Abstract
Marek's disease virus (MDV) causes a common lymphomatous and neuropathic disease in domestic chickens and, less commonly, turkeys and quail. It is a member of the α-herpesviruses and until now was considered to be strongly cell associated. In 1991, MDV was suggested to be the causative infectious agent of multiple sclerosis (MS) in humans. In a previous study, we investigated the leukocytes of 107 well-defined MS patients for the presence of MDV DNA but were unable to confirm a role for MDV in the pathogenesis of MS. A recent report (S. Laurent, E. Esnault, G. Dambrine, A. Goudeau, D. Choudat, and D. Rasschaert, J. Gen. Virol. 82:233-240, 2001) described the detection of MDV DNA in 20% of 202 human serum samples, regardless of whether the individuals were exposed to poultry. The detection of MDV DNA in chicken serum samples was reported as well. The aim of the present study was to investigate whether we can confirm the presence of MDV DNA in chickens and humans if we use plasma as the source for nucleic acid isolation. Leukocytes and plasma specimens from 16 chickens experimentally infected with MDV serotype 1 and plasma specimens from 300 volunteer blood donors were tested for MDV DNA by two different TaqMan PCR assays. MDV DNA was repeatedly found in the leukocytes as well as in the plasma specimens of all 16 animals. All human samples analyzed, however, tested negative by both assays. Accordingly, Marek's disease in chickens can be diagnosed by detection of MDV DNA in plasma as well as in leukocytes. Once again, we found no evidence for the spread of MDV to humans.
Marek's disease (MD) is a common lymphomatous and neuropathic disease of the domestic chicken and, less commonly, of turkey and quail. It is named after the Hungarian pathologist Jozsef Marek, who in 1907 first published his observations of a paralytic disease affecting four cocks. In addition to this classical neuronal form, a visceral T-cell-based lymphomatosis has been observed. In particular, the T-cell lymphomas, which affect virtually all inner organs and the skin, have caused serious economic losses to the poultry industry worldwide for many years. In 1967, the agent of MD was identified as a herpesvirus (1), and 2 years later the first successful vaccine against a herpesviral disease and, simultaneously, the first vaccine against a malignancy were described (2). Despite vaccination programs, which greatly reduced rates of mortality and the economic losses caused by MD, vaccination failures due to the evolution of more virulent strains of MD virus (MDV) have been observed over and over again since the late 1970s. A recent report provided evidence for MDV infection in turkeys in Germany (16), and failure of a highly potent vaccine has been observed in both Europe and the United States (14, 17).
In 1991, MDV was suggested to be the causative infectious agent of multiple sclerosis (MS) in humans (8). This was based on an epidemiological study which showed an unusually high prevalence of MS in Key West, Fla. (3). Then it was postulated that seabirds were likely vectors of an environmental pathogen which could have caused this disease (9). A very high percentage of sera from MS patients (91%) were shown to be reactive with the MDV antigen, but it remained unclear if these results were specific for MDV antibodies or were caused by cross-reacting antibodies against Epstein-Barr virus (10, 11). The leukocytes of 107 well-defined MS patients were therefore investigated for the presence of MDV DNA by a highly sensitive real-time PCR technique, but MDV-related sequences could not be found in any of the patients (6). Thus, the results did not suggest a role for MDV in the pathogenesis of MS, nor did they indicate the spread of MDV among humans.
In a recent report, Laurent et al. (7) described the detection of MDV DNA in 41 (20%) of 202 human serum samples. However, they did not find any difference in the prevalence of MDV DNA between a group of individuals constantly exposed to poultry and another group of office staff. Simultaneously, they detected MDV DNA from five serum samples from chickens with clinical signs of MD and provided the alignment of the MDV gD sequences from all 46 serum samples. That report prompted us to perform a new study on the question of the possible spread of MDV to humans, although, so far, it has generally been accepted that MDV is strongly cell associated. The aim of this study was to investigate if we can confirm the detection of MDV DNA in chickens and humans if we use plasma as the source for nucleic acid isolation.
MATERIALS AND METHODS
Chicken blood specimens.
Whole-blood specimens (500 μl in 100 μl of 2% sodium citrate) were collected from the wing veins of 16 animals on days 9 and 12 after they were experimentally infected with highly virulent MDV serotype 1 (MDV-1) strain EU1 (14) and were subjected to low-speed centrifugation (500 × g for 12 min). Buffy coats were isolated with an automatic pipette, and the leukocytes were counted. DNA from approximately 106 leukocytes of each chicken was isolated with the QIAamp DNA Blood Mini kit (QIAGEN GmbH, Hilden, Germany) and eluted in 100 μl of distilled water. DNA from 80 μl of plasma was isolated with the High Pure Viral Nucleic Acid kit (Roche Diagnostics GmbH, Mannheim, Germany) and eluted in 50 μl of elution buffer.
Human blood specimens.
Whole-blood samples were collected from 300 volunteer blood donors and placed in 5.5-ml tubes containing potassium-EDTA at a concentration of 1.6 mg of EDTA per ml blood (Monovette; Sarstedt, Nümbrecht, Germany). Those samples were centrifuged at 3,291 × g for 4 min, and the plasma was separated within 24 h. Plasma specimens were stored at −50°C until nucleic acid preparation. The sex and age distributions of the blood donor population was as follows: women ages 18 to 24 years, 28.52%; women ages 25 to 34 years, 12.03%; women ages 35 to 54 years, 13.61%; women ages 55 to 65 years, 1.72%; men ages 18 to 24 years, 18.48%; men ages 25 to 34 years, 12.09%; men ages 35 to 54 years, 11.30%; men ages 55 to 65 years, 2.24%. It was not investigated whether the volunteers had been exposed to chickens.
DNA was prepared from 2 ml of human EDTA-plasma specimens with a NucliSens extractor (BioMérieux Deutschland GmbH, Nürtingen, Germany) (15). To increase the nucleic acid yield, we added a first incubation step in which the samples were incubated with lysis buffer at 60°C with horizontal shaking at 110 rpm for 30 min. The further isolation procedure was performed according to the protocol of the manufacturer for sample volumes between 200 and 2,000 μl. Total nucleic acid from 2 ml of plasma was eluted in 50 μl of elution buffer.
TaqMan PCR.
An initial test for amplification of the DNA sequence of the MDV UL48 gene and simultaneous detection of PCR products was described previously (6). For the present study, we developed a second approach based on the TaqMan Universal PCR Master Mix (manufactured by Roche Molecular Systems, Branchburg, N.J., and distributed by Applied Biosystems, Weiterstadt, Germany) on the ABI Prism 7700 SDS instrument (Applied Biosystems). The sequences of the primers and the fluorogenic TaqMan probe (Table 1) were chosen so that they were homologous to all 46 MDV gD sequences provided by Laurent et al. (7). In addition, we performed a standard nucleotide-nucleotide search on the Internet with the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST), in which each of the oligonucleotides chosen showed 100% homology to all four MDV-specific sequences but to no other organisms at a 53% or greater level of homology. The MDV-specific TaqMan probe was labeled with 6-carboxyfluorescein (FAM) as the reporter dye and 6-carboxytetramethylrhodamine (TAMRA) as the quencher dye and was custom synthesized (Applied Biosystems); the MDV primers were synthesized elsewhere (TIB Molbiol, Berlin, Germany). A human genomic sequence which could be detected in human plasma was coamplified separately as a PCR control to prevent any false-negative results due to failure of nucleic acid isolation or PCR inhibition (TaqMan β-actin control reagents; PE Biosystems, Foster City, Calif.). PCR experiments were carried out in special optical tubes (MicroAmp Optical Tubes/Caps; PE Applied Biosystems) in a total volume of 50 μl. The final concentrations of the MDV-specific TaqMan probe and primers were optimized to 300 nM each. The concentration of the β-actin-specific probe labeled with FAM and TAMRA was 200 nM, whereas the concentrations of the β-actin-specific primers were 300 nM each. Thermal cycler conditions were 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C.
TABLE 1.
MDV primer and probe sequences
| MDV-specific oligonucleotide | Sequence (5′ → 3′) | G+C content (%)a | Tm (°C)b |
|---|---|---|---|
| Forward primer | TGG GAC GAC GCA AAT ATG ATG | 48 | 60 |
| TaqMan probe | FAM-CAT GGT TTG TCT TGG GCA GAG CAT GTG-TAMRA | 52 | 70 |
| Reverse primer | AAT GGT TCA TTA GTA GAG CAG TTG GC | 42 | 60 |
G+C content; percentage of G and C residues in the respective oligonucleotide.
Tm, melting temperature of the oligonucleotides.
All human DNA samples and the DNA specimens from chicken leukocytes were tested twice by the new method (method 2) and twice by method 1, which was described previously (6). Due to the small amount of chicken plasma, the chicken plasma samples were tested twice only by method 2. In each MDV PCR experiment with the chicken plasma samples, 20 μl of eluate from the respective nucleic acid isolation was studied. A total of 15 μl of the human nucleic acid template was tested. For coamplification of human β-actin DNA, 3 μl of the corresponding template was investigated separately. DNA isolated from chicken embryo fibroblasts infected with virulent MDV-1 strain GA (3 μl of a 1:1,000 dilution) served as a positive control in each run. Threshold values were calculated as the upper 10-fold standard deviation of the background fluorescence signal measured over the baseline from cycle 3 to the cycle before the exponential increase in the first PCR kinetics was observed. The threshold cycle number (CT) was defined as the fractional cycle number at which the reporter fluorescence generated by cleavage of the probe passes this threshold. Results were interpreted as follows: CT values of <40 were considered a positive result, and CT values of 40 were considered a negative result.
RESULTS
Sensitivity of TaqMan MDV PCR.
To determine the 95% detection limit of the new TaqMan MDV PCR (method 2), we investigated semilogarithmic dilutions of the MDV-1 BAC20 DNA described by Schumacher et al. (13). This standard preparation was diluted to 102.5, 102, 101.5, 10, 100.5, and 1 MDV genome equivalents (geq). Twenty-two samples with each concentration were processed in five consecutive runs of the TaqMan MDV PCR. The 95% detection limit was calculated by probit analysis with SPSS software for Windows (version 9.0) and amounted to 13.3 geq per PCR.
Chicken blood specimens.
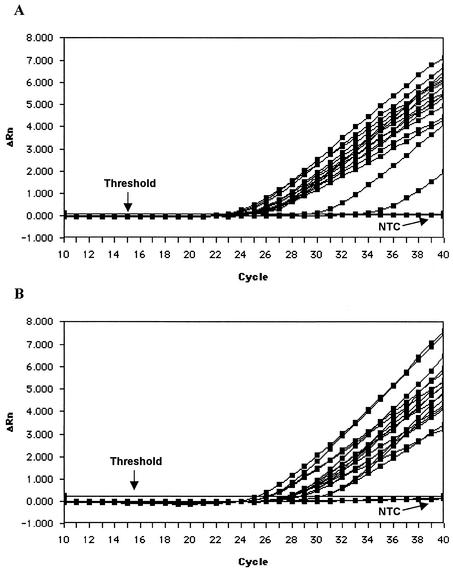
Blood specimens from 16 chickens were investigated for the presence of MDV DNA. In the single PCR experiment, total DNA from approximately 2 × 105 leukocytes or 32 μl of plasma was studied. MDV DNA was repeatedly detected in the leukocytes in both TaqMan PCR tests as well as in the plasma of all 16 animals by method 2. The original amplification plots of the experiments conducted by method 2 are provided in Fig. 1A and B. The viral loads in the cells of 14 chickens were comparable, whereas the MDV DNA levels in 2 animals were markedly lower. This may have been due to a reduced viral load or to a lower efficiency of nucleic acid isolation for these two animals. The comparison of methods 1 and 2 showed that method 2 had a slightly higher sensitivity. The mean CT values were 26.31 by method 1 and 24.81 by method 2 (Fig. 2). Thus, MDV DNA was detected 1.5 PCR cycles earlier by method 2 than by method 1.
FIG. 1.
Amplification plots from the TaqMan MDV PCR (method 2) with chicken leukocytes (A) and chicken plasma specimens (B) (n = 16 each), as well as with one no-template control (NTC). The relative fluorescence units (ΔRn) for the MDV reporter dye over the course of cycles 10 to 40 are shown. The results presented here, as well as those presented in Fig. 2 and 3, are screen shots of the original experiments.
FIG. 2.
Comparison of TaqMan MDV PCR methods 1 and 2 performed with DNA isolated from the leukocytes of 14 of 16 chickens. For a clear arrangement, the results for two chickens with markedly higher CT values were excluded (see Fig. 1B). The amplification plots of methods 1 and 2 are divided by the unlabeled arrow. Method 2 had lower CT values and higher relative fluorescence units (ΔRn) and thus had a higher sensitivity than method 1. NTC, no-template control.
Human blood specimens.
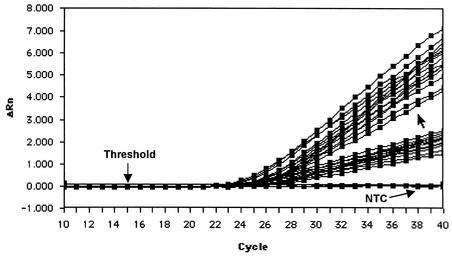
Plasma specimens from a total of 300 volunteer blood donors were tested for MDV DNA by both TaqMan PCR tests. In each single PCR, the total DNA from 600 μl of plasma was studied. All samples tested negative. Separately, the human β-actin genomic sequences were detected in each specimen (Fig. 3). By using the upper limits of 1 − α confidence intervals of the binomial distribution, for an α level of 5% we obtained the value 0.0099; i.e., the probability of the appearance of MDV in our human population was lower than 1%.
FIG. 3.
Amplification plots of a control TaqMan PCR for detection of human β-actin genomic sequences. The results of a representative experiment are shown. Plasma samples from 18 human blood donors that tested negative for MDV DNA by both PCR tests were analyzed. NTC, no-template control; ΔRn, relative fluorescence units.
DISCUSSION
The aim of the present study was to clarify the discrepancies between the observations reported by Laurent et al. (7) and those reported in another paper (6) concerning the detection of MDV-specific sequences in the human population. Because a low sensitivity of the TaqMan assay and/or the sample characteristics might have been reasons for the failure to detect MDV-specific DNA in previous attempts (6), we first determined the sensitivity of our new TaqMan MDV PCR method. The 95% detection limit amounted to 13.3 geq per PCR and was deemed acceptable, especially if we assume that up to 10 genomes are present in each cell latently infected with MDV (12). Consequently, we were able to detect 6 to 7 infected cells among a total of 106 cells.
We were able to show that MDV DNA can be detected in leukocytes as well as in plasma specimens from chickens. Given that the efficiency of nucleic acid isolation was 100%, we investigated the total DNA from only 32 μl of plasma in a single PCR experiment. Use of the amount of MDV DNA present in 32 μl of plasma was sufficient for DNA to be detected in all samples investigated. This is a very interesting observation, because MDV has so far been considered to be strongly cell associated. The detection of viral DNA can be explained by the release of noninfectious virus particles from lytically infected B or T cells or by the presence of MDV virions after destruction of these infected cells (1).
We were unable to confirm the presence of any MDV-related DNA sequences in plasma from our human blood donor population. The human EDTA-treated whole-blood samples, the plasma separation, and the nucleic acid isolation procedures have been shown to be suitable for the detection of hepatitis C virus RNA and hepatitis B virus DNA in blood donors (4, 5). Theoretically, the viral yield from plasma should be higher than that from serum, since during the separation of serum a slight loss of virus particles within the clot could be assumed. Based on a plasma input of 2 ml, we investigated total DNA extracted from 600 μl of human plasma in each PCR experiment. Thus, the method with human plasma should be 19 times more sensitive than the method with chicken plasma. In comparison to the study of Laurent et al. (7), the amount of DNA from human plasma which we investigated in one PCR was 12 times higher, since in our experiment the amount of plasma from which nucleic acid was isolated was four times higher and the amount of template in the PCR master mix was three times higher.
All 46 new MDV gD sequences provided by Laurent et al. (7) were taken into consideration in the oligonucleotide design of method 2 to avoid false-negative results due to any mutation. Otherwise, false-positive results due to contamination with PCR products were practically excluded in our laboratory, since no post-PCR steps were necessary with the TaqMan PCR assays and the closed PCR tubes were discarded.
There is an enormous difference between the finding of MDV DNA in 20% of human serum samples in the French population and the calculated probability of finding MDV DNA in less than 1% in the German population reported here. This fact could indicate that MDV has crossed from chickens to humans in a particular French population, but epidemiological criteria would demand that the prevalence of MDV DNA be higher in poultry workers than in office staff. Laurent et al. (7), however, described MDV DNA prevalences of 19 to 21% in the poultry-exposed group and 21 to 26% in the unexposed group. In our view, it would be of great interest to perform a second study with samples from the same French population in another laboratory in order to confirm these findings.
In conclusion, MD can be diagnosed in chickens by detection of MDV DNA in plasma as well as in leukocytes. Once again, however, we found no evidence for the spread of MDV to humans.
Acknowledgments
We thank Paul Sondermeijer (Intervet International, Boxmeer, The Netherlands) for providing MDV strain GA DNA. Furthermore, we acknowledge the excellent technical assistance of Diana Sander, Andrea Reimer, and Kerstin Wink. Thanks are also due to Una Doherty for assisting us in editing the English form of the manuscript.
REFERENCES
- 1.Churchill, A. E., and P. M. Biggs. 1967. Agent of Marek's disease in tissue culture. Nature 215:528-530. [DOI] [PubMed] [Google Scholar]
- 2.Churchill, A. E., L. N. Payne, and R. C. Chubb. 1969. Immunization against Marek's disease using a live attenuated virus. Nature 221:744-747. [DOI] [PubMed] [Google Scholar]
- 3.Helmick, C. G., J. M. Wrigley, M. M. Zack, W. J. Bigler, J. L. Lehman, R. S. Janssen, E. C. Hartwig, and J. J. Witte. 1989. Multiple sclerosis in Key West, Florida. Am. J. Epidemiol. 130:935-949. [DOI] [PubMed] [Google Scholar]
- 4.Hennig, H., J. Luhm, D. Hartwig, H. Kluter, and H. Kirchner. 2001. A novel RT-PCR for reliable and rapid HCV RNA screening of blood donations. Transfusion 41:1100-1106. [DOI] [PubMed] [Google Scholar]
- 5.Hennig, H., I. Puchta, J. Luhm, P. Schlenke, S. Goerg, and H. Kirchner. 2002. Frequency and load of hepatitis B virus DNA in first-time blood donors with antibodies to hepatitis B core antigen. Blood 100:2637-2641. [DOI] [PubMed] [Google Scholar]
- 6.Hennig, H., K. Wessel, P. Sondermeijer, H. Kirchner, and K. P. Wandinger. 1998. Lack of evidence for Marek's disease virus genomic sequences in leukocyte DNA from multiple sclerosis patients in Germany. Neurosci. Lett. 250:138-140. [DOI] [PubMed] [Google Scholar]
- 7.Laurent, S., E. Esnault, G. Dambrine, A. Goudeau, D. Choudat, and D. Rasschaert. 2001. Detection of avian oncogenic Marek's disease herpesvirus DNA in human sera. J. Gen. Virol. 82:233-240. [DOI] [PubMed] [Google Scholar]
- 8.MacGregor, H. S. 1991. Multiple sclerosis clusters in Florida. J. Epidemiol. Community Health 45:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacGregor, H. S., and Q. I. Latiwonk. 1992. Search for the origin of multiple sclerosis by first identifying the vector. Med. Hypotheses 37:67-73. [DOI] [PubMed] [Google Scholar]
- 10.MacGregor, H. S., and Q. I. Latiwonk. 1993. Complex role of gamma-herpesviruses in multiple sclerosis and infectious mononucleosis. Neurol. Res. 15:391-394. [DOI] [PubMed] [Google Scholar]
- 11.McHatters, G. R., and R. G. Scham. 1995. Bird viruses in multiple sclerosis: combination of viruses or Marek's alone? Neurosci. Lett. 188:75-76. [DOI] [PubMed] [Google Scholar]
- 12.Morgan, R. W., Q. Xie, J. L. Cantello, A. M. Miles, E. L. Bernberg, J. Kent, and A. Anderson. 2001. Marek's disease virus latency. Curr. Top. Microbiol. Immunol. 255:223-243. [PubMed] [Google Scholar]
- 13.Schumacher, D., B. K. Tischer, W. Fuchs, and N. Osterrieder. 2000. Reconstitution of Marek's disease virus serotype 1 (MDV-1) from DNA cloned as a bacterial artificial chromosome and characterization of a glycoprotein B-negative MDV-1 mutant. J. Virol. 74:11088-11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schumacher, D., B. K. Tischer, J. P. Teifke, K. Wink, and N. Osterrieder. 2002. Generation of a permanent cell line that supports efficient growth of Marek's disease virus (MDV) by constitutive expression of MDV glycoprotein E. J. Gen. Virol. 83:1987-1992. [DOI] [PubMed] [Google Scholar]
- 15.van Buul, C., H. Cuypers, P. Lelie, M. Chudy, M. Nübling, R. Melsert, A. Nabbe, and P. Oudshoorn. 1998. The NucliSens extractor for automated nucleic acid isolation. Infusionsther. Transfusionsmed. 25:147-151. [Google Scholar]
- 16.Voelckel, K., E. Bertram, I. Gimeno, U. Neumann, and E. F. Kaleta. 1999. Evidence for Marek's disease in turkeys in Germany: detection of MDV-1 using the polymerase chain reaction. Acta Virol. 43:143-147. [PubMed] [Google Scholar]
- 17.Witter, R. L. 1997. Increased virulence of Marek's disease virus field isolates. Avian Dis. 41:149-163. [PubMed] [Google Scholar]