Abstract
Using J774 macrophages, the intracellular activities of gentamicin, azithromycin, telithromycin, ciprofloxacin, moxifloxacin, and oritavancin (LY333328) against Staphylococcus aureus (strain ATCC 25923) have been quantitatively assessed in a 24-h model. S. aureus was positively localized in phagolysosomes by confocal and electron microscopy, and extracellular growth was prevented with 0.5 mg of gentamicin/liter (1× MIC) in controls. When tested at extracellular concentrations equivalent to their maximum concentrations in human serum, all antibiotics except azithromycin caused a significant reduction of the postphagocytosis inoculum within 24 h, albeit to markedly different extents (telithromycin [2 mg/liter], 0.60 log; ciprofloxacin [4.3 mg/liter], 0.81 log; gentamicin [18 mg/liter], 1.21 log; moxifloxacin [4 mg/liter], 1.51 log; oritavancin [25 mg/liter], 3.49 log). Intracellular activities were not systematically related to drug accumulation (apparent cellular-to-extracellular concentration ratios in infected cells: ciprofloxacin, 3.2; gentamicin, 6.8; telithromycin, 8.7; moxifloxacin, 13.4; azithromycin, 50; oritavancin, 348). Intracellular activity was not directly correlated to extracellular activity as measured in broth. Conditions of pH 5 (i.e., mimicking that of phagolysosomes) markedly reduced the activity of gentamicin, azithromycin, and telithromycin (≥32×) and fairly extensively reduced that of ciprofloxacin and moxifloxacin (≥4×) but did not affect oritavancin activity. We conclude that the cellular accumulation of antibiotics is not the only parameter to take into account for intracellular activity but that local environmental conditions (such as pH) and other factors can also prove critical.
Staphylococcus aureus, a ubiquitous pyogenic bacterium, is causing severe infections in humans as well as in animals (63). S. aureus adheres to and easily invades nonprofessional as well as professional phagocytes (1, 33). In the latter cells, S. aureus tends to be restricted to the phagolysosomal compartment, where it largely escapes destruction and survives in a semiquiescent state for prolonged periods (29, 52). These intraphagocytic forms are considered responsible for the well-known recurrent character of staphylococcal infections as well as for the many failures of apparently appropriate antibiotic treatments (16, 27, 36). The main and most current treatment option for S. aureus infections is the administration of a β-lactam resistant to β-lactamases (often combined with an aminoglycoside and/or rifampin) (61). Clindamycin and fusidic acid are second-line antibiotics for treatment of these infections. Glycopeptides, oxazolidinones, or synergistins are recommended for multiresistant strains only (47). Yet it is usually recognized that β-lactams are poorly active against the intracellular organisms because of their lack of cellular accumulation (56, 59). A number of models have therefore been developed to assess the potential of other antibiotics, such as rifampin, clindamycin, or glycopeptides (2, 12, 34), against intracellular S. aureus. Antibiotics with known cellular accumulation such as macrolides (23, 38, 48, 60), synergistins (17), or fluoroquinolones (3, 4, 10, 46, 50) have also been studied. While providing useful information, these models have so far been used to examine only short periods of incubation and have not fully taken into account (i) the slow rate of intracellular accumulation of some antibiotics (see reference 57 for comments) and (ii) the reduced growth rate of intracellular S. aureus in comparison with that of bacteria in broth or other more favorable media (29). Moreover, contamination by bacteria growing extracellularly has often proven difficult to control (36).
We present here data obtained with a model of S. aureus-infected J774 mouse macrophages in which the intracellular growth of the bacteria and the influence of antibiotics has been monitored for 24 h. These cells were selected because they are quite permissive towards a variety of intracellular infectious agents, allowing detailed analysis of the effects of antibiotics without too much interference from the host-derived mechanisms of defense. We selected commonly used drugs from three different classes of antibiotics on the basis of their contrasting behaviors concerning pharmacodynamics, cellular accumulation, and distribution properties. Thus, we chose (i) the aminoglycoside gentamicin, which is a drug characterized by a marked, intense concentration-dependent bactericidal activity (5) and a poor cellular accumulation but a preferential accumulation in lysosomes (reviewed in reference 56); (ii) ciprofloxacin and moxifloxacin, which are examples of fluoroquinolones that, like aminoglycosides, show a marked concentration-dependent bactericidal effect (5) but accumulate quickly in cells and distribute in the cytosol (10); and (iii) azithromycin and telithromycin (two macrolides), which are essentially bacteriostatic and time-dependent antibiotics (5), accumulate in cells to a large extent, and are largely localized in acidic vacuoles (9, 40). We added oritavancin (LY333328 [15]), a newly developed glycopeptide which shows an intense, concentration-dependent bactericidal activity (8), after we discovered that this antibiotic accumulates to high levels in macrophages (this study). All drugs were used throughout the present study at clinically pertinent concentrations to allow for chemotherapeutically meaningful comparisons. Dose effects were correlated with cellular accumulation to delineate the intracellular pharmacodynamic properties of each drug in comparison with what was known or could be observed of their activities towards extracellular bacteria.
MATERIALS AND METHODS
Cells.
We used J774 macrophages, a continuous reticulosarcoma cell line of murine origin, which were maintained exactly as previously described (53, 57). These cells are permissive towards a large number of intracellular bacteria. Viability upon exposure to antibiotics (at the maximal concentration tested and for up to 24 h) was assessed by measuring the release of lactate dehydrogenase in the supernatant at the end of the experiments (less than 10% of the total cell content).
Bacterial strain and susceptibility testing.
S. aureus strain ATCC 25923 was used for all experiments. MICs were determined according to the recommendations of the U.S. National Committee for Clinical Laboratory Standards (1999). Minimum bactericidal concentrations (MBCs) were defined as the lowest concentration of each drug causing >99% reduction in growth. MICs and MBCs were measured (i) without adjustment of the broth pH (standard values; measured pH, 7.3) and (ii) in broth adjusted to pH 6 and pH 5 to mimic the pH conditions of endosomes and lysosomes, respectively.
Time and dose-kill curve studies with broth.
Bacteria exhibiting logarithmic growth were resuspended at a density of 106 CFU per ml in Mueller-Hinton broth. The number of viable bacteria was determined after incubation at 37°C with antibiotics for suitable times (up to 24 h) by plate assays with appropriately diluted samples. We checked that the amount of carried-over antibiotic, taking into account the dilution of samples and the amount plated on the dish (50 μl for 15 ml of agar), was insufficient to impair bacterial growth (the final concentration of each antibiotic being each time severalfold lower than its MIC).
Setting up of a 24-h model of intracellular infection.
Bacterial cultures exhibiting logarithmic growth were centrifuged at 14,000 rpm (5415 centrifuge; Gerätebau Eppendorf GmbH, Engelsdorf, Germany) for 4 min, and the pelleted bacteria were resuspended in RPMI 1640 supplemented with 10% fresh human serum (obtained from healthy volunteers as pooled samples and stored in aliquots at −70°C until use) and incubated for 30 min at 37°C to allow for opsonization. Bacteria were then adjusted to a concentration of 3.3 × 105 CFU per ml of culture medium on the basis of measurement of absorbance at 620 nm with a preestablished calibration curve. This suspension was then used to replace the culture medium of macrophages to yield an initial bacterium-to-macrophage ratio of 0.5, and phagocytosis was allowed to occur at 37°C for 1 h. Macrophages were then washed twice with prewarmed phosphate-buffered saline (PBS). Different protocols were then tested for the ability to support intracellular growth over a 24-h period without contamination of the extracellular medium (as assessed by optic microscopy and by plating samples from culture medium on tryptic soy agar). In a first approach, infected cells were exposed to either gentamicin (50 mg/liter) or lysostaphin (0.4 unit/ml) for 1 h at 37°C after phagocytosis before being washed and returned to gentamicin- or lysostaphin-free medium. Bacteria were seen to contaminate the extracellular milieu after approximately 5 h, causing a marked acidification and subsequent cell death. In a second approach, cell sheets were subjected every 4 h to extensive washing followed by 1 h of incubation with gentamicin (50 mg/liter) before being returned to gentamicin-free medium. Although this procedure allowed the sterility of the extracellular medium to be maintained for up to 24 h, it caused variable and difficult-to-control cell loss (at least 30%, as assessed on the basis of determinations of protein levels). In a third approach, cells were exposed continuously to lysostaphin (0.4 units/ml) during the whole 24-h postphagocytosis period. This also allowed maintenance of the sterility of the extracellular medium, but cell lysates (20 μl [approximately 4 μg of cell protein]) plated on Micrococcus luteus-seeded agar showed a definite inhibition zone that was not seen in controls, indicating that significant amounts of lysostaphin had been internalized. Moreover, we noted a rather important irreproducibility of the infection (based on the enumeration of cell-associated CFU), suggesting that lysostaphin was interfering with the assay. In a fourth approach, infected cells were incubated continuously with 0.5 mg of gentamicin/liter (i.e., the gentamicin MIC in broth). This prevented all extracellular bacterial growth up to 24 h. In contrast to what was seen with lysostaphin, (i) no inhibition of M. luteus growth was seen with lysates of cells collected after 24 h of incubation with this concentration of gentamicin (lower limit of detection for gentamicin, 0.25 mg/liter); (ii) infection and intracellular bacterial growth were obtained in a reproducible fashion (approximately 1.5 log10 increase in CFU in 24 h; see below); and (iii) the number of cells was also kept constant and reproducible from one experiment to another (941 ± 116 μg of cell protein/dish). This fourth protocol was then used as the standard method for all conditions in which no antibiotic was being tested (controls). When infected cells were exposed to the tested antibiotics, however, the addition of gentamicin at its MIC during the incubation was omitted. We established indeed that (i) all antibiotics under investigation were providing adequate protection against the extracellular growth of S. aureus when present at an extracellular concentration equal to or larger than their MICs, making their combination with gentamicin superfluous, and that (ii) the addition of gentamicin (0.5 mg/liter) only marginally modified the results obtained for these antibiotics in its absence.
Intracellular activity of antibiotics.
Cells were collected by washing with PBS (three times) and scraping from the culture dish with a Teflon spatula followed by resuspension in 5 ml of sterile water. This suspension was then subjected to vigorous shaking for 30 seconds (Vortex-2; Scientific Industries, Inc., Bohemia, N.Y.). Aliquots (50 μl) were then plated on tryptic soy agar after suitable dilution for determination of CFU by colony counting after 24 h of incubation at 37°C. In parallel, samples were subjected to sonication (Labsonic L; B. Braun Biotech International GmbH, Melsungen, Germany) (15 s at 100 W) to achieve homogeneity and were then used for protein determination (32) with bovine serum albumin as a standard. All results were then calculated as CFU per milligram of cell protein.
Determination of cellular antibiotic accumulation.
Accumulation studies were performed following the standard procedure described in previous publications by Carlier et al., Carryn et al., and Seral et al. (9-11, 13, 53). Briefly, cells (infected or uninfected) were incubated with antibiotics for 24 h. The medium was then decanted, and the cell sheets were quickly washed three times with ice-cold PBS (this effectively removes all of the antibiotic not tightly cell bound, and we checked for the absence of detectable antibiotic in the final wash). Cells were then collected by scraping in 1 ml of distilled water and subjected to sonication to achieve homogeneity. Azithromycin, telithromycin, and gentamicin were assayed by a microbiological assay exactly as described previously (53) (we checked in previous studies with azithromycin that this bioassay gave consistent results compared with radiolabeled and high-performance liquid chromatography-based assays [13]). Ciprofloxacin and moxifloxacin were measured by a fluorometric assay (13, 41, 51) with excitation and emission wavelengths (λex and λem) set at 275 and 450 nm and at 298 and 504 nm, respectively. In preliminary experiments, we checked that this assay method gave values that consistently matched those determined with a microbiological assay. Oritavancin was assayed by scintillation counting using 14C-labeled drug. Since oritavancin diffuses very poorly in agar, no direct comparison could be made between the values obtained by the radiochemical assay and those from a conventional microbiological disk-plate assay. Using Enterococcus faecalis ATCC 29212, we therefore devised a broth-based kill curve assay yielding a linear relationship between the log of the variation of the number of CFU and the concentration in a narrow but usable range (0.08 to 0.25 mg/liter). This method allowed us to confirm that the radiochemical assay measured bioactive drug levels.
All cell drug contents were systematically expressed by reference to the protein content of the corresponding samples (32). The apparent cellular-to-extracellular concentration ratio was then calculated by using a conversion factor of 3.8 μl of cell volume per mg of cell protein. This factor was adopted on the basis of a series of detailed volume/protein ratio measurements using the urea/sucrose partition method (44). The method provides direct information concerning the aqueous volume accessible to diffusible solutes. It is based on the same principle and provides the same information as the [3H]water/[14C]inulin (or [14C]polyethylene glycol) partition method applied to cells pelleted through a silicone oil barrier, as used by many other investigators.
Morphological studies.
Confocal microscopy was performed with S. aureus labeled with fluorescein-isothiocyanate (FITC) [5-(((2(carbohydrazino)methyl)-thio)acetyl)amino-fluorescein; Molecular Probes, Eugene, Oreg.]. Bacteria were incubated overnight with 0.5 mg of FITC/ml in Mueller-Hinton broth followed by sedimentation at 14,000 rpm for 1 min at 4°C and washing in PBS. Infection was carried out at a bacterium/macrophage ratio of approximately 50 (to allow for visualization of a sufficiently large number of bacteria), but all other conditions were otherwise unchanged. At appropriate times, cells were washed, fixed in 3.7% formaldehyde in PBS for 15 min at room temperature, permeabilized by exposure to Triton X-100 (0.2%), and stained for actin with 1.7 × 10−7 M rhodamine phalloidin (Molecular Probes). After washing, specimens were dried and mounted in 2.5% 1,4-diacylbicyclo(2,2,2)octane (Dabco; Sigma Chemical Co., St. Louis, Mo.) in Mowiol (Calbiochem-Novabiochem GmbH, Bad Soden, Germany). Observations were made under oil immersion conditions with a 40× or 63× objective mounted on an MRC1024 confocal microscope (Bio-Rad Laboratories, Richmond, Calif.). Images were digitally recorded with a Focus Graphics image recorder and used for direct computer-assisted reproduction with an ink-jet photo printer. For electron microscopy, infection of macrophages was carried out as described above but with unlabeled bacteria. Cells were fixed and prepared as described previously (57).
Antibiotics.
The following antibiotics were obtained as pure substances for microbiological standards from their corresponding manufacturers: azithromycin (dihydrate salt; potency, 94.4%) from Pfizer Inc., Groton, Conn.; telithromycin (potency, 99.3%) from Aventis Pharma, Romainville, France; ciprofloxacin (potency 85%) and moxifloxacin (potency 99.8%) from Bayer AG, Wuppertal, Germany; oritavancin (as LY333328; potency, 80.6%) and [14C]oritavancin (specific activity, 3.5 μCi/mg) from Eli Lilly & Co., Indianapolis, Ind. Gentamicin was procured as Geomycin (the commercial products registered for clinical use in Belgium and supplied by GlaxoSmithKline Belgium on behalf of Schering-Plough Belgium).
Other reagents.
Unless stated otherwise, all other reagents were of analytic grade and were purchased from E. Merck AG (Darmstadt, Germany).
Statistical analysis.
Unless specified otherwise, all data points presented were obtained from experiments made in triplicate and results are shown as means ± standard deviations (SD). When appropriate, the statistical significance of the differences observed between treated groups and controls, or between pertinent groups, was analyzed with the Student t test and Prism version 2.01 software (GraphPad Software, San Diego, Calif.).
RESULTS
Susceptibility testing.
The MICs and MBCs at neutral and acid pH are shown in Table 1 for the main antibiotics studied. As anticipated, acid pH markedly reduced the activity of all antibiotics (especially that of azithromycin and telithromycin), with the notable exception of oritavancin, which was as active at pH 7.4 and pH 5 (the latter being taken as the value prevailing in phagolysosomes [45]).
TABLE 1.
MICs and MBCs of the antibiotics used in the present study for S. aureus ATCC 25923 in broth at pH 7.3, pH 6, and pH 5a
| Antibiotic | Concn (mg/liter)
|
||||||
|---|---|---|---|---|---|---|---|
| pH 7.3
|
pH 6
|
pH 5
|
Cmaxb | ||||
| MIC | MBC | MIC | MBC | MIC | MBC | ||
| Gentamicin | 0.5 | 2 | 4 | 32 | 16 | 64 | 18 |
| Azithromycin | 0.5 | 8 | 32 | 64 | 512 | 512 | 0.4 |
| Telithromycin | 0.06 | 2 | 0.25 | 8 | 4 | 8 | 2 |
| Ciprofloxacin | 0.125 | 1 | 0.5 | 2 | 1 | 2 | 4.3 |
| Moxifloxacin | 0.06 | 0.06 | 0.06 | 0.25 | 0.25 | 1 | 4 |
| Oritavancin | 0.25 | 1 | 0.25 | 1 | 0.25 | 1 | 25 |
For comparison, the table shows also Cmax values. These concentrations were also the maximal concentrations tested in the present study, except in the case of azithromycin, for which the maximal concentration tested was 10 mg/liter.
Concentrations corresponding to the peak concentrations of the respective antibiotics in serum after administration of currently used and/or approved doses for humans, as indicated in references 26 (gentamicin), 24 (azithromycin), 42 (telithromycin), 6 (ciprofloxacin), and 54 (moxifloxacin) and D.K. Braun et al., Clin. Microbiol. Infect. 7(Suppl. 1):P434, 2001.
Characterization of the model.
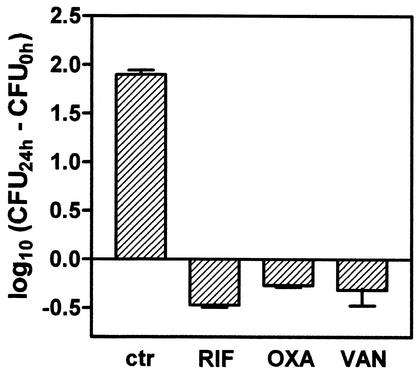
A detailed intracellular growth kinetics experiment allowed us to determine that phagocytosed S. aureus stayed apparently quiescent for about 8 to 12 h and then started to multiply exponentially to reach almost 2 log10 growth over the next 12 h. Confocal and electron microscopy was used to ascertain the localization of the bacteria. As shown in Fig. 1 and 2, S. aureus clearly appeared intracellular and restricted to the vacuolar apparatus. In parallel, we checked for the presence of small colony variants, a phenotype that can be induced by the intracellular environment (1, 62). These were not detected upon close examination of the colonies obtained from cell samples. The activity of three antibiotics commonly used in the treatment of staphylococcal infections (rifampin, oxacillin, and vancomycin) was assessed at fixed extracellular concentrations (corresponding to the maximum concentration of drug in serum (Cmax) for humans; Table 1) for 24 h. Only a modest reduction of the original inoculum (0.2 to 0.3 log10) was seen, whereas controls showed approximately 2 log10 growth (Fig. 3).
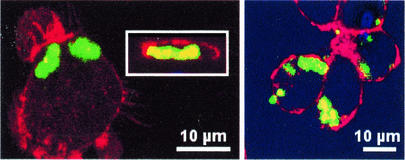
FIG. 1.
Confocal microscopy of J774 macrophages observed 5 h after phagocytosis of opsonized S. aureus. Cell actin (located mostly on the inner face of the pericellular membrane) was labeled with rhodamine-phalloidin (red signal), and bacteria were labeled with FITC (green signal). (Left panel) Incubation was performed in the absence of any antibiotic (in the inset, a vertical section of the cell shown in the main panel is depicted; actin surrounds the bacteria, demonstrating its intracellular localization). (Right panel) Incubation performed in the presence of 0.5 mg of gentamicin/liter. No difference was seen with cells incubated without gentamicin.
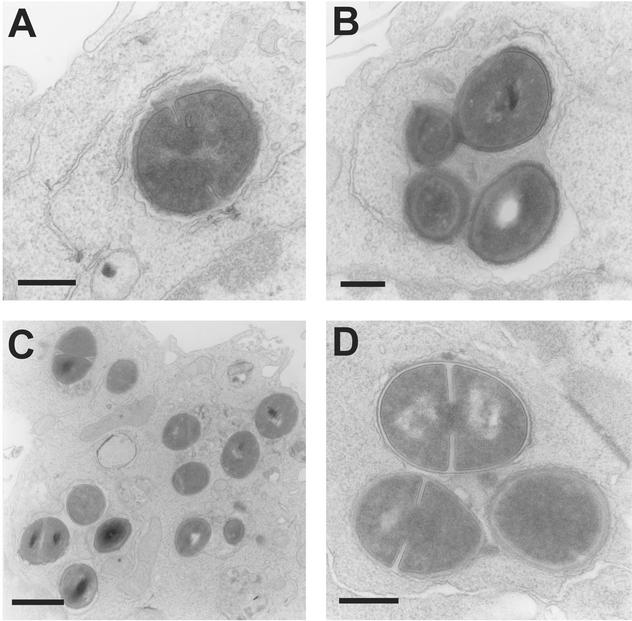
FIG. 2.
Electron microscopy of J774 macrophages fixed 1 h (A and B) or 24 h (C and D) after phagocytosis of opsonized S. aureus. In both cases, incubation was carried out in the presence of 0.5 mg of gentamicin/liter. Bacteria appeared isolated (A) or sometimes in clusters (B) at 1 h after phagocytosis, without evidence of damage but with no sign of division. In contrast, most bacteria observed at 24 h were in the active process of division. In both cases, all bacteria were seen in membrane-bounded structures with no evidence of transfer to cytosol. Bars are 0.3 μm (A, B, and D) and 1 μm (C).
FIG. 3.
Variations in the number of CFU per milligram of protein (± SD; n = 3) collected from J774 macrophages after 24 h of incubation postphagocytosis compared to that seen with the original postphagocytosis inoculum. ctr, control cells incubated with 0.5 mg of gentamicin/liter; RIF, cells incubated with rifampin (4 mg/liter); OXA, cells incubated with oxacillin (6 mg/liter); VAN, cells incubated with vancomycin (50 mg/liter). These concentrations correspond to peak concentrations in serum observed for patients after administration of conventional doses of oxacillin and vancomycin (14, 22) and to the low side of the range of peak concentrations in serum observed for rifampin (21). They also represent values 250 fold (rifampin) and 50 fold (oxacillin and vancomycin) larger than the MIC (in broth) of the corresponding antibiotic for the strain of S. aureus used in the present study.
Extracellular and intracellular activities: time and concentration dependency.
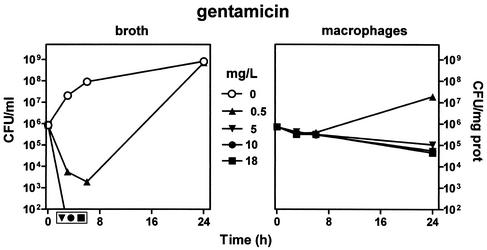
The influence of time and concentrations of all antibiotics on bacteria growing in broth in comparison with that on intracellular bacteria was then systematically investigated. Incubation was carried out for 24 h at drug concentrations from the MIC (as determined in broth at pH 7.3) to multiples thereof and up to the Cmax (except for azithromycin, for which another scale had to be chosen since its Cmax was below its MIC [see values in the corresponding figure]). Gentamicin (Fig. 4) displayed a fast-developing and profound bactericidal activity on bacteria in broth at all concentrations exceeding its MIC. Against intracellular bacteria, gentamicin was ineffective at its MIC but became slightly and slowly bactericidal at 5 mg/liter (10 × its MIC) with, however, no improvement at higher concentrations (up to 18 mg/liter). This pattern did not result from a slow drug accumulation, since it was also observed when cells had been preincubated with gentamicin (at 18 mg/liter) for 24 h prior to phagocytosis (the incubation of the cells being made in the continuous presence of gentamicin at 18 mg/liter after phagocytosis and washing of the extracellular bacteria).
FIG. 4.
Variations in the number of CFU upon incubation for up to 24 h with increasing concentrations of gentamicin. (Left panel) Results for bacteria in broth (initial pH, 7.3). (Right panel) Results for bacteria collected from J774 macrophages (after phagocytosis). The lowest drug concentration (0.5 mg/liter) corresponds to the MIC (in broth) of gentamicin for the strain of S. aureus used in this study; the highest concentration (18 mg/liter) corresponds to the Cmax observed for patients after the administration of conventional doses of gentamicin (26). Each point corresponds to the mean value for three independent determinations. SD values were calculated, but the corresponding bars are smaller than the symbols in many cases. For the results obtained with broth, symbols placed outside of the bottom of the graph indicate samples in which no or only a few colonies were observed (i.e., numbers were too close to the lowest limit of detection [102 CFU/ml] to be fully reliable). Cells incubated with 0.5 mg of gentamicin/liter served as controls for all subsequent experiments.
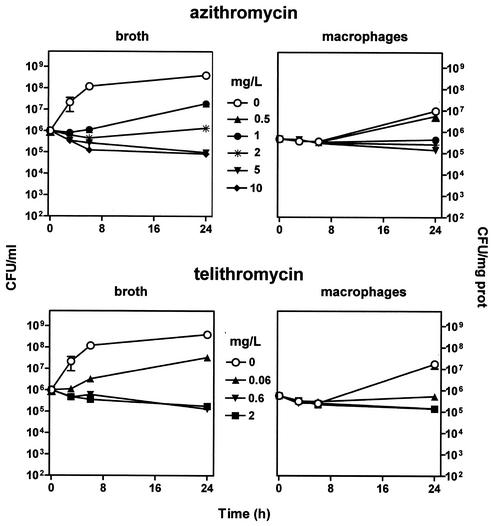
Figure 5 shows that azithromycin and telithromycin were essentially bacteriostatic against bacteria in broth (with only a marginal bactericidal effect and with no influence seen at concentrations above 10 times the MIC). Both antibiotics exerted only a modest effect on intracellular bacteria, with a maximal decrease in bacterial counts of approximately 0.6 log10 at extracellular concentrations of 10 times their MIC or above.
FIG. 5.
Variations in the number of CFU upon incubation for up to 24 h with increasing concentrations of azithromycin (top two panels) or telithromycin (bottom two panels). (Left two panels) Results for bacteria in broth (initial pH, 7.3). (Right two panels) Results for bacteria collected from J774 macrophages (after phagocytosis). The lowest drug concentrations investigated (0.5 mg/liter for azithromycin, 0.06 mg/liter for telithromycin) correspond to the MICs (in broth) of the drugs for the strain of S. aureus used in this study; note that for azithromycin, the peak concentration observed for patients receiving a conventional dose (500 mg) did not exceed 0.4 mg/liter (24); for telithromycin, the highest concentration investigated (2 mg/liter) corresponds to the Cmax observed for patients after administration of a conventional dose (800 mg) (42). Each point corresponds to the mean value for three independent determinations. SD values were calculated, but the corresponding bars are smaller than the symbols in many cases. Note that cells incubated in the absence of azithromycin or telithromycin were maintained in the presence of 0.5 mg of gentamicin/liter to prevent extracellular growth of S. aureus.
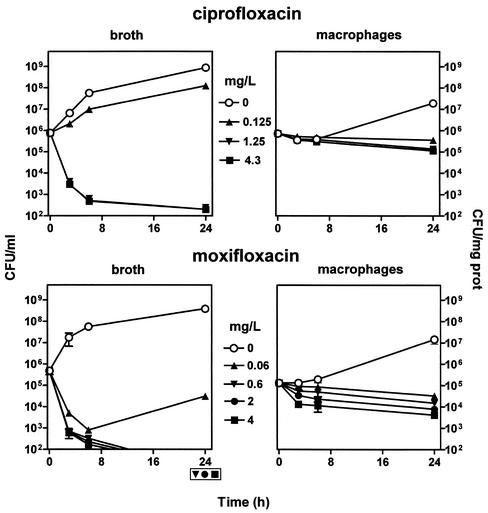
Figure 6 shows that ciprofloxacin and moxifloxacin exhibited a marked and fast-developing bactericidal effect against bacteria in broth which was maximal at 10 times the MIC for both drugs (the steepness of the curves prevented us from analyzing in greater detail the concentration dependency of this bacterial killing in the time frame chosen). In contrast, ciprofloxacin exerted only a slowly developing and poorly concentration-dependent bactericidal effect towards intracellular S. aureus (0.8 log10 reduction at 24 h at 10 times the MIC). Moxifloxacin showed also a slowly developing bactericidal effect towards intracellular S. aureus, but this effect was markedly concentration dependent (reaching a 1.5 log10 reduction in CFU compared to that seen with the original inoculum for cells incubated with the largest concentration tested [Cmax, 4 mg/liter]).
FIG. 6.
Variations in the number of CFU upon incubation for up to 24 h with increasing concentrations of ciprofloxacin and moxifloxacin. (Left two panels) Results for bacteria in broth (initial pH, 7.3). (Right two panels) Results for bacteria collected from J774 macrophages (after phagocytosis). The lowest drug concentrations investigated (0.125 mg/liter for ciprofloxacin, 0.06 mg/liter for moxifloxacin) correspond to the MICs (in broth) of the drugs for the strain of S. aureus used in this study; the highest concentrations (4.3 mg/liter for ciprofloxacin, 4 mg/liter for moxifloxacin) correspond to the Cmax observed for patients after administration of the currently recommended doses of each of these two fluoroquinolones (reference 54 and U.S. ciprofloxacin package insert, Bayer Corporation, West Haven, Conn.). Each point corresponds to the mean value for three independent determinations. SD values were calculated, but the corresponding bars are smaller than the symbols in many cases. Symbols placed outside of the bottom of the lower left graph indicate samples in which no or only a few colonies were observed (i.e., numbers were too close to the lowest limit of detection [102 CFU/ml] to be fully reliable). Note that cells incubated in the absence of ciprofloxacin or moxifloxacin were maintained in the presence of 0.5 mg of gentamicin/liter to prevent extracellular growth of S. aureus.
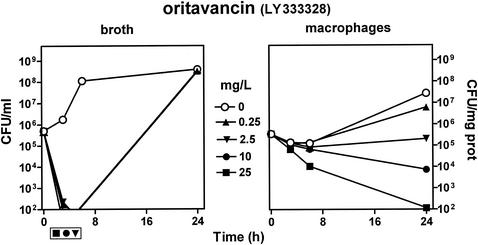
Figure 7 shows that oritavancin displayed an extremely fast and profound bactericidal effect on bacteria in broth (with bacterial counts falling below the detection level in less than 2 h). Intracellular bacteria were almost unaffected by oritavancin when cells were incubated with a drug concentration equal to 1× MIC in broth. Yet in contrast with all other antibiotics examined so far, larger extracellular concentrations of oritavancin caused a marked reduction in CFU which developed on both a time- and concentration-dependent manner, with the drug becoming clearly bactericidal at an extracellular concentration of 10 mg/liter (40 times its MIC). At its Cmax (25 mg/liter), oritavancin caused a 1.5 log10 reduction in CFU in 6 h and a 3.5 log10 decrease (0.03% survival) after 24 h.
FIG. 7.
Variations in the number of CFU upon incubation for up to 24 h with increasing concentrations of oritavancin. (Left two panels) Results for bacteria in broth (initial pH, 7.3). (Right two panels) Results for bacteria collected from J774 macrophages after phagocytosis. The lowest drug concentration investigated (0.25 mg/liter) corresponds to the MIC (in broth) of oritavancin for the strain of S. aureus used in this study, and the highest concentration investigated (25 mg/liter) corresponds to the Cmax observed for patients after administration of the dose currently used in the clinical development program of this antibiotic (D. K. Braun et al., Abstracts of the 11th European Congress of Clinical Microbiology and Infectious Diseases, Clin. Microbiol. Infect., 7[Suppl. 1]:P434, 2001). Each point corresponds to the mean value of three independent determinations. SD values were calculated, but the corresponding bars are smaller than the symbols in many cases. Symbols placed outside of the bottom of the lower left graph indicate samples in which no or only a few colonies were observed (i.e., numbers were too close to the lowest limit of detection [102 CFU/ml] to be fully reliable). Note that cells incubated in the absence of oritavancin were maintained in the presence of 0.5 mg of gentamicin/liter to prevent extracellular growth of S. aureus.
Accumulation of antibiotics.
Table 2 shows the values obtained at 24 h with cells incubated with an extracellular drug concentration equivalent to the Cmax (except for telithromycin and azithromycin, for which higher concentrations needed to be used [6 and 10 mg/liter, respectively] because of the insensitivity of the corresponding microbiological assay). We also compared infected and uninfected cells in the same experiment. Ciprofloxacin, gentamicin, telithromycin, and moxifloxacin achieved cellular-to-extracellular concentration ratios ranging from only 3.2 to 11.4, whereas azithromycin reached an approximately 50-fold accumulation and oritavancin peaked at an approximately 340-fold accumulation. No statistically significant difference was seen between infected and uninfected cells.
TABLE 2.
Accumulation of antibiotics in macrophages incubated for 24 h with the concentrations of antibiotics indicated (these concentrations correspond to the Cmax value as defined in except for those for azithromycin and telithromycin)
| Antibiotic | Extracellular concn (mg/liter) | Cellular-to-extracellular concn ratioa
|
|
|---|---|---|---|
| Noninfected cells | Infected cells | ||
| Gentamicin | 18 | 6.3 ± 0.7 | 6.8 ± 1.7 |
| Azithromycin | 10 | 45.0 ± 5.1 | 50.0 ± 7.5 |
| Telithromycin | 6 | 8.6 ± 1.2 | 8.7 ± 1.1 |
| Ciprofloxacin | 4.3 | 3.2 ± 0.6 | 3.2 ± 0.3 |
| Moxifloxacin | 4 | 11.4 ± 0.8 | 13.4 ± 0.4 |
| Oritavancin | 25 | 336.3 ± 13.6 | 343.8 ± 10.6 |
Calculated from the determinations of the drug content per milligram of cell protein and using a conversion factor of 3.8 μl/mg of cell protein. All values are means of three determinations ± SD. In statistical analysis (Student's t test), all differences between infected and uninfected cells were nonsignificant (P > 0.05) except those seen with moxifloxacin, for which the P value was 0.02.
DISCUSSION
The present study was one of the first systematic attempts to examine the activity of different classes of antibiotics against S. aureus in macrophages in long-term (24 h) experiments. It was designed to allow a direct comparison between extra- and intracellular activities against a single well-characterized strain of S. aureus and was conducted at drug extracellular concentrations that are directly clinically relevant.
The morphological observations indicated that we were dealing with a truly phagolysosomal infection, in contrast to what takes place in epithelial cells where S. aureus can gain access to the cytosol (1, 28). The bacteria were therefore expected to become exposed to nonspecific cell defense mechanisms, which probably explains the very characteristic lag period (about 12 h) before they eventually started actively multiplying. This delay in growth has also been observed in P388D1 murine macrophage-like cells (35) and in CFT-1 epithelial cells (28) and was not due the presence of small colony variants in detectable amounts. The model needed the continuing presence of gentamicin in the medium (in the absence of other antibiotics) to prevent the extracellular growth of the bacteria released from macrophages 6 to 8 h after phagocytosis. These bacteria must indeed be of intracellular origin, since otherwise growth would have taken place much earlier (gentamicin has only a short postantibiotic effect towards S. aureus [18]). This use of gentamicin in controls could be criticized on the basis that the drug can impair the intracellular growth of S. aureus, thereby reducing the difference seen between controls (made in the presence of gentamicin) and antibiotic-treated cells. The model is nevertheless correct if activity is defined as a reduction in CFU in comparison with the original inoculum. Our analysis will, therefore, be limited to the examination of this parameter.
The main and probably most critical finding made here is that the intracellular activities observed were considerably lower than would be expected on the basis of their activity in broth and the levels of their cellular accumulation. This finding is particularly impressive when examining the activity of bactericidal, concentration-dependent antibiotics such as gentamicin, fluoroquinolones, or oritavancin. It has long been suggested that intracellular accumulation of antibiotics would be conducive to intracellular activity (56, 59). Yet several authors have now offered experimental evidence that there is no simple and direct correlation between accumulation and activity for antibiotics like fluoroquinolones (3, 25, 43, 46, 50, 64) or macrolides (25, 39, 48, 49). We have confirmed this conclusion and extend it to other antibiotics. This lack of or decreased expression of the activity of antibiotics in the intracellular milieu is probably multifactorial. For bacteriostatic antibiotics such as macrolides, it can simply be argued that these drugs reach their maximal antibacterial effect at a relatively low concentration and that accumulation at higher levels is without therapeutic benefit. For macrolides and aminoglycosides, we also see that acid pH conditions markedly decrease their activity (alkalinization of phagolysosomes markedly increases the intracellular activity of amikacin [35]). The activity of fluoroquinolones is, however, less affected by acid pH, and that of oritavancin is not affected at all.
The rate of uptake and the subcellular localization could also, a priori, play a major role. The uptake rate is, however, probably not a limiting factor in the present long-term model. Macrolides and aminoglycosides concentrate in lysosomes of uninfected cells (9, 40, 55), whereas fluoroquinolones are found in the soluble fraction of cell extracts (10) and are considered to be highly diffusible. The subcellular localization of oritavancin is not known, but vancomycin was found to slowly accumulate in the lysosomes of renal cells (7) and preliminary data suggest that oritavancin also localizes in lysosomes (F. Van Bambeke et al., unpublished data). Thus, localization per se may not be predictive of activity. Another and perhaps more important parameter which needs to be considered is the binding of the antibiotics to proteins or other intracellular constituents. We unfortunately lack direct information in this context, especially concerning infected cells. Yet we know that macrolides and aminoglycosides which are sequestered in lysosomes are likely to bind to phospholipids through electrostatic interactions (31, 58) and to be therefore largely unavailable. The same phenomenon could take place for oritavancin, which is a basic compound. Concerning fluoroquinolones, no molecular explanation has been given so far concerning the mechanism of their accumulation in cells but available data (10, 19) suggest that it results from loose (nonspecific?) binding to cell constituents. Finally, parameters related to the bacteria may also be critical. The intracellular milieu may indeed be directly responsible for the modulation of metabolism towards a stage of decreased sensitivity. Moreover, the slower multiplication rate of intracellular bacteria may play a major role (6, 19, 20, 30). Slow growth, however, does not seem to adversely affect the activity of oritavancin (37).
Whatever the various mechanisms explaining the loss of activity of antibiotics against intracellular S. aureus, the type of model developed here may prove useful for comparisons of drugs in this context. Indeed, it clearly appears that gentamicin, macrolides, and ciprofloxacin are largely ineffective, raising questions about the true usefulness of these antibiotics for acting upon and for eradicating intracellular forms of S. aureus in vivo. Conversely, moxifloxacin and (to a greater degree) oritavancin appear to be very effective. Our data, therefore, open interesting perspectives and suggest useful animal and humans trials with these drugs. Our results also show that intracellular activity of new antibiotics should be experimentally addressed early on during the selection and development process.
Acknowledgments
Jean-Michel Michot determined the volume/cell protein ratio of J774 macrophages as used in this paper. F. Renoird-Andries provided skillful help for the electron microscopy studies, and M. C. Cambier provided skillful help for cell culture maintenance.
C.S. was Chercheur post-doctoral of the Belgian Fonds de la Recherche Scientifique Médicale (fellowship 3.4.549.00). F.V.B. is Chercheur Qualifié of the Belgian Fonds National de la Recherche Scientifique. This work was supported by the Belgian Fonds de la Recherche Scientifique Médicale (grant 3.4.549.00). We thank Eli Lilly Benelux, Brussels, Belgium, for a grant-in-aid and Eli Lilly & Co., Indianapolis, Ind., for providing us with 14C-labeled oritavancin. We thank the other manufacturers for the kind gift of their corresponding antibiotics.
REFERENCES
- 1.Alexander, E. H., and M. C. Hudson. 2001. Factors influencing the internalization of Staphylococcus aureus and impacts on the course of infections in humans. Appl. Microbiol. Biotechnol. 56:361-366. [DOI] [PubMed] [Google Scholar]
- 2.al Nawas, B., and P. M. Shah. 1998. Intracellular activity of vancomycin and Ly333328, a new semisynthetic glycopeptide, against methicillin-resistant Staphylococcus aureus. Infection 26:165-167. [DOI] [PubMed] [Google Scholar]
- 3.al Nawas, B., and P. M. Shah. 1998. Intracellular activity of ciprofloxacin and moxifloxacin, a new 8-methoxyquinolone, against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 41:655-658. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, R., and G. K. Joone. 1993. In vitro investigation of the intraphagocytic bioactivities of ciprofloxacin and the new fluoroquinolone agents, clinafloxacin (CI- 960) and PD 131628. Chemotherapy 39:424-431. [DOI] [PubMed] [Google Scholar]
- 5.Andes, D., and W. A. Craig. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261-268. [DOI] [PubMed] [Google Scholar]
- 6.Bahl, D., D. A. Miller, I. Leviton, P. Gialanella, M. J. Wolin, W. Liu, R. Perkins, and M. H. Miller. 1997. In vitro activities of ciprofloxacin and rifampin alone and in combination against growing and nongrowing strains of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 41:1293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beauchamp, D., P. Gourde, M. Simard, and M. G. Bergeron. 1992. Subcellular localization of tobramycin and vancomycin given alone and in combination in proximal tubular cells, determined by immunogold labeling. Antimicrob. Agents Chemother. 36:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biavasco, F., C. Vignaroli, R. Lupidi, E. Manso, B. Facinelli, and P. E. Varaldo. 1997. In vitro antibacterial activity of LY333328, a new semisynthetic glycopeptide. Antimicrob. Agents Chemother. 41:2165-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlier, M. B., I. Garcia-Luque, J. P. Montenez, P. M. Tulkens, and J. Piret. 1994. Accumulation, release and subcellular localization of azithromycin in phagocytic and non-phagocytic cells in culture. Int. J. Tissue React. 16:211-220. [PubMed] [Google Scholar]
- 10.Carlier, M. B., B. Scorneaux, A. Zenebergh, J. F. Desnottes, and P. M. Tulkens. 1990. Cellular uptake, localization and activity of fluoroquinolones in uninfected and infected macrophages. J. Antimicrob. Chemother. 26(Suppl. B):27-39. [DOI] [PubMed] [Google Scholar]
- 11.Carlier, M. B., A. Zenebergh, and P. M. Tulkens. 1987. Cellular uptake and subcellular distribution of roxithromycin and erythromycin in phagocytic cells. J. Antimicrob. Chemother. 20(Suppl. B):47-56. [DOI] [PubMed] [Google Scholar]
- 12.Carlone, N. A., A. M. Cuffini, M. Ferrero, V. Tullio, and G. Avetta. 1989. Cellular uptake, and intracellular bactericidal activity of teicoplanin in human macrophages. J. Antimicrob. Chemother. 23:849-859. [DOI] [PubMed] [Google Scholar]
- 13.Carryn, S., F. Van Bambeke, M.-P. Mingeot-Leclercq, and P. M. Tulkens. 2002. Comparative intracellular (THP-1 macrophages) and extracellular activities of β-lactams, azithromycin, gentamicin, and fluoroquinolones against Listeria monocytogenes at clinically relevant concentrations. Antimicrob. Agents Chemother. 46:2095-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers, H. F. 2000. Penicillins, p. 261-274. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious disease. Churchill Livingstone, Philadelphia, Pa.
- 15.Cooper, R. D., N. J. Snyder, M. J. Zweifel, M. A. Staszak, S. C. Wilkie, T. I. Nicas, D. L. Mullen, T. F. Butler, M. J. Rodriguez, B. E. Huff, and R. C. Thompson. 1996. Reductive alkylation of glycopeptide antibiotics: synthesis and antibacterial activity. J. Antibiot. (Tokyo) 49:575-581. [DOI] [PubMed] [Google Scholar]
- 16.Craven, N., and J. C. Anderson. 1984. Phagocytosis of Staphylococcus aureus by bovine mammary gland macrophages and intracellular protection from antibiotic action in vitro and in vivo. J. Dairy Res. 51:513-523. [DOI] [PubMed] [Google Scholar]
- 17.Desnottes, J. F., and N. Diallo. 1992. Cellular uptake and intracellular bactericidal activity of RP 59500 in murine macrophages. J. Antimicrob. Chemother. 30(Suppl. A):107-115. [DOI] [PubMed] [Google Scholar]
- 18.Dominguez, M. C., R. M. de La, and M. V. Borobio. 2001. Application of a spectrophotometric method for the determination of post-antibiotic effect and comparison with viable counts in agar. J. Antimicrob. Chemother. 47:391-398. [DOI] [PubMed] [Google Scholar]
- 19.Easmon, C. S., and J. P. Crane. 1985. Uptake of ciprofloxacin by macrophages. J. Clin. Pathol. 38:442-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eng, R. H., F. T. Padberg, S. M. Smith, E. N. Tan, and C. E. Cherubin. 1991. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob. Agents Chemother. 35:1824-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farr, B. M. 2000. Rifamycins, p. 348-361. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious disease. Churchill Livingstone, Philadelphia, Pa.
- 22.Fekety, R. 2000. Vancomycin, teicoplanin, and the streptogramins: quinupristin and dalfopristin, p. 382-392. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious disease. Churchill Livingstone, Philadelphia, Pa.
- 23.Fietta, A., C. Merlini, and G. G. Gialdroni. 1997. Inhibition of intracellular growth of Staphylococcus aureus by exposure of infected human monocytes to clarithromycin and azithromycin. J. Chemother. 9:17-22. [DOI] [PubMed] [Google Scholar]
- 24.Foulds, G., R. M. Shepard, and R. B. Johnson. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 25(Suppl. A):73-82. [DOI] [PubMed] [Google Scholar]
- 25.Garcia, I., A. Pascual, S. Ballesta, and E. J. Perea. 2000. Uptake and intracellular activity of ofloxacin isomers in human phagocytic and non-phagocytic cells. Int. J. Antimicrob. Agents 15:201-205. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert, D. N. 2000. Aminoglycosides, p. 307-336. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious disease. Churchill Livingstone, Philadelphia, Pa.
- 27.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713-3722. [DOI] [PubMed] [Google Scholar]
- 28.Kahl, B. C., M. Goulian, W. van Wamel, M. Herrmann, S. M. Simon, G. Kaplan, G. Peters, and A. L. Cheung. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect. Immun. 68:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapral, F. A., and M. G. Shayegani. 1959. Intracellular survival of staphylococci. J. Exp. Med. 110:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamp, K. C., M. J. Rybak, E. M. Bailey, and G. W. Kaatz. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob. Agents Chemother. 36:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurent, G., M. B. Carlier, B. Rollman, F. Van Hoof, and P. Tulkens. 1982. Mechanism of aminoglycoside-induced lysosomal phospholipidosis: in vitro and in vivo studies with gentamicin and amikacin. Biochem. Pharmacol. 31:3861-3870. [DOI] [PubMed] [Google Scholar]
- 32.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 33.Lowy, F. D. 2000. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 8:341-343. [DOI] [PubMed] [Google Scholar]
- 34.Mandell, G. L., and T. K. Vest. 1972. Killing of intraleukocytic Staphylococcus aureus by rifampin: in-vitro and in-vivo studies. J. Infect. Dis. 125:486-490. [DOI] [PubMed] [Google Scholar]
- 35.Maurin, M., and D. Raoult. 1994. Phagolysosomal alkalinization and intracellular killing of Staphylococcus aureus by amikacin. J. Infect. Dis. 169:330-336. [DOI] [PubMed] [Google Scholar]
- 36.Maurin, M., and D. Raoult. 2001. Use of aminoglycosides in treatment of infections due to intracellular bacteria. Antimicrob. Agents Chemother. 45:2977-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercier, R. C., C. Stumpo, and M. J. Rybak. 2002. Effect of growth phase and pH on the in vitro activity of a new glycopeptide, oritavancin (LY333328), against Staphylococcus aureus and Enterococcus faecium. J. Antimicrob. Chemother. 50:19-24. [DOI] [PubMed] [Google Scholar]
- 38.Meyer, A. P., C. Bril-Bazuin, H. Mattie, and P. J. van den Broek. 1993. Uptake of azithromycin by human monocytes and enhanced intracellular antibacterial activity against Staphylococcus aureus. Antimicrob. Agents Chemother. 37:2318-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milatovic, D. 1990. Intraphagocytic activity of erythromycin, roxithromycin and azithromycin. Eur. J. Clin. Microbiol. Infect. Dis. 9:33-35. [DOI] [PubMed] [Google Scholar]
- 40.Miossec-Bartoli, C., L. Pilatre, P. Peyron, E.-N. N′Diaye, V. Collart-Dutilleul, I. Maridonneau-Parini, and A. Diu-Hercend. 1999. The new ketolide HMR3647 accumulates in the azurophil granules of human polymorphonuclear cells. Antimicrob. Agents Chemother. 43:2457-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mortimer, P. G., and L. J. Piddock. 1991. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J. Antimicrob. Chemother. 28:639-653. [DOI] [PubMed] [Google Scholar]
- 42.Namour, F., D. H. Wessels, M. H. Pascual, D. Reynolds, E. Sultan, and B. Lenfant. 2001. Pharmacokinetics of the new ketolide telithromycin (HMR 3647) administered in ascending single and multiple doses. Antimicrob. Agents Chemother. 45:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen, S. L., N. Obel, M. Storgaard, and P. L. Andersen. 1997. The effect of quinolones on the intracellular killing of Staphylococcus aureus in neutrophil granulocytes. J. Antimicrob. Chemother. 39:617-622. [DOI] [PubMed] [Google Scholar]
- 44.O'Donnell, M. E. 1993. Role of Na-K-Cl cotransport in vascular endothelial cell volume regulation. Am. J. Physiol. 264:C1316-C1326. [DOI] [PubMed] [Google Scholar]
- 45.Ohkuma, S., and B. Poole. 1978. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. USA 75:3327-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paillard, D., J. Grellet, V. Dubois, M. C. Saux, and C. Quentin. 2002. Discrepancy between uptake and intracellular activity of moxifloxacin in a Staphylococcus aureus-human THP-1 monocytic cell model. Antimicrob. Agents Chemother. 46:288-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paradisi, F., G. Corti, and D. Messeri. 2001. Antistaphylococcal (MSSA, MRSA, MSSE, MRSE) antibiotics. Med. Clin. N. Am. 85:1-17. [DOI] [PubMed] [Google Scholar]
- 48.Pascual, A., S. Ballesta, I. Garcia, and E. J. Perea. 2001. Uptake and intracellular activity of ketolide HMR 3647 in human phagocytic and non-phagocytic cells. Clin. Microbiol. Infect. 7:65-69. [DOI] [PubMed] [Google Scholar]
- 49.Pascual, A., M. C. Conejo, I. Garcia, and E. J. Perea. 1995. Factors affecting the intracellular accumulation and activity of azithromycin. J. Antimicrob. Chemother. 35:85-93. [DOI] [PubMed] [Google Scholar]
- 50.Pascual, A., I. Garcia, S. Ballesta, and E. J. Perea. 1997. Uptake and intracellular activity of trovafloxacin in human phagocytes and tissue-cultured epithelial cells. Antimicrob. Agents Chemother. 41:274-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piddock, L. J., and Y. F. Jin. 1999. Antimicrobial activity and accumulation of moxifloxacin in quinolone-susceptible bacteria. J. Antimicrob. Chemother. 43(Suppl. B):39-42. [DOI] [PubMed] [Google Scholar]
- 52.Rogers, D. E., and R. Tompsett. 1952. The survival of staphylococci within human leukocytes. J. Exp. Med. 95:209-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seral, C., J.-M. Michot, H. Chanteux, M.-P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2003. Influence of P-glycoprotein inhibitors on accumulation of macrolides in j774 murine macrophages. Antimicrob. Agents Chemother. 47:1047-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sullivan, J. T., M. Woodruff, J. Lettieri, V. Agarwal, G. J. Krol, P. T. Leese, S. Watson, and A. H. Heller. 1999. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12- 8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob. Agents Chemother. 43:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tulkens, P., and A. Trouet. 1978. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem. Pharmacol. 27:415-424. [DOI] [PubMed] [Google Scholar]
- 56.Tulkens, P. M. 1991. Intracellular distribution and activity of antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 10:100-106. [DOI] [PubMed] [Google Scholar]
- 57.Tyteca, D., P. Van Der Smissen, M. Mettlen, F. Van Bambeke, P. M. Tulkens, M. P. Mingeot-Leclercq, and P. J. Courtoy. 2002. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp. Cell Res. 281:86-100. [DOI] [PubMed] [Google Scholar]
- 58.Van Bambeke, F., J. P. Montenez, J. Piret, P. M. Tulkens, P. J. Courtoy, and M. P. Mingeot-Leclercq. 1996. Interaction of the macrolide azithromycin with phospholipids. I. Inhibition of lysosomal phospholipase A1 activity. Eur. J. Pharmacol. 314:203-214. [DOI] [PubMed] [Google Scholar]
- 59.van den Broek, P. J. 1991. Activity of antibiotics against microorganisms ingested by mononuclear phagocytes. Eur. J. Clin. Microbiol. Infect. Dis. 10:114-118. [DOI] [PubMed] [Google Scholar]
- 60.Vazifeh, D., H. Abdelghaffar, and M. T. Labro. 2002. Effect of telithromycin (HMR 3647) on polymorphonuclear neutrophil killing of Staphylococcus aureus in comparison with roxithromycin. Antimicrob. Agents Chemother. 46:1364-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verhoef, J., and F. J. Schmitz. 1999. Staphylococci and other micrococceae, p. 13.1-13.12. In D. Armstrong and J. Cohen (ed.), Infectious diseases. Harcourt Publishers Ltd., London, United Kingdom.
- 62.Vesga, O., M. C. Groeschel, M. F. Otten, D. W. Brar, J. M. Vann, and R. A. Proctor. 1996. Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J. Infect. Dis. 173:739-742. [DOI] [PubMed] [Google Scholar]
- 63.Waldvogel, F. A. 2000. Staphylococcus aureus, p. 2069-2092. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, Philadelphia, Pa.
- 64.Yamamoto, T., H. Kusajima, M. Hosaka, and H. Shinoda. 1995. Uptake and intracellular activity of fleroxacin in phagocytic cells. Chemotherapy 41:353-359. [DOI] [PubMed] [Google Scholar]