Abstract
We investigated the influence of various clinical prognostic factors in patients with glioblastoma multiforme (GBM) treated with a combined modality approach. A total of 175 patients with GBM was treated in four consecutive prospective phase II studies using surgery, hyperfractionted or accelerated hyperfractionated radiotherapy (RT) and either adjuvant or concurrent or pre-irradiation chemotherapy (CHT) between Janaury 1988 and December 1993. The median survival time for all 175 patients was 14 months and 1–3-year survival (OS) rates were 57%, 34% and 24%, respectively. The median time to tumour progression was 12 months, and 1–3-year progression-free survival (PFS) rates were 43%, 11% and 7%, respectively. Survival analysis showed that of all investigated prognostic factors, only gender did not influence survival. Patients ≤55 years did better than those >55 years; patients with KPS 80–100 did better than those with KPS 50–70; patients with frontal tumours did better than those with tumours in other locations; patients with tumours up to 4 cm did better than those with larger tumours, as did patients with either subtotal or gross total tumour resection when compared to those undergoing biopsy only. Multivariate analysis showed that gender and tumour location did not independently influence survival. When PFS was used as the endpoint, only gender did not influence PFS, as confirmed by multivariate analysis.
Keywords: Prognostic factors, Glioblastoma multiforme, Radiation therapy, Surgery, Chemotherapy
Introduction
The vast majority of malignant gliomas are glioblastoma multiforme (GBM), which represent one of the frustrating challenges in neuro-oncology. The widely accepted standard treatment approach in these patients includes surgery and postoperative radiotherapy (RT) to a tumour dose of 60 Gy in 30 daily fractions [1, 2, 3]. With this approach, however, results are poor, and the vast majority of patients still die within a few years of the initial diagnosis, with long-term survival (e.g., 5 years) being an anecdotal event.
In order to improve these poor results, numerous attempts were made in the last few decades, including high-dose RT [4], chemical modifiers [3], heavy particles [5], adjuvant chemotherapy (CHT) [1, 2, 6, 7] and altered fractionated regimens [8, 9, 10]. In recent years interstitial brachytherapy [11], radiosurgery [12, 13, 14], stereotactic fractionated RT (SFRT) [15, 16] and intensity-modulated RT (IMRT) [17, 18] were all used to enable highly precise delivery of the radiation dose to the target volume. Since the pattern of failure still points to local recurrence being the dominant event, one of the most important limitations of newer techniques is the failure to define the tumour extension precisely. While the standard investigations in the diagnosis and treatment planning of brain gliomas are CT and MRI, recent efforts seem to have focused on the impact of functional imaging such as Single Photon Emission Computerised Tomography (SPECT), Positron Emission Tomography (PET) or Magnetic Resonance Spectroscopy (MRS) both on more precise identification of residual, post-surgical tumour volume and on treatment planning after surgical resection of gliomas [19, 20, 21, 22, 23, 24].
A number of variables that could influence treatment outcome in patients with GBM have been investigated in the past. Gender, age, performance status, neurologic function, tumour size and site, midline shift, extent of surgery and RT dose, among other factors, were frequently evaluated. However, these, as well as many other variables, have not been consistently examined in all or even the majority of existing studies. The importance of these variables is, therefore, rather difficult to evaluate without verification in multiple studies due to the fact that some of them may be significant by random chance alone. Since the characteristics of patients can influence their clinical course [25, 26], this particularly relates to the design and execution of clinical trials and the evaluation of the treatment outcome. It was also observed that the magnitude of the difference in outcome for categories of the strongest prognostic factors is larger than that for the type of therapy used [27]. Therefore, separation of patients into distinct prognostic subgroups should represent an important contribution to the design and stratification of malignant glioma trials and should enable the accrual of the individual patients into the more appropriate treatment groups or studies. Finally, this also may help correctly interpret the results of studies comparing different treatment regimens and help assess the potential of new treatment approaches.
Having continuous interest in the treatment of these tumours, we undertook the present analysis in order to identify potential prognostic factors in patients with GBM treated with a combined modality approach in a single institution over a period of 6 years. We have focused on clinical pretreatment prognostic factors because they are easy to observe and note, and they represent the factors that can be collected not only early in the course of the disease or diagnosis, well before we decide upon the "optimal" treatment approach in this disease, but during the time of follow-up as well, regardless of the treatment itself.
Material and methods
Over a 6-year period (January 1988 to December 1993), we conducted four prospective phase II studies in patients with malignant glioma. Pretreatment and treatment considerations and treatment results were published previously in detail [9, 10, 28, 29]. Besides patients with anaplastic astrocytoma, patients with glioblastoma multiforme were included in these studies, and they constituted the cohort for this report. During study I [9], surgery was followed by postoperative high-dose hyperfractionated radiation therapy (72 Gy in 60 fractions for 30 days over 6 weeks) and adjuvant chemotherapy (CHT), consisting of BCNU, vincristine, procarbazine and cisplatin (BOPP), which started 4 weeks after completion of Hfx RT and lasted up to a maximum of six cycles. During study II [10], surgery was followed by accelerated hyperfractionated RT (66 Gy in 44 fractions for 22 treatment days over 4.5 weeks) and concurrent CHT consisting of BCNU and hydroxyurea given once weekly during RT. During study III [28], 3 weeks after surgery, two cycles of CHT consisting of carboplatin and etoposide and then accelerated hyperfractionated RT (60 Gy in 40 fractions for 20 days over 4 weeks) followed. In study IV [29], surgery was followed by accelerated hyperfractionated RT (60 Gy in 40 fractions for 20 days over 4 weeks) and concurrent carboplatin and etoposide given weekly during the RT course. During the study period RT characteristics varied only regarding the treatment volume, with the use of more limited volumes in recent studies defined as contrast enhancing volume on the pre-surgical CT scan plus a 2–3-cm margin.
Follow-up was done in regular intervals, being the same for all patients using the same diagnostic tools (standard laboratory tests, physical and neurological examination, brain CT). These investigations were also carried out in any cases of signs of tumour progression.
The Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–3 was used during studies I and II, while the Karnofsky performance status (KPS) score 50–100 was used during studies III and IV. Since not all patients with ECOG PS 3 (those corresponding to KPS of 40) had their KPS counterparts in studies III and IV (the latter two studies allowed only KPS 50–100), we have recalculated ECOG PS according to KPS by equalling ECOG 0 to KPS 100, ECOG 1 to KPS 80–90 and ECOG 2 to KPS 60–70. We excluded a total of seven patients (study I, n=4 and study II, n=3, respectively) with ECOG PS of 3 that would correspond to KPS of 40 to facilitate subsequent analyses regarding PS.
In all of these phase II studies the primary endpoint was survival. Survival was calculated from the first date of RT/CHT, and progression-free survival (PFS) was calculated from the first date of RT/CHT until tumour progression or death (by any cause), whichever happened first, both using the Kaplan-Meier method. The differences between survival curves were analysed by the log-rank test. The interaction of each prognostic factor and its effect on either overall survival or PFS were analysed using the Cox proportional hazards model. All these statistical analyses have been done using the SPSS statistical software.
Results
Clinical pretreatment variables included in this study were: (1) gender, (2) age, (3) Karnofsky performance status, (4) tumour size, (5) tumour site and (6) extent of surgery. A total of 175 patients with malignant glioma having a KPS of 50–100 were used for this analysis. Patients' characteristics per study group are given in Table 1. The four studies had similar patient populations, except significantly more patients with KPS 80–100 were observed in studies III and IV. Median survival time (MST) for all 175 patients was 12 months, and 1–3-year survival rates were 44%, 13% and 6%, respectively. Median time to tumour progression was 12 months, and 1–3-year PFS rates were 43%, 11% and 7%, respectively. Gender did not affect survival (P=0.40), while age did (P=0.0000). Patients with higher KPS did better than those with lower KPS (P=0.0000), as did patients having smaller preoperative tumours (P=0.0001). Also, patients with frontal tumours did significantly better than those with tumours in other locations (P=0.0001), and patients undergoing more extensive surgery did significantly better than those undergoing surgery (P=0.0000) (Table 2). Next, the Cox proportionate hazards model was used to identify those factors that could have independently influenced survival. On univariate Cox analysis, only gender did not impact survival (P=0.41), but when a multivariate analysis was done, besides gender, also tumour location (P=0.0656) was shown not to influence survival. All other variables were prognosticators of survival.
Table 1.
Patient characteristics by study group. M male, F female, KPS Karnofsky performance status, F frontal, B biopsy, STR subtotal tumour resection, GTR gross total tumour resection
| Characteristic | Study I | Study II | Study III | Study IV | Total | |
|---|---|---|---|---|---|---|
| Age (years) | ≤55 | 16 | 20 | 15 | 28 | 79 |
| >55 | 17 | 26 | 20 | 33 | 96 | |
| Sex | M | 22 | 24 | 30 | 52 | 128 |
| F | 11 | 22 | 5 | 9 | 47 | |
| KPS | 50–70 | 20 | 28 | 11 | 15 | 74 |
| 80–100 | 13 | 18 | 24 | 46 | 101 | |
| Size (cm) | ≤4 | 19 | 27 | 19 | 34 | 99 |
| >4 | 14 | 19 | 16 | 27 | 76 | |
| Location | F | 10 | 15 | 11 | 13 | 49 |
| Other | 23 | 31 | 24 | 48 | 126 | |
| Surgery | B | 10 | 9 | 11 | 22 | 52 |
| STR/GTR | 23 | 37 | 24 | 39 | 123 |
Table 2.
Prognostic factors influencing overall survival. M male, F female, KPS Karnofsky performance status, F frontal, B biopsy, STR subtotal tumor resection, GTR gross total tumor resection
| Characteristic | n | MST | Survival | Cox model | |||||
|---|---|---|---|---|---|---|---|---|---|
| (Month) | 1 year | 2 years | 3 years | P | Univariate P | Multivariate P | |||
| Age (years) | ≤55 | 79 | 15 | 63% | 23% | 16% | 0.0000 | 0.0000 | 0.0052 |
| >55 | 96 | 10 | 28% | 5% | 0% | ||||
| Sex | M | 128 | 12 | 43% | 11% | 4% | 0.40 | 0.41 | – |
| F | 47 | 12 | 44% | 18% | 15% | ||||
| KPS | 50–70 | 74 | 9 | 20% | 1% | 0% | 0.0000 | 0.0000 | 0.0048 |
| 80–100 | 101 | 14 | 61% | 21% | 11% | ||||
| Size (cm) | ≤4 | 99 | 14 | 59% | 14% | 4% | 0.0001 | 0.0001 | 0.0069 |
| >4 | 76 | 9 | 25% | 10% | 8% | ||||
| Location | F | 49 | 18 | 61% | 34% | 15% | 0.0001 | 0.0001 | 0.0656 |
| Other | 126 | 11 | 37% | 5% | 3% | ||||
| Surgery | B | 52 | 7 | 13% | 2% | 0% | 0.0000 | 0.0000 | 0.0000 |
| STR/GTR | 123 | 13 | 57% | 17% | 11% | ||||
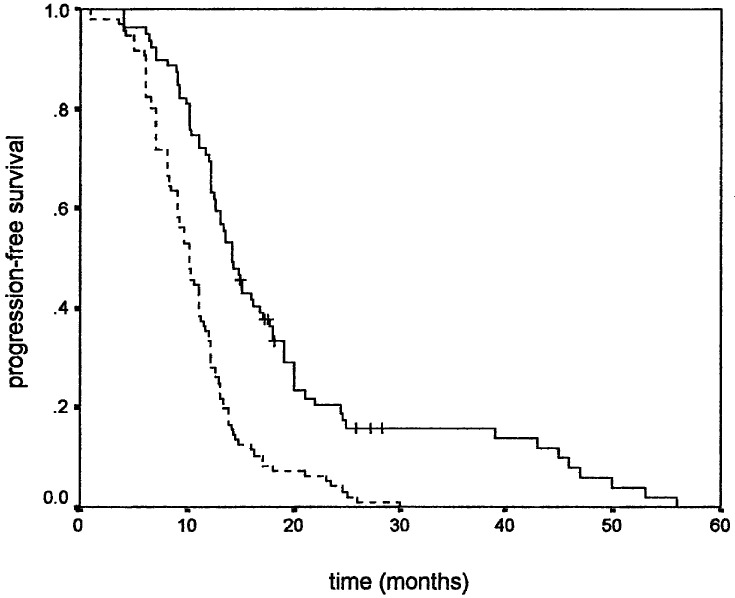
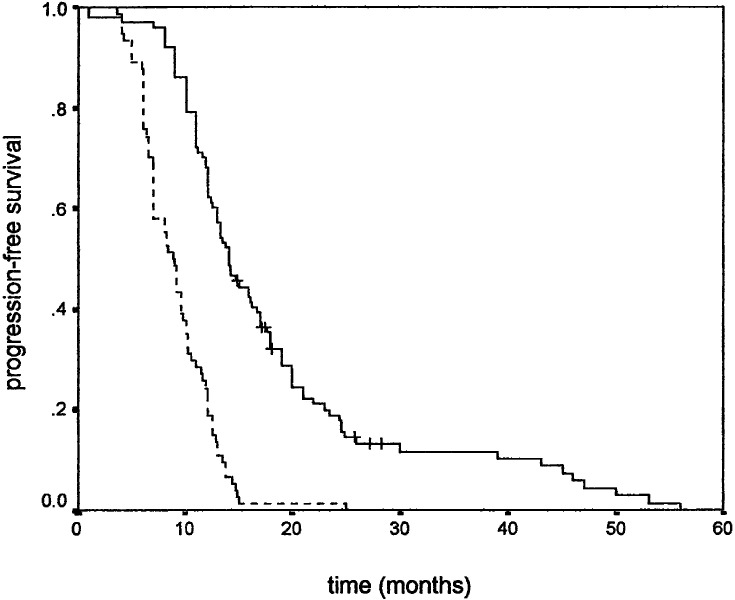
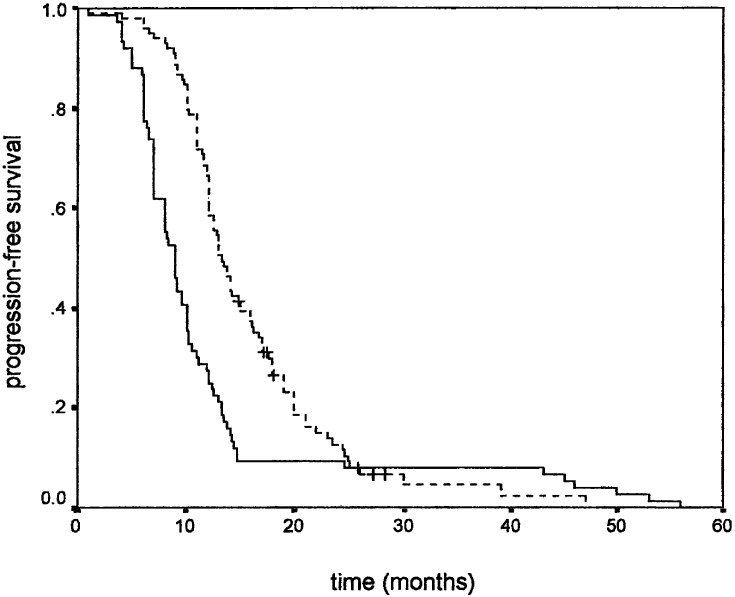
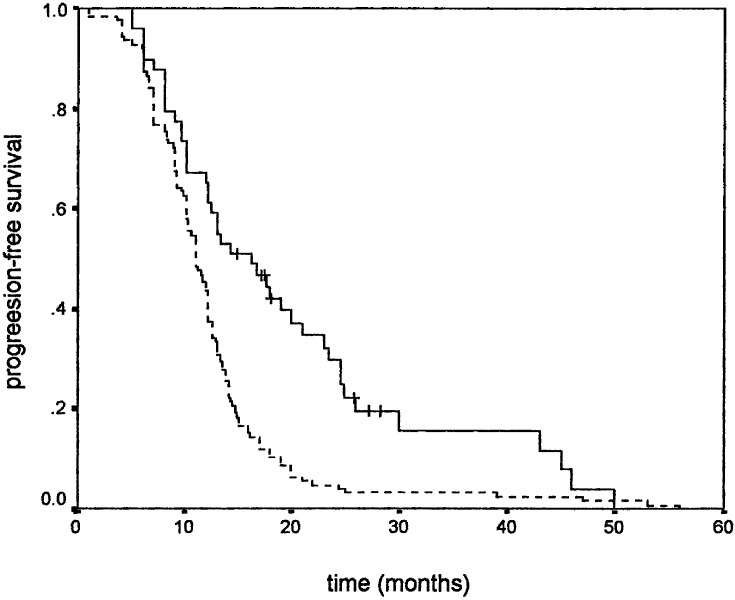
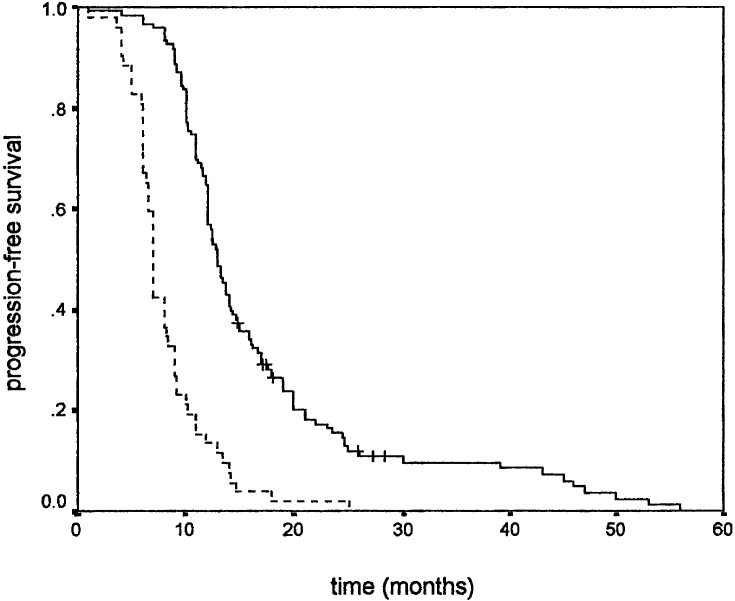
Similar results were observed when PFS was used as an endpoint. Younger age positively influenced PFS (Fig. 1), while gender did not. Higher KPS positively correlated with PFS (Fig. 2). Tumour size also correlated with PFS, favouring patients with smaller tumour size (Fig. 3). As in overall survival, frontal tumour location also carried better PFS (Fig. 4). Finally, patients undergoing more radical tumour resection had better PFS than those with biopsy only (Fig. 5). On univariate analysis, only gender did not impact PFS (P=0.44), which was confirmed by univariate Cox analysis (P=0.41) and a multivariate analysis showing that all other variables were independent prognosticators of PFS (Table 3).
Fig. 1.
Progression-free survival according to age: ≤55 years (___) and >55 years (- - - -)
Fig. 2.
Progression-free survival according to KPS: 80–100 (____) and 50–70 (- - - -)
Fig. 3.
Progression-free survival according to tumour size: ≤4 cm (- - - -) and >4 cm (___)
Fig. 4.
Progression-free survival according to tumour location: frontal (____) and other (- - - -)
Fig. 5.
Progression-free survival according to extent of surgery: subtotal resection or gross total resection (____) and biopsy (- - - -)
Table 3.
Prognostic factors influencing progression-free survival. M male, F female, KPS Karnofsky performance status, F frontal, B biopsy, STR subtotal tumor resection, GTR gross total tumor resection
| Characteristic | n | MTP | Progression-free | Cox model | |||||
|---|---|---|---|---|---|---|---|---|---|
| (Month) | 1 year | 2 year | 3 years | P | Univariate P | Multivariate P | |||
| Age (years) | ≤55 | 79 | 14 | 62% | 19% | 16% | 0.0000 | 0.0000 | 0.0031 |
| >55 | 96 | 10 | 28% | 4% | 0% | ||||
| Sex | M | 128 | 11 | 42% | 9% | 5% | 0.44 | 0.41 | – |
| F | 47 | 12 | 47% | 16% | 11% | ||||
| KPS | 50–70 | 74 | 9 | 19% | 1% | 0% | 0.0000 | 0.0000 | 0.0030 |
| 80–100 | 101 | 14 | 61% | 18% | 12% | ||||
| Size (cm) | ≤4 | 99 | 13 | 59% | 12% | 5% | 0.0001 | 0.0001 | 0.0163 |
| >4 | 76 | 9 | 24% | 9% | 8% | ||||
| Location | F | 49 | 16 | 59% | 30% | 16% | 0.0001 | 0.0002 | 0.0449 |
| Other | 126 | 11 | 37% | 4% | 3% | ||||
| Surgery | B | 52 | 7 | 13% | 2% | 0% | 0.0000 | 0.0000 | 0.0000 |
| STR/GTR | 123 | 13 | 56% | 15% | 10% | ||||
The strongest prognosticators of both survival/PFS were, in descending order, surgery, KPS, age and tumour size, while tumour location exerted its influence only on PFS but not on overall survival.
Discussion
During the current analysis only gender was not shown to be of prognostic importance for both survival and PFS in patients with glioblastoma multiforme. Sometimes it was not included in an analysis of prognostic factors [30, 31], but when it was, invariably it did not impact the treatment outcome [32, 33, 34, 35]. In the current study we also could not document its impact, which is not surprising due to the negative results in each of the initial investigations [9, 10, 28, 29] we combined to make a basis for the current study.
Tumour location exhibited a rather unclear effect in the current study. We have used the designation of a particular tumour site according to the major tumour bulk, particularly with tumours crossing boundaries. We compared frontal tumour location to other tumour locations, since frontal tumours may be easier to resect and, therefore, may have a favourable prognosis. While tumour location was not shown to be an independent prognosticator of survival (multivariate, P=0.0656), it was shown to predict PFS (multivariate, P=0.0449) in the current study. Results from the literature are conflicting, at least in part due to both the different designations of tumour location used as well as the fact that some of the studies did not take it into account [30, 34, 36]. Of those that did, some used the separation between frontal, parietal, temporal and occipital [9, 10, 28, 29, 37], while others used the separation between superficial and deep-seated [38, 39]. While some [28, 29, 33, 35] did not observe its influence on treatment outcome, Simpson et al. [31] found an independent influence of frontal tumour location, as we did in our previous studies [9, 10], while superficial tumour location was an independent factor in the series of Jelsma and Bucy [38, 39]. These authors speculated about the accessibility of superficial or frontal tumour locations, although with sophisticated neurosurgical approaches, this should be of no major importance today, especially in light of adjuvant treatments. To better integrate tumour location into a framework of factors influencing the selection of patients with glioblastoma multiforme for gross total tumour resection, Shinoda et al. [35] created a topographical staging system taking into account tumour location, size and eloquence of the adjacent brain based on MRI. The three stages (I–III) created that way correlated with both survival and the complication rate
All other factors proved to be independent prognosticators in the current study. The strongest one was the extent of surgery. It is usually considered an important initial attack in the management of glioblastoma multiforme in providing a histologic diagnosis and decreasing elevated intracranial pressure. However, the influence of the extent of surgery on treatment outcome in this patient population is still open to debate. The definition of the extent of resection depending on the neurosurgeon's impression of "resectability" [40, 41, 42, 43], inaccurate estimates of both pre- and post-surgical tumour burden, as well as retrospective evaluation of the extent of surgery by describing it from the original patients' charts/operative reports of other neurosurgeons [40, 42, 43, 44], co-morbidity and other less well-defined factors were frequently cited as possible reasons for this. The extent of resection was not clearly stated in all studies that evaluated outcome. In those that did, it was not done in a uniform manner. Regardless of the extent, resection was compared either to biopsy [40, 44] or to biopsied and untreated patients grouped together [39, 44]. Contrarily, some studies provided the survival data according to the extent of surgical resection [9, 10, 28, 29, 37, 41, 42, 43, 45].
Another problem is the presumed "oncological" nature of resection, since more radical tumour resection should theoretically lead to better results from a cytokinetic point of view [46]. When coupled with inaccuracy to delineate "all" of the existing tumour, it becomes more clear why conflicting results concerning a benefit for more radically resected patients exist. Indeed, several decades ago Cushing [47] as well as Jelsma and Bucy [38, 39] both noted that patients with more radical resection fared better and had a lower operative mortality. Several other large series of >200 patients [44, 48, 49], including all malignant glioma patients, reached the same conclusion about long-term survival. Also, in his review in 1985, Salcman [42] used the data on 603 patients to find that those patients who had resection consistently survived longer. Contrarily, two large reviews at the beginning of the 1990s, including all malignant glioma patients, concluded quite the opposite. Quigley and Maroon [50] noted that 16 of 20 studies (involving 5,691 patients) observed no relationship between the extent of surgery and survival, while only four studies found this relationship to be of either minor or unspecified significance. Nazzaro and Neuwelt [51] reviewed more than 100 studies to find no justification for relating longer patient survival to more radical resections. The last decade also brought conflicting evidence regarding this matter [31, 34, 52, 53]. With time, however, it became not only possible to use quantitative imaging to more precisely determine the extent of tumour resection [45, 54, 55], but to use different imaging modalities, different methods to quantify tumour volumes as well as irregular timing of post-surgical imaging, making any conclusion unreliable. Using MRI in resected patients with gliomas, residual tumour cannot be differentiated from unspecific postoperative changes. With the widespread use of functional imaging, however, using fusion images, the anatomic information of MRI can be combined with the biological information obtained by SPECT, PET or MRS. These functional images should provide a biological definition of the tumour remnant and can be used not only for quantification of the real amount of the residual tumour, but for better definition of the target volume for the high precision RT techniques such as SRS, SFRT or IMRT. While some of the largest series [34, 54] noted that more radical resection correlated with longer survival, others did not [53].
Perhaps the ongoing controversy on the impact of the extent of surgery in malignant glioma rests on our inability to precisely explain (understand) how this attack works. Kreth et al. [33] recently opened a debate speculating that two possible ways tumour resection works were either by creating a space (decompressive surgery) or by significantly removing tumour cells (cytoreductive surgery) [32, 51, 53]. As stated by the authors, unfortunately, no clear separation between the two ways of acting is possible nowadays, simply because tumour resection accomplishes both of these aims. The results of the current study support the view that more radical surgery offers survival/PFS advantages over the less-radical procedures. This prognostic factor in our study proved to be the strongest one, although it has been frequently emphasised that its impact is largely shadowed by the influence of stronger prognosticators such as age and KPS.
In our study, KPS was the second strongest prognosticator. In numerous studies it was almost always observed that better performance status has a favourable effect on survival [9, 27, 28, 29, 40, 48, 55], although many problems with the interpretation of the data exist. One of the problems is the different scoring systems used. The majority of studies used KPS, but there were numerous patient stratifications (from as low as 10), and some studies limited accrual only to higher KPS values (e.g., 70). Another problem was the timing of the evaluation: pre-treatment, post-surgery or immediately pre-RT [45, 48, 55]. Additionally, some studies did not include it for consideration [42, 43, 56], while others observed its impact on survival only when evaluated as an independent variable [57, 58]. Nevertheless, there is substantial evidence that performance status is an independent prognosticator of treatment outcome in patients with malignant glioma, an observation frequently made in the last decade [30, 40, 57, 59, 60, 61]. The results of the current study fit well with this observation. Patients with a higher KPS achieved significantly higher MST/MTP and superior survival/PFS.
Age was investigated for several decades as a prognostic factor in glioblastoma multiforme. While some studies included paediatric cases [49, 57], others included the elderly up to 85 years of age [41]. Also, while some authors did not provide an analysis of the treatment outcome according to age [48, 49], others found its impact on survival only when analysed as an independent variable, but did not observe this when a multivariate analysis was used [39, 40, 57]. Regardless of potential problems inherent to any retrospective study, numerous studies found that younger age correlates with longer survival/PFS in patients with glioblastoma multiforme, sometimes included into the cohorts of patients with malignant glioma [40, 44, 48, 55, 58, 62].
What could the rational for such an observation be? Some suggested that the advantageous effect of younger age might lie in the greater proliferative potential of malignant glioma in older patients. Hoshino et al. [63] found that when tumours from patients >50 years were compared with those from patients <50 years, the former had a significantly higher mean labelling index, considered as the measure for more accurately predicting the prognosis than the histopathological diagnosis. Indeed, these observations go well with the clinical observations of poorer survival rates in older patients. Age may also influence the selection of patients for specific surgical therapies [44], with younger patients likely to undertake more extensive tumour resections.
The last decade definitely established the role of age in predicting treatment outcome in these patients. Using recursive partitioning and amalgamation on the data of 1,578 patients with malignant glioma treated in several Radiation Therapy Oncology Group (RTOG) studies, Curran et al. [27] identified older age (>50 years) as the most important prognostic factor negatively correlating with survival. This was also observed by other investigators [29, 30, 31, 33], including the current study in which patients <55 years achieved significantly higher MST/MTP as well as overall survival/PFS than their older counterparts.
Preoperative tumour size was not frequently included in an analysis of prognosticators [9, 27, 32, 34, 35]. When it was included, however, different observations were made. Simpson et al. [31], Kreth et al. [33] and Hulshof et al. [30] did not find its influence on treatment outcome; Jeremic et al. [10, 28] also did not. Contrarily, in an another study, Jeremic et al. [29] observed its prognostic value. There are numerous explanations for such a discrepancy, including the small number of patients evaluated and rapid tumour growth immediately prior to presentation [59] leading to the short time it takes for the tumour to grow from a lesion of a small size to a large size when compared to the overall time from diagnosis to death. A possible explanation could also include the lesser importance of the size than the location of the tumour [60, 61] and the fact that the effect of treatment received after the initial scan outweighs any that preoperative tumour size may have. During the current study, we found an independent influence of tumour size (cut-off value of 4 cm) on both PFS and survival. Unfortunately, no volumetric analysis was made during this study to enable better insight into this matter, and no functional imaging was available at that time.
In conclusion, this multivariate analysis showed that age, KPS, size and extent of surgery were independent prognosticators of survival/PFS. Also, while tumour location was predictive of PFS, it was not so of survival. These results support the findings of previous studies that identification of prognostic factors is an important goal in clinical research of glioblastoma multiforme treated with a combined modality approach.
References
- 1.Deutch M, Green SB, Strike TA, et al (1989) Results of randomized trial comparing BCNU plus radiotherapy, streptozocin plus radiotherapy, BCNU plus hyperfractionated radiotherapy, and BCNU following misonidasole plus radiotherapy in the post-operative treatment of malignant glioma. Int J Radiat Oncol Biol Phys 16:1389–1396 [DOI] [PubMed] [Google Scholar]
- 2.Kristiansen K, Hagen S, Kollevold T, et al (1981) Combined modality therapy of operated astrocytomas Grade III and IV. Confirmation of the value of postoperative irradiation and lack of potentiation of bleomycin on survival time: a prospective multicenter trial of the Scandinavian Glioblastoma Study group. Cancer 47:649–652 [DOI] [PubMed] [Google Scholar]
- 3.Nelson DF, Diener-West M, Weinstein AS, et al (1986) A randomized comparison of misonidasole sensitized radiotherapy plus BCNU and radiotherapy plus BCNU for treatment of malignant glioma after surgery: final report of an RTOG study. Int J Radiat Oncol Biol Phys 12:1793–1800 [DOI] [PubMed] [Google Scholar]
- 4.Salazar OM, Rubin P, Feldstein ML, Pizzutiello R (1979) High dose radiation therapy in the treatment of malignant gliomas: final report. Int J Radiat Oncol Biol Phys 5:1733–1740 [DOI] [PubMed] [Google Scholar]
- 5.Kolker JD, Halpern HJ, Krishnasamy S, et al (1990) "Instant-mix" whole brain photon with neutron boost radiotherapy for malignant gliomas. Int J Radiat Oncol Biol Phys 19:409–414 [DOI] [PubMed] [Google Scholar]
- 6.Jeremic B, Jovanovic D, Djuric L, Jevremovic S, Mijatovic L (1992) Advantage of post-radiotherapy chemotherapy with CCNU, procarbazine and vincristine (mPCV) over chemotherapy with VM-26 and CCNU. J Chemother 4:123–126 [DOI] [PubMed] [Google Scholar]
- 7.Levin VA, Silver P, Hannigan J, et al (1980) Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine and vincristine (PCV) over BCNU for anaplastic astrocytoma: NCOG 6G91 final report. Int J Radiat Oncol Biol Phys 18:321–324 [DOI] [PubMed] [Google Scholar]
- 8.Jeremic B, Grujicic D, Antunovic V, Djuric Lj, Stojanovic M, Shibamoto Y (1994) Hyperfractionated radiation therapy (HFX RT) followed by multiagent chemotherapy (CHT) in patients with malignant glioma: a phase II study. Int J Radiat Oncol Biol Phys 30:1179–1185 [DOI] [PubMed] [Google Scholar]
- 9.Jeremic B, Grujicic D, Antunovic V, Djuric Lj, Shibamoto Y (1995) Accelerated hyperfractionated radiation therapy for malignant glioma. A phase II study. Am J Clin Oncol (CCT) 18:449–453 [DOI] [PubMed] [Google Scholar]
- 10.Nelson DF, Curran WJ Jr, Scott CB, et al (1993) Hyperfractionated radiation therapy and bis-chlorethyl nitrosourea in the treatment of malignant glioma: possible advantage observed at 72.0 Gy in 1.2 Gy b.i.d. fractions: report of the Radiation Therapy Oncology Group protocol 8302. Int J Radiat Oncol Biol Phys 25:193–207 [DOI] [PubMed] [Google Scholar]
- 11.Sneed PK, Gutin PH, Larson DA, et al (1994) Patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int J Radiat Oncol Biol Phys 29:719–727 [DOI] [PubMed] [Google Scholar]
- 12.Mehta MP, Masciopinto J, Rozental J, et al (1994) Stereotactic radiosurgery for glioblastoma multiforme: report of a prospective study evaluating prognostic factors and analyzing long-term survival advantage. Int J Radiat Oncol Biol Phys 30:541–549 [DOI] [PubMed] [Google Scholar]
- 13.Shrieve DC, Alexander E 3rd, Black PM, Wen PY, Fine HA, Kooy HM, Loeffler JS (1994) Treatment of patients with primary glioblastoma multiforme with standard postoperative radiotherapy and radiosurgical boost: prognostic factors and long-term outcome. J Neurosurg 90:72–77 [DOI] [PubMed] [Google Scholar]
- 14.Regine WF, Patchell RA, Strottmann JM, Meigooni A, Sanders M, Young AB (2000) Preliminary report of a phase I study of combined fractionated stereotactic radiosurgery and conventional external beam radiation therapy for unfavourable gliomas. Int J Radiat Oncol Biol Phys 48:421–426 [DOI] [PubMed] [Google Scholar]
- 15.Cardinale RM, Schmidt-Ullrich RK, Benedict SH, Zwicker RD, Han DC, Broaddus WC (1998) Accelerated radiotherapy regimens for malignant gliomas using stereotactic concomitant boosts for dose escalation. Radiat Oncol Investigat:175–181 [DOI] [PubMed] [Google Scholar]
- 16.Shiu A, Kooy H, Ewton J (1997) Comparison of miniature multileaf collimation (MMLC) with circular collimation for stereotactic treatment. Int J Radiat Oncol Biol Phys 37:679–688 [DOI] [PubMed] [Google Scholar]
- 17.Khoo VS, Oldham M, Adams EJ, Bedford JL, Webb S, Brada M (1999) Comparison of intensity-modulated tomotherapy with stereotactically guided conformal radiotherapy for brain tumors. Int J Radiat Oncol Biol Phys 45:415–425 [DOI] [PubMed] [Google Scholar]
- 18.Woo SY, Granbt III WH, Belleza D (1996) A comparison of intensity modulated conformal therapy with aconventional external beam stereotactic radiosurgery system for the treatment of single and multiple intracranial lesions. Int J Radiat Oncol Biol Phys 35:593–597 [DOI] [PubMed] [Google Scholar]
- 19.Pirzkall A, McKnight TR, Graves EE et al (2001) MR-spectroscopy guided target delineation for high-grade gliomas. Int J Radiat Oncol Biol Phys 50:915–928 [DOI] [PubMed] [Google Scholar]
- 20.Biersack HJ, Coenen HH, Stocklin G, Reichmann K, Bockisch A, Oehr P, Kashab M, Rollmann (1989) Imaging of brain tumors with L-3-(123I)iodo-alpha-methyl-L-tirosine. J Nucl Med 30:110–112 [PubMed] [Google Scholar]
- 21.Weber W, Bartenstein P, Gross MW, Kinzel D, Daschner D, Feldmann HJ, Reidel G, Ziegler SI, Lumenta C, Molls M, Schwaiger M (1997) Fluorine-18-FDG PET and iodine-123-IMT SPECT in the evaluation of brain tumors. J Nucl Med 38:802–808 [PubMed] [Google Scholar]
- 22.Gross MW, Weber W, Feldmann HJ, Bartenstein P, Schwaiger M, Molls M (1998) The value of F-18-fluorodeoxyglucose PET for the 3-D radiation treatmnt planning of malignant gliomas. Int J Radiat Oncol Biol Phys 41:989–995 [DOI] [PubMed] [Google Scholar]
- 23.Grosu A-L, Weber W, Feldmann HJ, Wuttke B, Bartenstein P, Gross MW, Lumenta C, Schwaiger M, Molls M (2000) First experience with I-123-alpha-methyl-tyrosine SPECT in the 3-D radiation treatment planning of brain gliomas. Int J Radiat Oncol Biol Phys 47:517–526 [DOI] [PubMed] [Google Scholar]
- 24.Grosu A-L, Feldmann HJ, Dick S, Dzewas B, Nieder C, Gumprecht H, Frank A, Schwaiger M, Molls M, Weber WA (2002) Implications of IMT-SPECT for postoperative radiotherapy planning in patients with gliomas. Int J Radiat Oncol Biol Phys 54:842–854 [DOI] [PubMed] [Google Scholar]
- 25.Glatstein E, Makuch RW (1984) Illusion and reality: practical pitfalls in interpreting clinical trials. J Clin Oncol 2:488–497 [DOI] [PubMed] [Google Scholar]
- 26.Simon R (1984) Importance of prognostic factors in cancer clinical trials. Cancer Treat Rep 68:185–192 [PubMed] [Google Scholar]
- 27.Curran WJ Jr, Scott CB, Horton J, et al (1993) Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 85:704–710 [DOI] [PubMed] [Google Scholar]
- 28.Jeremic B, Shibamoto Y, Grujicic D, et al (1999) Preirradiation carboplatin and etoposide and accelerated hyperfractionated radiation therapy in patients with high-grade astrocytomas. A phase II study. Radiother Oncol 51:27–33 [DOI] [PubMed] [Google Scholar]
- 29.Jeremic B, Shibamoto Y, Grujicic D, et al (2001) Concurrent accelerated hyperfractionated radiation therapy and carboplatin/etoposide in patients with malignant glioma: long-term results of a phase II study. J Neuro-Oncol 51:133–141 [DOI] [PubMed] [Google Scholar]
- 30.Hulshof MCCM, Schimmel EC, Bosch AD, Gonzalez DG (2000) Hypofractionation in glioblastoma multiforme. Radiother Oncol 54:143–148 [DOI] [PubMed] [Google Scholar]
- 31.Simpson JR, Horton J, Scott C, et al (1993) Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys 26:239–244 [DOI] [PubMed] [Google Scholar]
- 32.Kreth FW, Warnke PC, Scheremet R, Ostertag CB (1993) Surgical resection and radiation therapy in the treatment of glioblastoma multiforme. J Neurosurg 78:762–766 [DOI] [PubMed] [Google Scholar]
- 33.Kreth FW, Berlis A, Spiropoulou V, et al (1999) The role of tumor resection in the treatment of glioblastoma multiforme in adults. Cancer 86:2117–2123 [PubMed] [Google Scholar]
- 34.Lacroix M, Abi-Said D, Fourney DR, et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme. J Neurosurg 95:190–198 [DOI] [PubMed] [Google Scholar]
- 35.Shinoda J, Sakai N, Murase S, Yano H, Matsuhisa T, Funakoshi T (2001) Selection of eligible patients with supratentorial glioblastoma multiforme for gross total resection. J Neurooncol 52:161–171 [DOI] [PubMed] [Google Scholar]
- 36.Gamburg ES, Regine WF, Patchell RA, Strotmann JM, Mohiuddin M, Young AB (2000) The prognostic significance of midline shift at presentation on survival in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys 48:1359–1362 [DOI] [PubMed] [Google Scholar]
- 37.Jeremic B, Grujicic D, Antunovic V, Stojanovic M, Shibamoto Y (1994) Influence of the extent of surgery and tumor location on treatment outcome of patients with glioblastoma multiforme treated with combined modality approach. J Neurooncol 21:177–185 [DOI] [PubMed] [Google Scholar]
- 38.Jelsma R, Bucy PC (1967) The treatment of glioblastoma multiforme of the brain. J Neurosurg 27:388–400 [DOI] [PubMed] [Google Scholar]
- 39.Jelsma R, Bucy PC (1969) Glioblastoma multiforme. Its treatment and some factors effecting survival. Arch Neurol 20:161–171 [DOI] [PubMed] [Google Scholar]
- 40.Kinsella TJ, Rowland CJ, Klecker R et al (1988) Pharmacology and phase I/II study of continuous intravenous infusions of iododeoxyuridine and hyperfractionated radiotherapy in patients with glioblastoma multiforme. J Clin Oncol 6:876–879 [DOI] [PubMed] [Google Scholar]
- 41.Newall J, Ranshoff J, Kaplan B (1988) Glioblastoma in the older patient: how long a course of radiotherapy is necessary? J Neurooncol 6:325–327 [DOI] [PubMed] [Google Scholar]
- 42.Salcman M (1985) Resection and reoperation in neuro-oncology. Rationale and approach. Neurol Clin 3:831–842 [PubMed] [Google Scholar]
- 43.Salcman M (1987) Surgical decision-making for malignant brain tumors. Clin Neurosurg 35:285–313 [PubMed] [Google Scholar]
- 44.Davis L, Martin J, Goldstein SL et al (1949) A study of 211 patients with verified glioblastoma multiforme. J Neurosurg 6:33–44 [DOI] [PubMed] [Google Scholar]
- 45.Andreou J, George AE, Wise A et al (1983) CT prognostic criteria of survival after malignant glioma surgery. AJNR 4:488–490 [PMC free article] [PubMed] [Google Scholar]
- 46.Hoshino T (1984) A commentary on the biology and growth kinetics of low-grade and high-grade gliomas. J Neurosurg 61:895–900 [DOI] [PubMed] [Google Scholar]
- 47.Cushing H (1932) Intracranial tumors. Notes upon a series of 2,000 verified cases with surgical mortality percentages pertaining thereto. Thomas, Springfield
- 48.Chang CH, Horton J, Schoenfeld D, et al (1983) Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. Cancer 52:997–1007 [DOI] [PubMed] [Google Scholar]
- 49.Walker MD, Alexander E, Hunt WE, Mahaley MS, Ranshoff J, Gehan EA (1978) Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. J Neurosurg 49:333–343 [DOI] [PubMed] [Google Scholar]
- 50.Quigley MR, Maroon JC (1991) The relationship between survival and the extent of resection in patients with supratentorial malignant gliomas. Neurosurgery 29:385–389 [DOI] [PubMed] [Google Scholar]
- 51.Nazzaro JM, Neuwelt EA (1990) The role of surgery in the management of supratentorial intermediate and high-grade astrocytomas in adults. J Neurosurg 73:331–334 [DOI] [PubMed] [Google Scholar]
- 52.Devaux BC, O'Fallon JR, Kelly PJ (1993) Resection, biopsy, and survival in malignant glial neoplasms. J Neurosurg 78:767–775 [DOI] [PubMed] [Google Scholar]
- 53.Kowalczuk A, Macdonald RL, Amidei C, et al (1997) Quantitative imaging study of extent of surgical resection and prognosis of malignant astrocytomas. Neurosurgery 41:1028–1038 [DOI] [PubMed] [Google Scholar]
- 54.Albert FK, Forsting M, Sartor K, Adams H-P, Kunze S (1994) Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 34:45–61 [DOI] [PubMed] [Google Scholar]
- 55.Coffey RJ, Lunsford LD, Taylor FH (1988) Survival after stereotactic biopsy of malignant gliomas. Neurosurgery 22:465–473 [DOI] [PubMed] [Google Scholar]
- 56.Wood JR, Green SB, Shapiro WR (1988) The prognostic importance of tumor size in malignant gliomas: a computed tomography scan study by the Brain Tumor Cooperative Group. J Clin Oncol 6:338–343 [DOI] [PubMed] [Google Scholar]
- 57.Hitchcock E, Sato F (1964) Treatment of malignant gliomata. J Neurosurg 21:497–505 [DOI] [PubMed] [Google Scholar]
- 58.Wurschmidt F, Buenemann H, Heilmann H-P (1995) Prognostic factors in high-grade malignant glioma. A multivariate analysis of 76 cases with postoperative radiotherapy. Strahlenther Onkol 171:315–321 [PubMed] [Google Scholar]
- 59.Hoshino T, Wilson CB, Rosenblum ML, et al (1975) Chemotherapeutic implications of growth and cell cycle time in glioblastomas. J Neurosurg 43:127–135 [DOI] [PubMed] [Google Scholar]
- 60.Onoyama Y, Abe M, Yabumoto E, et al (1976) Radiation therapy in the treatment of glioblastoma. Am J Roentgenol 126:481–492 [DOI] [PubMed] [Google Scholar]
- 61.Ramsay RG, Brand WN (1973) Radiotherapy of glioblastoma multiforme. J Neurosurg 39:197–202 [DOI] [PubMed] [Google Scholar]
- 62.Burger PC, Green SB (1987) Patient age, histologic features, and length of survival in patients with glioblastoma multiforme. Cancer 59:1617–1625 [DOI] [PubMed] [Google Scholar]
- 63.Hoshino T, Prados M, Wilson CB, et al (1989) Prognostic significance of the bromodeoxyuridine labeling index of human gliomas. J Neurosurg 71:335–341 [DOI] [PubMed] [Google Scholar]