Abstract
Peroxisome proliferator-activated receptor α (PPARα) regulates the expression of the key genes involved in lipid metabolism following activation of this receptor by various ligands. Ginseng, a highly valuable medicine in oriental societies, is also reported to modulate lipid metabolism, although the mechanism of its action remains unknown. In order to test our hypothesis that ginseng exerts its effects by altering PPARα-mediated pathways, the effects of Korean red ginseng on PPARα function and serum lipid profiles were investigated using in vivo and in vitro approaches.
In vivo administration of ginseng extract (GE) and ginsenosides (GS) not only inhibited mRNA levels of acyl-CoA oxidase, a rate-limiting enzyme for PPARα-mediated peroxisomal fatty acid β-oxidation, induced by the potent PPARα ligand Wy14,643 in a dose- and time-dependent manner, but also inhibited the induction of PPARα target genes expected following treatment with Wy14,643.
Consistent with the in vivo data, both GE and GS caused dose-dependent decreases in the endogenous expression of a luciferase reporter gene containing the PPAR responsive element (PPRE), while GS significantly decreased the magnitude of reporter gene activation in the presence of Wy14,643.
Serological studies demonstrated that, compared with vehicle-treated mice, treatment with GS significantly increased serum concentrations of total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol. Compared to groups treated with Wy14,643 alone, which significantly decreased serum triglyceride and HDL cholesterol levels versus controls, coadministration of either GE or GS with Wy14,643 modestly increased serum triglycerides and HDL cholesterol.
These results indicate that the effects of ginseng on serum lipid profiles may be mediated by changes in the expression of PPARα target genes, providing the first evidence that in vivo and in vitro treatments of ginseng modulate PPARα action. In addition, these data suggest that ginseng can act as an inhibitor of PPARα function, which may have therapeutic implications.
Keywords: Ginseng; ginsenosides; lipid metabolism; PPARα; Wy14,643
Introduction
Ginseng is widely used in oriental societies as one of the most valuable medicines. There are extensive reports that ginseng has many pharmacological effects on the CNS, endocrine, immune, and cardiovascular systems (Kenarova et al., 1990; Nah et al., 1995; Wen et al., 1996; Gillis, 1997; Attele et al., 1999). In contrast, there are only a few studies providing evidence that ginseng may modulate lipid metabolism and are limited to examination of effects at the serological level. Moreover, these latter studies are inconsistent and contradictory in that some suggest that ginseng exhibits hypolipidemic action in experimental animal systems, such as hypercholesterolemic rabbits, high-cholesterol diet-fed rats, and patients with hyperlipidemia (Yamamoto et al., 1983; Cui et al., 1998; Inoue et al., 1999), whereas another reports that ginseng administration fails to exert any beneficial effects on hypercholesterolemia in rabbits (Ismail et al., 1999). In addition, all of the above studies fail to provide any evidence, genetic, molecular, or biochemical, for a mechanistic explanation of ginseng's reported effects.
The major metabolites of ginseng are ginsenosides (GS), also referred to as steroidal saponins, which comprise about 3–6% of ginseng components and contain most of the pharmacological activity of ginseng (Huang, 1999). Ginseng has over 30 GS, each of which has been shown to produce multiple effects (Odashima et al., 1985; Tsang et al., 1985). One potential mechanism of ginseng action can be explained by the structural characteristics of GS. GS belong to a family of steroids and share structural characteristics with steroid hormones (Ota et al., 1987; Banthorpe, 1994; Kim et al., 1998), such as a four-ring, steroid-like structure with attached sugar moieties (Kaku et al., 1975). Given this structural similarity, we hypothesized that GS may bind to nuclear receptors and alter gene expression, similar to the actions of steroid hormones.
Peroxisome proliferator-activated receptor α (PPARα) is a member of the steroid/thyroid hormone receptor superfamily that regulates the expression of a number of genes critical for lipid and lipoprotein metabolism. PPARα is well characterized to initiate triglyceride-lowering effects through transcriptional activation of peroxisomal, microsomal, and mitochondrial fatty acid-metabolizing enzymes (Aoyama et al., 1998; Kroetz et al., 1998; Yan et al., 1998), as well as lipoprotein lipase and apolipoprotein CIII (Hertz et al., 1995; Auwerx et al., 1996). PPARα is also well known to increase the circulating amounts of high-density lipoprotein (HDL) levels through induction of apolipoprotein AI and AII gene expressions (Vu-Dac et al., 1995; Staels & Auwerx, 1998). The absence of PPARα results in abnormalities in triglycerides and cholesterol metabolism because of reduced lipoprotein and fatty acid metabolism (Lemberger et al., 1996; Peters et al., 1997; Costet et al., 1998). Based on the well-documented regulation of lipid metabolism by PPARα, it would seem to be a logical target if ginseng and its pharmacologically active GS exert their effects on serum lipids as a result of interactions with nuclear receptors.
The objective of this study was to not only determine the actual effect of ginseng with respect to the regulation of lipid metabolism, but also examine whether its mechanism of action involves PPARα. Using in vivo and in vitro approaches, we examined whether ginseng extract (GE) and GS can regulate the transcription of PPARα target enzymes of the fatty acid β-oxidation system, leading to changes in lipid profiles. We here show that ginseng, and its pharmacologically active GS, inhibited transcriptional activation of PPARα target genes, thereby elevating serum levels of total cholesterol, triglycerides, and HDL cholesterol. To our knowledge, this is the first study to explore the mechanism of ginseng-mediated effects on lipid metabolism, and is the first to demonstrate that ginseng interacts with the nuclear receptor PPARα to exert these effects.
Methods
Animal treatments
For all experiments, 8-week-old male mice (C57BL/6J) were used and bred at the Korea Research Institute of Bioscience and Biotechnology under specific pathogen-free conditions and a standard 12-h light/dark cycle. In one series of experiments, the effects of GE or GS were analyzed. In the first study, mice received once daily intraperitoneal injections of GE or GS at indicated doses for 10 days followed by a diet containing 0.1% (w w−1) (4-chloro-6-(2,3-xylidine)-pyrimidinylthio)acetic acid (Wy14,643) for 1 day. Chow diet-fed control mice were administered saline. In a subsequent experiment, mice were treated with GE (5 mg per mouse) or GS (0.5 mg per mouse) for the indicated period of time followed by Wy14,643 for 1 day. In a third study, mice received intraperitoneal injections of either saline (control), GE (5 mg), and GS (0.5 mg) for 10 days, or a 0.1% Wy14,643 diet for 1 day. For cotreatment studies, mice were treated with either GE (5 mg) for 10 days followed by a 0.1% Wy14,643 diet for 1 day, or GS (0.5 mg) for 10 days followed by a 0.1% Wy14,643 diet for 1 day. At the end of each study, animals were killed by cervical dislocation, tissues were harvested, weighed and snap frozen in liquid nitrogen and stored at −80°C until use. Serum total cholesterol, triglycerides, and HDL cholesterol were measured using an automatic blood chemical analyzer (CIBA Corning, OH, U.S.A.).
Drugs
Wy-14,643, a powerful PPARα ligand, was purchased commercially (ChemSyn Science Laboratories, Lenexa, KS, U.S.A.). Korean red GE powder was commercially prepared from ginsengs cultivated with care in well-fertilized fields for 6 years (Korea Ginseng Corp.). GS were obtained from extractions of the GE powder. Briefly, ginseng powder (100 g) is placed into a 1000 ml flask with a refluxing condenser and extracted twice with 500 ml of water-saturated 1-butanol for 1 h at 80°C. The extracted solution is passed through a Whatman filter paper (No. 41) after being cooled. The process is repeated twice more. The residue and filter paper are washed with 100 ml of water-saturated 1-butanol and then the filtrate is washed twice with 100 ml of water in a 2000 ml separating funnel. The butanol layer is then evaporated to dryness. The concentrate is extracted to remove any traces of fat with 100 ml of diethyl ether for 30 min at 36°C in a flask with a refluxing condenser, following which the ether solution is decanted. The residue is dried at 50°C and weighed.
Analysis of target gene expression
Total RNA was prepared using Trizol reagent (Gibco-BRL, Grand Island, NY, U.S.A.) and analyzed by electrophoresis on 1.2% agarose gels containing 0.22 M formaldehyde. The separated RNA was transferred to Nytran membranes (Schneicher & Schuell, Inc., Dassel, Germany) by downward capillary transfer in the presence of 20 × SSC buffer (3 M NaCl, 0.3 M sodium citrate, pH 7.0), then UV-crosslinked, and baked for 2 h at 80°C. Probe hybridization and washing were performed using standard techniques. Blots were exposed to phosphorimager screen cassettes and were visualized using a Molecular Dynamics Storm 860 PhosphorImager system (Sunnyvale, CA, U.S.A.). The probes used in this study were 32P-labeled by the random-primer method using a Ready-to-Go DNA Labeling kit (Amersham-Pharmacia Biotech, Piscataway, NJ, U.S.A.), as previously described (Sinal et al., 2001). Densitometric analysis of the mRNA signals was performed using ImageQuant image analysis software (Molecular Dynamics, Sunnyvale, CA, U.S.A.).
Transient transfection assay
The expression vectors, pSG5-mPPARα and PPRE3-tk-luc reporter gene were generously provided by Dr Frank J. Gonzalez (National Cancer Institute, NIH, Bethesda, MD, U.S.A.). Murine liver cell line NMu2Li cells were routinely cultured in DMEM containing 10% fetal bovine serum (Gibco-Brl, Grand Island, NY, U.S.A.), penicillin G (100 U ml−1), streptomycin sulfate (100 μg ml−1), amphotericin B (0.25 μg ml−1), and 2-mercaptoethanol (50 μM). Cells were seeded in six-well tissue culture plates (2 × 104 cells per well) 24 h prior to transfection. For all transfections, 200 ng per well of each of the appropriate plasmids was used. Transfections were performed using GeneSHUTTLE-40 (Q·Biogene, Carlsbad, CA, U.S.A.) according to the manufacturer's instructions. After 6 h, the culture medium was changed and the test compounds, Wy14,643, GE or GS were added. After incubation for 24 h in the presence of the aforementioned chemicals, cells were washed twice with PBS and assayed for luciferase and β-galactosidase activity using commercial kits according to the manufacturer's instructions (Promega, Madison, WI, U.S.A.).
Statistics
Unless otherwise noted, all values are expressed as mean±standard deviation (s.d.). All data were analyzed by the unpaired, Student's t-test for significant differences between the mean values of each group using SigmaPlot 2001 (SPSS Inc., Chicago, IL, U.S.A.).
Results
GE and GS decrease Wy14,643-induced hepatic mRNA expression of PPARa target genes
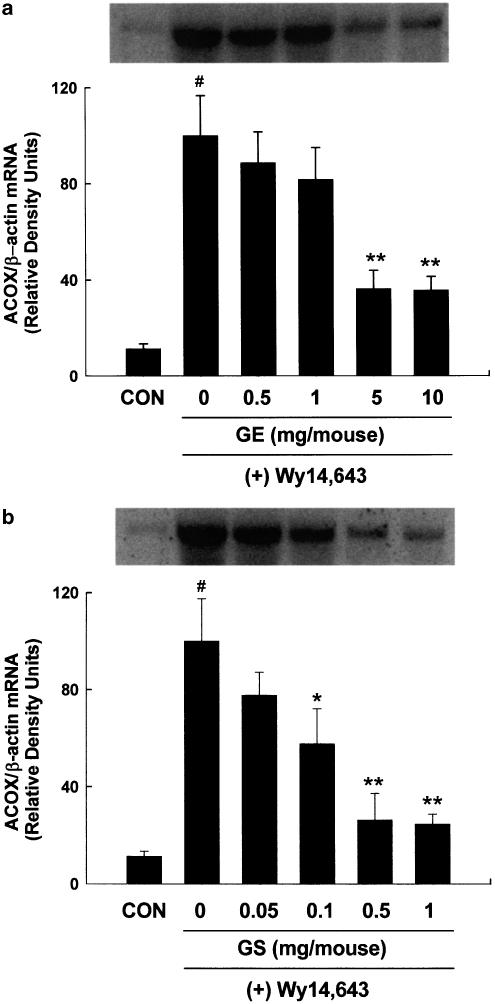
To study the effects of pharmacological doses of GE and GS on PPARα target gene expression, 8-week-old male mice were treated once daily with GE or GS for 10 days followed by a diet supplemented with 0.1% Wy14,643 for 1 day in a dose-dependent experiment. PPARα is activated by a number of structurally diverse compounds such as clofibrate, bezafibrate, ciprofibrate, and the potent PPARα agonist Wy14,643. Compared with chow-fed control mice, mice fed a diet containing 0.1% (w w−1) Wy14,643 for 1 day exhibited significant increases in mRNA levels of acyl-CoA oxidase (ACOX) (P<0.001). However, compared to Wy14,643 alone, both GS and GE at all doses caused inhibition of the Wy14,643-induced hepatic mRNA expression of ACOX, known to be a first and rate-limiting enzyme for PPARα-mediated fatty acid β-oxidation pathway (Figure 1), being more pronounced by GS. Maximal inhibition of ACOX mRNA expression was achieved at doses of 5 and 10 mg per mouse for GE, and 0.5 and 1 mg per mouse for GS, which resulted in a 2.7-fold decrease for GE and a 3.7-fold decrease for GS when compared to Wy14,643-only (P<0.005). However, the reduction in hepatic mRNA expression of ACOX by GE and GS was not observed after feeding Wy14,643 for periods longer than 1 day (unpublished data).
Figure 1.
Dose–response effects of (a) GE and (b) GS on ACOX mRNA levels by Wy14,643. Adult male mice received intraperitoneal injections of GE or GS at the indicated doses for 10 days followed by a diet containing 0.1% (w w−1) Wy14,643 for 1 day. Chow diet-fed control mice (CON) were administered saline. At the end of the experiment, samples were collected and analyzed as described under ‘Methods'. The mean±s.d. for three animals is shown and all values are expressed as relative density units using β-actin as reference. Insets show representative autoradiograms of Northern blots used for quantitation. #Significantly different versus control, P<0.001. *Significantly different versus Wy14,643 diet-only, P<0.05. **Significantly different versus Wy14,643 diet-only, P<0.005.
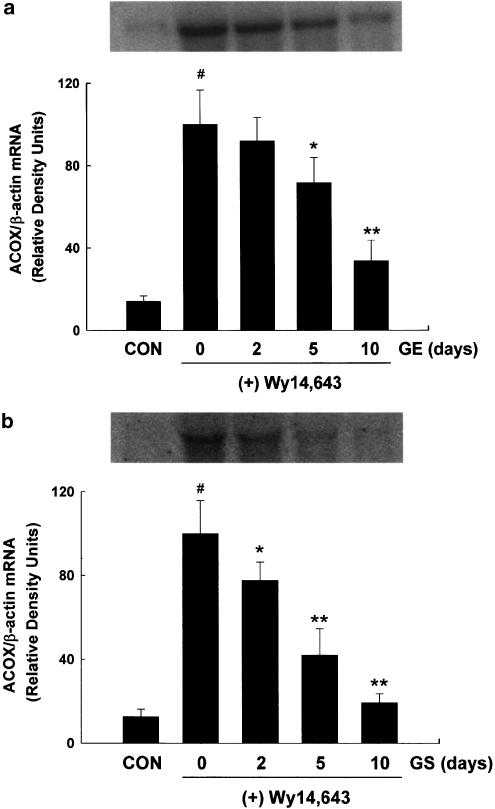
To determine the time-dependent effects of GE and GS on ACOX mRNA levels, ACOX gene expression was measured after administration of GS (0.5 mg per mouse) or GE (5 mg per mouse) for 2, 5, or 10 days followed by treatment of Wy14,643 for 1 day. Significant decreases of ACOX mRNA expression by Wy14,643 were shown in a time-dependent manner, as evidenced by the complete inhibition of gene expression following 10-day treatments of GE and GS (P<0.001) (Figure 2).
Figure 2.
Time-dependent effects of (a) GE and (b) GS on ACOX mRNA levels by Wy14,643. Adult male mice received intraperitoneal injections of GE or GS at the indicated doses for 10 days followed by a diet containing 0.1% (w w−1) Wy14,643 for 1 day. Chow diet-fed control mice (CON) were administered saline. At the end of the experiment, samples were collected and analyzed as described under ‘Methods'. All values are expressed as the mean±s.d. of three animals and are expressed as relative density units using β-actin as reference. #Significantly different versus control, P<0.001. *Significantly different versus Wy14,643 diet-only, P<0.05. **Significantly different versus Wy14,643 diet-only, P<0.005.
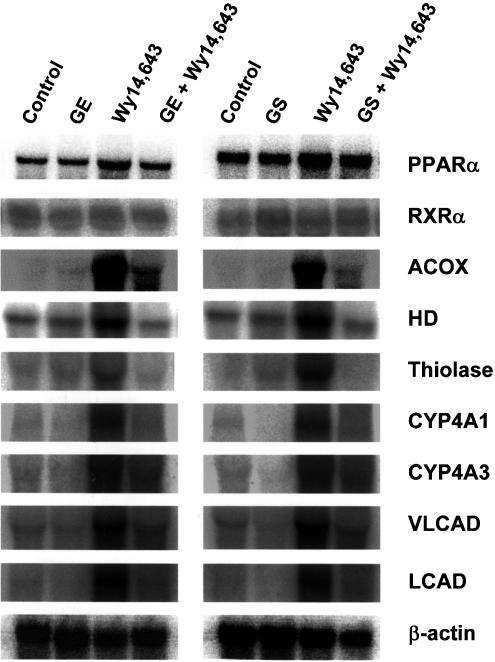
We next studied the effects of GE and GS on the mRNA levels of the several PPARα target genes, such as ACOX, enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase, 3-ketoacyl-CoA thiolase, cytochrome P450 4A1 and 4A3, and very-long-chain and long-chain acyl-CoA dehydrogenases, in mice fed a diet containing 0.1% Wy14,643 (Figure 3). In vivo treatment for 10 days with either GE (5 mg per mouse) or GS (0.5 mg per mouse), followed by a 1-day treatment of 0.1% Wy14,643 resulted in significantly reduced hepatic induction in all the target genes tested, compared with Wy14,643 treatment alone. Analysis of the hepatic mRNA expression of PPARα and its heterodimerization partner retionoid × receptor α (RXRα) revealed no significant effects by any dietary regimens. These results suggest that, compared with Wy14,643-only treated mice, both GE and GS may significantly reduce Wy14,643-induced mRNA levels of PPARα target genes.
Figure 3.
Inhibition of Wy14,643-induced mRNA levels of PPARα target genes by GE and GS. Mice received intraperitoneal injections of either saline (control), GE (5 mg), and GS (0.5 mg) for 10 days, or a 0.1% Wy14,643 diet for 1 day. For cotreatment studies, mice were treated with either GE (5 mg) for 10 days followed by a 0.1% Wy14,643 diet for 1 day, or GS (0.5 mg) for 10 days followed by a 0.1% Wy14,643 diet for 1 day. At the end of the experiment, samples were collected and analyzed as described under ‘Methods'. PPARα, peroxisome proliferator-activated receptor α; RXRα, retinoid X receptor α; ACOX, acyl-CoA oxidase; HD, enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase; thiolase, 3-ketoacyl-CoA thiolase; CYP4A1, cytochrome P450 4A1; CYP4A3, cytochrome P450 4A3; VLCAD, very-long-chain acyl-CoA dehydrogenase; LCAD, long-chain acyl-CoA dehydrogenase.
GE and GS inhibit PPARa reporter gene expression
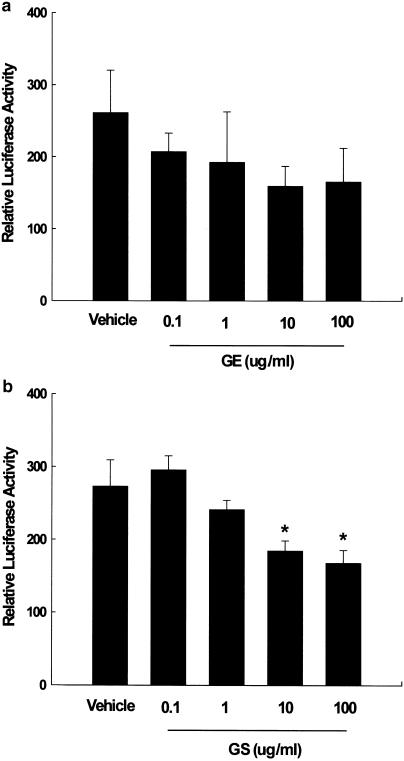
In order to examine the mechanism by which ginseng inhibited the Wy14,643-mediated induction of PPARα target genes, NMu2Li cells were cotransfected with PPARα and RXRα expression constructs as well as a luciferase reporter construct (PPRE3-tk-luc) containing three copies of the PPAR responsive element (PPRE) from the rat ACOX gene. Transfected cells were treated with GE and GS at doses that did not show any cytotoxic effects as measured by trypan blue exclusion. Treatment of the transfected cells with GE caused a dose-dependent decrease of luciferase activity (Figure 4a). GS also caused a dose-dependent decrease in reporter gene activation and significant decreases in the magnitude of reporter gene activation at doses of 10 or 100 μg ml−1 (P<0.05) (Figure 4b).
Figure 4.
Dose-dependent inhibitions of PPARα reporter gene expression by (a) GE and (b) GS. NMu2Li cells were transiently transfected with expression plasmids for PPARα, a luciferase reporter gene construct containing three copies of the PPRE from the rat ACOX gene and β-galactosidase gene. Cells were treated with various concentrations of GE and GS at the initial time of culture. Following incubation for 24 h, cells were harvested, lysed, and subsequently assayed for luciferase and β-galactosidase activities. All values are expressed as the mean±s.d. of relative luciferase units/β-galactosidase activity. Experiments were performed at least three times. *Significantly different versus vehicle, P<0.05.
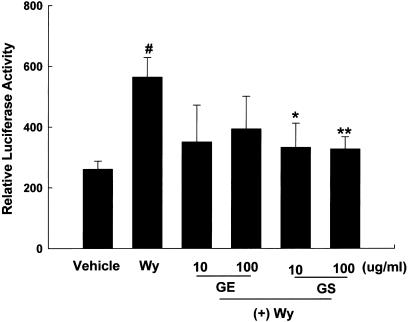
To determine if GE and GS also interfered with Wy14,643-mediated PPARα transactivation, experiments were performed using cells treated with a combination of ginseng and Wy14,643. As shown in Figure 5, treatment of PPARα-transfected cells with 10 μM Wy14,643 caused a two-fold increase of reporter gene activation compared with vehicle-treated cells (P<0.01). Conversely, cotreatment of Wy14,643 (10 μM) with either GE or GS at doses of 10 or 100 μg ml−1 decreased reporter gene activation, significantly so for GS at both doses (P<0.05 and P<0.01, respectively).
Figure 5.
Inhibition of Wy14,643-induced PPARα reporter gene expression by GE and GS. NMu2Li cells were transiently transfected with pSG5-mPPARα, reporter plasmid PPRE-TK-Luc, β-galactosidase gene, and pBSK. Cells were treated with 10 μM Wy-14,643 (Wy) alone or concomitantly treated with Wy and GE or Wy and GS. After incubation for 24 h, cells were harvested, lysed and subsequently assayed for luciferase and β-galactosidase activities. All values are expressed as the mean±s.d. of relative luciferase units/β-galactosidase activity. Experiments were performed at least three times. #Significantly different versus vehicle, P<0.01. *Significantly different versus Wy, P<0.05. **Significantly different versus Wy, P<0.01.
GE and GS regulate serum lipid levels
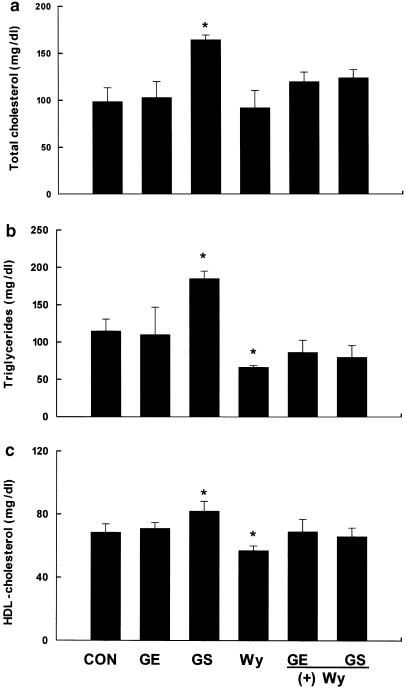
Mice treated with GS had significantly higher serum concentrations of total cholesterol, triglycerides, and HDL cholesterol compared with vehicle-treated mice (Figure 6). Compared to vehicle-treated mice, GS-treated mice had serum total cholesterol, triglycerides, and HDL cholesterol that were respectively 61, 67, and 20% higher (P<0.05). However, compared to controls, Wy14,643 treatment slightly decreased serum total cholesterol, but significantly reduced serum triglyceride and HDL cholesterol levels by 42 and 17%, respectively (P<0.05). Compared to the Wy14,643-alone treatment group, concomitant treatment with Wy14,643/GE or Wy14,643/GS showed a trend towards increasing serum concentrations of total cholesterol, triglycerides, and HDL cholesterol. In addition, the changes in serum levels of total cholesterol were well correlated with those in circulating HDL cholesterol following GE and GS treatments, confirming that HDL is the major cholesterol-containing lipoprotein in mice.
Figure 6.
Changes in circulating (a) total cholesterol, (b) triglycerides, and (c) HDL cholesterol by GE and GS. Mice received intraperitoneal injections of either saline (control), GE (5 mg), and GS (0.5 mg) for 10 days, or a 0.1% Wy14,643 (Wy) diet for 1 day. For cotreatment studies, mice were treated with either GE (5 mg) for 10 days followed by a 0.1% Wy diet for 1 day, or GS (0.5 mg) for 10 days followed by a 0.1% Wy diet for 1 day. Serum concentrations of total cholesterol, triglycerides, and HDL cholesterol were measured, and all values are expressed as the mean±s.d. of four animals. *Significantly different versus control, P<0.05.
Discussion
Although public enthusiasm for herbal remedies continues to grow in the Far East and around the world, the absence of scientific analysis in the literature of these compounds makes it difficult to determine not only the validity of their effects but also their mechanisms of action. Much research effort has focused on the mechanism of the action of ginseng in experimental animals during the last decade, but many of these studies rarely permit the evaluation of qualitative and quantitative data on GE and GS. In addition, since there are only a few reports concerning regulation of lipid profiles by ginseng, and all of which are limited in scope to examination at the serological level, a study examining ginseng-mediated effects on lipid metabolism at the molecular level would seem warranted. Based on the suggestion in oriental medicine that ginseng modulates lipid metabolism, the structural similarities of GS to steroidal hormones, as well as the documented role of PPARα in the expression of genes encoding mitochondrial and peroxisomal β-oxidizing enzymes, microsomal ω-oxidizing enzymes, and apolipoproteins, resulting in the regulation of lipid and lipoprotein metabolism, we hypothesized an interaction between ginseng and PPARα in the control of lipid homeostasis.
To understand the role of PPARα in ginseng-mediated lipid metabolism, we studied the effects of GE and GS at pharmacological doses on the expression of PPARα target genes involved in the fatty acid oxidation in the liver. Our in vivo results demonstrate that GE and GS not only cause a dose-dependent reduction in the expression of ACOX mRNA but also inhibit the transcriptional activation of PPARα target genes by the potent PPARα ligand Wy14,643. Our data show that GS was more effective in the inhibition of PPARα target expression than GE, which is perhaps not surprising in light of the fact that GS is the major pharmacologically active metabolite of ginseng (Gillis, 1997; Attele et al., 1999). Consistent with the in vivo data, GE and GS decreased the expression of a PPRE-luciferase reporter construct in transactivation assays. GE cotreatment reduced, while GS cotreatment significantly reduced Wy14,643-initiated luciferase activity by 41% (P<0.05) and 42% (P<0.001) at doses of 10 and 100 μg ml−1, respectively, compared with reporter gene activation of cells treated with Wy14,643 alone, suggesting that ginseng can negatively modulate the action of PPARα.
Serum concentrations of total cholesterol, triglycerides, and HDL cholesterol were significantly increased by 61, 67, and 20%, respectively, following GS treatment (P<0.05). Moreover, cotreatment of GE or GS with Wy14,643 modestly elevated the circulating levels of total cholesterol, triglycerides, and HDL cholesterol that were lowered by Wy14,643 alone. Together, these results suggest that these increases are, at least in part, due to the inhibition by ginseng of PPARα-regulated transactivation of target genes encoding fatty-acid-metabolizing enzymes. PPARα stimulates the catabolism of fatty acid and cholesterol metabolism by increasing the transcription of rate-limiting enzymes in mitochondrial and peroxisomal β-oxidation, and microsomal ω-oxidation (Schoonjans et al., 1996; Aoyama et al., 1998; Staels et al., 1998). Therefore, our data suggest that ginseng's inhibition of transcriptional activation of PPARα target enzymes increases the intracellular fatty acid levels available for triglyceride synthesis, which leads to a rise in serum triglycerides (Kalderon et al., 1992; Rustan et al., 1992). This result is in contrast with the expectation that ginseng can stimulate PPARα function since the majority of the limited studies to date on ginseng's effects in the serum suggest it has beneficial effects on lipid metabolism. In contrast to data presented in this study, these previous reports show that ginseng decreased serum triglycerides and cholesterol in diet- or cyclophosphamide-induced hyperlipidemic rabbits and patients with hyperlipidemia (Qureshi et al., 1983; Yamamoto et al., 1983; Cui et al., 1998; Inoue et al., 1999). The discrepancy between the former reports and our study may be because of variations in the metabolism of ginseng leading to different pharmacologically active metabolites and the animals used for analysis. It is well known that there are species differences in the expression of metabolizing enzymes and in the control of metabolism. Therefore, metabolism in different species may produce differences in lipid profiles by ginseng seen in this work using mice when compared with the former study using rabbits and man. According to a review by Gillis (1997), ginseng preparations may have different pharmacological effects as a result of a number of factors including the differences in GS content in ginseng root extracts, the method of extraction, subsequent treatment following cultivation, age and part of the plant extracted, and even the season of its collection (Soldati and Sticher, 1980; Shibata et al., 1985; Gillis, 1997). Thus, the source and preparation of previous ginseng used in the former studies may have led to different results from those seen here. However, given we prepared the GS compound from ginseng ourselves and used it directly along with its parent form, the above factors would not have contributed to any significant degree to the lipid metabolism and PPARα target gene differences seen between GE and GS in our study.
There is evidence to suggest that ginseng can interact with nuclear receptors including PPARα. GS, called steroidal saponins, are the main components of ginseng and most pharmacological actions of ginseng are attributed to GS (Banthorpe, 1994; Kim et al., 1998; Attele et al., 1999; Huang, 1999). Our results are supported by the findings that Rg1, one of the GS, is a functional ligand of the nuclear glucocorticoid receptor, and can activate a reporter gene containing a glucocorticoid responsive element (GRE) in a concentration-dependent manner (Lee et al., 1997; Chung et al., 1998). In combination with our in vivo data that show that the GE- and GS-mediated inhibition of PPARα target gene expression is not associated with the suppression of either PPARα or RXRα mRNA, our reporter assay data also suggest that GE and GS may exert their inhibitory functions on PPARα-mediated transactivation by interfering with the binding of agonistic ligands to PPARα (Seol et al., 1998; Shiau et al., 1998; Lee et al., 2000; Sinal et al., 2001). Since GS have structural similarities to steroidal hormones, and our data show induction of total cholesterol and triglycerides, as well as concomitant inhibition of Wy14,643-mediated effects, it seems more likely that both GE and GS may exert their effects by disrupting the activation of PPARα, thus acting as competitive inhibitors.
In conclusion, the results of this study provide the first direct evidence that in vivo and in vitro treatments of ginseng, especially GS, suppress PPARα target gene expression, thereby providing a molecular mechanism by which ginseng modulates alterations in serum lipid profiles. Furthermore, ginseng may cause significant impairment of PPARα-dependent activation of genes involved in the fatty-acid β-oxidation, suggesting that the use of ginseng be limited under certain pathophysiological conditions such as hypercholesterolemia and hypertriglyceridemia. Further studies will be necessary not only to determine the mechanism by which PPARα action is inhibited by ginseng, but also to identify and characterize each individual GS for their effects on PPARα function for potential therapeutic implications.
Acknowledgments
This research was supported by Grant No. R05-2002-001099-0 from the Basic Research Program of the Korea Science & Engineering Foundation, and 21st Century Frontier Biological Modulators Project (CBM-02-B-7) from the Korea Ministry of Science & Technology.
Abbreviations
- ACOX
acyl-CoA oxidase
- GE
ginseng extract
- GS
ginsenosides
- HDL
high-density lipoprotein
- PPARα
peroxisome proliferator-activated receptor α
- PPRE
PPAR responsive element
- RXRα
retinoid X receptor α
- Wy14,643
(4-chloro-6-(2,3-xylidine)-pyrimidinylthio)acetic acid
References
- AOYAMA T., PETERS J.M., IRITANI N., NAKAJIMA T., FURIHATA K., HASHIMOTO T., GONZALEZ F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα) J. Biol. Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- ATTELE A.S., WU J.A., YUAN C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem. Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- AUWERX J., SCHOONJANS K., FRUCHART J.C., STAELS B. Transcriptional control of triglyceride metabolism: fibrates and fatty acids change the expression of the LPL and apo C-III genes by activating the nuclear receptor PPAR. Atherosclerosis. 1996;124 Suppl:S29–S37. doi: 10.1016/0021-9150(96)05854-6. [DOI] [PubMed] [Google Scholar]
- BANTHORPE D.V.Terpenoids Natural Products 1994Essex: Longman Scientific and Technical; 331–339.ed. Mann, J. pp [Google Scholar]
- CHUNG E., LEE K.Y., LEE Y.J., LEE Y.H., LEE S.K. Ginsenoside Rg1 down-regulates glucocorticoid receptor and displays synergistic effects with cAMP. Steroids. 1998;63:421–424. doi: 10.1016/s0039-128x(98)00043-9. [DOI] [PubMed] [Google Scholar]
- COSTET P., LEGENDRE C., MORE J., EDGAR A., GALTIER P., PINEAU T. Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J. Biol. Chem. 1998;273:29577–29585. doi: 10.1074/jbc.273.45.29577. [DOI] [PubMed] [Google Scholar]
- CUI X., SAKAGUCHI T., ISHIZUKA D., TSUKADA K., HATAKEYAMA K. Orally administered ginseng extract reduces serum total cholesterol and triglycerides that induce fatty liver in 66% hepatectomized rats. J. Int. Med. Res. 1998;26:181–187. doi: 10.1177/030006059802600402. [DOI] [PubMed] [Google Scholar]
- GILLIS C.N. Panax ginseng pharmacology: a nitric oxide link. Biochem. Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- HERTZ R., BISHARA-SHIEBAN J., BAR-TANA Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. J. Biol. Chem. 1995;270:13470–13475. doi: 10.1074/jbc.270.22.13470. [DOI] [PubMed] [Google Scholar]
- HUANG K.C. The Pharmacology of Chinese Herbs. Boca Raton, FL: CRC Press; 1999. [Google Scholar]
- INOUE M., WU C.Z., DOU D.Q., CHEN Y.J., OGIHARA Y. Lipoprotein lipase activation by red ginseng saponins in hyperlipidemia model animals. Phytomedicine. 1999;6:257–265. doi: 10.1016/S0944-7113(99)80018-X. [DOI] [PubMed] [Google Scholar]
- ISMAIL M.F., GAD M.Z., HAMDY M.A. Study of the hypolipidemic properties of pectin, garlic and ginseng in hypercholesterolemic rabbits. Pharmacol. Res. 1999;39:157–166. doi: 10.1006/phrs.1998.0421. [DOI] [PubMed] [Google Scholar]
- KAKU T., MIYATA T., URUNO T., SAKO I., KINOSITA Y. Chemico-pharmacological studies on saponins of Panax ginseng C. A. Meyer. I. Chemical part. Arzneimitted-Forschung. 1975;25:343–347. [PubMed] [Google Scholar]
- KALDERON B., HERTZ R., BAR-TANA J. Tissue selective modulation of redox and phosphate potentials by beta, beta'-methyl-substituted hexadecanedioic acid. Endocrinology. 1992;131:1629–1635. doi: 10.1210/endo.131.4.1396307. [DOI] [PubMed] [Google Scholar]
- KENAROVA B., NEYCHEV H., HADJIIVANOVA C., PETKOV V.D. Immunomodulating activity of ginsenoside Rg1 from Panax ginseng. Jpn. J. Pharmacol. 1990;54:447–454. doi: 10.1254/jjp.54.447. [DOI] [PubMed] [Google Scholar]
- KIM Y.S., KIM D.S., KIM S.I. Ginsenoside Rh2 and Rh3 induce differentiation of HL-60 cells into granulocytes: modulation of protein kinase C isoforms during differentiation by ginsenoside Rh2. Int. J. Biochem. Cell Biol. 1998;30:327–338. doi: 10.1016/s1357-2725(97)00141-6. [DOI] [PubMed] [Google Scholar]
- KROETZ D.L., YOOK P., COSTET P., BIANCHI P., PINEAU T. Peroxisome proliferator-activated receptor alpha controls the hepatic CYP4A induction adaptive response to starvation and diabetes. J. Biol. Chem. 1998;273:31581–31589. doi: 10.1074/jbc.273.47.31581. [DOI] [PubMed] [Google Scholar]
- LEE Y.J., CHUNG E., LEE K.Y., LEE Y.H., HUH B., LEE S.K. Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol. Cell. Endocrinol. 1997;133:135–140. doi: 10.1016/s0303-7207(97)00160-3. [DOI] [PubMed] [Google Scholar]
- LEE Y.K., DELL H., DOWHAN D.H., HADZOPOULOU-CLADARAS M., MOORE D.D. The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression. Mol. Cell Biol. 2000;20:187–195. doi: 10.1128/mcb.20.1.187-195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMBERGER T., DESVERGNE B., WAHLI W. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Annu. Rev. Cell Dev. Biol. 1996;12:335–363. doi: 10.1146/annurev.cellbio.12.1.335. [DOI] [PubMed] [Google Scholar]
- NAH S.Y., PARK H.J., MCCLESKEY E.W. A trace component of ginseng that inhibits Ca2+ channels through a pertussis toxin-sensitive G protein. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8739–8743. doi: 10.1073/pnas.92.19.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ODASHIMA S., OTA T., KOHNO H., MATSUDA T., KITAGAWA I., ABE H., ARICHI S. Control of phenotypic expression of cultured B16 melanoma cells by plant glycosides. Cancer Res. 1985;45:2781–2784. [PubMed] [Google Scholar]
- OTA T., FUJIKAWA-YAMAMOTO K., ZONG Z.P., YAMAZAKI M., ODASHIMA S., KITAGAWA I., ABE H., ARICHI S. Plant-glycoside modulation of cell surface related to control of differentiation in cultured B16 melanoma cells. Cancer Res. 1987;47:3863–3867. [PubMed] [Google Scholar]
- PETERS J.M., HENNUYER N., STAELS B., FRUCHART J.C., FIEVET C., GONZALEZ F.J., AUWERX J. Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor alpha-deficient mice. J. Biol. Chem. 1997;272:27307–27312. doi: 10.1074/jbc.272.43.27307. [DOI] [PubMed] [Google Scholar]
- QURESHI A.A., ABUIRMEILEH N., DIN Z.Z., AHMAD Y., BURGER W.C., ELSON C.E. Suppression of cholesterogenesis and reduction of LDL cholesterol by dietary ginseng and its fractions in chicken liver. Atherosclerosis. 1983;48:81–94. doi: 10.1016/0021-9150(83)90019-9. [DOI] [PubMed] [Google Scholar]
- RUSTAN A.C., CHRISTIANSEN E.N., DREVOR C.A. Serum lipids, hepatic glycerolipid metabolism and peroxisomal fatty acid oxidation in rats fed omega-3 and omega-6 fatty acids. Biochem. J. 1992;283:333–339. doi: 10.1042/bj2830333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOONJANS K., STAELS B., AUWERX J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J. Lipid Res. 1996;37:907–925. [PubMed] [Google Scholar]
- SEOL W., HANSTEIN B., BROWN M., MOORE D.D. Inhibition of estrogen receptor action by the orphan receptor SHP (short heterodimer partner) Mol. Endocrinol. 1998;12:1551–1557. doi: 10.1210/mend.12.10.0184. [DOI] [PubMed] [Google Scholar]
- SHIAU A.K., BARSTAD D., LORIA P.M., CHENG L., KUSHNER P.J., AGARD D.A., GREENE G.L. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- SHIBATA S., TANAKA O., SHOJI J., SAITO H.Chemistry and pharmacology of Panax Economic and Medicinal Plant Research 1985New York: Academic Press; 217–284.ed. Wagner, H., Hikino, H. & Farnsworth, N.R. Vol. 1. pp [Google Scholar]
- SINAL C.J., YOON M., GONZALEZ F.J. Antagonism of the actions of peroxisome proliferator activated receptor-alpha by bile acids. J. Biol. Chem. 2001;276:47154–47162. doi: 10.1074/jbc.M107000200. [DOI] [PubMed] [Google Scholar]
- SOLDATI F., STICHER O. HPLC separation and quantitative determination of ginsenosides from Panax ginseng, Panax quinquefolium and from ginseng drug preparations. 2nd communication. Planta Med. 1980;39:348–357. doi: 10.1055/s-2008-1074929. [DOI] [PubMed] [Google Scholar]
- STAELS B., AUWERX J. Regulation of apo A-I gene expression by fibrates. Atherosclerosis. 1998;137 Suppl:S19–S23. doi: 10.1016/s0021-9150(97)00313-4. [DOI] [PubMed] [Google Scholar]
- STAELS B., DALLONGEVILLE J., AUWERX J., SCHOONJANS K., LEITERSDORF E., FRUCHART J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- TSANG D., YEUNG H.W., TSO W.W., PECK H. Ginseng saponins: influence on neurotransmitter uptake in rat brain synaptosomes. Planta Med. 1985;3:221–224. doi: 10.1055/s-2007-969463. [DOI] [PubMed] [Google Scholar]
- VU-DAC N., SCHOONJANS K., KOSYKH V., DALLONGEVILLE J., FRUCHART J.C., STAELS B., AUWERX J. Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. J. Clin. Invest. 1995;96:741–750. doi: 10.1172/JCI118118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEN T.C., YOSHIMURA H., MATSUDA S., LIM J.H., SAKANAKA M. Ginseng root prevents learning disability and neuronal loss in gerbils with 5-minute forebrain ischemia. Acta Neuropathol. (Berl.). 1996;91:15–22. doi: 10.1007/s004010050387. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO M., UEMURA T., NAKAMA S., UEMIYA M., KUMAGAI A. Serum HDL-cholesterol-increasing and fatty liver-improving actions of Panax ginseng in high cholesterol diet-fed rats with clinical effect on hyperlipidemia in man. Am. J. Chin. Med. 1983;11:96–101. doi: 10.1142/S0192415X83000161. [DOI] [PubMed] [Google Scholar]
- YAN Z.H., KARAM W.G., STAUDINGER J.L., MEDVEDEV A., GHANAYEM B.I., JETTEN A.M. Regulation of peroxisome proliferator-activated receptor alpha-induced transactivation by the nuclear orphan receptor TAK1/TR4. J. Biol. Chem. 1998;273:10948–10957. doi: 10.1074/jbc.273.18.10948. [DOI] [PubMed] [Google Scholar]