Abstract
A genome scan was performed on 164 Dutch affected sib pairs (ASPs) with attention-deficit/hyperactivity disorder (ADHD). All subjects were white and of Dutch descent and were phenotyped according to criteria set out in the Diagnostic and Statistical Manual Of Mental Disorders, 4th edition. Initially, a narrow phenotype was defined, in which all the sib pairs met the full ADHD criteria (117 ASPs). In a broad phenotype, additional sib pairs were included, in which one child had an autistic-spectrum disorder but also met the full ADHD criteria (164 ASPs). A set of 402 polymorphic microsatellite markers with an average intermarker distance of 10 cM was genotyped and analyzed using the Mapmaker/sibs program. Regions with multipoint maximum likelihood scores (MLSs) >1.5 in both phenotypes were fine mapped with additional markers. This genome scan indicated several regions of interest, two of which showed suggestive evidence for linkage. The most promising chromosome region was located at 15q, with an MLS of 3.54 under the broad phenotype definition. This region was previously implicated in reading disability and autism. In addition, MLSs of 3.04 and 2.05 were found for chromosome regions 7p and 9q in the narrow phenotype. Except for a region on chromosome 5, no overlap was found with regions mentioned in the only other independent genome scan in ADHD reported to date.
Introduction
Attention-deficit/hyperactivity disorder (ADHD [MIM 143465]) is a highly heritable psychiatric disorder that affects ∼4%–5% of children in Western countries (Buitelaar 2002). The syndrome persists into adulthood in about one-third of the cases (Spencer et al. 1998) and affects ∼0.5%–2% of adults (Buitelaar 2002).
According to the criteria of the Diagnostic and Statistical Manual Of Mental Disorders, 4th edition (DSM-IV), a diagnosis of ADHD requires that a child meet six of nine criteria for inattention (inattentive subtype), six of nine criteria for hyperactivity/impulsivity (hyperactive/impulsive subtype), or both (combined subtype). Furthermore, the behavioral problems must have started before the age of 7 years and must have persisted for at least 6 mo in at least two different settings (e.g., school and home) (American Psychiatric Association 1994).
There is a substantial difference between the sexes in the prevalence of childhood ADHD, with boys being affected 3 times more often than girls in the general population (Gaub and Carlson 1997) and 10 times more often in clinical settings (Arnold 1996). In adults this ratio becomes 2:1 in the general population (Biederman 1998). High levels of comorbidity with other psychiatric disorders are common in both sexes (Angold et al. 1999). When viewed either categorically or on a continuous scale, ADHD has an estimated heritability of 75%–91% (Levy 1997). The relative risk for siblings of affected children (λs) is increased approximately fivefold (Biederman et al. 1992). On the basis of the relative risk for first- (λ=5–8) and second-degree family members (λ=2), a model of multiple genes interacting in an additive manner was proposed elsewhere (Smalley 1997). Despite much research, the exact etiology of ADHD has still not been clarified.
Because methylphenidate, the most widely prescribed drug for treating ADHD, mainly blocks the dopamine transporter, most genetic research has focused on the dopaminergic system. Several studies have found an association with the seven-repeat allele of a 48-bp repeat in the dopamine 4 receptor (DRD4) gene (Faraone et al. 2001). Similar results were reported for the 10-repeat allele of a 40-bp repeat in the dopamine transporter (DAT1) gene (Cook et al. 1995; Gill et al. 1997; Waldman et al. 1998; Daly et al. 1999; Barr et al. 2001). A number of studies have failed to replicate these findings, possibly because of their small sample size or the fact that ADHD is a genetically heterogeneous disorder (Palmer et al. 1999; Holmes et al. 2000; Kotler et al. 2000; Jorm et al. 2001; Roman et al. 2001).
The relative risk of developing ADHD for a carrier of a DAT1 or DRD4 risk allele is ∼1.3 and 1.4, respectively (Faraone et al. 2001; Maher et al. 2002). When the syndrome is viewed as the extreme of a continuous trait, these genes explain only 2%–3% of the difference in symptom severity (Waldman et al. 1998; Schmidt et al. 2001). Accordingly, it is assumed that there are other, as-yet-unidentified genes that play a major role in ADHD. It is possible that some of these genes are involved in serotonergic and noradrenergic neurotransmission (Gainetdinov et al. 1999). Genomewide linkage analysis provides a means of identifying chromosome regions containing susceptibility genes, without an a priori hypothesis about their function. In addition, studies involving affected sib pairs (ASPs) require no assumptions about the mode of inheritance. Recently, the results of the first whole-genome screen in American ASPs were published (Fisher et al. 2002). However, under a broad, as well as a narrow, definition of the ADHD phenotype, no chromosomal regions were found that were suggestive for linkage, according to recently suggested criteria for the interpretation of linkage results (Lander and Kruglyak 1995). In a follow-up study in an extended sample, significant linkage was found on chromosome 16p13, in a region already implicated in autism (Smalley et al. 2002). It has been suggested that ADHD and autistic-spectrum disorders, which also have a high heritability (Bailey et al. 1995), have common genetic factors (Luteijn et al. 2000; Smalley et al. 2002). According to the DSM-IV classification, an autistic-spectrum disorder rules out the diagnosis of ADHD; however, a substantial number of patients with ADHD have mild problems in social interactions and communication that are rather similar to the symptoms of autism. (Clark et al. 1999; Luteijn et al. 2000). A subgroup of autistic children also showed high levels of inattention, hyperactivity, and impulsivity (Jaselskis et al. 1992; Aman and Langworthy 2000; Noterdaeme et al. 2001).
The present article reports the results, using a broad and a narrow phenotype, of a whole-genome scan in 164 ASPs with ADHD who were white and of Dutch descent. We detected three loci with MLSs of 3.54, 3.04, and 2.05 on chromosome regions 15q, 7p, and 9q, respectively.
Subjects and Methods
Ascertainment
The sample in the present study consists of 238 children in 106 families, whose diagnoses were assigned according to DSM-IV criteria. The families were recruited from three academic child psychiatric outpatient clinics, in Utrecht (n=24), Groningen (n=25), and Amsterdam (n=3). Other families (n=54) were recruited through a patient organization and by placing advertisements in journals and on the Internet. The Medical Ethical Committee of the Utrecht University Medical Center approved the study, and all parents gave written informed consent.
Instruments and Procedures
The children and their parents were invited to participate in extensive diagnostic evaluations that lasted ∼1 d. After evaluation, ASPs were included in the study if they met the following five criteria: (1) at least one member of each ASP met all criteria for the DSM-IV-defined combined, inattentive, or hyperactive/impulsive type of ADHD; (2a) the other member of the ASP met the same criteria, (2b) the other member met at least five of nine DSM-IV criteria for inattention and/or five of nine criteria for hyperactivity/impulsivity (“subthreshold” ADHD), or (2c) the other member met all DSM-IV criteria for ADHD but had been found to have an autistic-spectrum disorder, which excludes an ADHD diagnosis; (3) both members were at least 3 years old but not >18 years old (in our sample of 238 children, 5% were <6 years of age); (4) for those with a history of educational problems, only children with an estimated full-scale IQ >80 on the Wechsler Intelligence Scale for Children–Revised or the Wechsler Preschool and Primary Scale of Intelligence were included in the analysis (Wechsler 1967; Wechsler 1974); and (5) all four grandparents were white and of Dutch descent, with the exception of two families, each with two affected siblings, in which one of the parents was not white.
Patients who had handicaps (e.g., deafness), other psychiatric disorders (e.g., schizophrenia) or genetic syndromes (e.g., fragile X syndrome) that could be related to behavioral problems were excluded from the analysis.
Relationships between siblings were verified using the program GRR (Abecasis et al. 2001). This resulted in the identification of two half-sibs, who were also excluded. In two families with MZ twins and additional affected siblings, one of the twins was excluded. In five families these twins constituted the only sib pair, which resulted in exclusion of the whole family.
Despite the fact that ADHD had been previously diagnosed by child psychiatrists or pediatricians in all children in this analysis, the diagnosis was verified in clinical interviews with the parents and the children. In addition, for all the patients the DSM-IV version of the Diagnostic Interview Schedule for Children (DISC) (Shaffer et al. 2000) was conducted with both parents by specially trained graduate students in medicine or child psychology. This instrument was also used to measure the presence of mood disorders, anxiety disorders (other than simple phobias), oppositional defiant disorder (ODD) and conduct disorder (CD). Finally, the Conners and Childhood Behavior Checklist/Teacher Report Form were collected from teachers and parents (Goyette et al. 1978; Achenbach and Ruffle 2000).
The final diagnosis of ADHD, which served as the primary basis for inclusion in the study, was determined using a best-estimate procedure (Leckman et al. 1982). To this end, the results of the medical history, clinical interview, DISC interview, information about social skills, and the scores on the Conners and Childhood Behavior Checklist/Teacher Report Form, as rated by the parents and teachers, were summarized by E.M.V. into a patient report (Goyette et al. 1978; Achenbach and Ruffle 2000; Shaffer et al. 2000). This report was discussed in regular case reviews with an experienced child psychiatrist (J.K.B.). The result was a consensus diagnosis. If a child showed severe social deficits, the possibility of an autistic-spectrum disorder was considered after collecting additional information on his or her language and social development and repertoire of activities and interests.
A narrow phenotype of probands and siblings with full ADHD symptoms according to the DSM-IV criteria was defined. The sample comprised 117 ASPs, according to an unweighted analysis that calculated all possible pairs in families with more than two affected siblings. In the same manner, a broad phenotype was defined, comprising the narrow phenotype plus an additional 47 ASPs with a broad phenotype (i.e., one member had full ADHD and the other met criteria 2b or 2c of the selection criteria). The broadly and narrowly defined samples are described in table 1.
Table 1.
Number of ASPs by Phenotype
|
Broad Phenotype |
Narrow Phenotype |
|||||
| No. of Children per Family | No. ofFamilies | No. ofASPsa | No. ofChildrenb | No. ofFamilies | No. ofASPsa | No. ofChildrenb |
| 2 | 85 | 85 (85) | 170 | 62 | 62 (62) | 124 |
| 3 | 17 | 51 (34) | 51 | 9 | 27 (18) | 27 |
| 4 | 3 | 18 (9) | 12 | 3 | 18 (9) | 12 |
| 5 | 1 |
10 (4) |
5 |
1 |
10 (4) |
5 |
| Total | 106 | 164 (132) | 238 | 75 | 117 (93) | 168 |
Total number of ASPs, calculated in two different ways. In the unweighted (all pairs) method, a family with n sibs contributes (n2 − n)/2 sib pairs. The number of independent sib pairs, in which a family with n sibs contributes (n − 1) pairs, is shown in parentheses.
Total number of children who make up the relevant sib pairs.
The mean age ± SD of the children was 10 years ± 3. Their clinical characteristics are shown in table 2. The parent with the highest educational level defined the socioeconomic status of the family unit. The following classifications were used: I, no or uncompleted high school; II, high school completed; III, some college education; or IV, college completed. Children with an autistic-spectrum disorder were divided into pervasive developmental disorder not otherwise specified (PDD-NOS) and autism/Asperger syndrome, according to the DSM-IV criteria. PDD-NOS is generally viewed as a less severe form of autism. Children with Asperger syndrome, unlike those with autism, have normal language skills.
Table 2.
Clinical Characteristics of Individual Children[Note]
|
No. (%) of Children with |
||
| Characteristic | Narrow Phenotype | Broad Phenotype |
| Sex: | ||
| Male | 167 (83.9) | 30 (76.9) |
| Female | 32 (16.1) | 9 (23.1) |
| Socioeconomic status: | ||
| I | 0 | 0 |
| II | 50 (25.1) | 3 (7.6) |
| III | 73 (36.7) | 18 (46.2) |
| IV | 76 (38.2) | 18 (46.2) |
| Comorbiditya: | ||
| Anxiety | 27 (13.8) | 10 (27.8) |
| Mood | 15 (7.7) | 2 (5.6) |
| ODD | 82 (41.8) | 9 (25.0) |
| CD | 14 (7.1) | 1 (2.8) |
| Broad phenotype: | ||
| Subthreshold ADHD | … | 13 (33.3) |
| PDD-NOS | … | 18 (46.2) |
| Autism/Asperger | … | 8 (20.5) |
| ADHD subtype: | ||
| Combined | 170 (85.4) | … |
| Inattentive | 25 (12.6) | … |
| Hyperactive/impulsive | 4 (2.0) |
… |
| Overall total | 199 (83.6) | 39 (16.4) |
Note.— In contrast to table 1, in which sib pairs are the point of reference, in this table the individual child is the point of reference. Thus, a child with ADHD who has an autistic sibling is now counted in the narrow phenotype. The total number of children in the narrow phenotype therefore differs from that in table 1.
In both narrow and broad phenotypes, comorbidity data from three children are missing.
The DSM-IV criteria distinguish three subtypes of ADHD: inattentive, hyperactive/impulsive, and combined (American Psychiatric Association 1994). In accordance with the results of most other studies, the majority of children in the present study had the combined subtype. In two families with both minor and adult siblings with ADHD, the adult siblings were also included in the analysis. Phenotypic characteristics of study samples were compared by means of a χ2 test.
Genotyping and Analysis
DNA was extracted from peripheral blood lymphocytes or buccal mucosa, using established procedures. Samples obtained from buccal mucosa were purified with an additional phenol extraction step. DNA concentration was measured with a spectrophotometer (Tecan), and samples were diluted with distilled water to a concentration of 15 ng/μl.
The Mammalian Genotyping Service of the Marshfield Medical Research Foundation performed the genotyping for the initial screen. The marker set was based on Marshfield Screening set 10 and consisted of 402 polymorphic microsatellite markers with an average spacing of 10 cM and an average heterozygosity of 0.75.
In the second stage of the screen, chromosomal regions were fine mapped with additional microsatellite markers from the Marshfield database. Marker positions in these regions were verified using the Ensembl, Celera, and Decode (Kong et al. 2002) human sequence databases. Either the forward or the reverse oligonucleotide primer was labeled with 6-FAM, HEX, or NED fluorescent dyes (Biolegio and Applied Biosystems). PCRs were performed in a 10-μl volume containing 30 ng of template DNA, 25 ng of each oligonucleotide primer, 0.2 mM of each dNTP, and 0.4 U AmpliTaq Gold (Applied Biosystems), in 1× PCR buffer II with 2.5 mM MgCl2 (Applied Biosystems). DNA was initially denatured at 94°C for 7 min and was then subjected to 33 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, followed by a final extension step of 30 min at 72°C. PCR products were diluted four times with distilled water, and 1 μl of diluted product was mixed with 4 μl of HiDi (Applied Biosystems) and 0.1 μl of GS-500 size standard (Applied Biosystems) and was separated in POP6 polymer on an ABI 3700 capillary DNA sequencer (Applied Biosystems).
Data were analyzed using Genescan 3.6 and Genotyper 3.5 for Windows NT (Applied Biosystems). Two independent raters genotyped the additional markers and checked results for inconsistency. If they disagreed, the genotypes were classified as unknown. In the initial screen, genotypes with a reported Marshfield quality score <0.99 were also recoded as unknown.
Inheritance within families was verified using the Pedcheck program (O’Connell and Weeks 1998). If there were inheritance errors, the complete family was excluded from the analysis for that marker. Allele frequencies were calculated from the parental genotypes.
Data were analyzed using the Mapmaker/sibs program (Kruglyak and Lander 1995). Maximum-likelihood-scores (MLSs) were determined in single-point and multipoint analyses and were calculated using the possible-triangle method (Holmans 1993), which makes no assumptions about the mode of inheritance. Dominance variance was allowed for in all analyses.
In multipoint analysis, MLSs were calculated at 10 intervals between two adjacent markers, with off-end ranges of 10 cM at both ends of the chromosome. All possible pairs within each family were used in the calculations. In those regions with an MLS >2, data were also analyzed by means of a weighted procedure. According to this method, all possible sib pair combinations in families with more than two affected siblings were taken into account but were weighted with a factor (2/n), where n was the number of affected sibs in the family. In the analysis of the X chromosome, the separate LOD scores for sister-sister, sister-brother, and brother-brother pairs were summed (Cordell et al. 1995).
CIs (95%) under linkage peaks were determined by calculating, and determining the map position of the point obtained by subtracting 1 from the maximum. Locus-specific λsib values were calculated by dividing the observed ZO value at the point of the maximal MLS by the expected value of 0.25 (Kruglyak and Lander 1995). Overall information content of the markers was obtained by calculating the average multipoint information content for all markers, including the 10-cM off-end scales. Exclusion mapping was performed for different relative risks, using the “exclude” option in Mapmaker/sibs (Kruglyak and Lander 1995).
Results
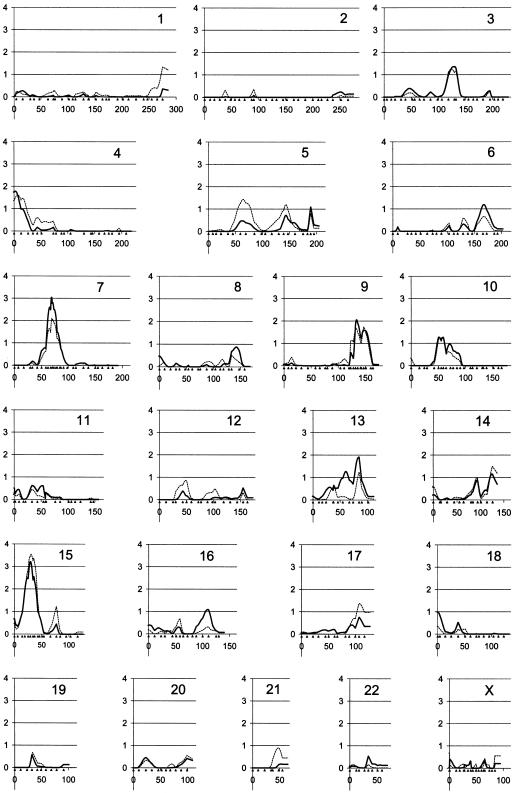
DNA was available from all the parents, except one missing father. In the initial screen, all families were genotyped using 402 polymorphic microsatellite markers with an average intermarker spacing of 10 cM. Average genotyping completeness was 97.3%, and the average marker information content across the genome was 0.72. The most promising regions on chromosomes 7, 9, 10, and 15 were fine mapped with additional markers, and genotyping in these areas was repeated for markers from the initial screen. This resulted in a resolution finer than 5 cM and a marker information content of 90%–95%. Multipoint MLSs for the broad and narrow phenotype groups are shown in figure 1. Table 3 summarizes all the genomic regions with multipoint MLSs >1. Single-point MLSs were in agreement with multipoint results (data not shown).
Figure 1.
Multipoint MLS for all chromosomes (Y-axis). MLS for the small phenotype are indicated with a solid line, and the dotted line represents MLS for the broad phenotype. Genetic distance (in cM) with marker positions indicated by solid triangles (X-axis). Chromosome numbers are listed in the upper right corner of each graph.
Table 3.
Chromosome Regions with Multipoint Maximum Likelihood Scores >1[Note]
|
Multipoint MLS |
||||
| ChromosomeRegion | Marker | Locationa(cM) | Narrow Phenotype | Broad Phenotype |
| 3q13.32 | D3S2460 | 134.6 | 1.36 |
1.25 |
| 4p16.3 | D4S3360 | .0 | 1.78 |
1.62 |
| 5p13.1 | D5S2500 | 69.2 | 0.47 | 1.43 |
| 6q26 | D6S305 | 166.4 | 1.19 |
0.66 |
| 7p13 | D7S1818 | 69.6 | 3.04 |
2.09 |
| 9q33.3 | D9S1825 | 136.5 | 2.05 |
1.68 |
| 10cen | D10S1426 | 59.0 | 1.26 |
1.25 |
| 13q33.3 | D13S796 | 93.5 | 1.91 |
1.25 |
| 15q15.1 | GATA50C03 | 34.8 | 3.21 | 3.54 |
Note.— MLSs for both phenotypes are given, with the highest score underlined. The marker closest to the maximum MLS is shown. Only those multipoint MLSs that were supported by multiple markers in single-point analysis are listed.
Locations correspond with the Marshfield genetic map, sex-averaged distances.
On chromosome region 7p, the MLS was 3.04, with a 95% CI of 16 cM (26.6 Mb). The estimated sibling relative risk of this locus would be 1.19. The relative risk of a locus on chromosome 15 was estimated to be 1.60 (with 1.32 being the lower limit of of the 95% CI). Here, the MLS was 3.54, and the 95% CI was 18 cM (16 Mb).
Because one-fifth of the families in our sample had more than two affected siblings, we investigated the effect of weighting multiple sibships in the regions with MLS >2. When this procedure was followed, the MLS on chromosomes 15q and 7p decreased to 2.49 and 2.27, respectively, and the MLS on chromosome 9q increased to 2.34. Exclusion mapping showed that the existence of a locus with a relative risk of 1.5 could be excluded only for 8.3% of the total genome. However, 53.9% and 87% of the genome could be excluded for loci with relative risk values of 2 and 3, respectively.
Discussion
Our genome scan in 164 ASPs with ADHD has identified regions on chromosomes 7p and 15q with maximum MLSs of 3.04 and 3.54, respectively, which can be regarded as suggestive for linkage, according to the criteria proposed by Lander and Kruglyak (1995). The study was based on a narrow phenotype of sib pairs, in which both children had ADHD according to the DSM-IV criteria. An additional broad phenotype was distinguished, in which the probands met the full ADHD criteria and the siblings had subthreshold ADHD or had received a diagnosis of an autistic-spectrum disorder and also met the full criteria for ADHD. The results for the broad and narrow phenotype groups were very similar. There were no unique areas of linkage restricted to a single phenotype. In most peak regions, MLSs were higher in the analysis of the narrow phenotype, even though the number of contributing sib pairs was reduced by almost 30%.
Following the method used in the first reported genome screen in ADHD (Fisher et al. 2002), we analyzed the results of the present study with the use of all possible sib pairs in each family. Because pairs in families with more than two affected siblings are not independent, the resulting MLS may be inflated. On the other hand, assigning a lower weight to sib pairs from families with more than two affected siblings is probably too conservative (Daly and Lander 1996). Even in the weighted analysis, the regions on chromosomes 15q and 7p can be classified as suggestive for linkage.
In their genome scan in an extended sample of 270 ASPs, Ogdie et al. (2003) identified regions with LOD scores >1 on chromosomes 5p13, 6q14, 11q25, 16p13, 17p11, and 20q13. It is remarkable that, when their data are compared with our results, none of these regions coincide with the regions with LOD scores >1 in the present study, with exception of the 5p region, which showed modest LOD scores in both studies. It is not yet clear to what extent these differences are due to spurious findings or to clinical or genetic heterogeneity. Our two groups of researchers are now investigating the possibility of performing a combined analysis of the two samples. However, such a study will require a detailed comparison of marker sets, as well as phenotypic characteristics.
Several differences between our sample and the one described by Ogdie et al. (2003) are apparent. First, the Dutch sample was specifically selected for a homogeneous ethnic background. With two exceptions, families were included only if all four grandparents of the ASPs were white and of Dutch descent. Since there was relatively little immigration to the Netherlands until the second half of the 20th century, our sample is more ethnically homogeneous than the American sib pair sample, which included 20% nonwhite children. Other differences are the sex ratio (17% girls in the Dutch sample vs. 28% in the American sample; P=.01) and the prevalence of the inattentive subtype of ADHD (13% in the Dutch sample vs. 55% in the American sample; P<.01).
Moreover, the Dutch sample is characterized by a lower level of conduct disorder (6% vs. 15%; P<.01) and a higher socioeconomic status (0% vs. 22% in the lowest socioeconomic class; P<.01). Social insurance and health care coverage levels in the Netherlands are higher, and inner city problems are less pronounced than in the United States. It is therefore possible that environmental factors accounted for some of the differences between the two studies; and, if we assume that there are at least several genes involved, different—and as yet partly unknown—environmental components could have led to the identification of different gene sets. Genetic linkage studies in a wide variety of complex disorders have generally demonstrated a lack of replication (Altmuller et al. 2001), and comparison of the genome screens conducted in ADHD indicates that linkage studies in ADHD could face similar difficulties.
ADHD is associated not only with autistic features but also with dyslexia and language disorders (Clark et al. 1999; Cohen et al. 2000; Kovac et al. 2001; Willcutt et al. 2002). It is interesting that, in both genome screens performed to date, the chromosome regions of maximum linkage coincide with linkage regions found in autism studies. In our study, the highest MLS was obtained on chromosome 15q in the broad phenotype group, which also included siblings with an autistic-spectrum disorder. In autism, the chromosome region 15q11-13 has repeatedly been suggested to harbor disease-susceptibility genes. Autism was shown to cosegregate with different chromosomal abnormalities in this region (Cook et al. 1997; Schroer et al. 1998; Wolpert et al. 2000), and several authors have reported evidence for linkage in this area (Philippe et al. 1999; Risch et al. 1999; Collaborative Linkage Study of Autism 2001; Gutknecht 2001; Shao et al. 2003). The highest MLS in our study was located ∼20 cM distal to the region most frequently reported, which falls just outside our 95% CI. One study, however, found the highest MLS at marker D15S118, which lies halfway between the markers that gave the highest MLSs in our study (Philippe et al. 1999). This sample of autistic children had a similar, mainly western European ethnic background.
The results of studies on the genetics of reading disability are also relevant for our findings in the 15q region. Two genome scans reported a susceptibility locus for reading disability in areas that fall within the 95% CI of our linkage peak on chromosome 15 (Grigorenko et al. 1997; Nothen et al. 1999). Moreover, reading disability was found to be associated with a three-marker haplotype that also included marker D15S994 (Morris et al. 2000). This marker had the highest single-point LOD score (3.37) in our study and contributed directly to the MLS peak. Interestingly, the findings in reading disability prompted an association study with markers in this region in families with ADHD. Significant association was found with marker D15S146, which is located 0.5 cM from D15S994 (Barr et al. 2002). Given these findings, we plan to collect detailed information on the reading abilities of the participants in our sample and to analyze the region on chromosome 15 in a further independent sample of families collected for genetic studies of reading disability. The results of the present study seem to lend further support to the hypothesis that a chromosome 15q locus plays a role in the etiology of genetically overlapping developmental disorders, including ADHD, autism and reading disability.
Most of the well-known ADHD candidate genes, including DAT1, DRD4, and DRD5, lay outside areas with elevated LOD scores, a point also raised by Fisher et al. (2002). It should be noted, however, that our study had limited power to detect loci with a low locus-specific relative risk. Recent meta-analyses suggest relative risk values for DAT1, DRD4, and DRD5 of 1.3, 1.4, and 1.6, respectively (Faraone et al. 2001; Maher et al. 2002). Although our study had a 97% power of finding a LOD score of 2.6 for a locus with a relative risk of 2.0, the chances of finding a similar LOD score for loci with a relative risk of 1.6 and 1.3 would have been 63% and 11%, respectively. Such loci were therefore unlikely to be detected.
Two regions in the present study harbor previously suggested ADHD candidate genes, both involved in dopaminergic neurotransmission. Dopa decarboxylase (DDC), also known as “aromatic L-amino acid decarboxylase,” is the enzyme that converts dopa into dopamine. Positron emission tomography showed an accumulation of [18F] fluorodopa in the right midbrain of children with ADHD, indicating an impaired function of DDC (Ernst et al. 1999). The gene is located on chromosome region 7p13, within 600 kb of the D7S2422 marker, which gave an MLS of 3.04 in the present study. In an Irish population of patients with ADHD, a haplotype consisting of the D7S2422 marker and a 4-bp deletion in exon 1 was transmitted more often to affected offspring, although these results were only marginally significant (Hawi et al. 2001). In the present study, we found evidence of an increased marker sharing in the DDC region relative to the ADHD phenotype. Taken together, these results indicate that the involvement of DDC in the etiology of ADHD deserves future investigation using fine-mapping techniques to define possible regions of linkage disequilibrium.
Another enzyme in the dopamine pathway is dopamine β-hydroxylase (DBH), which converts dopamine to norepinephrine. The gene on chromosome region 9q34 is located within the MLS peak found in our study. Several studies have reported an association between restriction site polymorphisms in this gene and ADHD (Daly et al. 1999; Roman et al. 2002; Wigg et al. 2002). A relationship between low DBH activity and symptoms of hyperactivity was reported several years ago (Rogeness et al. 1989).
In conclusion, this whole-genome scan in ADHD has located several susceptibility loci, two of which—on chromosome regions 7p and 15q—are suggestive for linkage. The chromosome 15 region is particularly interesting, since it has been implicated in autism and reading disability. These results may provide new directions in the search for specific genetic determinants of ADHD.
Acknowledgments
This study is dedicated to the memory of L. A. Sandkuijl (1953–2002), who died shortly after its completion. We thank him for his invaluable help as a statistician and a teacher. We gratefully acknowledge the support of the Mammalian Genotyping Service of the Marshfield Medical Research Foundation. We would also like to thank C. Wijmenga, for helpful discussions; J. L. Senior, for critically reading the manuscript; and all the families, for their willingness to participate in this study. This project was supported by the Makaria Foundation, the UMC Utrecht’s Genvlag program, and the Catharijne Foundation. E.M.V. was supported by the Drie Lichten Foundation, the Ter Meulen Foundation, the Koningsheide Foundation, the Yale Child Study Center, and the Harvard Unit of Neurodevelopment
Electronic-Database Information
URLs for data presented herein are as follows:
- Celera, http://www.celera.com/
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://research.marshfieldclinic.org/genetics/
- Ensembl Genome Browser, http://www.ensembl.org/
- GRR, http://qtl.well.ox.ac.uk/GRR/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for ADHD) [PubMed]
References
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2001) GRR: graphical representation of relationship errors. Bioinformatics 17:742–743 [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Ruffle TM (2000) The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev 21:265–271 [DOI] [PubMed] [Google Scholar]
- Altmuller J, Palmer LJ, Fischer G, Scherb H, Wjst M (2001) Genomewide scans of complex human diseases: true linkage is hard to find. Am J Hum Genet 69:936–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MG, Langworthy KS (2000) Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. J Autism Dev Disord 30:451–459 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th ed. American Psychiatric Association, Washington, DC [Google Scholar]
- Angold A, Costello EJ, Erkanli A (1999) Comorbidity. J Child Psychol Psychiatry 40:57–87 [PubMed] [Google Scholar]
- Arnold LE (1996) Sex differences in ADHD: conference summary. J Abnorm Child Psychol 24:555–569 [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M (1995) Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25:63–77 [DOI] [PubMed] [Google Scholar]
- Barr CL, Feng Y, Anderson B, Crosbie J, Roberts W, Malone M, Ickowicz A, Schachar R, Tannock R, Lovett M, Humphries T, Kennedy JL (2002) Significant evidence for linkage of attention-deficit hyperactivity disorder to the chromosome 15q region. Am J Med Genet 114:P154 [Google Scholar]
- Barr CL, Xu C, Kroft J, Feng Y, Wigg K, Zai G, Tannock R, Schachar R, Malone M, Roberts W, Nothen MM, Grunhage F, Vandenbergh DJ, Uhl G, Sunohara G, King N, Kennedy JL (2001) Haplotype study of three polymorphisms at the dopamine transporter locus confirms linkage to attention-deficit/hyperactivity disorder. Biol Psychiatry 49:333–339 [DOI] [PubMed] [Google Scholar]
- Biederman J (1998) Attention-deficit/hyperactivity disorder: a life-span perspective. J Clin Psychiatry 59 Suppl 7:4–16 [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Keenan K, Benjamin J, Krifcher B, Moore C, Sprich-Buckminster S, Ugaglia K, Jellinek MS, Steingard R, et al (1992) Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder: patterns of comorbidity in probands and relatives psychiatrically and pediatrically referred samples. Arch Gen Psychiatry 49:728–738 [DOI] [PubMed] [Google Scholar]
- Buitelaar JK (2002) Epidemiology: what have we learned over the last decade? In: Sandberg S (ed) Hyperactivity and attention-deficit disorders. Cambridge University Press, Cambridge, pp 30–63 [Google Scholar]
- Clark T, Feehan C, Tinline C, Vostanis P (1999) Autistic symptoms in children with attention deficit-hyperactivity disorder. Eur Child Adolesc Psychiatry 8:50–55 [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Vallance DD, Barwick M, Im N, Menna R, Horodezky NB, Isaacson L (2000) The interface between ADHD and language impairment: an examination of language, achievement, and cognitive processing. J Child Psychol Psychiatry 41:353–362 [PubMed] [Google Scholar]
- Collaborative Linkage Study of Autism (2001) An autosomal genomic screen for autism. Am J Med Genet 105:609–615 [DOI] [PubMed] [Google Scholar]
- Cook EH Jr, Lindgren V, Leventhal BL, Courchesne R, Lincoln A, Shulman C, Lord C, Courchesne E (1997) Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. Am J Hum Genet 60:928–934 [PMC free article] [PubMed] [Google Scholar]
- Cook EH Jr, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL (1995) Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet 56:993–998 [PMC free article] [PubMed] [Google Scholar]
- Cordell HJ, Kawaguchi Y, Todd JA, Farrall M (1995) An extension of the maximum lod score method to X-linked loci. Ann Hum Genet 59:435–449 [DOI] [PubMed] [Google Scholar]
- Daly G, Hawi Z, Fitzgerald M, Gill M (1999) Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol Psychiatry 4:192–196 [DOI] [PubMed] [Google Scholar]
- Daly MJ, Lander ES (1996) The importance of being independent: sib pair analysis in diabetes. Nat Genet 14:131–132 [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM (1999) High midbrain [18F] DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry 156:1209–1215 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Doyle AE, Mick E, Biederman J (2001b) Meta-analysis of the association between the 7-repeat allele of the dopamine D(4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry 158:1052–1057 [DOI] [PubMed] [Google Scholar]
- Fisher SE, Francks C, McCracken JT, McGough JJ, Marlow AJ, MacPhie IL, Newbury DF, Crawford LR, Palmer CG, Woodward JA, Del’Homme M, Cantwell DP, Nelson SF, Monaco AP, Smalley SL (2002) A genomewide scan for loci involved in attention-deficit/hyperactivity disorder. Am J Hum Genet 70:1183–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG (1999) Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283:397–401 [DOI] [PubMed] [Google Scholar]
- Gaub M, Carlson CL (1997) Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry 36:1036–1045 [DOI] [PubMed] [Google Scholar]
- Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M (1997) Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Mol Psychiatry 2:311–313 [DOI] [PubMed] [Google Scholar]
- Goyette CH, Conners CK, Ulrich RF (1978) Normative data on revised Conners Parent and Teacher Rating Scales. J Abnorm Child Psychol 6:221–236 [DOI] [PubMed] [Google Scholar]
- Grigorenko EL, Wood FB, Meyer MS, Hart LA, Speed WC, Shuster A, Pauls DL (1997) Susceptibility loci for distinct components of developmental dyslexia on chromosomes 6 and 15. Am J Hum Genet 60:27–39 [PMC free article] [PubMed] [Google Scholar]
- Gutknecht L (2001) Full-genome scans with autistic disorder: a review. Behav Genet 31:113–123 [DOI] [PubMed] [Google Scholar]
- Hawi Z, Foley D, Kirley A, McCarron M, Fitzgerald M, Gill M (2001) Dopa decarboxylase gene polymorphisms and attention deficit hyperactivity disorder (ADHD): no evidence for association in the Irish population. Mol Psychiatry 6:420–424 [DOI] [PubMed] [Google Scholar]
- Holmans P (1993) Asymptotic properties of affected-sib-pair linkage analysis. Am J Hum Genet 52:362–374 [PMC free article] [PubMed] [Google Scholar]
- Holmes J, Payton A, Barrett JH, Hever T, Fitzpatrick H, Trumper AL, Harrington R, McGuffin P, Owen M, Ollier W, Worthington J, Thapar A (2000) A family-based and case-control association study of the dopamine D4 receptor gene and dopamine transporter gene in attention deficit hyperactivity disorder. Mol Psychiatry 5:523–530 [DOI] [PubMed] [Google Scholar]
- Jaselskis CA, Cook EH, Jr., Fletcher KE, Leventhal BL (1992) Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 12:322–327 [PubMed] [Google Scholar]
- Jorm AF, Prior M, Sanson A, Smart D, Zhang Y, Easteal S (2001) Association of a polymorphism of the dopamine transporter gene with externalizing behavior problems and associated temperament traits: a longitudinal study from infancy to the mid-teens. Am J Med Genet 105:346–350 [DOI] [PubMed] [Google Scholar]
- Kong A, Gudbjartsson DF, Sainz J, Jonsdottir GM, Gudjonsson SA, Richardsson B, Sigurdardottir S, Barnard J, Hallbeck B, Masson G, Shlien A, Palsson ST, Frigge ML, Thorgeirsson TE, Gulcher JR, Stefansson K (2002) A high-resolution recombination map of the human genome. Nat Genet 31:241–247 [DOI] [PubMed] [Google Scholar]
- Kotler M, Manor I, Sever Y, Eisenberg J, Cohen H, Ebstein RP, Tyano S (2000) Failure to replicate an excess of the long dopamine D4 exon III repeat polymorphism in ADHD in a family-based study. Am J Med Genet 96:278–281 [DOI] [PubMed] [Google Scholar]
- Kovac I, Garabedian B, Du Souich C, Palmour RM (2001) Attention deficity/hyperactivity in SLI children increases risk of speech/language disorders in first-degree relatives: a preliminary report. J Commun Disord 34:339–354 [DOI] [PubMed] [Google Scholar]
- Kruglyak L, Lander ES (1995) Complete multipoint sib-pair analysis of qualitative and quantitative traits. Am J Hum Genet 57:439–454 [PMC free article] [PubMed] [Google Scholar]
- Lander E, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results [see comments]. Nat Genet 11:241–247 [DOI] [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM (1982) Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry 39:879–883 [DOI] [PubMed] [Google Scholar]
- Levy F (1997) Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. J Am Acad Child Adolesc Psychiatry 36:737–744 [DOI] [PubMed] [Google Scholar]
- Luteijn EF, Serra M, Jackson S, Steenhuis MP, Althaus M, Volkmar F, Minderaa R (2000) How unspecified are disorders of children with a pervasive developmental disorder not otherwise specified? A study of social problems in children with PDD-NOS and ADHD. Eur Child Adolesc Psychiatry 9:168–179 [DOI] [PubMed] [Google Scholar]
- Maher BS, Marazita ML, Ferrell RE, Vanyukov MM (2002) Dopamine system genes and attention deficit hyperactivity disorder: a meta-analysis. Psychiatr Genet 12:207–215 [DOI] [PubMed] [Google Scholar]
- Morris DW, Robinson L, Turic D, Duke M, Webb V, Milham C, Hopkin E, Pound K, Fernando S, Easton M, Hamshere M, Williams N, McGuffin P, Stevenson J, Krawczak M, Owen MJ, O’Donovan MC, Williams J (2000) Family-based association mapping provides evidence for a gene for reading disability on chromosome 15q. Hum Mol Genet 9:843–848 [DOI] [PubMed] [Google Scholar]
- Noterdaeme M, Amorosa H, Mildenberger K, Sitter S, Minow F (2001) Evaluation of attention problems in children with autism and children with a specific language disorder. Eur Child Adolesc Psychiatry 10:58–66 [DOI] [PubMed] [Google Scholar]
- Nothen MM, Schulte-Korne G, Grimm T, Cichon S, Vogt IR, Muller-Myhsok B, Propping P, Remschmidt H (1999) Genetic linkage analysis with dyslexia: evidence for linkage of spelling disability to chromosome 15. Eur Child Adolesc Psychiatry Suppl 3 8:56–59 [DOI] [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE (1998) PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogdie MN, Macphie IL, Minassian SL, Yang M, Fisher SE, Francks C, Cantor RM, McCracken JT, McGough JJ, Nelson SF, Monaco AP, Smalley SL (2003) A genomewide scan for attention-deficit/hyperactivity disorder in an extended sample: suggestive linkage on 17p11. Am J Hum Genet 72:1268–1279 (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CG, Bailey JN, Ramsey C, Cantwell D, Sinsheimer JS, Del’Homme M, McGough J, Woodward JA, Asarnow R, Asarnow J, Nelson S, Smalley SL (1999) No evidence of linkage or linkage disequilibrium between DAT1 and attention deficit hyperactivity disorder in a large sample. Psychiatr Genet 9:157–160 [DOI] [PubMed] [Google Scholar]
- Philippe A, Martinez M, Guilloud-Bataille M, Gillberg C, Rastam M, Sponheim E, Coleman M, Zappella M, Aschauer H, Van Maldergem L, Penet C, Feingold J, Brice A, Leboyer M, van Malldergerme L (1999) Genome-wide scan for autism susceptibility genes. Paris Autism Research International Sibpair Study. Hum Mol Genet 8:805–812 [DOI] [PubMed] [Google Scholar]
- Risch N, Spiker D, Lotspeich L, Nouri N, Hinds D, Hallmayer J, Kalaydjieva L, et al (1999) A genomic screen of autism: evidence for a multilocus etiology. Am J Hum Genet 65:493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogeness GA, Maas JW, Javors MA, Macedo CA, Fischer C, Harris WR (1989) Attention deficit disorder symptoms and urine catecholamines. Psychiatry Res 27:241–251 [DOI] [PubMed] [Google Scholar]
- Roman T, Schmitz M, Polanczyk G, Eizirik M, Rohde LA, Hutz MH (2001) Attention-deficit hyperactivity disorder: a study of association with both the dopamine transporter gene and the dopamine D4 receptor gene. Am J Med Genet 105:471–478 [DOI] [PubMed] [Google Scholar]
- ——— (2002) Further evidence for the association between attention-deficit/hyperactivity disorder and the dopamine-beta-hydroxylase gene. Am J Med Genet 114:154–158 [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Perez-Edgar K, Hu S, Hamer DH (2001) Association of DRD4 with attention problems in normal childhood development. Psychiatr Genet 11:25–29 [DOI] [PubMed] [Google Scholar]
- Schroer RJ, Phelan MC, Michaelis RC, Crawford EC, Skinner SA, Cuccaro M, Simensen RJ, Bishop J, Skinner C, Fender D, Stevenson RE (1998) Autism and maternally derived aberrations of chromosome 15q. Am J Med Genet 76:327–336 [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME (2000) NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 39:28–38 [DOI] [PubMed] [Google Scholar]
- Shao Y, Cuccaro ML, Hauser ER, Raiford KL, Menold MM, Wolpert CM, Ravan SA, Elston L, Decena K, Donnelly SL, Abramson RK, Wright HH, DeLong GR, Gilbert JR, Pericak-Vance MA (2003) Fine mapping of autistic disorder to chromosome 15q11-q13 by use of phenotypic subtypes. Am J Hum Genet 72:539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley SL (1997) Genetic influences in childhood-onset psychiatric disorders: autism and attention-deficit/hyperactivity disorder. Am J Hum Genet 60:1276–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley SL, Kustanovich V, Minassian SL, Stone JL, Ogdie MN, McGough JJ, McCracken JT, MacPhie IL, Francks C, Fisher SE, Cantor RM, Monaco AP, Nelson SF (2002) Genetic linkage of attention-deficit/hyperactivity disorder on chromosome 16p13, in a region implicated in autism. Am J Hum Genet 71:959–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens TE, Faraone SV (1998) Adults with attention-deficit/hyperactivity disorder: a controversial diagnosis. J Clin Psychiatry Suppl 7 59:59–68 [PubMed] [Google Scholar]
- Waldman ID, Rowe DC, Abramowitz A, Kozel ST, Mohr JH, Sherman SL, Cleveland HH, Sanders ML, Gard JM, Stever C (1998) Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: heterogeneity owing to diagnostic subtype and severity. Am J Hum Genet 63:1767–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1967) Wechsler Preschool and Primary Scale of Intelligence. Psychological Corporation, New York [Google Scholar]
- ——— (1974) WISC-R manual: Wechsler Intelligence Scale for Children-Revised Manual. Psychological Corporation, New York [Google Scholar]
- Wigg K, Zai G, Schachar R, Tannock R, Roberts W, Malone M, Kennedy JL, Barr CL (2002) Attention deficit hyperactivity disorder and the gene for dopamine beta-hydroxylase. Am J Psychiatry 159:1046–1048 [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Smith SD, Cardon LR, Gayan J, Knopik VS, Olson RK, DeFries JC (2002) Quantitative trait locus for reading disability on chromosome 6p is pleiotropic for attention-deficit/hyperactivity disorder. Am J Med Genet 114:260–268 [DOI] [PubMed] [Google Scholar]
- Wolpert CM, Menold MM, Bass MP, Qumsiyeh MB, Donnelly SL, Ravan SA, Vance JM, Gilbert JR, Abramson RK, Wright HH, Cuccaro ML, Pericak-Vance MA (2000) Three probands with autistic disorder and isodicentric chromosome 15. Am J Med Genet 96:365–372 [DOI] [PubMed] [Google Scholar]