Abstract
The study was undertaken to investigate the 5-HT receptor mediating the inhibitory effect of 5-HT on peristalsis in the guinea-pig isolated ileum. The facilitatory and inhibitory effects were measured as the decrease and increase, respectively, in the intraluminal pressure required to trigger peristalsis. In the presence of 5-HT2/3&4 receptor antagonists ketanserin (0.1 μM), granisetron (1 μM) and SB-204070 (1 μM), a cumulative addition (0.1–100 μM) of 5-HT or 5-carboxamidotryptamine, but not 2-methyl-5-HT produced a concentration-dependent increase in the threshold required to trigger peristalsis. The 5-HT7 receptor selective antagonist SB-269970-A (0.01–1 μM) or methiothepin (0.01–0.1 μM) concentration-dependently antagonised this response to 5-HT. SB-269970-A (1 μM) and methiothepin (1 μM) were also able to restore peristalsis in tissues in which peristalsis was inhibited by a prior addition of 30 μM of 5-HT. The results indicate an involvement of 5-HT7 receptors in the inhibitory effect of 5-HT on peristalsis in the guinea-pig ileum.
Keywords: 5-HT, 5-HT7 receptor, peristalsis, guinea-pig ileum, SB-269970-A, methiothepin
Introduction
5-Hydroxytryptamine (5-HT) is known to produce both a facilitation (Bulbring & Crema, 1958) and inhibition (Kosterlitz & Robinson, 1957) of peristalsis in the guinea-pig ileum. The facilitatory effects of 5-HT have been shown to be mediated via 5-HT3 (Tuladhar et al., 1995) and 5-HT4 receptors (Craig & Clarke, 1991; Costall et al., 1993). However, the receptor mediating the inhibitory effect of 5-HT has not been studied so far. The present study was undertaken to investigate the 5-HT receptor mediating the inhibitory effect of 5-HT on peristalsis in the guinea-pig ileum.
Methods
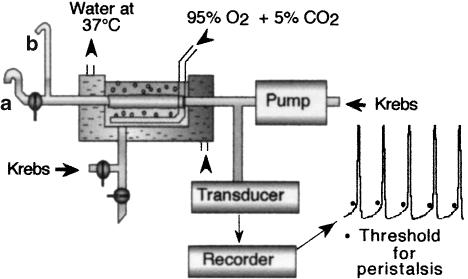
Peristalsis was measured and recorded using the methodology we have previously described (Costall et al., 1993). Briefly, up to four segments of ileum (taken 5–30 cm from the ileo-caecal junction) were obtained from guinea-pigs (Dunkin Hartley) of either sex (500–1000 g) and cannulated at the oral and aboral ends and secured horizontally in a bath containing Krebs–Henseleit solution kept at 37°C and oxygenated with 95% O2 and 5% CO2 (Figure 1). The outflow tube at the aboral end had two outlets, one at the level of the fluid in the bath (A) and the other 4 cm above the fluid in the bath (B). The second tube was narrow ensuring that fluid regurgitated into the system at the end of the peristaltic stroke was minimised without producing a high resistance. Each segment was perfused from the oral side at a constant rate which varied in different tissues from 1 to 1.5 ml min−1. The intraluminal pressure was recorded from the oral side. When the lower outlet was closed, the pressure increased slowly as the level of fluid increased in the narrow arm of the upper outlet B resulting in the distension of the ileum. On reaching the threshold pressure, peristalsis was triggered, starting as a contraction of the circular muscle travelling from the oral side to the aboral side. This contraction of circular muscle forced the intraluminal contents out of the upper outlet B. At the start of each peristalsis, the transducer recorded a sudden rise in pressure and at the end, the pressure returned to the baseline, starting another cycle. The pressure when a sudden increase was recorded was taken as the threshold pressure. The facilitatory effect of 5-HT and other 5-HT receptor agonists on peristalsis was characterised by a decrease in the threshold pressure. Conversely, the inhibitory effect on peristalsis was characterised by an increase in the threshold pressure, which eventually led to a complete loss of peristalsis in many tissues. When peristalsis was abolished, the ileum remained distended and the fluid entering through the inlet tube passively exited the upper outlet. For calculation purpose, the threshold is taken as 40 mm (the height of the upper outlet) when peristalsis was abolished.
Figure 1.
Schematic diagram of the apparatus used to study peristalsis.
All drugs were added to the serosal side, that is, into the bath. Cumulative concentration response curves to 5-HT and other 5-HT receptor agonists were constructed by adding increasing concentrations of the agonist; a higher concentration of the agonist was added once the maximum inhibitory effect of the previous concentration had achieved. In these experiments, the antagonists, if required, were added to the reservoir and equilibrated for at least 30 min before the addition of the agonist. Only one curve was obtained per tissue, as responses were absent or greatly reduced in the second and subsequent runs. In some experiments, a single concentration of 5-HT (30 μM) or 5-carboxamidotryptamine (30 μM) was added and 5-HT7 receptor antagonists SB-269970-A (1 μM) or methiothepin (1 μM) were added once the maximum inhibitory effect was obtained with 5-HT or 5- carboxamidotryptamine.
The data are expressed as mean±s.e.m of n experiments. Data were statistically analysed using ANOVA followed by Dunnett's t-test for multiple comparison. Granisetron, SB-204070 and SB-269970-A were gifts from GlaxoSmithKline, Harlow, U.K. All other substances and reagents were from Sigma or Tocris.
Results
As reported previously (Costall et al., 1993; Tuladhar et al., 1995), an addition of 5-HT (0.3–100 μM) to the serosal side of the ileum produced an initial facilitation of the peristalsis characterised by a lowering of the threshold. This effect of 5-HT was followed by an inhibition of peristalsis characterised by an increase in the threshold pressure and decrease in the rate of peristalsis. Inclusion of the 5-HT2 receptor antagonist ketanserin (0.1 μM), the 5-HT3 receptor antagonist granisetron (1 μM) and together with the 5-HT4 receptor antagonist SB-204070 (1 μM) in the bathing solution abolished the facilitatory effect of the lower concentrations (up to 10 μM) of 5-HT and greatly reduced the effect of higher concentrations (>10 μM) of 5-HT. The inclusion of these 5-HT receptor antagonists also caused a statistically insignificant shift to the left of the concentration–response curve of 5-HT to inhibit peristalsis (data not shown).
In the presence of ketanserin, granisetron and SB-204070, a cumulative addition (0.1–100 μM) of 5-HT or the 5-HT1/4/7 receptor agonist 5-carboxamidotryptamine, but not the 5-HT3 receptor agonist 2-methyl-5-HT, produced a concentration-dependent increase in the threshold required to trigger peristalsis (Figure 2 and Figure 4). The 5-HT7 receptor selective antagonist SB-269970-A (0.01–1 μM, Figure 4b) or methiothepin (0.01–0.1 μM, Figure 4c) concentration-dependently antagonised this response to 5-HT. SB-269970-A (1 μM) and methiothepin (1 μM) also restored peristalsis in tissues in which peristalsis was inhibited by a prior addition of 30 μM of 5-HT (Figure 3, 4d and e).
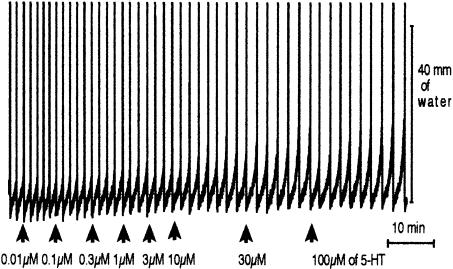
Figure 2.
A trace illustrating the inhibitory effect of a cumulative addition of 5-HT on peristalsis in the presence of ketanserin (0.1 μM), granisetron (1 μM) and SB-204070 (1 μM).
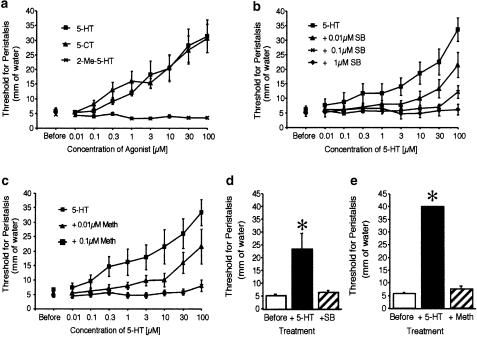
Figure 4.
(a) Concentration–response curves to 5-HT, 5-carboxamidotryptamine (5-CT) and 2-methyl-5-HT (2-Me-5-HT) to inhibit peristalsis in the guinea-pig ileum. The threshold for peristalsis before and after the cumulative addition of the drugs to the serosal side are shown. (b) Effect of an increasing concentration of SB-269970-A (SB) on the inhibitory effect of 5-HT on peristalsis. (c) Effect of an increasing concentration of methiothepin (Meth) on the inhibitory effects of 5-HT on peristalsis. The buffer routinely contained ketanserin (0.1 μM), granisetron (1 μM) and SB-204070 (1 μM) in these experiments (d and e). The inhibitory effect of 30 μM of 5-HT on peristalsis in the guinea-pig ileum and the reversal of the effect by a further addition of 1 μM of SB-269970-A (SB) (d) and methiothepin (Meth) (e). The buffer did not contain ketanserin, granisetron (1 μM) or SB-204070 in these experiments. *P<0.001 compared to threshold before treatment with 5-HT. All points indicate the means and vertical bars the s.e.mean of six separate experiments.
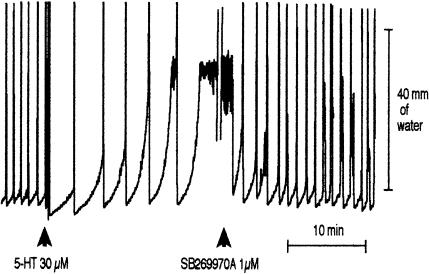
Figure 3.
A trace illustrating the effect of 30 μM 5-HT on peristalsis and the reversal of the inhibitory effect of 5-HT on peristalsis by 1 μM SB-269970-A. Ketanserin, granisetron or SB-204070 was not present in these experiments.
Discussion
Both the facilitatory and the inhibitory effects of 5-HT on peristalsis in the guinea-pig ileum have been known for many years (Kosterlitz & Robinson, 1957; Bulbring & Crema, 1958). The 5-HT3 and 5-HT4 receptors have recently been shown to be responsible for mediating the facilitatory effect of 5-HT in the guinea-pig ileum (see Section Introduction). However, the receptor mediating the inhibitory effect, which was the first effect of 5-HT on peristalsis to be reported (Kosterlitz & Robinson, 1957), has not been described so far. Indeed, some recent investigators have been unable to observe the inhibitory response of 5-HT on peristalsis (Craig & Clarke, 1991). The failure to observe the inhibitory effects of 5-HT in the latter study can be attributed to the methodology employed. In this regard, we have shown previously that the inhibitory effect of 5-HT can be studied using the experimental arrangement described in this study (Costall et al., 1993). However, the characterisation of the 5-HT receptor mediating the inhibitory effect was hampered by the lack of selective 5-HT receptor antagonists acting at the 5-HT7 receptors. The recent introduction of SB-269970-A enabled us to re-examine the receptor mediating the inhibitory effect of 5-HT in this tissue.
The facilitatory effect of 5-HT was prevented or greatly reduced in the presence of ketanserin, granisetron and SB-204070 in the bathing medium, which supports the previous findings that 5-HT3/5-HT4 receptors are involved in the facilitatory effects of 5-HT on peristalsis (Craig & Clarke, 1991; Costall et al., 1993; Tuladhar et al., 1995). The ability of 5-HT to inhibit peristalsis in the presence of these antagonists also excludes the possibility of 5-HT2, 5-HT3 and 5-HT4 receptors in the inhibitory effect of 5-HT. 5-HT1 receptors and particularly 5-HT1A receptors have been implicated in many inhibitory effects of 5-HT in the guinea-pig ileum (Galligan, 1992; Pan & Galligan, 1995). However, the involvement of 5-HT1A receptors in the present inhibitory effect of 5-HT is unlikely as the response was inhibited by submicromolar concentrations of SB-269970-A; the compound is reported to have no affinity for 5-HT1A receptor at these concentrations (pKi value <5 for 5-HT1A receptors) (Lovell et al., 2000). Furthermore, in our preliminary studies the 5-HT1A receptor selective antagonist WAY-100635, with an apparent pKB value of 9.73 to antagonise 5-HT1A receptor-mediated electrically evoked contractions in the guinea-pig ileum (Forster et al., 1995), failed to affect significantly the inhibitory response of 5-HT (30 μM) at 0.1 μM (our unpublished observation). Nevertheless, WAY-100635 reduced the 5-HT (30 μM) induced inhibitory effect on peristalsis at 1 μM, the observation that originally led us to suspect the involvement of the 5-HT7 receptor, as the compound is known to have affinity for the 5-HT7 receptors at higher concentrations (Forster et al., 1995).
SB-269970-A is a selective antagonist of 5-HT7 receptors with a pKi value of 8.3 at the 5-HT7 receptors in guinea-pig cortex (Hagan et al., 2000). The concentration range of SB-269970-A that was able to antagonise the inhibitory effect of 5-HT on peristalsis in the present study strongly indicates the involvement of the 5-HT7 receptor in inhibiting peristalsis. The ability of low concentrations of methiothepin, another potent but nonselective 5-HT7 receptor antagonist, to antagonise the 5-HT effect and the agonist activity of 5-carboxamodotryptamine but not 2-methyl 5-HT, further support the involvement of 5-HT7 receptors in this response (Vanhoenacker et al., 2000).
The potency of 5-HT and 5-carboxamidotryptamine to inhibit peristalsis in the present study is also comparable to the potency reported in other functional studies on 5-HT7 receptors. Thus, the threshold concentrations at which 5-HT begins to relax the guinea-pig ileum (Feniuk et al., 1983) and human colon (Prins et al., 1999) were in the range of 0.1–0.3 μM, the concentration range at which the inhibition of the peristalsis commenced in the present study. Nevertheless, the potency of the agonists observed in this study (and other functional studies) is lower than that expected by the affinity estimated from the binding studies on the receptor. Such discrepancy between the potency observed in the functional studies and affinity estimates from the binding studies are well known with the 5-HT7 receptors (Eglen et al., 1997) and may be a result of difficulty of the agonists to access the receptors in the whole tissues.
It should be noted that although concentration-related increases in the pressure threshold required to trigger peristalsis were seen with both 5-HT and 5-carboxamidotryptamine, the response curves were shallow and failed to plateau even at 100 μM (a concentration 300 to 1000-fold higher than the threshold concentration of 0.1–0.3 μM). This may be attributed to the failure of the antagonists in the bathing medium to antagonise fully the facilitatory effects at higher concentrations of the agonists and to a degree of tachyphylaxis occurring at those concentrations on the cumulative addition of the drugs. Furthermore, the existence of different splice variants of the 5-HT7 receptors are known, which although do not seems to differ in their pharmacological properties (Vanhoenacker et al., 2000) may differ in their sensitivity to desensitisation. Further studies are required to confirm or deny the above hypothesis.
The presence of 5-HT7 receptors in the gastrointestinal tract has been demonstrated in many species including the guinea-pig and human tissues using different techniques, the expression of mRNA, binding and functional studies (Feniuk et al., 1983; Eglen et al., 1997; Hemedah et al., 1999; Prins et al., 1999; Krobert et al., 2001; Liu et al., 2001). However, an immunohistochemical study to elucidate the cellular location of the receptor in the gastrointestinal tract is still lacking. The functional studies carried out so far on intestinal tissues indicate their smooth muscle location and a relaxant role (Feniuk et al., 1983; Eglen et al., 1997; Prins et al., 1999). Thus, it is likely that in the peristaltic circuitry, the 5-HT7 receptors are located at the level of the smooth muscles. However, this hypothesis cannot be tested using tetrodotoxin in the present peristalsis study and so another possibility, that the receptors in the circuitry may be located on the enteric neurons, cannot be ignored. Indeed, Bulbring & Crema (1958) believed the effect to be due to the blockade of ganglionic transmission. Furthermore, many of the neuronally mediated inhibitory effects on the guinea-pig ileum previously described as mediated via 5-HT1A receptors were based on the use of 8-OH-DPAT, a compound that is now known to have additional agonist activity at 5-HT7 receptors (Eglen et al., 1997).
Although the exact location of the 5-HT7 receptors in the peristaltic circuitry cannot be ascertained, the receptors (like 5-HT3 and HT4 receptors) are unlikely to be involved in the ‘peristalsis circuitry proper' but could provide inhibitory modulation (unlike 5-HT3 and HT4 receptors that provide facilitatory modulation; see Costall et al., 1993) of the peristalsis circuitry. These conclusions are based on the observations that SB-269970-A on its own did not affect peristalsis and peristalsis can be restored following tachyphylaxis of the inhibitory effect (Bulbring & Crema, 1958). The physiological and/or pathological source of 5-HT to influence these receptors is also unknown. However, it is likely to be the enteric serotonergic neurons rather than the mucosal enterochromaffin cells as 5-HT added into the lumen does not inhibit peristalsis (Bulbring & Crema, 1958; Tuladhar et al., 1997).
Peristalsis in the guinea-pig ileum has been a useful model to study 5-HT effects on gastrointestinal motility. The ligands acting on 5-HT3/5-HT4 receptors – the stimulation of which results in the facilitation of peristalsis – have been shown to be effective clinical agents to treat gastrointestinal motility disorders including Irritable Bowel Syndrome (Camilleri et al., 2000; Beglinger, 2002). IBS is a common gastrointestinal disorder, the pathophysiology of which is multifactorial and only partially understood (De Ponti & Tonini, 2001). The patients suffer from abdominal pain, diarrhoea and/or constipation. An involvement of an excessive level of 5-HT in the pathophysiology of at least a subset of people suffering from IBS can be argued from the proven clinical effectiveness of a 5-HT3 antagonist in the disease (Camilleri et al., 2000). If an excessive release of 5-HT occurs in IBS, it is most likely that 5-HT will act not only on the 5-HT3 or 5-HT4 receptors but also on the 5-HT7 receptors, which has a higher affinity for 5-HT than the 5-HT3 receptors. Thus, the end effect on motility would be complex because of the antagonising effects of 5-HT7 receptors over that of 5-HT3/5-HT4 receptors on peristalsis. Additional complication may arise owing to a propensity of all these receptors to desensitisation. Analogous events occur in IBS where diarrhoea and constipation vary among patients and over time. So far, only partial agonists acting on 5-HT4 receptors have been studied for use in constipation predominant IBS patients (De Ponti & Tonini, 2001). The current study predicts that 5-HT7 receptor antagonists may be another useful group of agents for the treatment of constipation predominant IBS. The 5-HT7 receptor antagonists, by preventing the inhibition of peristalsis, are expected to increase motility and prevent accumulation and consequent dilatation of the intestine, which may even aid in relieving the pain of IBS. An alteration in the 5-HT7 receptor function may also be an underlying cause of many other GI motility disorders such as idiopathic constipation, 5-HT7 ligands may also prove to be useful in these conditions.
In conclusion, the present study indicates that 5-HT7 receptors are involved in the inhibitory effect of 5-HT on peristalsis in the guinea-pig ileum and that the ligands acting on the receptor may prove to be useful agents to treat gastrointestinal motility disorders such as Irritable Bowel Syndrome.
Acknowledgments
We thank GlaxoSmithKline for the gift of drugs.
Abbreviations
- 5-HT
5-hydroxytryptamine
- SB-204070
8-amino-7-chloro(N-butyl-4-piperidyl) methylbenzo-1,4-di-oxan-5-carboxylate hydrochloride
- SB-269970-A
(((R)-3-(2-(2-(4-methyl-piperidin-1-yl)ethyl)-pyrrolidine-1-sulphonyl)-phenol)),N-[4-methoxy-3-(4-methyl-1-piperazinyl)phenyl]-3-methyl-4-(4-pyridinyl)benzamide)
References
- BEGLINGER C. Tegaserod: a novel, selective 5-HT4 receptor partial agonist for irritable bowel syndrome. Int. J. Clin. Prac. 2002;56:47–51. [PubMed] [Google Scholar]
- BULBRING E., CREMA A. Observations concerning the action of 5-hydroxytryptamine on the peristaltic reflex. Br. J. Pharmacol. 1958;13:444–457. doi: 10.1111/j.1476-5381.1958.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMILLERI M., NORTHCUTT A.R., KONG S., DUKES G.E., MCSORLEY D., MANGEL A.W. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- COSTALL B., NAYLOR R.J., TULADHAR B.R. 5-HT4 receptor mediated facilitation of the emptying phase of the peristaltic reflex in the guinea-pig isolated ileum. Br. J. Pharmacol. 1993;110:1572–1578. doi: 10.1111/j.1476-5381.1993.tb14003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAIG D.A., CLARKE D.E. Peristalisis evoked by 5-HT and renzapride: evidence for putative 5-HT4 receptor activation. Br. J. Pharmacol. 1991;102:563–564. doi: 10.1111/j.1476-5381.1991.tb12211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PONTI F., TONINI M. Irritable Bowel Syndrome: new agents targeting serotonin receptor subtypes. Drugs. 2001;61:317–332. doi: 10.2165/00003495-200161030-00001. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., JASPER J.R., CHANG D.J., MARTIN G.R. The 5-HT7 receptor: orphan found. Trends Pharmacol. Sci. 1997;18:104–107. doi: 10.1016/s0165-6147(97)01043-2. [DOI] [PubMed] [Google Scholar]
- FENIUK W., HUMPHREY P.P.A., WATTS A.D. 5-Hydroxytryptamine-induced relaxation of isolated mammalian smooth muscle. Eur. J. Pharmacol. 1983;96:71–78. doi: 10.1016/0014-2999(83)90530-7. [DOI] [PubMed] [Google Scholar]
- FORSTER E.A., CLIFFE I.A., BILL D.J., DOVER G.M., JONES D., REILLY Y., FLETCHER A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur. J. Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- GALLIGAN J.J. Differential inhibition of cholinergic and noncholinergic neurogenic contractions by 5-hydroxytryptamine1A receptor agonists in guinea pig ileum. J. Pharmacol. Exp. Therap. 1992;260:306–312. [PubMed] [Google Scholar]
- HAGAN J.J., PRICE G.W., JEFFREY P., DEEKS N.J., STEAN T., PIPER D., SMITH M.I., UPTON N., MEDHURST A.D., MIDDLEMISS D.N., RILEY G.J., LOVELL P.J., BROMIDGE S.M., THOMAS D.R. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br. J. Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEMEDAH M., COUPAR I.M., MITCHELSON F.J. [3H]-Mesulergine labels 5-HT7 sites in rat brain and guinea-pig ileum but not rat jejunum. Br. J. Pharmacol. 1999;126:179–188. doi: 10.1038/sj.bjp.0702293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSTERLITZ H.W., ROBINSON J.A. Inhibition of the peristaltic reflex of the isolated guinea-pig ileum. J. Physiol. 1957;136:249–262. doi: 10.1113/jphysiol.1957.sp005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROBERT K.A., BACH T., SYVERSVEEN T., KVINGEDAL A.M., LEVY F.O. The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn-Schmiedebergs Arch. Pharmacol. 2001;363:620–632. doi: 10.1007/s002100000369. [DOI] [PubMed] [Google Scholar]
- LIU H., IRVING H.R., COUPAR I.M. Expression patterns of 5-HT7 receptor isoforms in the rat digestive tract. Life Sci. 2001;69:2467–2475. doi: 10.1016/s0024-3205(01)01318-2. [DOI] [PubMed] [Google Scholar]
- LOVELL P.J., BROMIDGE S.M., DABBS S., DUCKWORTH D.M., FORBES I.T., JENNINGS A.J., KING F.D., MIDDLEMISS D.N., RAHMAN S.K., SAUNDERS D.V., COLLIN L.L., HAGAN J.J., RILEY G.J., THOMAS D.R. A novel, potent, and selective 5-HT7 antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)-ethyl)pyrrolidine-1-sulfonyl)phenol (SB-269970) J. Med. Chem. 2000;43:342–345. doi: 10.1021/jm991151j. [DOI] [PubMed] [Google Scholar]
- PAN H., GALLIGAN J.J. Effects of 5-HT1A and 5-HT4 receptor agonists on slow synaptic potentials in enteric neurons. Eur. J. Pharmacol. 1995;278:67–74. doi: 10.1016/0014-2999(95)00101-p. [DOI] [PubMed] [Google Scholar]
- PRINS N.H., BRIEJER M.R., VAN BERGEN P.J., AKKERMANS L.M., SCHUURKES J.A. Evidence for 5-HT7 receptors mediating relaxation of human colonic circular smooth muscle. Br. J. Pharmacol. 1999;128:849–852. doi: 10.1038/sj.bjp.0702762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TULADHAR B.R., COSTALL B., NAYLOR R.J. Evidences for a 5-HT3 receptor-mediated facilitation of the emptying phase of the peristaltic reflex in the isolated guinea-pig ileum. Br. J. Pharmacol. 1995;114:374P. doi: 10.1111/j.1476-5381.1993.tb14003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TULADHAR B.R., KAISAR M., NAYLOR R.J. Evidence for a 5-HT3 receptor involvement in the facilitation of peristalsis on mucosal application of 5-HT in the guinea pig isolated ileum. Br. J. Pharmacol. 1997;122:1174–1178. doi: 10.1038/sj.bjp.0701503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHOENACKER P., HAEGEMAN G., LEYSEN J.E. 5-HT7 receptors: current knowledge and future prospects. Trends Pharmacol. Sci. 2000;21:70–77. doi: 10.1016/s0165-6147(99)01432-7. [DOI] [PubMed] [Google Scholar]