Abstract
The proton acceptor group in the recently described retinal protein, proteorhodopsin has an unusually high pKa of 7.1. It was shown that at pH above this pKa, illumination initiates a photocycle similar to that of bacteriorhodopsin, and the protein transports proton across the cell membrane. Recently it was reported that proteorhodopsin, unlike bacteriorhodopsin, transports protons at pH below the pKa of the proton acceptor, and this transport is in the reverse direction. We have investigated the photocycle of proteorhodopsin at such low pH. At pH 5, three spectrally distinct intermediates K, L, and N, and another spectrally silent one, PR′, could be identified, but a deprotonated Schiff base containing M-like intermediate, characteristic for proton pumping activity, does not accumulate. All the reactions between the intermediates are close to equilibrium, except the last transition from PR′ to PR, when the protein returns to its initial unexcited state in a quasiunidirectional reaction. The electric signal measurements indicate that although charge motions are detected inside the protein, their net dislocation is zero, indicating that contrary to the earlier reported, at low pH no charged particle is transported across the membrane.
INTRODUCTION
The recently identified light-activated proton pump, proteorhodopsin (PR), belongs to the retinal protein family type 1, the archaeal type rhodopsin (Spudich et al., 2000). It was discovered in the uncultivated marine γ-proteobacterium of the SAR 86 (Beja et al., 2000, 2001).
Similar to other retinal proteins, PR is formed from seven transmembrane α-helices. The early studies suggested that the chromophore in the protein is an all-trans retinal covalently bound via lysine-Schiff base to helix G (Beja et al., 2000). Later it was shown that, as in bacteriorhodopsin (BR), the retinal could be both in all-trans and 13-cis form. While some authors at low pH report only small amount of 13-cis, 15-anti form (Dioumaev et al., 2002) others determine a content of ∼20% of it (Friedich et al., 2002). The amino acid sequence, the deduced structure, the transport function, and the photocycle of proteorhodopsin at high pH all show great similarities to that of bacteriorhodopsin.
Comparing the PR and BR sequences, the putative proton acceptor and donor groups in PR were identified as Asp-97 and Glu-108, respectively. This result was confirmed with visible and FTIR spectroscopy (Dioumaev et al., 2002). The pKa of the proton acceptor Asp-97 was determined by spectral titration. Although one group measured a pKa of 7.1 (Dioumaev et al., 2002), others determined it to be 7.68 (Friedich et al., 2002). In the photocycle at pH 9.5, the intermediates were designated as K, M, N, and O, (Beja et al., 2001; Dioumaev et al., 2002; Friedich et al., 2002). During this BR-like photocycle, a proton is transported from the cytoplasmic to the extracellular side of the membrane (Beja et al., 2000; Dioumaev et al., 2002; Váró et al., 2002; Krebs et al., 2002).
In BR, the pKa of the proton acceptor Asp-85 is ∼2.5 (Balashov et al., 1996). At pH below 2.5, the proton acceptor is not available, and in the photocycle of the “blue membrane”, the retinal Schiff base does not deprotonate (Váró and Lanyi, 1989) and transport is blocked (Dér et al., 1991). It was unexpected, therefore, that PR was reported to transport at pH below 7 (Friedich et al., 2002). Even more unusual was that this transport was in the opposite direction from the transport at high pH. In this paper we describe the photocycle of proteorhodopsin at low pH. Time-resolved spectroscopy in the visible as well as absorption kinetic and electric signal measurements reveal the details of the photocycle in which the proton acceptor is already protonated in the nonilluminated state. Three spectral and four kinetic intermediates were observed. The electric signal measurements reveal that although in the early steps of the photocycle charge motions were observed, the overall charge shift across the membrane, during the whole photocycle, is practically zero. This means that contrary to the earlier finding (Friedich et al., 2002), this photocycle does not transport charge across the membrane.
MATERIALS AND METHODS
Wild-type PR was expressed in Escherichia coli (strain UT5600), as described before (Beja et al., 2000; Dioumaev et al., 2002). The cells were broken using an Aminco French press at 12 MPa. The membranes were purified by centrifugation in distilled water and on a sucrose gradient.
The measuring and analysis techniques were the same as described earlier (Kulcsár et al., 2000). The absorption kinetic and transient spectroscopy measurements were carried out on acrylamide gel samples, following the procedure described elsewhere (Mowery et al., 1979). For electric signal measurements, oriented gel samples were prepared (Dér et al., 1985). During the sample preparation, no buffer or salt was used to avoid the aggregation of the membranes. The gels were equilibrated with a bathing solution containing 100 mM NaCl and 50 mM MES (2-[N-morpholino]ethanesulfonic acid) buffer; if not mentioned otherwise, the pH was set to 5, and the temperature of the sample was 20°C.
A 250-W halogen lamp with a heat filter and monochromator provided the continuous measuring light. Laser excitation was with a frequency-doubled Nd-YAG laser (Surelite 10, λ = 532 nm, Continuum, Santa Clara, CA). Time-resolved difference spectra were measured with a gated optical multichannel analyzer (Zimányi et al., 1989) and the absolute spectra of intermediates were calculated as before (Gergely et al., 1997). Absorption kinetic signals were recorded at several wavelengths with a transient recorder card (NI-DAQ PCI-5102, National Instruments, Austin, TX) with 16 MB memory and the signals fitted with RATE and EYRING programs as described before (Kulcsár et al., 2000). Electric signals were measured on the earlier described setup (Gergely et al., 1993).
RESULTS AND DISCUSSION
Spectral titration of proteorhodopsin
Determination of the pKa of the wild-type PR was from spectral titration in the pH range 4.5–10 (Fig. 1 A). As reported before (Dioumaev et al., 2002), the spectrum of PR shifted from 546 nm to 517 nm with increasing pH. In the difference spectra, after subtracting the spectrum at pH 4.5 from the others, an absorption maximum at 500 nm and a minimum at 570 nm can be observed (data not shown). The relative shifts of spectra and the difference amplitudes yield the same titration curve. On a freshly prepared gel sample, the fitted apparent pKa was 7.1 ± 0.1, with a Hill coefficient of 0.8 ± 0.05 protons (Fig. 1 B), as was previously determined (Dioumaev et al., 2002). The points on Fig. 1 B corresponding to the relative shift of the spectra indicate a slightly lower pKa at ∼7, but this value is within the error. If the sample was kept at room temperature for several days, the measured pKa shifted toward higher pH values as reported before (Friedich et al., 2002). We suspect that this is caused by oxidation of one or more of the three cysteines in the protein, as this effect is missing from the triple cysteine mutant (unpublished results). The importance of the cysteine mutants was already reported (Krebs et al., 2002). To avoid the shift of the pKa, we used freshly prepared samples in all measurements.
FIGURE 1.
The spectral titration of the proteorhodopsin at 20°C in 100 mM NaCl, 25 mM MES, and 25 mM TRIS in the pH range 4.5–10. (A) The changes in the spectrum. (B) The titration curve. The apparent pKa is 7.1.
Absorption kinetic measurements were performed at four wavelengths at both high and low pH (Fig. 2). An M-like absorption signal could be observed at high pH as before (Dioumaev et al., 2002). At pH = 5, the absorption kinetic traces were dramatically different. No M-like positive absorption change appeared at 410 nm, indicating the absence of an intermediate with deprotonated Schiff base (Dioumaev et al., 2002; Friedich et al., 2002). As the pH was raised, a small positive absorption change already could be observed at pH 5.5 (not shown). During the following analyses of the low pH photocycle, all the measurement were carried out at pH 5. A fraction of the unexcited PR has the chromophore in 13-cis form (Dioumaev et al., 2002; Friedich et al., 2002). It is possible that this protein has a different photocycle from that, which contains all-trans retinal. Although during the following analyses the existence of an additional, parallel photocycle was not detected, this possibility is not excluded.
FIGURE 2.
Absorption kinetic signals measured at several characteristic wavelengths at pH 5 and 9.5. Measuring conditions were the same as in Fig. 1.
It is known that the proton pumping BR at low pH has a changed photocycle, which does not contain intermediate M, and transports chloride ion, instead of proton (Dér et al., 1989). The chloride ion pumping halorhodopsin (HR), when the bathing solution contains azide, has an M-like intermediate and transports proton, instead of chloride (Váró et al., 1996; Váró, 2000). To compare the PR-low pH photocycle to that of the BR-low pH and HR photocycle, the effect of different salts was investigated. Replacing the NaCl with Na2SO4 or NaN3, the absorption kinetic signals did not change both at high and low pH (data not shown); indicating that there is no alteration in the photocycle and transport activity in the presence of different salts.
The spectra of the intermediates
The spectra of intermediates of the low pH photocycle were determined with transient optical multichannel spectroscopy. The methodology of the measurements for eliminating the possible artifacts was as described earlier (Váró et al., 2002). In short: the difference spectra measured at various times after the flash photoexcitation were submitted to singular value decomposition (SVD) (Golub and Kahan, 1992; Gergely et al., 1997). To judge the importance of the spectral components, it was taken into account the weight factor of that spectrum and the product of the autocorrelation calculated from the basis spectrum and its amplitude component. The first three spectra had weight factors of 1.17, 0.14, and 0.04, and autocorrelation product of 0.948, 0.884, and 0.129, respectively. All the other weight factors and autocorrelation products were smaller. Based on these, the first two basis spectra were considered to be different from noise. The difference spectra were reconstructed from the SVD components (Fig. 3). In addition to noise filtering, this analyzes gives the minimum number of spectrally different intermediates. In this photocycle it was two. The complex shape of basis spectra and the amplitude components suggest the presence of more intermediates, however. Neither the row difference spectra (not shown) nor the reconstructed set (Fig. 3) have significant absorption change in the short wavelength region, characteristic for intermediate M. This corroborates the result based on absorption kinetic measurement that no deprotonated Schiff base appears during the low pH photocycle of the PR. Lack of an intermediate with deprotonated Schiff base would contradict the observation of transport unless this state decays much more rapidly than it is formed (Friedich et al., 2002). This possibility will be explored in our future studies.
FIGURE 3.
(SVD)-filtered difference spectra of the proteorhodopsin, measured at the indicated time delay after the laser excitation. The measuring conditions: 100 mM NaCl, 50 mM MES, pH 5, and 20°C.
A model-independent search for the spectra of intermediates was performed with the method already described (Gergely et al., 1997). With two intermediates it was impossible to get spectra with only single maximum. After introducing a third intermediate, the spectra had acceptable shapes (Fig. 4). The introduction of a fourth component failed to give spectra with the required characteristics. Three spectrally independent intermediates should be considered in the model construction of the PR-low pH photocycle, although kinetically there could be more, spectrally silent components. In analogy with the BR intermediates, we named these intermediates K, L, and N.
FIGURE 4.
Spectra of intermediates of the proteorhodopsin photocycle at low pH, calculated from the difference spectra in Fig. 3.
Photocycle model
The multiphasic decay of the kinetic signal (Fig. 5 A) indicated the existence of a spectrally silent intermediate PR′, with spectrum similar to that of PR as it was observed also in the case of HR (Váró et al., 1995) or PR at high pH (Váró et al., 2002). Various sequential and parallel models were fitted to the absorption kinetic signals with the RATE program (Ludmann et al., 1998), with each transition but the last as equilibrium reactions as follows.
- Model with sequential reactions:
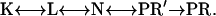
- Models with two parallel photocycles, such as:


- Or model with a branch:


The requirement for accepting the model was that it should hold up under all measuring conditions and fit the measured electric signal as well. Whereas under some measuring conditions all models could be made to fit the absorption kinetic signal, the sequential model was the only one that gave good fits (Fig. 5 B) at all measured temperature. Absorption kinetic measurements were performed between 30°C and 5°C at three different wavelengths (Fig. 6). The lifetimes of the fitted sequential model at 20°C are presented in Table 1. The linearity of the Eyring plots of the rate constants (ln k versus 1/T) is the most stringent criterion. The thermodynamic parameters calculated from the Eyring plots allow drawing the energetic picture of the photocycle (Fig. 7). The free energy levels of the K, L, N, and PR′ intermediates have almost the same value, resulting in a nearly equilibrium level between these intermediates. From the enthalpy and entropy diagram we can see that the photocycle is entropy driven.
FIGURE 5.
Absorption kinetic signal measured at three wavelengths (A, solid lines), the fit to the model with sequential equilibrium reactions (A, broken lines), and the time-dependent concentration of the intermediates of the fitted photocycle. Measuring conditions were the same as in Fig. 3.
FIGURE 6.
Absorption kinetic signals measured at three wavelengths at temperature 5, 10, 15, 20, 25, and 30°C, from right to left. The other measuring conditions are the same as in Fig. 3.
TABLE 1.
Lifetimes of the model with sequential reactions, fitted to the absorption kinetic signals of the proteorhodopsin
| Transition | K-L | L-K | L-N | N-L | N-PR′ | PR′-N | PR′-PR |
|---|---|---|---|---|---|---|---|
| Lifetime (ms) | 0.32 | 0.14 | 1.52 | 5.53 | 0.12 | 0.26 | 27.7 |
Measuring conditions: 100 mM NaCl, 50 mM MES, pH 5, and 20°C.
FIGURE 7.
Free energy, enthalpy, and entropy of the low pH photocycle of the proteorhodopsin, calculated from the temperature-dependent absorption kinetic signals shown in Fig. 6.
The main difference between the above described model and that reported earlier (Friedich et al., 2002) is the introduction of the intermediate L. This difference could be due to the fact that in the above-mentioned paper, there was no direct fit of the intermediate kinetics to the measured data. Instead the authors tried to correlate the six fitted exponential to the observable mixture of intermediates. In their model the late intermediate was named O, whereas we used N, as it is not shifted strongly toward the red.
Charge motions
The charge motions during the photocycle were determined by electric signal measurements on oriented gel samples. Comparing the current signal measured at pH = 5.0 (Fig. 8 A, solid line), with that measured at pH = 9.5 (Fig. 8 A, broken line), it can be seen, that at high pH when there is transport, the fast negative component is followed by a positive one. This component is missing from that of low pH signal. The voltage signal, calculated from the integral of the current signal, shows even better the differences between the high and low pH events. The signal measured at pH = 9.5 (Fig. 8 bottom, broken line) ends with a large positive component, as expected for translocation of a proton. In contrast, at low pH the signal up to 100 ms, a time when the photocycle is nearly finished (cf. Fig. 5), is approximately zero (Fig. 8 bottom, solid line). The current signal and its integral, the voltage, were reproducible in the time interval of 100 to 100 ms both high and low pH measurements. As only a small fraction of PR′ to PR transition occurs after 100 ms and no other intermediate is present, it is difficult to imagine that the transfer of a full charge across the membrane could happen in this time domain. Thus, the data indicate that although the high pH photocycle transports a charge across the membrane, this kind of transport is missing from the low pH photocycle. This is contrary to the earlier reported transport activity at low pH, from photocurrents measured in PR-containing liposomes attached to a planar lipid film (Friedich et al., 2002). The discrepancy between the two conclusions is puzzling and will need to be explored in further experiments.
FIGURE 8.
The current (A) and voltage signal (bottom) measured on oriented gel sample at pH 5 (solid lines) and pH 9.5 (broken lines).
Acknowledgments
National Science Research Fund of Hungary grant OTKA T034788 and National Institutes of Health grant GM29498 supported this work.
References
- Balashov, S. P., E. S. Imasheva, R. Govindjee, and T. G. Ebrey. 1996. Titration of aspartate-85 in bacteriorhodopsin: What it says about chromophore isomerization and proton release. Biophys. J. 70:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beja, O., L. Aravind, E. V. Koonin, T. Suzuki, A. Hadd, L. P. Nguyen, S. B. Jovanovich, C. M. Gates, R. A. Feldman, J. L. Spudich, E. N. Spudich, and E. F. DeLong. 2000. Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science. 289:1902–1906. [DOI] [PubMed] [Google Scholar]
- Beja, O., E. N. Spudich, J. L. Spudich, M. Leclerc, and E. F. DeLong. 2001. Proteorhodopsin phototrophy in the ocean. Nature. 411:786–789. [DOI] [PubMed] [Google Scholar]
- Dér, A., P. Hargittai, and J. Simon. 1985. Time-resolved photoelectric and absorption signals from oriented purple membranes immobilized in gel. J. Biochem. Biophys. Methods. 10:295–300. [DOI] [PubMed] [Google Scholar]
- Dér, A., S. Száraz, R. Tóth-Boconádi, Z. Tokaji, L. Keszthelyi, and W. Stoeckenius. 1991. Alternative translocation of protons and halide ions by bacteriorhodopsin. Proc. Natl. Acad. Sci. USA. 88:4751–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dér, A., R. Tóth-Boconádi, and L. Keszthelyi. 1989. Bacteriorhodopsin as a possible chloride pump. FEBS Lett. 259:24–26. [Google Scholar]
- Dioumaev, A. K., L. S. Brown, J. Shih, E. N. Spudich, J. L. Spudich, and J. K. Lanyi. 2002. Proton transfers in the photochemical reaction cycle of proteorhodopsin. Biochemistry. 41:5348–5358. [DOI] [PubMed] [Google Scholar]
- Friedich, T., S. Geibel, R. Kalmbach, I. Chizhov, K. Ataka, J. Heberle, M. Engelhard, and E. Bamberg. 2002. Proteorhodopsin is a light-driven proton pump with variable vectoriality. J. Mol. Biol. 321:821–838. [DOI] [PubMed] [Google Scholar]
- Gergely, C., C. Ganea, G. I. Groma, and G. Váró. 1993. Study of the photocycle and charge motions of the bacteriorhodopsin mutant D96N. Biophys. J. 65:2478–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely, C., L. Zimányi, and G. Váró. 1997. Bacteriorhodopsin intermediate spectra determined over a wide pH range. J. Phys. Chem. B. 101:9390–9395. [Google Scholar]
- Golub, G., and W. Kahan. 1992. Calculating the singular values and pseudo-inverse of a matrix. SIAM J. Num. Anal. 2:205–224. [Google Scholar]
- Krebs, R., U. Alexiev, R. Partha, A. M. Devita, and M. S. Braiman. 2002. Detection of fast light-activated H+ release and M intermediate formation from proteorhodopsin. BMC Physiol. 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulcsár, A., G. I. Groma, J. K. Lanyi, and G. Váró. 2000. Characterization of the proton transporting photocycle of pharaonis halorhodopsin. Biophys. J. 79:2705–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludmann, K., C. Gergely, and G. Váró. 1998. Kinetic and thermodynamic study of the bacteriorhodopsin photocycle over a wide pH range. Biophys. J. 75:3110–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowery, P. C., R. H. Lozier, Q. Chae, Y. W. Tseng, M. Taylor, and W. Stoeckenius. 1979. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 18:4100–4107. [DOI] [PubMed] [Google Scholar]
- Spudich, J. L., C. S. Yang, K. H. Jung, and E. N. Spudich. 2000. Retinylidene proteins: structures and functions form archea to humans. Annu. Rev. Cell Dev. Biol. 16:365–392. [DOI] [PubMed] [Google Scholar]
- Váró, G. 2000. Analogies between halorhodopsin and bacteriorhodopsin. Biochim. Biophys. Acta. Bio-Energetics. 1460:220–229. [DOI] [PubMed] [Google Scholar]
- Váró, G., L. S. Brown, M. Lakatos, and J. K. Lanyi. 2002. Characterization of the photochemical reaction cycle of proteorhodopsin. Biophys. J. In press. [DOI] [PMC free article] [PubMed]
- Váró, G., L. S. Brown, R. Needleman, and J. K. Lanyi. 1996. Proton transport by halorhodopsin. Biochemistry. 35:6604–6611. [DOI] [PubMed] [Google Scholar]
- Váró, G., L. S. Brown, N. Sasaki, H. Kandori, A. Maeda, R. Needleman, and J. K. Lanyi. 1995. Light-driven chloride ion transport by halorhodopsin from Natronobacterium pharaonis 1. The photochemical cycle. Biochemistry. 34:14490–14499. [DOI] [PubMed] [Google Scholar]
- Váró, G., and J. K. Lanyi. 1989. Photoreactions of bacteriorhodopsin at acid pH. Biophys. J. 56:1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimányi, L., L. Keszthelyi, and J. K. Lanyi. 1989. Transient spectroscopy of bacterial rhodopsins with optical multichannel analyzer. 1. Comparison of the photocycles of bacteriorhodopsin and halorhodopsin. Biochemistry. 28:5165–5172. [DOI] [PubMed] [Google Scholar]










