. 1993 May;105(5):823–832. [PubMed] [Google Scholar]
- Sirimanne E. S., Blumberg R. M., Bossano D., Gunning M., Edwards A. D., Gluckman P. D., Williams C. E. The effect of prolonged modification of cerebral temperature on outcome after hypoxic-ischemic brain injury in the infant rat. Pediatr Res. 1996 Apr;39(4 Pt 1):591–597. doi: 10.1203/00006450-199604000-00005. [DOI] [PubMed] [Google Scholar]
- Stolar C. J., Snedecor S. M., Bartlett R. H. Extracorporeal membrane oxygenation and neonatal respiratory failure: experience from the extracorporeal life support organization. J Pediatr Surg. 1991 May;26(5):563–571. doi: 10.1016/0022-3468(91)90708-2. [DOI] [PubMed] [Google Scholar]
- Thoresen M., Bågenholm R., Løberg E. M., Apricena F., Kjellmer I. Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child Fetal Neonatal Ed. 1996 Jan;74(1):F3–F9. doi: 10.1136/fn.74.1.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoresen M., Penrice J., Lorek A., Cady E. B., Wylezinska M., Kirkbride V., Cooper C. E., Brown G. C., Edwards A. D., Wyatt J. S. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995 May;37(5):667–670. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]




