Abstract
In this study we compared the protein kinase dependent regulation of gastric H,K-ATPase and Na,K-ATPase. The protein kinase A/protein kinase C (PKA/PKC) phosphorylation profile of H,K-ATPase was very similar to the one found in the Na,K-ATPase. PKC phosphorylation was taking place in the N-terminal part of the α-subunit with a stoichiometry of ∼0.6 mol Pi/mole α-subunit. PKA phosphorylation was in the C-terminal part and required detergent, as is also found for the Na,K-ATPase. The stoichiometry of PKA-induced phosphorylation was ∼0.7 mol Pi/mole α-subunit. Controlled proteolysis of the N-terminus abolished PKC phosphorylation of native H,K-ATPase. However, after detergent treatment additional C-terminal PKC sites became exposed located at the beginning of the M5M6 hairpin and at the cytoplasmic L89 loop close to the inner face of the plasma membrane. N-terminal PKC phosphorylation of native H,K-ATPase α-subunit was found to stimulate the maximal enzyme activity by 40–80% at saturating ATP, depending on pH. Thus, a direct modulation of enzyme activity by PKC phosphorylation could be demonstrated that may be additional to the well-known regulation of acid secretion by recruitment of H,K-ATPase to the apical membranes of the parietal cells. Moreover, a distinct difference in the regulation of H,K-ATPase and Na,K-ATPase is the apparent absence of any small regulatory proteins associated with the H,K-ATPase.
INTRODUCTION
The gastric H,K-ATPase is, like the Na,K-ATPase, a member of the class 2C P-type ion-transport ATPases that is present in the apical membranes of the parietal cells and required for acid secretion. The H,K-ATPase couples the free energy of ATP hydrolysis to the establishment of the electrochemical gradients for H+ across the plasma membrane. The catalytic coupling of ATP hydrolysis to ion transport includes the following steps:
E2(K2) + ATP + 2 Hcyt+ ↔ E1ATP·H2 + 2 Kcyt+
E1ATP·H2 ↔ E1∼P(H2) + ADP
E1∼P(H2) ↔ E2−P + 2 Hext+
E2−P + 2 Kext+ ↔ E2(K2) + Pi
(1), The first step is the ATP accelerated release of two K+ at the cytoplasmic face of the enzyme and binding of two cytoplasmic H+. This step is associated with a conformational change from an E2-form with cation sites exposed to the cytoplasm to the E1-form with cation sites exposed to the luminar side. (2), Then follows phosphorylation of the enzyme and the occlusion of H+. (3), Via a conformational change from E1 to E2, the two H+ are released to the extracellular side. (4), Finally, two K+ are bound, and dephosphorylation leads to the occlusion of the bound K+. Reaction steps 1 and 3 are associated with thermally driven transitions, i.e., conformational changes, and are considered rate-limiting steps of the reaction cycle.
Histamine stimulation of acid secretion is mediated by elevated cAMP in the parietal cells (Chew et al., 1980) and cholinergic stimulation is mediated via an increase in the intracellular Ca2+ (Chew and Brown, 1986), suggesting that the acute regulation of H,K-ATPase in the cell takes place by signal transduction mechanisms via protein kinase phosphorylation, as is the case for the closely related Na,K-ATPase (for review, see Therien and Blostein, 2000). Several studies have demonstrated that the gastric H,K-ATPase α-subunit is phosphorylated in vitro by intrinsic protein kinases at the N-terminal segment (Togawa et al., 1995; Togawa et al., 1996; Kanagawa et al., 2000). Phosphoprotein mapping of the N-terminus suggested that two tyrosine residues (Tyr-7 and Tyr-10) and a serine residue (Ser-27) are involved (Togawa et al., 1996). Apparently, both the cAMP-activated protein kinase A (PKA) and the Ca2+/phospholipids-activated protein kinase C (PKC) were able to phosphorylate the conserved Ser-27 in this preparation (Togawa et al., 1996). Furthermore, in the presence of detergent PKA phosphorylation of microsomes from rabbit gastric mucosa has been demonstrated in vitro (Murtazina et al., 1999) and is suggested to represent phosphorylation of H,K-ATPase at a C-terminal site, possibly Ser-952 located within the small cytoplasmic L89 loop between the M8 and M9 transmembrane segments, which is conserved in all known H,K-ATPase and Na,K-ATPase isoforms. Therefore, the protein kinase phosphorylation pattern of H,K-ATPase may resemble qualitatively that of the Na,K-ATPase with N-terminal PKC sites and C-terminal PKA sites. However, the phosphorylation stoichiometry was determined neither for PKC nor for PKA phosphorylation.
The main regulatory process associated with stimulation of acid secretion involves recruitment to the apical membranes of the parietal cells of H,K-ATPase sequestered in turbulovesicles (for review, see Dunbar and Caplan, 2001). The signal for initiation of this membrane trafficking is not known in any detail but is probably mediated by a combination of second messengers released upon stimulation of acid secretion, e.g., cAMP, Ca2+, and diacylglycerol. After cessation of acid secretion, the H,K-ATPase is reinternalized by endocytosis, which depends on a tyrosine-based motif contained in the β-subunit (Courtois-Coutry et al., 1997).
The Na,K-ATPase is also known to be regulated by membrane trafficking controlled by N-terminal PKC phosphorylation (for review, see Pedemonte and Bertorello, 2001) through a mechanism that involves binding of phosphoinositide 3-kinase to a polyproline motif in the N-terminus (Yudowski et al., 2000). Furthermore, protein kinase phosphorylation also seems to involve direct modification of Na,K-ATPase catalytic activity both in vitro and in vivo, at least in some systems (reviewed in Therien and Blostein, 2000). Similar direct effects on H,K-ATPase activity by protein kinase phosphorylation have not yet been observed.
The Na,K-ATPase function is known to be modified by associated small regulatory proteins, like the γ-subunit in kidney (for references, see Therien and Blostein, 2000). This regulatory mechanism now seems to extend to other members of small hydrophobic proteins, the so-called FXYD protein family with a common FXYD motif (Sweadner and Rael, 2000). For example, this applies to FXYD4 (CHIF, channel-inducing factor), FXYD1 (PLM, phospholemman), and FXYD7 (not characterized at the protein level) (Beguin et al., 2001; Garty et al., 2002; Crambert et al., 2002; Beguin et al., 2002; Mahmmoud et al., 2000). Some FXYD proteins, like the phospholemman-like protein from shark (PLMS), seem to interact with the Na,K-ATPase depending on PKA/PKC phosphorylation of its C-terminal motif (Mahmmoud et al., 2000; Cornelius et al., 2001) in a mechanism resembling the phospholamban (PLN) regulation of sarco(endo)plasmic reticulum Ca-ATPase, SERCA (Simmerman and Jones, 1998). In both SERCA and Na,K-ATPase, structural coupling between the small regulatory proteins and the enzyme involves contact interactions at both the cytoplasmic and the transmembrane domains (Asahi et al., 1999; Tatulian et al., 2002). For both PLN and PLMS, interaction with the α-subunit inhibits the enzyme, which can be relieved by structural rearrangements accomplished either by protein kinase phosphorylation (Negash et al., 2000) or by breaking experimentally the hydrophobic interaction in the transmembrane domain by addition of detergents (Mahmmoud et al., 2000). To date, no small regulatory proteins have been identified in gastric H,K-ATPase preparations. There are, however, two previous reports of the isolation of a 17.5-kDa protein from rabbit gastric mucosa, which is a substrate for PKA and stimulates H,K-ATPase (Sack, 1992; 1993). The question is, therefore, if small proteins associate with the H,K-ATPase and are involved in regulation of acid secretion.
Considering the lack of information about alternative modes to achieve acute regulation of gastric H,K-ATPase in addition to the well-known regulation by membrane trafficking, we decided first to characterize the PKA and PKC phosphorylation pattern including both localization and phosphorylation intensity, and compare this to the one found in Na,K-ATPase (Mahmmoud and Cornelius, 2002). Second, we investigated whether or not N-terminal PKC phosphorylation leads to any functional changes in the reaction mechanism of the H,K-ATPase. Finally, we have screened the gastric H,K-ATPase membrane preparation to identify a possible presence of small hydrophobic proteins associated with the H,K-ATPase that could be involved in regulation of acid secretion.
Experimental procedures
H,K-ATPase preparation
Preparation of pig H,K-ATPase-enriched membrane vesicles was performed as described by Sachs et al. (Sachs et al., 1976). Protein concentration was determined using Peterson's modification (Peterson, 1977) of the Lowry method, using bovine serum albumin as a standard.
The maximum hydrolytic activity (Vmax) of gastric H,K-ATPase was measured using the method of Baginski et al. (1967) in a reaction mixture containing 3–5 μg H,K-ATPase in 30 mM histidine, pH. 7.2, 4 mM MgCl2, 20 mM KCl, and 3 mM ATP and 7.6·10−7 M nigericin (Cornelius, 1988) to allow fast K+ equilibration in the H,K-ATPase vesicles. Vmax was ∼2 units/mg at 30°C (1 unit = 1 μmole Pi/min). With a phosphorylation level (EP) of ∼2 nmol/mg this corresponds to a maximum turnover number, kcat ∼10 s−1 (30°C). A more sensitive method of Lindberg and Ernster (1956) was used to assess submaximal ATPase activity at variable K+-concentrations and pH. In this case, the reaction mixture contained 0.3 μg H,K-ATPase in 30 mM histidine, 1 mM MgCl2, 10 μM ATP (Na+-salt) containing 0.03 μCi/pmol [32P]ATP. The liberated 32Pi was measured by scintillation counting.
Controlled proteolysis of the α-subunit. N-terminal truncation of the H,K-ATPase α-subunit was performed by incubating membrane-bound enzyme with trypsin (trypsin to protein ratio of 1:100 (w/w)) for 10 min on ice in the presence of 30 mM histidine, pH 7.00 or 6.2, in the presence or absence of 20 mM KCl. In all cases 1 mM EDTA was present during proteolysis. Truncation of the N-terminus was confirmed by the increase in the mobility of the α-subunit band in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) from an apparent molecular mass of ∼94 to 90 kDa. After incubation with trypsin, the mixture was diluted 10 times with ice-cold imidazole buffer (25 mM, pH 7.4) and the membranes were sedimented in a Beckman centrifuge at 170,000 g for 1 h. Finally, the membranes were homogenized in 30 mM histidine, pH 7.00 containing 25% glycerol. Extensive trypsinization of H,K-ATPase was performed as first described by Karlish et al. (1991), by incubation of the membranes with a trypsin/protein ratio of 1:5 at 37°C for 1 h in the presence of 20 mM KCl and 2 mM EDTA. Trypsin treatment was terminated by addition of excess soybean trypsin inhibitor. The mixture was centrifuged and washed as above and the “19-kDa membranes” were suspended in histidine-glycerol buffer at −20°C.
Proteolytic fingerprinting
Proteolytic fingerprinting was performed essentially as described earlier (Sweadner, 1991). Membranes (containing 5 μg protein) suspended in electrophoresis sample buffer (Laemmli, 1970) were treated with 5 μg soybean trypsin inhibitor. Shortly before loading to gel, 0.8 μg of trypsin was added (trypsin to protein ratio of 1:5 (w/w)), and volumes containing 2 μg of protein were loaded onto SDS-gels. In control samples, the same volume of buffer without trypsin was added. Electrophoresis was run for 10–16 h, then the samples were transferred to PVDF membranes and stained with Coomassie blue, or washed with phosphate-buffered saline for subsequent antibody staining (see below).
PKA and PKC phosphorylation of H,K-ATPase membranes
PKA phoshorylation was performed in a reaction mixture containing 50 mM Hepes (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 0.1 mM ATP (Tris-salt), 4 μg protein, a detergent concentration as indicated in legends to figures, and 3 units of PKA. PKC phosphorylation was performed in a typical assay mixture containing 50 mM Hepes (pH 7.5), 10 mM MgCl2, 0.5 mM CaCl2, 0.02 mM L-α phosphatidylserine (Avanti Polar Lipids, Alabaster, AL), 0.01 mM Dioleoyl 1,2-sn-glycerol (Sigma, St. Louis, MO), 0.1 mM ATP (Tris-salt), 4 μg protein, and 0.13 μg of PKC. PKC phosphorylation in mixed micelle assay contained the same ligands plus detergent concentrations as indicated in legends to figures. The phosphorylation reaction for both kinases was initiated by the addition of ATP (containing 3 μCi/pmol 32P-[ATP]), allowed to proceed for 30 min at 24°C, and terminated by the addition of 16 μl sample buffer (Laemmli, 1970). The phosphorylation stoichiometry was calculated after autoradiography from the radioactivity associated with the α-band in the SDS-PAGE and the enzyme purity determined from the maximum phosphoenzyme level (∼2 nmol/mg), as previously described (Cornelius and Logvinenko, 1996).
SDS-PAGE and immunoblotting
The phosphorylated proteins were separated using SDS-PAGE (3% stacking gel, 9% intermediate, and 16% resolving gels, unless otherwise indicated). The gels were stained with Coomassie blue, destained and dried, then analyzed by autoradiography overnight at −80°C. The phosphorylated bands corresponding to the α-subunit were excised from the gels and the radioactivity measured in a scintillation counter. The phosphorylation stoichiometry was calculated from the radioactivity associated with the α-subunits and the amount of the H,K-ATPase protein in the preparation deduced from measurements of the phosphoenzyme (EP) level as previously described (Cornelius and Logvinenko, 1996).
For immunoblotting after electrophoresis, proteins were transferred to PVDF membranes, then washed three times for 20 min with PBS and incubated overnight at room temperature with the primary antibody, as described in the results and in legends to figures. The PVDF membranes were washed again with PBS and incubated with goat anti-rabbit antibody for 2 h. After washing, the proteins were detected using enhanced chemiluminescence reagents (Amersham Pharmacia, Biotech, UK).
N-terminal sequence analysis
Membranes were separated with SDS-PAGE. Excised bands from PVDF membranes after electroblotting of SDS-PAGE were analyzed by N-terminal Edman degradation performed by the Protein Structure Core Facility, University of Nebraska Medical Center with an Applied Biosystems Procise sequencer.
Materials
The catalytic subunit of PKA was purchased from Sigma (St. Louis, MO, USA). PKC was from CalBiochem (La Jolla, CA, USA) and contained the Ca2+-dependent isoforms. C-terminal specific antibodies to the Na,K-ATPase α-subunit (C1002–1016 and C815–828) were kindly provided by J. V. Møller (University of Aarhus, Denmark). Monoclonal antibody to the gastric H,K-ATPase α-subunit (Smolka et al., 1991) was from Calbiochem.
Results
The microsome fraction from pig gastric mucosa is enriched in H,K-ATPase (Sachs et al., 1976), and a band migrating at an apparent molecular mass of ∼94 kDa in SDS-PAGE was detected in immunoblots by a specific H,K-ATPase α-subunit antibody (cf. Fig. 6 A). This antibody reacts with an epitope in the C-terminal end of the large cytoplasmic loop of the H,K-ATPase α-subunit with the sequence D682MDPSEL688 (Smolka et al., 1991). No contamination from Na,K-ATPase could be detected in the preparation as indicated by immunoblotting using two different sequence specific antibodies to Na,K-ATPase (not shown). Second, no inhibition of ATPase activity could be detected using ouabain (1 mM). Finally, ATP hydrolysis was stimulated by Na+ with a very low K0.5 ∼100 mM.
FIGURE 6.
Proteolytic fingerprinting to localize C-terminal PKC phosphorylation. (A) An immunoblot using a specific H,K-ATPase α-subunit antibody of intact (left lane) and extensively trypsin treated (right lane) H,K-ATPase. Nearly all H,K-ATPase α-subunit is cleaved and several low molecular weight proteolytic products are probed with the antibody. (B) An autoradiogram showing proteolytic fingerprinting of PKC phosphorylated H,K-ATPase in the absence (left lane) or in the presence (right lane) of 0.1% TX-100. In the absence of TX-100 PKC phosphorylation is mainly associated with the 32-kDa N-terminal fragment, whereas in the presence of TX-100 additional phosphorylated fragments appear, most notably one at 19 kDa. A faint band migrating at an apparent molecular mass of 12 kDa is also observed. (C) Autoradiogram comparing PKA phosphorylation and PKC phosphorylation of H,K-ATPase in the absence (lanes 1 and 3) and presence (lanes 2 and 4) of 0.1% TX-100 after proteolytic fingerprinting. As seen, PKA and PKC phosphorylation are detected at a 19-kDa fragment and only in the presence of TX-100.
The H,K-ATPase in the microsomal vesicles were oriented preferentially with the ATP substrate site exposed; accordingly, hydrolytic activity was measured after inclusion of the H+/K+-ionophore nigericin in the test medium to eliminate effect of limiting internal K+. The maximum hydrolytic activity of the enzyme was 75–100 μmol·mg−1·h−1 at 10 mM K+, 3 mM ATP and pH 7.2.
Protein kinase phosphorylation profile
Fig. 1 shows an autoradiogram of PKA and PKC phosphorylation of gastric H,K-ATPase after SDS-PAGE. PKA phosphorylation is evident only in the presence of Triton X-100 (lane 2), similar to PKA phosphorylation of the Na,K-ATPase α-subunit (Feschenko and Sweadner, 1994). The stoichiometry of phosphorylation by PKA was ∼0.7 mol Pi/mol α-subunit.
FIGURE 1.
PKA and PKC phosphorylation profiles of gastric H,K-ATPase-containing microsomes. Autoradiogram of 32P-labeled proteins after SDS-gel electrophoresis showing PKA (lanes 1 and 2) and PKC (lanes 3 and 4) phosphorylation of pig gastric microsomes in the absence (lanes 1 and 3) or in the presence (lanes 2 and 4) of 0.1% Triton X-100. Membranes were incubated with protein kinase buffer, in the presence of 3 μCi/pmol [32P]ATP, as mentioned in Experimental Procedures. A total of 2 μg of phosphorylated proteins was separated by SDS-PAGE and visualized by autoradiography. The H,K-ATPase α-subunit was excised from the gel and the associated radioactivity measured by scintillation counting (Cornelius and Logvinenko, 1996). In the PKA profile with TX-100 (lane 2) the H,K-ATPase α-subunit, Rap 1A, and histone H3 are identified. In the PKC profile the H,K-ATPase α-subunit, PKC, and histone H1 are phosphorylated both in the absence and presence of Triton X-100, although the phosphorylation intensity of the H,K-ATPase α-subunit and the autophosphorylation of PKC are enhanced by detergent (see Fig. 4).
In contrast to PKA phosphorylation, PKC phosphorylation occurred in the absence of Triton X-100 with a stoichiometry of ∼0.6 mol Pi/mol α-subunit. However, the phosphorylation stoichiometry increased significantly to ∼1.2 mol Pi/mol α-subunit in the presence of 0.1% Triton X-100. This twofold stimulation may be due to detergent activation of PKC, as indicated by the increased PKC autophosphorylation (Fig. 1, the band labeled PKC), and/or to the exposure of additional sites in the α-subunit, as previously found for the Na,K-ATPase (Mahmmoud and Cornelius, 2002). It should also be mentioned that in PKC phosphorylation assays performed in the presence of detergent, a small fraction of right-side out oriented pumps may contribute to the increased PKC phosphorylation.
Small regulatory proteins
In the closely related Ca-ATPase and Na,K-ATPase, acute regulation is achieved in part by associated small hydrophobic proteins. In the case of SERCA both sarcolipin (Odermatt et al., 1998) and PLN are well known examples. A family of small hydrophobic proteins called the FXYD protein family (see Introduction) (Sweadner and Rael, 2000) has been shown to be tissue specific regulators of Na,K-ATPase (Mahmmoud et al., 2000, Beguin et al., 2001; Garty et al., 2002; Crambert et al., 2002; Beguin et al., 2002). Some of the FXYD proteins, like PLM and PLMS, contain a multisite phosphorylation motif for PKA and PKC phosphorylations (Mahmmoud et al., 2000, Palmer et al., 1991); others lack such phosphorylation motifs, e.g., γ and CHIF. This is also the case for the small regulatory proteins of SERCA where PLN can be phosphorylated by PKA, whereas sarcolipin cannot.
Our first approach was therefore to see if any low molecular weight proteins were substrate for protein kinase phosphorylation in the H,H-ATPase membrane preparation. As seen from the autoradiogram, several small proteins in the H,K-ATPase membranes were phosphorylated by protein kinases (Fig. 1).
After phosphorylation by PKA a band migrating at a molecular mass of ∼22 kDa contained a protein sequence, which was identified by N-terminal Edman degradation as homologous to the Ras-related GTPases Rap 1A (sequence read: [M]REYKLV[V]LGX). The phosphorylated band located at ∼18 kDa (PKA lane) was identified as homologous to members of the histone H3 family (sequence read: [H]E[T]KQTAXK[P]TGGKAXLXQXA). After PKC phosphorylation a band migrating at an apparent molecular mass of 11 kDa was found homologous to members of the histone H1 family (sequence read: XKASGPXVS[E/F]). The band migrating at ∼24 kDa was not consistently phosphorylated by PKC in all autoradiograms. Therefore, none of the low MW bands that is phosphorylated by PKA or PKC and consistently observed in the autoradiogram of Fig. 1 seem to represent FXYD proteins.
Using immunochemical methods, we then tried to detect FXYD proteins in the H,K-ATPase membrane preparation. Experiments using Western blots probed by a PLM antibody against the TYDY motif (kindly provided by L. R. Jones, Indiana University School of Medicine, Indianapolis, IN, USA), anti-PLMS antiserum, or anti-γ antiserum (kindly provided by S. J. D. Karlish, Weizmann Institute of Science, Rehovot, Israel) were all negative.
Using a method for extraction of small hydrophobic proteins (MacLennan, 1974) PLMS and γ could be isolated from either shark rectal or pig renal Na,K-ATPase, and detected by Coomassie and silver staining, or by immunoblots. However, similar extraction procedures were unable to isolate any small hydrophobic proteins from H,K-ATPase membrane preparations.
Together, the results thus indicate the absence of any homologous γ-like or PLMS-like proteins, or other FXYD proteins containing either the TYDY motif, or a multisite PKA/PKC phosphorylation motif associated with the H,K-ATPase.
Finally, shark rectal and pig renal Na,K-ATPase are both activated by sub-cmc concentrations of the nonionic detergent C12E8 (Huang et al., 1985; Mahmmoud et al., 2000) that partition into the membrane phase interfering with the hydrophobic interaction between the α-subunit and the FXYD protein (PLMS and γ, respectively). However, as shown in Fig. 2 sub-cmc concentrations of C12E8 (20 μM) did not affect enzyme activity gastric H,K-ATPase, indicating the absence of any small regulatory proteins hydrophobically associated with the H,K-ATPase and affecting its activity. This is in accord with a previous finding that neither γ nor CHIF from Na,K-ATPase associates with gastric H,K-ATPase (Beguin et al., 1997; Beguin et al., 2001).
FIGURE 2.
Comparison of the effects of sub-cmc concentrations of C12E8 on catalytic activity of Na,K-ATPase and H,K-ATPase. Pig stomach membranes were treated with 20 μM of C12E8 and the K+-activation curves were measured for control and C12E8 treated-membranes. The hydrolytic activity was measured as described in Methods. The reaction mixture contained 30 mM histidine, pH 7.00, 1 mM MgCl2, 0.3 μg protein, and the indicated KCl concentrations. Activities are expressed as percentage of maximum activity in controls.
Location of PKA and PKC phosphorylation sites
PKC phosphorylation of the native α-subunit of Na,K-ATPase in the absence of detergent is restricted to the N-terminus, because truncation of the N-terminus completely abolishes PKC phosphorylation (Feschenko and Sweadner, 1995). However, after mild detergent treatment additional C-terminal PKC sites are uncovered at the beginning of the M5M6 hairpin and the L89 cytoplasmic loop close to the membrane face (Mahmmoud and Cornelius, 2002).
To test whether the detergent-induced doubling of PKC phosphorylation stoichiometry of the H,K-ATPase α-subunit occurred exclusively at the N-terminus, a series of experiments were performed comparing native and N-terminal truncated H,K-ATPase phosphorylated in the presence and absence of detergent.
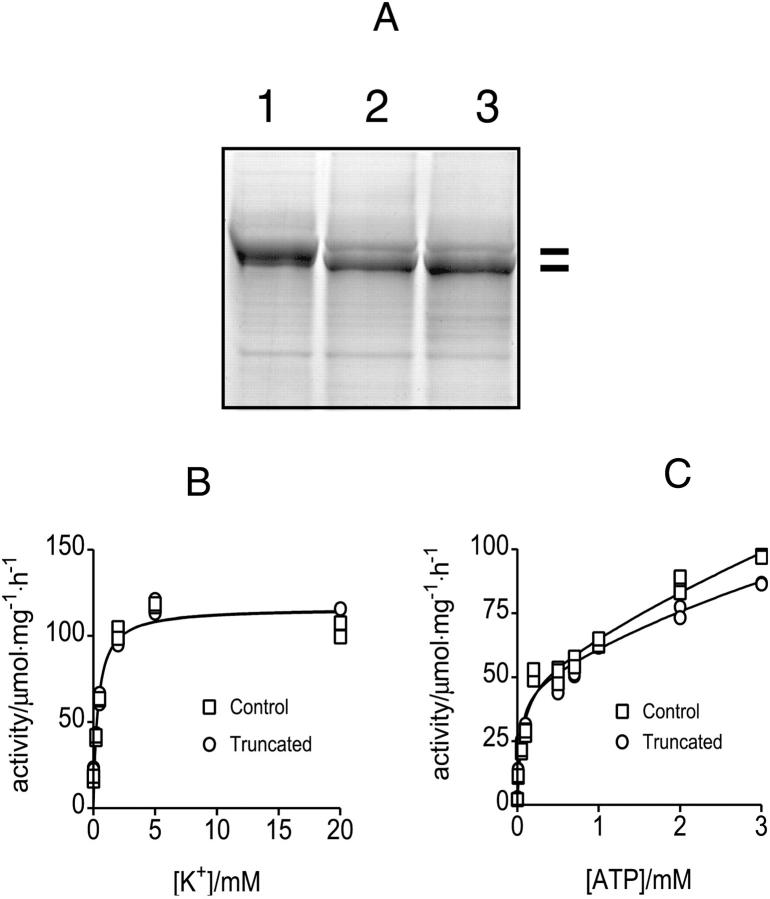
Fig. 3 A shows a representative result where N-terminal truncation was performed by mild proteolysis performed for 10 min at 0°C at different conditions followed by SDS-PAGE analysis of the proteolytic products. In contrast with control (lane 1), incubation of H,K-ATPase membranes either in the presence (lane 2) or absence (lane 3) of 20 mM K+ resulted in a 2 kDa shift of the mobility of the α-subunit in SDS-PAGE, most likely as a result of truncation of the small N-terminus. In analogy with the Na,K-ATPase, truncation was maximal in histidine buffer, pH 6.2, and in the absence of K+ (lane 3). It should be noted that truncation of the α-subunit at the specified conditions generated fully functional enzymes without significant differences in the affinities for K+ (Fig. 3 B) or ATP (Fig. 3 C), or in the maximum hydrolytic activity, Vmax. This is in contrast to the findings in Na,K-ATPase, where N-terminal truncation results in a significant inhibition of the enzyme (Jørgensen and Collins, 1986) and a shift in the E1/E2 conformational equilibrium toward E1 (Segall et al., 2002).
FIGURE 3.
N-terminal truncation of H,K-ATPase. A shows a Coomassie-stained SDS-PAGE demonstrating N-terminal truncation of the H,K-ATPase α-subunit by controlled proteolysis. Microsomes were incubated without (lane 1) or with (lanes 2 and 3) trypsin as described in Experimental Procedures. Lane 1, control; Lane 2, membranes were incubated in a proteolysis buffer, pH 7.2 containing 20 mM K+; Lane 3, membranes were incubated in a proteolysis buffer, pH 6.2, and in the absence of K+. Washed membranes were subjected to SDS-PAGE and the gel was stained with Coomassie blue. Representation of two independent experiments is shown. As seen, after mild proteolysis the mobility increases as a result of the N-terminal truncation. B shows K+ activation of control and N-terminal truncated enzyme in the presence of 3 mM ATP, pH 7.2. The fitted curves are hyperbolic with apparent K+ affinities of 0.36 ± 0.08 mM and 0.38 ± 0.09 mM, respectively. C shows ATP activation of H,K-ATPase before and after truncation at 20 mM K+, pH 7.2. In both cases the activation is biphasic with high-affinity apparent K0.5 ∼40 μM, and low-affinity components which saturated in the mM range.
In Fig. 4 PKC and PKA phosphorylation of control and N-terminal truncated H,K-ATPase was compared. As seen, PKC phosphorylation is evident in control enzyme irrespective of the presence of TX-100 (contr-TX and contr+TX), whereas after N-terminal truncation PKC phosphorylation is observed only in the presence of TX-100 (trunc+TX) indicating exposure of C-terminal PKC sites by detergent.
FIGURE 4.
PKA and PKC phosphorylation profiles of native and N-terminal truncated H,K-ATPase in the absence or presence of 0.1% Triton X-100. The upper panel shows autoradiogram of PKA phosphorylated H,K-ATPase preparations and the lower panel PKC phosphorylated H,K-ATPase preparations. Phosphorylated proteins were separated by SDS-PAGE and analyzed by autoradiography. The radioactivity associated with the α-subunits was measured by scintillation counting and the phosphorylation stoichiometry calculated as previously described (Cornelius and Logvinenko, 1996). For PKA phosphorylation the following phosphorylation stoichiometries were found (mol Pi/mol α): control-TX-100, 0.09 ± 0.014; control+TX-100, 0.71 ± 0.04; Truncated-TX-100, 0.018 ± 0.005; Truncated+TX-100, 0.30 ± 0.034. For PKC phosphorylation the stoichiometries were control-TX-100, 0.63 ± 0.01; control+TX-100, 1.16 ± 0.012; Truncated-TX-100, 0.13 ± 0.014; Truncated+TX-100, 0.43 ± 0.017.
PKA phosphorylation was present in native enzyme only to a very low stoichiometry; however, it was significantly increased after the addition of TX-100. After N-terminal truncation PKA phosphorylation was abolished in the absence of detergent but occurred after the addition of detergent, suggesting that PKA phosphorylation takes place primarily at the C-terminal site (Ser-953 in pig).
The N-terminus of the H,K-ATPase α-subunit contains a highly conserved PKC consensus site (Ser-27), as seen in Fig. 5. However, because N-terminal truncated H,K-ATPase can be phosphorylated by PKC after detergent treatment, other sites in addition to the N-terminal one must become available. To locate such sites we performed proteolytic fingerprinting. As seen from the immunoblot in Fig. 6 A, the extensive proteolysis generated several low molecular weight bands that are probed with the H,K-ATPase antibody. The autoradiogram of PKC phosphorylated H,K-ATPase in the absence or presence of 0.1% Triton X-100 after extensive proteolytic digestion is shown in Fig. 6 B. As seen, in the absence of detergent phosphorylation is mainly occurring at the 32 kDa N-terminal proteolytic fragment, whereas in the presence of detergent a 32P-labeled band at 19 kDa appears and also a less intensively labeled 12-kDa band can be observed. The phosphorylated 19-kDa band could be identified as identical to the band including the PKA site in the L89 loop by comparing autoradiograms after PKC and PKA phosphorylation (Fig. 6 C).
FIGURE 5.
Alignment of amino acid sequences of H,K-ATPase α-subunit N-terminus (upper) and C-terminus (lower) from different species. Putative phosphorylation sites are labeled with the respective amino acid number in the full-length mature protein. Sequences were obtained form GenBank. In the N-terminus a PKC site at Ser-27 is conserved. In the C-terminus, PKC sites at the M5M6 hairpin (Ser-785) and at the M8M9 loop (Thr-949) are conserved and in close apposition to the likewise conserved PKA site at Ser-953.
Functional effects
In previous investigations (Kanagawa et al., 2000) the presence of Ser/Thr phosphatase activity has been demonstrated in a gastric H,K-ATPase preparation comparable to the one used in the present investigation. Therefore, to investigate whether PKC phosphorylation affected the functional properties of H,K-ATPase, the time course of PKC phosphorylation of H,K-ATPase was initially determined to ensure that functional tests were performed at a maximal and constant phosphorylation level. Fig. 7 A shows that a steady protein kinase phosphorylation level of H,K-ATPase was attained after 25 min and was constant for at least an hour indicating the absence of Ser/Thr phosphatase activity in the H,K-ATPase membrane preparation. This was confirmed by the autoradiogram shown in Fig. 7 B where membranes phosphorylated for 30 min by PKC in the presence of hot ATP to achieve maximum phosphorylation were subsequently mixed with 2.5 mM cold ATP and increasing amount of unphosphorylated H,K-ATPase membranes followed by an additional 30 min incubation. The fact that no dephosphorylation is observed strongly indicates the absence of phosphatase activity in the H,K-ATPase membrane preparation. Because Ser/Thr phosphatases are soluble, this may indicate differences in the washing or storage procedures of the preparations used in the two investigations.
FIGURE 7.
(A) Time course of PKC phosphorylation of H, K-ATPase. The figure shows the phosphorylation stoichiometry of PKC phosphorylation of H, K-ATPase as a function of time. PKC phosphorylation was performed as described in Methods except that the reaction was terminated after the indicated time intervals with SDS sample buffer containing 0.5% TCA. Volumes containing 2 μg protein were loaded onto the gel. After electrophoresis and autoradiography (shown as inset to the figure), the α bands were cut from the gel and counted for radioactivity. The phosphorylation stoichiometry was calculated as described in Methods. (B) Autoradiogram showing absence of endogenous phosphatase activity in H,K-ATPase preparation. A total of 4 μg of H, K-ATPase membranes were phosphorylated by PKC for 30 min at 24°C in the presence of 32P[ATP] as described in Methods. The reactions were then quenched with 2.5 mM cold ATP and the phosphorylated membranes mixed with increasing concentrations of unphosphorylated membranes (0, 1, 2, 3, and 4 μg as indicated in the figure) and incubated again for 30 min. The samples were then treated with SDS sample buffer and subjected to SDS-PAGE and autoradiography. The constant level of phosphorylation indicates the absence of phosphatase activity.
PKC phosphorylation of rat renal Na,K-ATPase at N-terminal serines (Ser-11 and Ser-18) is involved in regulation of Na,K-ATPase localization via membrane trafficking (Pedemonte and Bertorello, 2001) but does not change the catalytic activity of the enzyme directly (Pedemonte et al., 1997). Phosphorylation of native H,K-ATPase by PKC in vitro does not require detergent. Therefore it is possible to investigate if N-terminal PKC phosphorylation causes any direct changes in the functional state of the H,K-ATPase without interference from added detergents.
The K+ activation of the H,K-ATPase was studied at both μM and mM ATP concentrations before and after PKC phosphorylation. Fig. 8 shows that the K+ activation of H,K-ATPase at 10 μM ATP before and after PKC phosphorylation at pH 6.5 (Fig. 8 A) and at pH 7.2 (Fig. 8 B). All activation curves appear hyperbolic with a slight deactivation at 20 mM K+. In the absence of protein kinase phosphorylation, the maximal hydrolytic activity increased from ∼7 μmol·mg−1·h−1 at pH 7.2 to ∼14 μmol·mg−1·h−1 at pH 6.5. The K0.5 for K+-activation increased in parallel from ∼0.08 mM at pH 7.2 to ∼0.23 mM at pH 6.5. Thus K0.5/Vmax (equal to the slope in the Lineweaver-Burk plots) did not change substantially with pH indicating no effect of pH in this range on the K+ affinity. Furthermore, at both pH values neither the maximal hydrolytic activity nor the apparent K+ affinity was affected by PKC phosphorylation.
FIGURE 8.
Steady-state K+ activation of hydrolytic activity at low ATP. H,K-ATPase membranes were incubated in PKC buffer at 24°C, pH 7.5, in the presence (□) or absence of PKC (○) for 30 min followed by measurement of the steady-state hydrolytic activity at variable K+ concentrations in the presence of 10 μM ATP in histidine buffer with the pH values adjusted to 6.5 or 7.2 as indicated. At both pH = 6.5 (A) and pH = 7.2 (B), identical K+ activation curves were obtained in control and after PKC phosphorylation. Note, however, the higher apparent K+-affinities (K0.5 ∼0.23 mM at pH 6.5 vs. 0.08 mM at pH 7.2) and the lower maximum hydrolytic activities (∼14 μmol·mg−1·h−1 at pH 6.5 vs. ∼7 μmol·mg−1·h−1 at pH 7.2) when pH increases.
Because the ATP-activation curve contains a low-affinity component (Wallmark et al., 1980) the PKC effects were also investigated at Vmax conditions of 3 mM ATP and different K+ concentrations at both pH 6.5 and 7.2 (Fig. 9 A and B). As seen from Fig. 9, the K+ activation pattern at 3 mM ATP changed dramatically compared to the one observed at low ATP concentrations: Increasing pH changed Vmax only slightly, whereas the apparent affinity for K+ changed from 0.8 mM at pH 7.2 to 2.5 mM at pH 6.5 (compare controls in Fig. 9 A and B). The ten times increase in the K0.5 value for K+-activation observed by increasing the ATP concentration is caused by the induced shift toward the E1 conformation. PKC significantly activated the maximum hydrolytic activity at both pH values: At pH 6.5 the activation was ∼30%, whereas at pH 7.2 the activation amounted to ∼80%. At both pH values the K0.5 value for K+-activation was unaffected by PKC phosphorylation, meaning that the ratio K0.5/Vmax was decreased by PKC phosphorylation indicating an increased apparent K+ affinity.
FIGURE 9.
Steady-state K+ activation of hydrolytic activity at high ATP. These experiments were performed by incubation of H,K-ATPase membranes in PKC buffer at 24°C in the presence (□) or absence of PKC (○) for 30 min followed by measurement of the maximum hydrolytic activity at variable K+ concentration at 3 mM ATP. Incubation of the enzyme with PKC at both pH 6.5 (A) and 7.2 (B) resulted in a significant activation of the hydrolytic activity. Curves represent fitting of the data with a hyperbolic binding curve. At pH 7.2 the maximum hydrolytic activity at 30°C was 63.5 ± 1.4 μmol·mg−1·h−1 in control experiments whereas in membranes pre-incubated with PKC the activity was 112.5 ± 1.2 μmol·mg−1·h−1 (P < 0.007). A similar activation was also observed at pH 6.2: 57.5 ± 1.9 μmol·mg−1·h−1 for control and 87.7 ± 1.4 μmol·mg−1·h−1 after PKC phosphorylation (P < 0.003). The fitted values for the K0.5 of K+-activation at pH 7.2 were 0.59 mM and 0.58 mM in control and after PKC phosphorylation, respectively. At pH 6.2 the K0.5 of K+-activation were identical 1.7 mM before and after PKC phosphorylation.
That PKC activation is observed only at high ATP concentrations indicates that it is not the ATP binding step itself (step 1 of the reaction scheme 1) that is affected by PKC but one of the subsequent steps. To achieve activation at Vmax conditions the affected step must be rate determining, which points to the E1P → E2P step (step 3).
SCHEME 1.
In Fig. 10, the ATP-activation curves were investigated before and after PKC-phosphorylation at 10 mM K+, pH = 7.2. As seen, the control activation curve is biphasic with a small high-affinity component (K0.5 ∼14 μM) and a larger low-affinity component that did not seem to saturate within the range of substrate concentration employed (K0.5 ∼6 mM). After PKC phosphorylation the ATP activation became hyperbolic with an apparent ATP affinity of 339 ± 44 μM. This effect is in accord with the observations that in the K+-activation curves PKC activation is observed only at mM ATP concentrations, but not at μM concentrations (cf. Figs. 8 and 9).
FIGURE 10.
The steady-state ATP activation curve obtained at [K+] = 10 mM, pH 7.2 in control H,K-ATPase membranes (○) and after PKC phosphorylation (□). In control the activation is biphasic, whereas after PKC phosphorylation it becomes hyperbolic and the maximum hydrolytic activity increased significantly.
Discussion
The present study demonstrates in vitro phosphorylation of H,K-ATPase by both PKA and PKC (Figs. 1 and 4). PKA phosphorylation to near-stoichiometric values required the presence of detergent as is the case also for Na,K-ATPase (Feschenko and Sweadner, 1994). Comparison of the phosphorylation profiles of native and N-terminal truncated H,K-ATPase together with proteolytic fingerprinting that demonstrated PKA phosphorylation of the 19-kDa fragment (Fig. 6 C) suggested the conserved C-terminal serine residue at the L89 loop (Ser-952 in rat H,K-ATPase α-subunit) as the main target for PKA (Fig. 5).
PKC phosphorylated the H,K-ATPase in the absence of detergent at the N-terminus to near-stoichiometric values where a conserved PKC site, Ser-27, is located. This residue has previously been reported to be phosphorylated in a pig gastric preparation by intrinsic membrane-bound kinase (Togawa et al., 1996) apparently representing a PKC-isoform (Kanagawa et al., 2000) and also by extrinsic PKC and PKA (Togawa et al., 1996). The absence of PKA phosphorylation after N-terminal truncation (Fig. 4) in the present investigation does not support, however, that the N-terminal Ser-27 of H,K-ATPase α-subunit is an efficient target for PKA phosphorylation in vitro. Thus, using purified protein kinases the main PKC phosphorylation site of native pig H,K-ATPase seems to be Ser-27, whereas the PKA phosphorylation site is Ser-953.
The finding of a doubling of the PKC phosphorylation stoichiometry by addition of detergents strongly indicated the presence of additional PKC sites, and the demonstration of detergent-supported PKC phosphorylation after N-terminal truncation indicated a C-terminal location of such sites (Fig. 4). The pattern of proteolytic fingerprinting (Fig. 6) after PKC phosphorylation closely compared with that found for the Na,K-ATPase (Mahmmoud and Cornelius, 2002), where a phosphorylated 19-kDa band corresponding to the proteolysis-resistant C-terminal fragment including the L89 loop and a 12-kDa band corresponding to the M5M6 hairpin could be identified by C-terminal specific antibodies. The identical phosphorylation pattern in trypsinized H,K-ATPase and Na,K-ATPase further indicates a C-terminal location of the detergent-exposed PKC sites also in H,K-ATPase. As seen from Fig. 5 the H,K-ATPase contains PKC consensus sites located at both the beginning of the M5M6 hairpin and the L89 cytoplasmic loop just as for the Na,K-ATPase. As with the Na,K-ATPase the latter site within the 19-kDa fragment is very close to the PKA site, and this close proximity of PKA and PKC phosphorylation sites in the same L89 cytoplasmic loop may be important as a target for cross talk between the signaling pathways of the two protein kinases, as recently indicated for shark Na,K-ATPase (Krüger et al., 2003). Both of these new putative C-terminal PKC sites are located close to the inner membrane face, and this could explain the requirement for detergents to expose them in vitro. On the other hand, the requirement for detergent makes it uncertain whether or not these membrane adjacent sites are physiologically important; a reservation, however, which also applies to the PKA site. The previously reported K+-dependent flexibility of the M5M6 hairpin of the H,K-ATPase and Na,K-ATPase α-subunits (Gatto et al., 1999; Lutsenko et al., 1995) suggests that phosphorylation at this site may in itself be regulated. At present we can only speculate on the significance of the alternative C-terminal PKC phosphorylation sites in regulation of gastric H,K-ATPase.
The very significant activation of catalytic activity produced by N-terminal PKC phosphorylation at physiological ATP concentrations (Figs. 9 and 10) indicates acceleration of a major rate-determining step in the H,K-ATPase reaction. Of the four steps indicated in the simplified version of the reaction mechanism (scheme 1), steps 1 and 3 are considered rate determining. The fact that PKC activation of catalytic activity is observed only at saturating ATP concentrations demonstrates, however, that the most likely step is the E1∼P → E2-P reaction. PKC phosphorylation at the N-terminal site seems, therefore, to function primarily as a conformational switching signal. Thus, for the first time a direct modification of the H,K-ATPase by PKC is demonstrated, which may have a potential role in the modulation of acid secretion and be additional to the well-known regulation by H,K-ATPase recruitment via membrane fusion events (see Dunbar and Caplan, 2001).
Screening of pig gastric H,K-ATPase membranes to identify the presence of small regulatory proteins homologous to the ones found in either of the two other members of the P2C ATPases, the SERCA and the Na,K-ATPase, was negative. Thus, a distinct difference in the regulation mechanisms of H,K-ATPase and Na,K-ATPase seems to be the absence of small regulatory proteins in H,K-ATPase. Previous reports indicating the presence of a 17.5-kDa protein that is a substrate for multisite PKA phosphorylation and important for the activation of rabbit H,K-ATPase (Sack, 1992; 1993) could not be confirmed using pig gastric microsomes. A protein migrating at 18 kDa and demonstrated to be a substrate for PKA was identified as a member of the histone H3 family. Whether or not this is caused by variable methods of membrane preparations or difference in species remains to be determined. It seems, therefore that P2C ATPases embody a range of regulatory mechanisms that either directly affect the activity of the enzyme, changing its localization by regulation of membrane trafficking, or indirectly change the enzyme activity by regulation of its association with specific regulatory proteins, like FXYD proteins. Although the regulation mechanisms vary in Na,K-ATPase and H,K-ATPase, they seem to be based on an amazingly similar protein kinase phosphorylation pattern.
Another distinct difference between the two closely related ATPases is the apparent unique functional significance of the Na,K-ATPase N-terminus as demonstrated by the truncation experiments (Fig. 3, B and C). In Na,K-ATPase, the N-terminus is important for the E1/E2 transition, and truncation of the N-terminal 32 amino acids results in significant inhibition of steady state catalytic activity both in renal α1 (Jørgensen and Collins, 1986) and in shark rectal α3 (Krüger et al., 2003), whereas this is not the case in H,K-ATPase. Recently, the N-terminal part of Na,K-ATPase has been proposed to play an important auto-regulatory role in Na,K-ATPase (Segall et al., 2002) that may be distinct from that in H,K-ATPase. Furthermore, in rat Na,K-ATPase the N-terminus is essential in regulation of membrane trafficking containing the two PKC sites, Ser-11 and Ser-18, together with the phosphoinositide 3-kinase polyproline motif necessary for controlling endocytosis (Pedemonte and Bertorello, 2001; Yudowski et al., 2000). In gastric H,K-ATPase the N-terminal PKC site seems to function as a conformational switching signal and the internalization signal motif is tyrosine-based and located to the β-subunit. Despite their close homology and the very similar protein kinase phosphorylation profile, H,K-ATPase and Na,K-ATPase seem to be regulated by quite different mechanisms.
Acknowledgments
The authors gratefully acknowledge Dr. I. Klodos for providing the pig H,K-ATPase preparation and also thank Lene Mauritsen for excellent technical assistance.
This work was supported in part by The Danish Medical Research Council, The NOVO foundation, and The A. P. Møller Foundation. The Carlsberg Foundation is acknowledged for supplying a postdoctoral fellowship to Y.A.M.
References
- Asahi, M., Y. Kimura, K. Kurzydlowski, M. Tada, and D. H. MacLennan. 1999. Transmembrane helix M6 in Sarco(endo)plasmic reticulum Ca2+-ATPase forms a functional site with phospholamban. Evidence for a physical interaction at other sites. J. Biol. Chem. 274:32855–32862. [DOI] [PubMed] [Google Scholar]
- Baginski, E. S., P. P. Foa, and B. Zak. 1967. Determination of phosphate: study of labile organic phosphate interference. Clin. Chim. Acta. 14:155–158. [Google Scholar]
- Beguin, P., G. Crambert, S. Guennoun, H. Garty, J.-D. Horisberger, and K. Geering. 2001. CHIF, a member of the FXYD protein family, is a regulator of Na,K-ATPase distinct from the γ-subunit. EMBO J. 20:3993–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin, P., G. Crambert, F. Monnet-Tschudi, M. Uldry, J.-D. Horisberger, H. Garty, and K. Geering. 2002. FXYD7 is a brain-specific regulator of Na,K-ATPase α1-β isozymes. EMBO J. 21:3264–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin, P., X. Wang, D. Firsov, A. Puoti, D. Claeys, J.-D. Horisberger, and K. Geering. 1997. The γ subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO J. 16:4250–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, C. S., and M. R. Brown. 1986. Release of intracellular Ca2+ and elevation of inositol triphosphate by secretagogues in parietal and chief cells isolated from rabbit gastric mucosa. Biochim. Biophys. Acta. 888:116–125. [DOI] [PubMed] [Google Scholar]
- Chew, C. S., S. J. Hersey, G. Sachs, and T. Berglinh. 1980. Histamine responsiveness of isolated gastric glands. Am. J. Physiol. 238:G312–G320. [DOI] [PubMed] [Google Scholar]
- Cornelius, F. 1988. Incorporation of C12E8-solubilized Na,K-ATPase into liposomes: determination of sidedness and orientation. Methods Enzymol. 156:156–167. [DOI] [PubMed] [Google Scholar]
- Cornelius, F., and N. Logvinenko. 1996. Functional regulation of reconstituted Na,K-ATPase by protein kinase A phosphorylation. FEBS Lett. 380:277–280. [DOI] [PubMed] [Google Scholar]
- Cornelius, F., Y. A. Mahmmoud, and H. R. Z. Christensen. 2001. Modulation of Na,K-ATPase by associated small transmembrane regulatory proteins and by lipids. J. Bioenerg. Biomembr. 33:417–425. [DOI] [PubMed] [Google Scholar]
- Courtois-Coutry, N., D. L. Roush, V. Rajendran, J. B. McCarthy, J. Geibel, M. Kashgarian, and M. J. Caplan. 1997. A tyrosine-based signal targets H/K-ATPase to a regulated compartment and is required for the cessation of gastric acid secretion. Cell. 90:501–510. [DOI] [PubMed] [Google Scholar]
- Crambert, G., M. Füzesi, H. Garty, S. Karlish, and K. Geering. 2002. Phospholemman (FXYD1) associates with Na,K-ATPase and regulates its transport properties. Proc. Natl. Acad. Sci. USA. 99:11476–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar, L. A., and M. J. Caplan. 2001. Ion pumps in polarized cells: sorting and regulation of the Na+, K+- and H+, K+-ATPases. J. Biol. Chem. 276:29617–29620. [DOI] [PubMed] [Google Scholar]
- Feschenko, M. S., and K. J. Sweadner. 1994. Conformation-dependent phosphorylation of Na,K-ATPase by protein kinase A and protein kinase C. J. Biol. Chem. 269:30436–30444. [PubMed] [Google Scholar]
- Feschenko, M. S., and K. J. Sweadner. 1995. Structural basis for species-specific differences in the phosphorylation of Na,K-ATPase by protein kinase C. J. Biol. Chem. 270:14072–14077. [DOI] [PubMed] [Google Scholar]
- Garty, H., M. Lindzen, R. Scanzano, R. Aizman, M. Füzesi, R. Goldshleger, N. Farman, R. Blostein, and S. J. D. Karlish. 2002. A functional interaction between CHIF and Na-K-ATPase: Implication for regulation by FXYD proteins. Am. J. Physiol. Renal Physiol. 283:F607–F615. [DOI] [PubMed] [Google Scholar]
- Gatto, C., S. Lutsenko, J. M. Shin, G. Sachs, and J. H. Kaplan. 1999. Stabilization of the H,K-ATPase M5M6 membrane hairpin by K+ ions. Mechanistic significance for P2-type ATPases. J. Biol. Chem. 274:13737–13740. [DOI] [PubMed] [Google Scholar]
- Huang, W.-H., S. S. Kakar, and A. Askari. 1985. Mechanisms of detergent effects on membrane-bound (Na+ + K+)-ATPase. J. Biol. Chem. 260:7356–7361. [PubMed] [Google Scholar]
- Jørgensen, P. L., and J. H. Collins. 1986. Tryptic and chymotryptic cleavage sites in sequence of alpha-subunit of (Na+ + K+)-ATPase from outer medulla of mammalian kidney. Biochim. Biophys. Acta. 860:570–576. [DOI] [PubMed] [Google Scholar]
- Kanagawa, M., S. Watanabe, S. Kaya, K. Togawa, T. Imagawa, A. Shimada, K. Kikuchi, and K. Taniguchi. 2000. Membrane enzyme systems responsible for the Ca2+-dependent phosphorylation of Ser27, the independent phosphorylation of Tyr10 and Tyr7, and the dephosphorylation of these phosphorylated residues in the α-chain of H/K-ATPase. J. Biochem. 127:821–828. [DOI] [PubMed] [Google Scholar]
- Karlish, S. J. D., R. Goldshleger, and W. D. Stein. 1991. A 19-kDa C-terminal tryptic fragment of the α chain of Na,K-ATPase is essential for occlusion and transport of cations. Proc. Natl. Acad. Sci. USA. 87:4566–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger, A., Y. A. Mahmmoud, and F. Cornelius. 2003. PKC phosphorylation directed at novel C-terminal sites affects the Na,K-ATPase activity. Ann. N. Y. Acad. Sci. In press. [DOI] [PubMed]
- Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. [DOI] [PubMed] [Google Scholar]
- Lindberg, O., and L. Ernster. 1956. Determination of organic phosphorus compounds by phosphate analysis. Methods Biochem. Anal. 3:1–12. [DOI] [PubMed] [Google Scholar]
- Lutsenko, S., R. Anderko, and J. H. Kaplan. 1995. Membrane disposition of the M5–M6 hairpin of Na+,K+-ATPase α-subunit is ligand dependent. Proc. Natl. Acad. Sci. USA. 92:7936–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan, D. H. 1974. Isolation of proteins of the Sarcoplasmic reticulum. Methods Enzymol. 32:291–302. [DOI] [PubMed] [Google Scholar]
- Mahmmoud, Y. A., and F. Cornelius. 2002. Protein kinase C phosphorylation of purified Na,K-ATPase: C-terminal phosphorylation sites at the α- and γ-subunits close to the inner face of the plasma membrane. Biophys. J. 82:1907–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmmoud, Y. A., H. Vorum, and F. Cornelius. 2000. Identification of a phospholemman-like protein from shark rectal glands. Evidence for indirect regulation of Na,K-ATPase by PKC via a novel member of the FXYD family. J. Biol. Chem. 275:35969–35977. [DOI] [PubMed] [Google Scholar]
- Murtazina, D., S. P. Petukhov, K. B. Storey, A. M. Rubtsov, and O. D. Lopina. 1999. Phosphorylation of H,K-ATPase α-subunit in microsomes from rabbit gastric mucosa by cAMP-dependent protein kinase. Biosci. Rep. 19:109–111. [DOI] [PubMed] [Google Scholar]
- Negash, S., H. Yao, J. Li, D. Bigelow, and T. C. Squier. 2000. Phospholamban remains associated with the Ca2+-and Mg2+-dependent ATPase following phosphorylation by cAMP-dependent protein kinase. Biochem. J. 351:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt, A., S. Becker, V. K. Khanna, K. Kurzydlowski, E. Leisner, D. Pette, and D. H. MacLennan. 1998. Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 273:12360–12369. [DOI] [PubMed] [Google Scholar]
- Palmer, C. J., B. T. Scott, and L. R. Jones. 1991. Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J. Biol. Chem. 266:11126–11130. [PubMed] [Google Scholar]
- Pedemonte, C. H., and A. M. Bertorello. 2001. Short-term regulation of proximal tubule Na,K-ATPase: Increased/decreased Na,K-ATPase activity mediated by protein kinase C isoforms. J. Bioenerg. Biomembr. 33:439–447. [DOI] [PubMed] [Google Scholar]
- Pedemonte, C. H., T. A. Pressley, M. F. Lokhandwala, and A. R. Cinelli. 1997. Regulation of Na,K-ATPase transport activity by protein kinase C. J. Membr. Biol. 155:219–227. [DOI] [PubMed] [Google Scholar]
- Peterson, G. L. 1977. A simplification of the protein assay method of Lowry et al., which is more generally applicable. Anal. Biochem. 83:346–444. [DOI] [PubMed] [Google Scholar]
- Sachs, G., H. H. Chang, E. Rabon, R. Schackman, M. Lewin, and G. Saccomani. 1976. A nonelectrogenic H+ pump in plasma membranes of hog stomach. J. Biol. Chem. 251:7690–7698. [PubMed] [Google Scholar]
- Sack, J. S. 1992. Isolation of a gastric phosphoprotein which stimulates acid secretion. J. Surg. Res. 53:166–169. [DOI] [PubMed] [Google Scholar]
- Sack, J. S. 1993. Regulation of gastric H+-K+-ATPase by cAMP-dependent protein kinase. J. Surg. Res. 55:223–227. [DOI] [PubMed] [Google Scholar]
- Segall, L., L. K. Lane, and R. Blostein. 2002. New insights into the role of the N terminus in conformational transitions of the Na,K-ATPase. J. Biol. Chem. 277:35202–35209. [DOI] [PubMed] [Google Scholar]
- Simmerman, H. K. B., and L. R. Jones. 1998. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol. Rev. 78:921–947. [DOI] [PubMed] [Google Scholar]
- Smolka, A., L. Alverson, R. Fritz, K. Swiger, and R. Swiger. 1991. Gastric H,K-ATPase topography: amino acids 888–907 are cytoplasmic. Biochem. Biophys. Res. Commun. 180:1356–1364. [DOI] [PubMed] [Google Scholar]
- Sweadner, K. J. 1991. Trypsin inhibitor paradoxically stabilizes trypsin activity in sodium dodecyl sulfate, facilitating proteolytic fingerprinting. Anal. Biochem. 194:130–135. [DOI] [PubMed] [Google Scholar]
- Sweadner, K. J., and E. Rael. 2000. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics. 68:41–56. [DOI] [PubMed] [Google Scholar]
- Therien, A. G., and R. Blostein. 2000. Mechanisms of sodium pump regulation. Am. J. Physiol. 279:C541–C566. [DOI] [PubMed] [Google Scholar]
- Togawa, K., T. Ishiguro, S. Kaya, A. Shimada, T. Imagawa, and K. Taniguchi. 1995. Reversible phosphorylation of both Tyr7 and Tyr10 in the α-chain of pig stomach H,K-ATPase by a membrane-bound kinase and a phosphatase. J. Biol. Chem. 270:15475–15478. [DOI] [PubMed] [Google Scholar]
- Togawa, K., S. Kaya, A. Shimada, T. Imagawa, S. Mårdh, J. Corbin, U. Kikkawa, and K. Taniguchi. 1996. Ser-27, Tyr-10, and Tyr-7 in the α-chain of pig stomach H,K-ATPase as Ca2+-dependent phosphorylatable sites by intrinsic and extrinsic protein kinases. Biochem. Biophys. Res. Commun. 227:810–815. [DOI] [PubMed] [Google Scholar]
- Tatulian, S. A., B. Chen, J. Li, S. Negash, C. R. Middaugh, D. Bigelow, and T. C. Squier. 2002. The inhibitory action of phospholamban involves stabilization of α-helices within the Ca-ATPase. Biochemistry. 41:741–751. [DOI] [PubMed] [Google Scholar]
- Wallmark, B., H. B. Stewart, E. Rabon, G. Saccomani, and G. Sachs. 1980. The catalytic cycle of gastric (H+ + K+)-ATPase. J. Biol. Chem. 255:5313–5319. [PubMed] [Google Scholar]
- Yudowski, G. A., R. Efendiev, C. H. Pedemonte, A. I. Katz, P.-O. Berggren, and A. M. Bertorello. 2000. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K+-ATPase alpha subunit and regulates its trafficking. Proc. Natl. Acad. Sci. USA. 97:6556–6561. [DOI] [PMC free article] [PubMed] [Google Scholar]