Abstract
Microorganisms were enumerated and isolated on selective solid media from a pilot-scale stirred-tank bioleaching operation in which a polymetallic sulfide concentrate was subjected to biologically accelerated oxidation at 45°C. Four distinct prokaryotes were isolated: three bacteria (an Acidithiobacillus caldus-like organism, a thermophilic Leptospirillum sp., and a Sulfobacillus sp.) and one archaeon (a Ferroplasma-like isolate). The relative numbers of these prokaryotes changed in the three reactors sampled, and the Ferroplasma isolate became increasingly dominant as mineral oxidation progressed, eventually accounting for >99% of plate isolates in the third of three in-line reactors. The identities of the isolates were confirmed by analyses of their 16S rRNA genes, and some key physiological traits (e.g., oxidation of iron and/or sulfur and autotrophy or heterotrophy) were examined. More detailed studies were carried out with the Leptospirillum and Ferroplasma isolates. The data presented here represent the first quantitative study of the microorganisms in a metal leaching situation and confirm that mixed cultures of iron- and sulfur-oxidizing prokaryotic acidophiles catalyze the accelerated dissolution of sulfidic minerals in industrial tank bioleaching operations. The results show that indigenous acidophilic microbial populations change as mineral dissolution becomes more extensive.
The use of microorganisms to recover metals from low-grade ores and mineral concentrates has developed into a successful and expanding area of biotechnology (32). The microbes catalyze metal recovery either by dissolution of metal-containing sulfide minerals, such as chalcocite (bioleaching), or by dissolving sulfidic minerals that are intimately associated with the native metal (biooxidation), such as gold in refractory ores, thereby allowing the metal to be extracted by conventional (chemical) means. Different engineering approaches have been used to facilitate microbial mineral processing; these approaches include in situ leaching, dump and heap leaching of low-grade ores, and aerated stirred tanks for microbial processing of mineral concentrates (3). Stirred tanks have several advantages, including the potential to control the bioleaching environment (e.g., pH and temperature) and much shorter turnover times (the times required for mineral processing to be effectively completed), although such systems have large capital and operating costs. Mineral processing operations that use bioreactors generally involve parallel and in-line (primary and secondary) oxidation tanks in order to maximize mineral dissolution.
Bioreactor mineral processing initially focused on the treatment of refractory gold sulfide ores. Successful commercial technologies include the BIOX process (5), which operates at ∼40°C by using mesophilic acidophiles and the Mintek/Bactech Bacox process, in which utilizes either mesophilic or moderately thermophilic cultures (26). However, the potential to recover other metals, such as Cu, Ni, Zn, and Co, was recognized later. In the late 1990s, a full-scale cobalt leaching operation in which bioreactors are used was commissioned in Uganda (6). Other examples include the Mintek/Bactech process to bioleach base metals (37), which operates at higher temperatures (∼45 to 50°C) with moderately thermophilic acidophiles, and the HIOX process (7), which operates at 78°C with extremely thermophilic acidophilic archaea. The advantage of using higher temperatures is that sulfide mineral oxidation is more rapid, and although mesophilic and thermophilic operations involve different populations of acidophilic microorganisms, the mechanisms involved in mineral dissolution are essentially the same.
Somewhat surprisingly, there have been relatively few studies on the composition of microbial populations in commercial mineral leaching operations (27). Goebel and Stackebrandt (13, 14) cloned 16S rRNA genes from acidophilic bacteria obtained from enrichment cultures of acidic runoff from a chalcocite overburden heap and a laboratory-scale mineral leaching bioreactor. Gene sequence data indicated that there was limited biodiversity in both situations, particularly the bioreactor leachate, in which the microflora appeared to comprise Leptospirillum ferrooxidans-, Sulfobacillus-, and Acidithiobacillus caldus-like bacteria. Pizarro et al. (31) used the different sizes of spacer regions between the 16S and 23S rRNA genes of acidophiles to identify microbial populations in a copper bioleaching system; Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans, as well as L. ferrooxidans, were identified by this approach. Differentiation of acidophilic microorganisms by analysis of restriction enzyme-digested 16S rRNA genes has also been described. Using this technique, Rawlings et al. (33) identified A. caldus and L. ferrooxidans in continuous-flow biooxidation tanks processing various metallic ores and operating at 40 to 55°C. Foucher et al. (12) analyzed microbial populations in a stirred-tank bioreactor and an aerated column reactor operating at 35°C and processing cobaltiferous pyrite by extracting DNA, amplifying 16S rRNA genes, and sequencing screened clones. Three bacteria were identified: L. ferrooxidans, A. caldus, and a gram-positive bacterium related to Sulfobacillus thermosulfidooxidans. The single-strand conformation polymorphism technique was used in the latter study to evaluate the proportions of the bacteria that were either planktonic or attached to mineral surfaces.
In this paper, the numerical abundance and diversity of microorganisms in pilot-scale stirred tanks in which a polymetallic sulfidic concentrate was subjected to biological oxidation at 45°C are reported. In addition, we describe the physiological characteristics of two of the indigenous microorganisms, a thermophilic Leptospirillum isolate and an archaeon belonging to the genus Ferroplasma.
MATERIALS AND METHODS
Pilot plant setup.
The pilot-scale biooxidation experiments from which the acidophilic prokaryotes were isolated were performed in three-stage continuously operated and mechanically agitated reactors (stirred tanks). The system consisted of a feed pulp tank and three reactors in series, and there was a container at the end for product collection. The pulp volumes were 20 liters in the first reactor and 10 liters in both the second and third reactors. The concentrate (fine milled to diameters of <45 μm) contained 22% (wt/wt) copper, 23% (wt/wt) iron, 8% (wt/wt) zinc, and 30% (wt/wt) sulfur, and the major minerals were chalcopyrite, sphalerite, and pyrite. The operating temperature was 45°C in each reactor, and the pH was controlled at ≤1.8. The concentrate was added as a homogeneous slurry (delivered from a stirred feed tank) with a pulp density of 7.5% (wt/vol), and it had an overall residence time of 6 days. The air supplied to the reactors was enriched with CO2 (0.2%, vol/vol) and was supplied to the reactors by means of a sparger situated below the impeller. The pilot plant was part of a bioleaching optimization program that had been running for more than 1 year, initially in the batch mode and later in the continuous mode. The system had been inoculated with an undefined culture of moderately thermophilic acidophiles that had previously been maintained on a similar concentrate. The samples used for microbiological analysis were taken a few months after continuous operation was started. The concentrations of soluble metals, pH values, and cumulative residence times for the three reactors are shown in Table 1.
TABLE 1.
Conditions in the reactors of the pilot-scale biooxidation plant
| Reactor | pH | Cumulative residence time (days) | Concn (g/liter) of:
|
|||
|---|---|---|---|---|---|---|
| Soluble Cu | Soluble Fea | Soluble Zn | Sulfate | |||
| 1 | 1.6 | 3 | 17 | 13 | 6.5 | 65 |
| 2 | 1.5 | 4.5 | 19 | 14 | 7 | 67 |
| 3 | 1.3-1.4 | 6 | 20 | 15 | 7 | 70 |
The iron was present predominantly as ferric iron.
Isolation and enumeration of acidophilic microorganisms.
The indigenous microfloras of leachate samples from each of the three reactors were analyzed by plating serially diluted samples onto overlaid solid media. These two-layer, agarose-gelled solid media included an acidophilic heterotroph (Acidiphilium sp. strain SJH) which removed the otherwise toxic products of agarose hydrolysis that were present at an acidic pH. Two variants were used, one containing ferrous sulfate (iron overlay) and the other containing both ferrous sulfate and potassium tetrathionate (iron-sulfur overlay); full descriptions of these solid media are given elsewhere (19). Overlay media are used extensively in our laboratory and in a number of other research laboratories that work with acidophilic microorganisms and have been shown to be both highly efficient and selective for cultivating both autotrophic and heterotrophic acidophiles. The mineral suspensions were vortexed thoroughly before dilution to promote detachment of cells adhering to the mineral surfaces. Inoculated plates were incubated at 45°C for up to 20 days and were inspected daily for microbial growth. Colonies that formed were examined with a stereo-scan microscope (Wild M3Z), and bacteria were examined with a phase-contrast microscope (Leitz Labolux fitted with Zenicke condenser and objective lenses). The counts for the various colony types that formed on the solid media were expressed as CFU per milliliter of leachate liquor. Single colonies were carefully removed and put into liquid medium containing 20 mM ferrous sulfate plus 2.5 mM potassium tetrathionate or into 10 mM ferrous sulfate-0.025% (wt/vol) yeast extract liquid medium (both at pH 2.0). The liquid medium used to culture the Ferroplasma-like isolates contained 50 mM ferrous sulfate, 50 mM potassium sulfate, 0.02% (wt/vol) yeast extract, and basal salts (pH 1.5), and all cultures were incubated at 45°C (19). Culture purity was checked by streaking liquid cultures onto solid media (with reselection of single colonies, if necessary).
Amplification and sequencing of 16S rRNA genes.
Isolates were grown in the appropriate medium to the late exponential phase or the early stationary phase, harvested, and washed once with 10 mM H2SO4 and then with TE buffer (10 mM Tris, 1 mM EDTA; pH 8.0). Cell pellets were resuspended in 20 μl of a lysis solution (0.05 M NaOH, 0.25% sodium dodecyl sulfate) and heated at 95°C for 10 min before 180 μl of autoclaved deionized water was added. Eubacterial 16S rRNA genes were amplified by PCR by using forward primer 5′-AGAGTTTGATCMTGGCTCAG-3′ and reverse primer (5′-TACGGYTACCTTGTTACGACTT-3′) complementing positions 8 to 27 and 1510 to 1492 of Escherichia coli 16S rRNA (23). Archaeal 16S rRNA genes were amplified with the same reverse primer and an archaeon-specific forward primer (5′-TCCGGTTGATCCYGCCRG-3′) (29). Touchdown PCR (8) was performed as follows: denaturation for 30 s at 95°C; annealing for 30 s at 55°C (57°C for archaea), with the temperature dropping by 1°C each 2 cycles for 20 cycles, followed by an additional 15 cycles of annealing at 45°C (47°C for archaea); elongation at 72°C for 90 s; and a final 10-min incubation period at 72°C. Dimethyl sulfoxide (2%, vol/vol) was routinely included in the PCR mixture, as this compound has been found to increase the reliability of 16S rRNA gene amplification with a some acidophilic microorganisms (Hallberg, unpublished data). PCR products were ligated to the pGEM-T Easy vector (Promega) according to the manufacturer's instructions, and the resulting plasmids were transformed into E. coli DH5α. Screening of clones with inserts (white colonies) was carried out by PCR by using primers M13 forward (5′-GTAAAACGACGGCCAG-3′) and M13 reverse (5′-CAGGAAACAGCTATGAC-3′) that are specific for the cloning vector. The resulting product was confirmed to be positive by restriction enzyme fragment analysis (double digestion with EcoRI and MspI) alongside the original PCR product used in the cloning. Plasmid DNA was purified by using the CONCERT rapid plasmid purification systems (GIBCO BRL) according to the manufacturer's instructions. The sequences of the inserts were determined commercially (MWG-Biotech, Ebersberg, Germany).
Phylogenetic affiliation of isolates.
The sequence data were compared with 16S rRNA sequences deposited in public databases by using the BLAST search program (1). The 16S rRNA gene sequences of various bacteria (including those closely related to the unknown sequences, as indicated by the BLAST search) obtained from the GenBank database were aligned with the new sequences by using ClustalW (36). The resulting alignments were then used to construct a distance matrix (21), which was followed by phylogenetic tree construction by neighbor joining (34). The algorithms provided by PHYLIP, version 3.5c (11), were used. Phylogenetic trees were constructed by using Treeview software (30).
Chromosomal base analysis.
Isolates MT6 and MT17 were grown in liquid media to the early stationary phase. Chromosomal DNA was purified by the technique described by Wilson (38), including cesium chloride gradient centrifugation. The DNA extracted from the gradients was dialyzed against three changes of 0.1× SSC (15 mM NaCl plus 1.5 mM Na3C6H5O7), and the ethidium bromide was extracted from the DNA with 0.1× SSC-saturated butanol.
Guanine plus cytosine (G+C) contents were determined from melting temperatures of the DNA, which were determined with a Hewlett-Packard HP 8453A UV-visible spectrophotometer connected to an HP 89090A Peltier temperature controller. The base composition of a DNA was determined from its melting point by using the equation G+C = 2.44[(Tm − 81.5) − (16.6log M)], where Tm is the melting temperature of the DNA and M is the molar concentration of the cations in the DNA suspension (25). The system was calibrated by using DNA having known base compositions (G+C contents) from several sources, including Micrococcus luteus (G+C content, 72 mol%), Acidocella facilis (64 mol%), Acidocella aminolytica (58 mol%), E. coli strain B (50 mol%), and calf thymus (42 mol%).
Physiological analyses.
Ferrous iron oxidation was monitored by measuring changes in the ferrous iron concentration by the ferrozine method (24) in cultures initially containing 25 mM FeSO4. Utilization of tetrathionate in cultures initially containing 5 mM S4O62− was determined by measuring changes in the concentration of this polythionate by cyanolysis (22) and also by measuring any decreases in the culture pH with a pHase electrode (BDH-Merck) coupled to an Accumet pH Meter 50. Autotrophic growth and heterotrophic growth were determined by culturing isolates in ferrous iron or tetrathionate medium with and without yeast extract (added at a concentration of 0.02% [wt/vol]) and assessing the biomass yield by examining bacterial counts (with a Thoma cell counting chamber) and total protein (2).
The pH and temperature optima of isolates MT6 and MT17 were determined in pH- and temperature-controlled cultures in a 2-liter bioreactor (model P350; Electrolab Ltd., Tewkesbury, United Kingdom). Isolate MT6 was grown in 25 mM ferrous sulfate medium with the culture maintained at pH 1.8 (to determine the optimum temperature) or at 43°C (to determine the optimum pH). Isolate MT17 was grown in 25 mM ferrous sulfate medium supplemented with yeast extract (0.02%, wt/vol), tryptone soya broth (0.0125%, wt/vol), and 0 to 300 mM potassium sulfate (to examine the effect of culture conductivity on growth). The cultures were maintained at pH 1.5 (to determine the optimum temperature) or at 37.5°C (to determine the optimum pH). The culture doubling times of MT6 and MT17 for each experimental run were determined from semilogarithmic plots of the amount of iron oxidized versus time.
Oxidation of pyrite by isolates MT6 and MT17 was assessed by growing cultures in 100-ml shake flasks (in triplicate), each of which contained 2% (wt/vol) ground rock pyrite (80% FeS2). For MT17, a second set of triplicate flasks was set up, to which yeast extract (final concentration, 0.02% [wt/vol]) was added. The inoculated flasks were incubated at 45°C (MT6) or 37°C (MT17) with shaking (150 rpm) for up to 60 days. Additional yeast extract (final concentration, 0.02% [wt/vol]) was added to the MT17 cultures grown on pyrite-yeast extract at day 42. Samples were removed at regular intervals, and the soluble total iron was analyzed by atomic absorption spectrometry.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the nucleotide sequences determined in this study are shown in Table 2.
TABLE 2.
Nearest relatives, based on 16S rRNA gene sequence homology, of the predominant isolates from the Mintek biooxidation plant
| Isolate | Length of 16S rRNA gene sequenced (bp) | GenBank accession no.a | Most closely related organism
|
||
|---|---|---|---|---|---|
| Strain | Accession no. | % Gene homology | |||
| MT1 | 1,462 | AF513711 | A. caldus type strain | Z29975 | 99.5 |
| MT2 | 496 | MT1 | 99.6 | ||
| MT6 | 1,484 | AF513709 | L. ferriphilum type strain | AF356829 | 99.5 |
| NC | 1,438 | AY121610 | “Sulfobacillus yellowstonensis” YTF1 | AY007665 | 98.9 |
| MT16 | 1,401 | F. acidiphilum type strain | AJ224936 | 99.6 | |
| MT17 | 1,400 | AF513710 | F. acidiphilum type strain | AJ224936 | 99.6 |
The genes from MT2 and MT16 are nearly identical to the genes from MT1 and MT17, respectively, and have not been deposited in the GenBank database.
RESULTS
Isolation and identification of indigenous acidophiles.
Colonies of acidophilic prokaryotes grew on both iron and iron-tetrathionate overlay plates when serially diluted samples from the in-line bioreactors were spread onto them. The organisms could be differentiated on the basis of size, morphology, and whether they were stained with ferric iron (iron oxidizers). Acidophilic prokaryotes generally form distinctive colonies on overlay plates, and this characteristic may be used as an initial criterion for distinguishing different genera and species (19). With the bioreactor inocula, four distinct colony forms were identified (Table 3). Three of these forms, groups I to III, were visible after 7 days of incubation at 45°C, while group IV colonies were apparent only after more protracted incubation (14 days). Groups I and IV grew only on iron-tetrathionate plates, group II grew only on iron overlay plates, and group III grew on both types of overlay plates.
TABLE 3.
Phenotypic characteristics of the six representative isolates obtained from the biooxidation reactors
| Group | Representative isolate(s) | Colony morphology | Cellular morphology | Carbon metabolism | Oxidation of:
|
Preliminary identification | ||
|---|---|---|---|---|---|---|---|---|
| Fe2+ | FeS2 | S4O62− | ||||||
| I | MT1, MT2 | Domed, entire, off-white | Motile straight rods | Autotrophic | − | − | + | A. caldus |
| II | MT6 | Flat, entire, small, orange-brown, Fe3+ encrusted | Motile curved rods | Autotrophic | + | + | − | Leptospirillum |
| III | NC | Domed, irregular, orange-brown centers with off-white peripheries (fried egg-like) | Nonmotile sporulating rods | Mixotrophic | +a | +a | +a | Sulfobacillus |
| IV | MT16, MT17 | Raised, entire, small, fried egg-like | Irregular cocci to pleomorphic | Heterotrophic | +a | +a | +a | Archaea |
Oxidation only in yeast extract-amended cultures.
Three of the four groups of isolates (groups II, III, and IV) were ferrous iron oxidizers, while group I isolates oxidized tetrathionate but not ferrous iron. The preliminary classification of the prokaryotes was based on their cellular and physiological characteristics (Table 3). Identities were later confirmed by phylogenetic analysis (16S rRNA gene sequence analysis) of the six representative isolates selected (Table 2). Although the tetrathionate-oxidizing isolates MT1 and MT2 displayed slightly different colony morphologies, the first 496 bp of the 16S rRNA gene of isolate MT2 was found to exhibit 99.6% homology with the same sequence of isolate MT1. Likewise, isolates MT16 (isolated from tank 3) and MT17 (isolated from tank 2) exhibited 99.6% homology, although they had some minor physiological differences. The phylogenetic relationships of the prokaryotes isolated from the bioreactors with known acidophilic microorganisms are shown in Fig. 1.
FIG. 1.
Neighbor-joining tree showing the relationship of the isolates from the Mintek biooxidation plant to other iron- and sulfur-oxidizing acidophiles. The topology of this tree is supported by DNA parsimony analysis. Scale bar = 0.1 nucleotide change per 100 for the horizontal branch lengths. S., Sulfolobus; Sb., Sulfobacillus.
Distribution of acidophilic prokaryotes.
The numbers of each of the four microbial genera isolated from leachate liquors from the three bioreactors are shown in Fig. 2. In the first tank, the most numerous cultivatable prokaryotes (65% of the CFU) were A. caldus-like bacteria, and Leptospirillum-like bacteria (29% of the CFU) were the dominant iron oxidizers, followed by Sulfobacillus-like bacteria (6% of the CFU). The indigenous microflora in the second tank was very different; Ferroplasma-like archaea (strains MT16 and MT17) accounted for about one-half of the total CFU, and most of the rest of the organisms were A. caldus. In tank 3, >99.9% of the CFU were Ferroplasma-like; colonies of A. caldus and Sulfobacillus (but not colonies of Leptospirillum) were also recovered, although only low numbers of colonies were obtained. The total numbers of acidophilic CFU isolated from the three bioreactors increased slightly as mineral oxidation occurred; the values were 5.2 × 105 CFU ml−1 in tank 1, 9.8 × 105 CFU ml−1 in tank 2, and 2.4 × 106 CFU ml−1 in tank 3.
FIG. 2.
Plate counts for acidophiles in each of the three in-line bioreactors. The microbes were grouped and tentatively identified by using the criteria shown in Tables 2 and 3.
Physiological characteristics of the Leptospirillum and Ferroplasma isolates.
Isolates MT6 and MT17 were selected for further study on the basis of their potential novelty; MT6 was an apparent thermophilic (or thermotolerant) Leptospirillum sp. isolate, while MT17 was a member of a species of the recently described archaeal genus Ferroplasma. The G+C content of the chromosomal DNA of Leptospirillum strain MT6 was 55 ± 0.3 mol%, while that of Ferroplasma strain MT17 was 37 ± 0.2 mol%; these values are similar to those obtained for Leptospirillum ferriphilum and Ferroplasma acidiphilum, respectively (4, 16).
Leptospirillum strain MT6 was found to have a minimum culture doubling time of about 2 h, a temperature optimum of 43°C, and a pH optimum of 1.5 (Fig. 3). The maximum temperature at which growth of Leptospirillum strain MT6 was observed was 50°C. Growth-coupled iron oxidation by Leptospirillum strain MT6 was observed at pH values between 0.8 and 2.0 (pH values above and below these values were not tested). Interestingly, culture pH had only a small impact on the doubling time of this isolate over this pH range.
FIG. 3.
Effects of temperature (a) and pH (b) on the growth of Leptospirillum strain MT6 (•) and Ferroplasma strain MT17 (▴). Each data point is the mean for two analyses (variation, <10%).
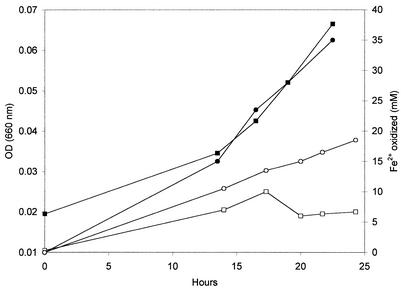
The Ferroplasma-like isolate, strain MT17, was less thermotolerant than Leptospirillum strain MT6, and its temperature optimum was 39°C. Its minimum culture doubling time (observed at pH 1.5) was about 7 h (Fig. 3). Ferrous iron oxidation was coupled to the growth of this archaeon under most conditions, although not at 50°C, at which the rate of ferrous iron oxidation (0.76 mM/h) was found to be arithmetic rather than logarithmic and there was no increase in cell number (Fig. 4). A similar scenario was observed at pH 0.55, although logarithmic growth of Ferroplasma strain MT17 was observed at pH 0.8. The concentrations of total dissolved ions (estimated from the medium conductivity) in the growth medium had little impact on the growth of Ferroplasma strain MT17 at values between 24 and 46 mS cm−1, although at medium conductivity values of 16 and 61 mS cm−1 ferrous iron oxidation by this archaeon was again not coupled to growth (data not shown). Ferroplasma strain MT17 was able to grow heterotrophically on yeast extract (in media in which ferric sulfate was substituted for ferrous sulfate) and to reduce ferric iron in yeast extract-containing media incubated anaerobically. Whether Ferroplasma strain MT17 could grow anaerobically by ferric iron respiration was not ascertained.
FIG. 4.
Growth (▪ and □) and ferrous iron oxidation (• and ○) by Ferroplasma strain MT17 at 45°C (• and ▪) and at 50°C (○ and □). Cultures were grown under controlled conditions (at pH 1.5) in a 2-liter bioreactor. OD (660 nm), optical density at 660 nm.
Leptospirillum strain MT6 was found to be obligately autotrophic, while no growth of Ferroplasma strain MT17 (or strain MT16) occurred in media that did not contain yeast extract. Inclusion of yeast extract at a concentration of >0.02% (wt/vol) did not result in increased growth yields of either of the Ferroplasma isolates, although the yields were significantly smaller in cultures that contained either 0.005 or 0.01% yeast extract than in cultures that contained the standard concentration (0.02%). Addition of glycerol (at a concentration of 10 mM) did not increase the growth yield of either of the archaea, although cultures of Ferroplasma strain MT16 (but not cultures of strain MT17) showed evidence of limited glucose utilization (resulting in greater protein yields) following repeated subculturing in media containing 10 mM glucose (data not shown).
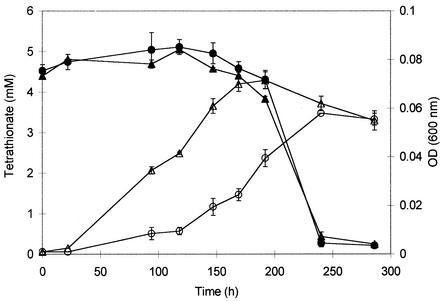
Both Leptospirillum strain MT6 and Ferroplasma strain MT17 were able to oxidize pyrite in pure cultures, although this was observed only in yeast extract-amended media in the case of the archaeon (Fig. 5). Whereas Leptospirillum strain MT6 (like other Leptospirillum spp.) was unable to oxidize tetrathionate, oxidation of this polythionate was observed in cultures of Ferroplasma strains MT16 and MT17 in 5 mM tetrathionate-0.02% yeast extract medium after prolonged lag periods of about 190 h (Fig. 6).
FIG. 5.
Leaching of pyrite by Leptospirillum strain MT6 (○) and by Ferroplasma strain MT17 (▵) in pyrite-basal salts medium and by Ferroplasma strain MT17 in the same medium amended with 0.02% (wt/vol) yeast extract (▴). The data points are the means for triplicate cultures, and the standard deviations were generally <5%. The arrow indicates the point at which additional yeast extract (0.02%, wt/vol) was added to the yeast extract-containing Ferroplasma cultures.
FIG. 6.
Growth, as measured by optical density at 600 nm [OD (600 nm)] (○ and ▵), and oxidation of tetrathionate (• and ▴) by Ferroplasma isolates MT16 (○ and •) and MT17 (▵ and ▴).
DISCUSSION
It is somewhat anomalous that commercial bioprocessing of sulfidic minerals has developed into an important and successful area of biotechnology with (in most cases) very limited data on the microbial populations that are present in bioleaching systems. One reason for this has been the lack of accurate and appropriate methods for analyzing populations of the extremophilic prokaryotes that are active in the metal-enriched, (thermo)acidic environments that constitute commercial leach liquors. Molecular techniques (gene libraries constructed from extracted DNA, amplified ribosomal DNA restriction enzyme analysis, fluorescence in situ hybridization, etc.) have been used to some effect in recent years (12, 13, 33). Some potential drawbacks of these techniques are (i) that problems with DNA extraction and other inherent biases in the techniques result in data that are not quantitative and may result in a failure to detect some microorganisms altogether; (ii) that the techniques may require preknowledge of the actual or expected indigenous microorganisms to be effective (e.g., fluorescence in situ hybridization); and (iii) that the indigenous microorganisms are not isolated. The plating technique used in the present study offers an alternative approach and has been shown to be highly effective (in terms of plating efficiency, etc.) for enumerating and isolating (often novel) acidophiles from acid mine drainage and other environmental samples (20). In the present study, limited biodiversity of moderately thermophilic or thermotolerant acidophiles was detected in a pilot-scale mineral leaching operation. While it is possible that other species of bacteria and archaea were present but were not isolated by the methods used, it has been shown in other work that all known species of mesophilic and moderately acidophilic mineral-oxidizing prokaryotes (and the majority of acidophilic heterotrophs) can be cultured on the two overlay plate variants used in the present study (19).
Data from the 45°C bioreactors used in this study showed that the indigenous mineral-oxidizing microfloras were both diverse and subject to change as leaching progressed. The primary microbial agents in mineral leaching are generally considered to be the organisms, such as Leptospirillum spp., that catalyze the dissimilatory oxidation of ferrous iron to ferric iron, since the latter is the prime oxidant of most sulfide minerals. In the case of pyrite (FeS2), ferric iron attack on a mineral produces ferrous iron (which is reoxidized by iron-oxidizing acidophiles) and reduced inorganic sulfur compounds (RISCs). The latter are metabolized by sulfur-oxidizing acidophiles, such as A. caldus. Somewhat paradoxically, since oxidation of RISCs yields considerably more energy than oxidation of ferrous iron, greater numbers of A. caldus than of iron-oxidizing prokaryotes are often present in mineral leachates (such as that in reactor 1 of the present study) even though this bacterium is unable to oxidize metal sulfides in pure culture (9, 17). Some bacteria, such as A. ferrooxidans and Sulfobacillus spp., are able to oxidize both ferrous iron and RISCs and may be perceived, therefore, to be better able to exploit mineral leaching environments than more specialized iron or sulfur oxidizers. However, other factors (such as relative substrate affinities and temperature and pH sensitivities) may have overriding effects, with the result that these bacteria have minor roles (at best) in commercial bioleaching operations.
Analysis of the microbial communities in the pilot-scale bioleaching plant was carried out on only one occasion. However, the fact that the plant had been operating for about 1 year before this analysis suggests that the system should have reached a steady state with regard to the prokaryotic populations present in the three in-line tanks. In addition, previously published data (31, 33) have shown that mineral bioleaching populations are often dominated by the sulfur-oxidizing organism A. caldus and Leptospirillum-like bacteria are the major iron-oxidizing bacteria. A similar scenario was found in the first of the in-line stirred tanks in the present study. The reasons why the microbial populations changed as leaching of the polymetallic sulfidic concentrate progressed (i.e., in reactors 1 to 3) are probably related to the evolution of the physicochemical conditions in the leach liquor. Mineral leaching is generally carried out in an essentially inorganic environment. No organic materials are added to the systems (although there may be some carryover of the flotation reagents use to concentrate the original ore), and carbon is often provided (as in this case) as carbon dioxide. Such conditions favor the growth of chemolithotrophic acidophiles, such as Leptospirillum spp. and A. caldus, which together accounted for 94% of the total CFU in reactor 1. Sulfobacillus spp. (such as isolate NC) are mixotrophic; their CO2 fixation systems tend to be less efficient than those of autotrophic and other mixotrophic acidophiles (such as Acidimicrobium ferrooxidans). This may account for the relatively low percentage of Sulfobacillus CFU recovered from reactor 1. Although greater numbers (about 50% greater) of A. caldus were found in leachate liquor from reactor 2 than in leachate liquor from reactor 1, the most numerous prokaryote was a Ferroplasma isolate. The emergence of this iron oxidizer coincided with decreases in the numbers of both Leptospirillum and Sulfobacillus. This trend continued into reactor 3, from which only Ferroplasma and A. caldus were recovered (the lower detection limit with the dilution series used was 102 CFU ml−1), and the number of A. caldus CFU in reactor 3 was about 0.1% of the numbers found in reactors 1 and 2. The leachate liquor became increasingly acidic and enriched with dissolved metals and sulfate with passage through the reactors. Both these factors are likely to favor Ferroplasma (10). However, the pH values in tanks 2 and 3 were well within the range tolerated by the Leptospirillum isolate (MT6) and known strains of A. caldus (17, 28). One other important factor that probably facilitated the growth of Ferroplasma in the last two reactors was organic carbon. Dissolved organic carbon (DOC) accumulates in cultures of autotrophic acidophiles, originating from both cell lysates and exudates (35). Although DOC was not measured in the leach liquors in the tanks, shake flask experiments with mixed cultures of Leptospirillum strain MT6 and A. caldus leaching pyrite have shown that up to 100 mg of DOC liter−1 may accumulate within 25 days (Okibe and Johnson, unpublished data).
Besides providing information on microbial communities in bioleaching liquors, this study led to isolation of two novel moderately thermophilic (or thermotolerant) iron oxidizers that may potentially be important in commercial bioleaching operations. Leptospirillum spp. are known to be the most significant iron-oxidizing microorganisms in bioleaching systems that operate at about 40°C (4). The isolation of a Leptospirillum-like bacterium from an operation that was maintained at 45°C was interesting, although Rawlings et al. (33) also reported that they found Leptospirillum-like bacteria in 45°C mineral leaching bioreactors. Currently, there are three recognized species of Leptospirillum: L. ferrooxidans, L. ferriphilum, and L. thermoferrooxidans (4, 18). All known strains of L. ferrooxidans (G+C content, 49 to 52 mol%) are mesophilic (upper temperature limit, <45°C), while some strains of L. ferriphilum (G+C content, 55 to 58 mol%) can oxidize iron at 45°C, although the temperature optima for growth tend to be several degrees lower (4). Unfortunately, the single isolate of L. thermoferrooxidans (15), which had a temperature optimum of 45 to 50°C and a temperature maximum of 55°C, has been lost (G. I. Karavaiko, personal communication). The 16S rRNA gene of this isolate was not sequenced, although its chromosomal base content (G+C content, 56 mol%) was similar to those of both L. ferriphilum (55 to 58 mol%) and isolate MT6 (55.2 mol%), and it is likely that this bacterium (like isolate MT6) is more closely related to L. ferriphilum than to L. ferrooxidans. On the other hand, the growth rate of Leptospirillum strain MT6 (minimum culture doubling time [td], ∼2 h) is much higher than those of both L. ferrooxidans and L. ferriphilum (td, 11 to 12 h), although it is similar to that of L. thermoferrooxidans (td, 1.7 h). Leptospirillum strain MT6, which has a minimum pH for growth of <0.8, compared with minimum pH values for growth of 1.1 to 1.3 for the species that have been characterized, is the most acidophilic Leptospirillum isolate yet characterized, although precise identification of this organism requires further work (e.g., DNA hybridization studies).
The situation with isolate MT17 (the more thoroughly studied of the two archaeal isolates) is slightly different, as it is only the third Ferroplasma strain to be characterized. There is currently only one recognized species of Ferroplasma, F. acidiphilum, which was also isolated from a bioleaching pilot plant (16), although a related archaeon with the proposed name “Ferroplasma acidarmanus” has also been described (10). Both organisms are pleomorphic and lack cell walls. F. acidiphilum was described as an obligate autotroph, although inclusion of yeast extract in culture media was found to be essential for growth of this prokaryote (16). “F. acidarmanus,” like isolate MT17, grows heterotrophically (10). There are also differences in the pH optima and minima of “F. acidarmanus,” isolate MT17, and F. acidiphilum (pH 1.2 and 0.0, pH 1.5 and 0.8, and pH 1.7 and 1.3, respectively). Ferroplasma strain MT17 was much less thermotolerant than Leptospirillum strain MT6 and, like the other Ferroplasma isolates, is a thermotolerant prokaryote rather than a moderately thermophilic prokaryote. All three isolates are also sensitive to dissolved solutes, as might be expected for organisms that lack cell walls; “F. acidarmanus” was found to dominate sites at Iron Mountain in California, where the solution conductivity was between 100 and 160 mS cm−1, whereas growth of isolate MT17 was optimum at a conductivity range of 24 to 46 mS cm−1. In contrast to these differences in physiological traits, the three Ferroplasma isolates have very similar (99.2 to 99.6%) 16S rRNA gene sequences, and F. acidiphilum and isolate MT17 have similar chromosomal base compositions (36.5 and 37.5 mol% G+C, respectively). It is quite possible, therefore, that F. acidiphilum, “F. acidarmanus,” and Ferroplasma strain MT17 are strains of the same species (F. acidiphilum) and that the variations described above are due to differences in the media used in the various studies.
The data obtained in this study represent the first quantitative study of the microorganisms in a metal leaching situation. Through the use of solid media, changes in the microbial population were observed throughout a pilot-scale biooxidation plant. In addition to providing quantitative data for the microbes, the solid media allowed isolation and subsequent characterization of novel acidophiles that may be important in commercial operations, which showed the importance of cultivation-based studies of acidic environments.
Acknowledgments
Naoko Okibe is grateful for financial assistance provided by The Institution of Mining and Metallurgy (United Kingdom), the Gen Foundation, and Glaxo Ltd.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Brierley, C. L. 1997. Mining biotechnology: research to commercial development and beyond, p. 3-17. In D. E. Rawlings (ed.), Biomining: theory, microbes and industrial processes. Springer-Verlag/Landes Bioscience, Georgetown, Tex.
- 4.Coram, N. J., and D. E. Rawlings. 2002. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates South African commercial biooxidation tanks that operate at 40°C. Appl. Environ. Microbiol. 68:838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dew, D. W., E. N. Lawson, and J. L. Broadhurst. 1997. The BIOX process for biooxidation of gold-bearing ores or concentrates, p. 45-80. In D. E. Rawlings (ed.), Biomining: theory, microbes and industrial processes. Springer-Verlag/Landes Bioscience, Georgetown, Tex.
- 6.d'Hugues, P., P. Cezac, F. Battaglia, and D. Morin. 1999. Bioleaching of a cobaltiferous pyrite at 20% solids: a continuous laboratory-scale study, p. 167-176. In R. Amils and A. Ballester (ed.), Biohydrometallurgy and the environment toward the mining of the 21st century, vol. 9A. Elsevier, Amsterdam, The Netherlands.
- 7.d'Hugues, P., D. Morin, and S. Foucher. 2001. HIOX project: a bioleaching process for the treatment of chalcopyrite concentrates using extreme thermophiles, p. 75-83. In V. S. T. Ciminelli and O. Garcia, Jr. (ed.), Biohydrometallurgy: fundamentals, technology and sustainable development, vol. 11A. Elsevier, Amsterdam, The Netherlands.
- 8.Don, R. H., P. T. Cox, B. J. Wainwright, K. Baker, and J. S. Mattick. 1991. Touchdown PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 19:4008. [DOI] [PMC free article] [PubMed]
- 9.Edwards, K. J., P. L. Bond, and J. F. Banfield. 2000. Characteristics of attachment and growth of Thiobacillus caldus on sulphide minerals: a chemotactic response to sulphur minerals? Environ. Microbiol. 2:324-332. [DOI] [PubMed] [Google Scholar]
- 10.Edwards, K. J., P. L. Bond, T. M. Gihring, and J. F. Banfield. 2000. An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 287:1796-1799. [DOI] [PubMed] [Google Scholar]
- 11.Felsenstein, J. 1989. PHYLIP—phylogeny inference package (version 3.5c). Caldistics 5:164-166. [Google Scholar]
- 12.Foucher, S., F. Battaglia-Brunet, P. d'Hugues, M. Clarens, J. J. Godon, and D. Morin. 2001. Evolution of the bacterial population during the batch bioleaching of a cobaltiferous pyrite in a suspended-solids bubble column, and comparison with a mechanically agitated reactor, p. 3-11. In V. S. T. Ciminelli and O. Garcia, Jr. (ed.), Biohydrometallurgy: fundamentals, technology and sustainable development, vol. 11A. Elsevier, Amsterdam, The Netherlands.
- 13.Goebel, B. M., and E. Stackebrandt. 1994. The biotechnological importance of molecular biodiversity studies for metal bioleaching. FEMS Symp. 75:259-273. [Google Scholar]
- 14.Goebel, B. M., and E. Stackebrandt. 1994. Cultural and phylogenetic analysis of mixed microbial populations found in natural and commercial bioleaching environments. Appl. Environ. Microbiol. 60:1614-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golovacheva, R. S., O. V. Golyshina, G. I. Karavaiko, A. G. Dorofeev, T. A. Pivovarova, and N. A. Chernykh. 1992. A new iron oxidizing bacterium, Leptospirillum thermoferrooxidans sp. nov. Microbiology (Engl. Transl. Mikrobiologiya) 61:744-750. [Google Scholar]
- 16.Golyshina, O. V., T. A. Pivovarova, G. I. Karavaiko, T. F. Kondrat'eva, E. R. B. Moore, W. R. Abraham, H. Lunsdorf, K. N. Timmis, M. M. Yakimov, and P. N. Golyshin. 2000. Ferroplasma acidiphilum gen. nov., sp. nov., an acidophilic, autotrophic, ferrous-iron-oxidizing, cell-wall-lacking, mesophilic member of the Ferroplasmaceae fam. nov., comprising a distinct lineage of the Archaea. Int. J. Syst. E vol. Microbiol. 50:997-1006. [DOI] [PubMed] [Google Scholar]
- 17.Hallberg, K. B., and E. B. Lindström. 1994. Characterization of Thiobacillus caldus, sp. nov., a moderately thermophilic acidophile. Microbiology 140:3451-3456. [DOI] [PubMed] [Google Scholar]
- 18.Hippe, H. 2000. Leptospirillum gen. nov. (ex Markoysan 1972), nom. rev., including Leptospirillum ferrooxidans sp. nov. (ex Markoysan 1972), nom. rev. and Leptospirillum thermoferrooxidans sp. nov. (Golovacheva et al. 1992). Int. J. Syst. E vol. Microbiol. 50:501-503. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, D. B. 1995. Selective solid media for isolating and enumerating acidophilic bacteria. J. Microbiol. Methods 23:205-218. [Google Scholar]
- 20.Johnson, D. B., S. Rolfe, K. B. Hallberg, and E. Iversen. 2001. Isolation and phylogenetic characterization of acidophilic microorganisms indigenous to acidic drainage waters at an abandoned Norwegian copper mine. Environ. Microbiol. 3:630-637. [DOI] [PubMed] [Google Scholar]
- 21.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, New York, N.Y.
- 22.Kelly, D. P., L. A. Chambers, and P. A. Trudinger. 1969. Cyanolysis and spectrophotometric estimation of trithionate in mixture with thiosulfate and tetrathionate. Anal. Chem. 41:898-902. [Google Scholar]
- 23.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. Wiley, New York, N.Y.
- 24.Lovley, D. R., and E. J. P. Phillips. 1987. Rapid assay for microbially reduced ferric iron in aquatic sediments. Appl. Environ. Microbiol. 53:1536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marmur, J., and P. Doty. 1962. Determination of base composition of deoxyribonucleic acid from its thermal denaturation temperature. J. Mol. Biol. 5:109-118. [DOI] [PubMed] [Google Scholar]
- 26.Miller, P. C. 1997. The design and operation practice of bacterial oxidation plant using moderate thermophiles (the BacTech process), p. 81-102. In D. E. Rawlings (ed.), Biomining: theory, microbes and industrial processes. Springer-Verlag/Landes Bioscience, Georgetown, Tex.
- 27.Norris, P. R., N. P. Burton, and N. A. M. Foulis. 2000. Acidophiles in bioreactor mineral processing. Extremophiles 4:71-76. [DOI] [PubMed] [Google Scholar]
- 28.Norris, P. R., R. M. Marsh, and E. B. Lindström. 1986. Growth of mesophilic and thermophilic acidophilic bacteria on sulfur and tetrathionate. Biotechnol. Appl. Biochem. 8:318-329. [Google Scholar]
- 29.Orphan, V. J., L. T. Taylor, D. Hafenbradl, and E. F. DeLong. 2000. Culture-dependent and culture-independent characterization of microbial assemblages associated with high-temperature petroleum reservoirs. Appl. Environ. Microbiol. 66:700-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Applic. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 31.Pizarro, J., E. Jedlicki, O. Orellana, J. Romero, and R. T. Espejo. 1996. Bacterial populations in samples of bioleached copper ore as revealed by analysis of DNA obtained before and after cultivation. Appl. Environ. Microbiol. 62:1323-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawlings, D. E. (ed.). 1997. Biomining: theory, microbes and industrial processes. Springer-Verlag/Landes Bioscience, Georgetown, Tex.
- 33.Rawlings, D. E., N. J. Coram, M. N. Gardner, and S. M. Deane. 1999. Thiobacillus caldus and Leptospirillum ferrooxidans are widely distributed in continuous flow biooxidation tanks used to treat a variety of metal containing ores and concentrates, p. 777-786. In R. Amils and A. Ballester (ed.), Biohydrometallurgy and the environment toward the mining of the 21st century, vol. 9A. Elsevier, Amsterdam, The Netherlands.
- 34.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 35.Schnaitman, C., and D. G. Lundgren. 1965. Organic compounds in the spent medium of Ferrobacillus ferrooxidans. Can. J. Microbiol. 11:23-27. [DOI] [PubMed] [Google Scholar]
- 36.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Staden, P. J., M. Rhodes, A. Pinches, and T. E. Martinez. 2000. Process engineering of base metal concentrate bioleaching, p. 127-129. In Randol Copper Hydromet Roundtable 2000, Randol International Ltd., Golden, Colo. Tucson, Ariz.
- 38.Wilson, K. 1987. Preparation of genomic DNA from bacteria, p. 2.4.1-2.4.5. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. A. Seidman, and K. Struhl (ed.), Current protocols in molecular biology. Green & Wiley Interscience, New York, N.Y. [DOI] [PubMed]