Abstract
Purpose
Serrated adenomas (SAs), which include a wide spectrum of lesions, can be broadly divided into two subtypes: type I, closely mimicking hyperplastic polyps (HPs), and type II, unequivocal adenomatous tumor. Our preliminary findings showed clinicopathologic differences between them. The present study was conducted to investigate apoptotic activity and expression of the cell cycle regulator proteins p21WAF1/CIP1 and p27Kip1 in type I and II SAs, as compared with traditional adenomas (TAs) and HPs.
Methods
Apoptotic activity was estimated in hematoxylin-eosin stained specimens, and p21WAF1/CIP1 or p27Kip1 immunoreactivity was determined in 62 SAs (19 type I and 43 type II), 50 TAs and 19 HPs. The numbers (percentages) of apoptotic or immunoreactive cells were counted per 1,000 epithelial cells in equally separated crypt zones (upper, middle, and lower thirds).
Results
The apoptotic activity in the middle, but not the upper or lower crypt zone was higher in type II SAs (median 0.2%, interquartile range 0.1–0.5%) than in HPs (0.1%, 0.1–0.2%, P<0.01), whereas it was lower in type I SAs (0.2%, 0.1–0.3%) than in TAs (0.5%, 0.2–0.6%, P<0.001). P21WAF1/CIP1 expression in the lower crypt zone was higher in both type I and type II SAs (19.8%, 7.0–33.2% and 20.4%, 3.9–47.8%, P<0.0001) than in TAs (1.2%, 0.6–5.2%), and a similar tendency was also observed for the middle crypt zone. p27Kip1 expression did not vary among the groups.
Conclusions
The differences in apoptotic activity and p21WAF1/CIP1 expression between SAs and TAs or HPs indicate that SA should be considered as a distinct subtype of colorectal neoplasm. The two subtypes of SA do not differ in these parameters despite specific clinicopathological features.
Keywords: Serrated adenoma, Colon, Apoptosis, p21WAF1/CIP1, p27Kip1
Introduction
The serrated adenoma (SA) is a recently described rare entity among colorectal neoplasms, sharing architectural features with hyperplastic polyps (HPs) but exhibiting distinct cytological atypia (Longacre and Fenoglio-Preiser 1990). SA includes a wide spectrum of lesions, from examples very similar to traditional hyperplastic polyps to others having typical adenomatous glands (Longacre and Fenoglio-Preiser 1990; Rubio and Jaramillo 1996; Ban 1999). In our previous study of 127 SAs, type II SAs were generally sessile or pedunculated polyps in the rectosigmoid colon whereas type I lesions tended to demonstrate a flat-elevated morphology and were found in the ascending colon and cecum. Type I SAs were smaller than their type II counterparts. In addition, co-existing invasive carcinomas were only detected with type II SAs (Sada et al., unpublished work).
Failure of apoptosis can cause reduced rates of cell cycle and can lead to pathologic accumulations of cells, resulting in the development of hyperplasia and neoplasia. The proteins encoded by p21WAF1/CIP1 and p27Kip1 as well as the p53 gene are important regulators of the cell cycle. p21WAF1/CIP1 and p27Kip1, sharing partial structural homology, function to inhibit cyclin-dependent kinase complexes required for the G1/S transition and DNA replication (Cordon-Cardo 1995). The p21WAF1/CIP1 regulatory protein can be transactivated in a p53-independent as well as a p53-dependent fashion (Michieli et al. 1994; Macleod et al. 1995) while p27Kip1 induces cell cycle arrest in response to extracellular anti-proliferative signals such as transforming growth factor-β (Polyak et al. 1994).
Several studies have focused on endoscopic (Jaramillo et al. 1996; Matsumoto et al. 1999b), clinicopathologic (Rubio and Jaramillo 1996; Matsumoto et al. 1999a), and oncogenic (Kang et al. 1997; Yao et al. 1999; Rubio and Rodensjö 1995b; Hiyama et al. 1998; Ajioka et al. 1998) characteristics of SAs. Furthermore, their proliferative activity has been investigated in comparison with hyperplastic polyps and traditional adenomas (Ban 1999; Rubio and Rodensjö 1995a; Fujishima 1996; Kang et al. 1997). However, apoptotic activity and levels of cell cycle regulator proteins in SAs, and in particular differences among these subtypes, have not been well described. Thus, we evaluated apoptosis and immunohistochemical expression of p21WAF1/CIP1 and p27Kip1 in a series of SAs (type I and II), as well as hyperplastic polyps (HPs) and traditional tubular or tubulovillous adenomas (TAs).
Materials and methods
Patients and specimens
Slides of colorectal polyps or tumors listed in the diagnostic files of the Division of Pathology at the Kitasato University East Hospital between June 1986 and December 1999 were reviewed by an experienced gastrointestinal pathologist (H.M.). All materials were fixed in 10% buffered formalin, routinely processed for embedding in paraffin wax, sectioned at 4 μm and stained with hematoxylin and eosin (H&E).
The diagnosis of SA was made on the basis of the criteria established by Longacre and Fenoglio-Preiser (1990): namely, the presence of a tumor with a resemblance to an HP at a scanning magnification, but with a serrated epithelium showing nuclear atypia with prominent nucleoli, nuclear stacking and mitotic figures in the upper parts of the crypts, and disordered maturation of the superficial epithelium. In addition, according to the previous descriptions (Rubio and Jaramillo 1996), SAs can be divided into type I, with dysplastic epithelium limited to the lower half of the serrated crypts, closely mimicking HP (Fig. 1), and type II, with dysplastic epithelium also present in the superficial half of serrated crypts, closely mimicking TA (Fig. 2). We applied the following criteria for distinction of type I SA from otherwise typical HP described by Torlakovic and Snover (1996) with some modification: dilatation of the crypts that is most prominent at the base; presence of horizontally oriented crypts; large areas without endocrine cells; focal mucus overproduction; and a proliferative zone basically situated at the base of the crypt with the presence of the numerous goblet cells in the base. Dysplasia of TAs and SAs was classified as low grade (mild or moderate) or high grade (severe), according to the WHO classification (Jass and Sobin 1989).
Fig. 1.
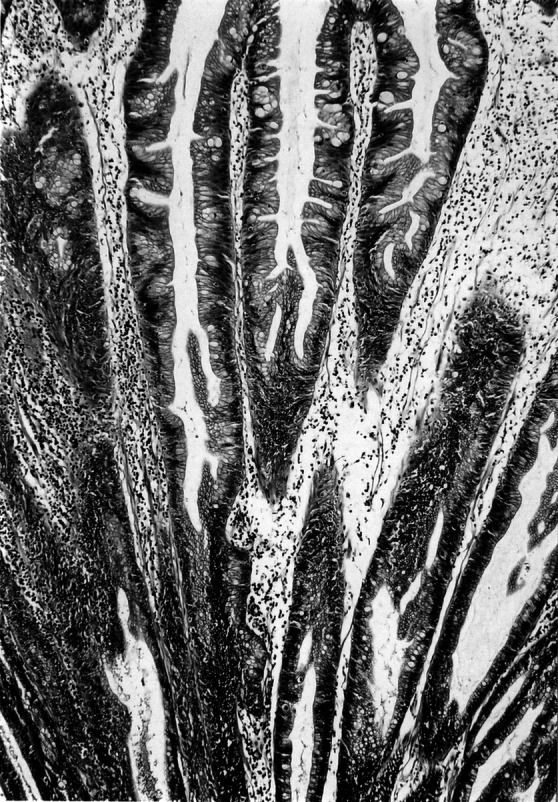
Type I SA demonstrating dysplastic nuclei present only in the lower halves of the serrated crypts (hematoxylin and eosin; original magnification ×80)
Fig. 2.
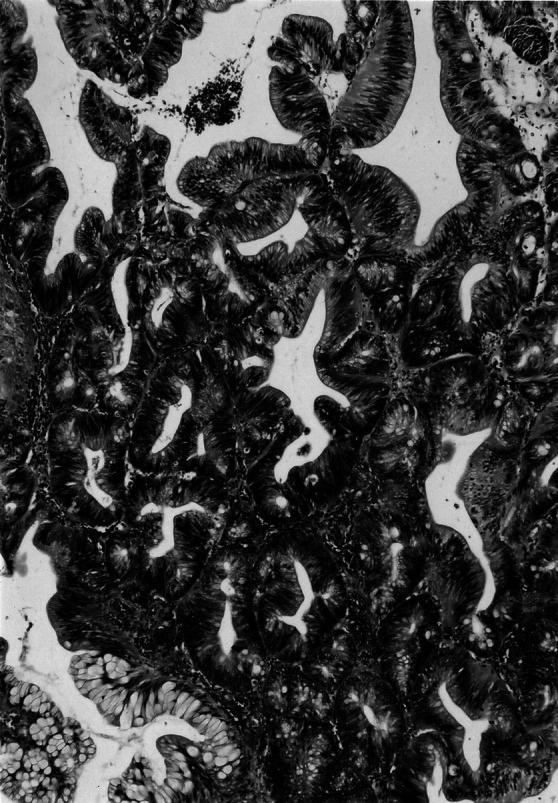
Type II SA having dysplastic nuclei even in the upper halves of the crypts (hematoxylin and eosin; original magnification ×100)
We randomly selected 62 SAs (19 type I and 43 type II) with low-grade dysplasia (LGD), 19 typical HPs and 50 TAs with LGD from our diagnostic files between 1986 and 1999. Cases of SA with high-grade dysplasia (HGD) or invasive carcinoma were excluded. Data for patient age, sex distribution, and size and location of the lesions are given in Table 1.
Table 1.
Clinical features of the hyperplastic polyp, traditional adenoma and serrated adenoma cases
| Hyperplastic polyp | Traditional adenoma | Serrated adenoma | ||
|---|---|---|---|---|
| Type I | Type II | |||
| (n=19) | (n=50) | (n=19) | (n=43) | |
| Sex (M:F) | 16:3 | 38:12 | 14:5 | 34:9 |
| Age (yr) | ||||
| Range | 35–71 | 26–80 | 34–71 | 23–82 |
| Mean | 52.8 | 56.5 | 54.5 | 57.8 |
| Size (mm) | ||||
| Range | 3–11 | 2–34 | 3–15 | 3–41 |
| Median | 4 | 7 | 6 | 12 |
| Location | ||||
| Rectosigmoid | 11 | 24 | 12 | 25 |
| Descending | 3 | 4 | 2 | 4 |
| Transverse | 4 | 8 | 1 | 10 |
| Ascending | 1 | 12 | 3 | 3 |
| Cecum | 0 | 2 | 1 | 1 |
Immunohistochemistry
One paraffin block was selected from each case, based on good morphological preservation. Immunostaining was performed using the labeled streptavidin-biotin (Histofine SAB-PO kit, Nichirei Co., Tokyo, Japan) technique. Antibodies employed were monoclonal anti-p21WAF1/CIP1 (clone Ab-1, 1:200 dilution, CALBIOCHEM, Cambridge, MA) and monoclonal anti-p27Kip1 (clone 57, 1:400 dilution, Transduction Laboratories, Lexington, KY). All staining was accomplished with a microwave antigen retrieval system.
Briefly, after deparaffinization, 4-μm-thick slides were heated in 10 mM citrate buffer (pH 6.0) for 15 min using a microwave oven and then incubated overnight at 4°C with an optimum dilution of primary antibodies. Negative controls were prepared by processing the sections in the same manner except for one point: omission of the primary antibodies. Hydrogen peroxide and diaminobenzidine were employed for the colorization step. Slides were faintly counterstained with Mayer's hematoxylin for microscopic examination. Only strong (definite) nuclear staining was considered a positive reaction for p21WAF1/CIP1 and p27Kip1. Cytoplasmic accumulation of the proteins was not taken into consideration.
Indices for apoptotic activity and p21WAF1/CIP1 and p27Kip1 immunoreactivity
Detection of apoptotic cells in H&E stained sections was performed under high-power view, applying the standard morphological criteria: overall shrinkage and homogeneously dark basophilic nuclei, presence of nuclear fragments (apoptotic bodies), sharply delineated cell borders surrounded by empty spaces and homogeneous eosinophilic cytoplasm (Fig. 3; Kerr et al. 1972). We chose not to use the terminal deoxynucleotidyl transferase-mediated dUTP (deoxyuridine triphosphate)-biotin nick end-labeling method (i.e., the TUNEL method) because it detects not only apoptotic but also necrotic cells (Gavrieli et al. 1992). The conventional morphologic criteria of apoptotic cells have also been reported to be more reliable (Ansari et al. 1993). In addition, our previous study (Akino et al. 2002) showed a significant positive correlation between immunohistochemical analysis of single-stranded DNA that detects early-stage apoptosis (Frankfurt et al. 1996) and the conventional morphologic assessment that we used here.
Fig. 3.
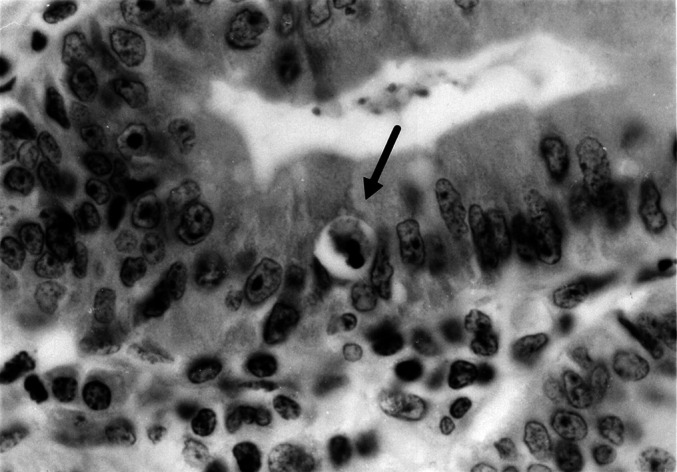
An apoptotic body (indicated by an arrow) in an SA (hematoxylin and eosin; original magnification ×400)
To assess the distribution of apoptotic or immunoreactive cells, the vertically oriented glands were separated into three equal portions [upper, middle, and lower thirds, representing respectively the maturation zone, the post-proliferative and early differentiative compartment, and the proliferative compartment of the colonic epithelium (Levine and Haggitt 1992)] using an ocular micrometer (scale: 10/10 mm squares). For evaluation of the apoptotic indices, and p21WAF1/CIP1 and p27Kip1 labeling indices, the numbers of apoptotic or immunoreactive cells were counted at ×400 magnification per 1,000 epithelial cells in at least five randomly chosen fields of each zone (totals of 3,000 cells counted).
All slides were independently evaluated twice by the same observer (M.S.), who was unaware of clinicopathological information and of the results of the other analyses. Subsequently, intra-observer disagreement (<10% of the case) was reviewed, followed by a conclusive judgment. Results are expressed as median percentages with interquartile ranges (IQRs).
Statistical analyses
Statview software (Abacus Concepts, Berkeley, CA) was used for all statistical analyses. Comparisons between groups were made with the Kruskal-Wallis test. Associations of pairs of indices among apoptotic activity and immunolabeling were analyzed using Spearman's correlation coefficient. A P value less than 0.05 was considered to indicate statistical significance.
Results
Apoptotic indices
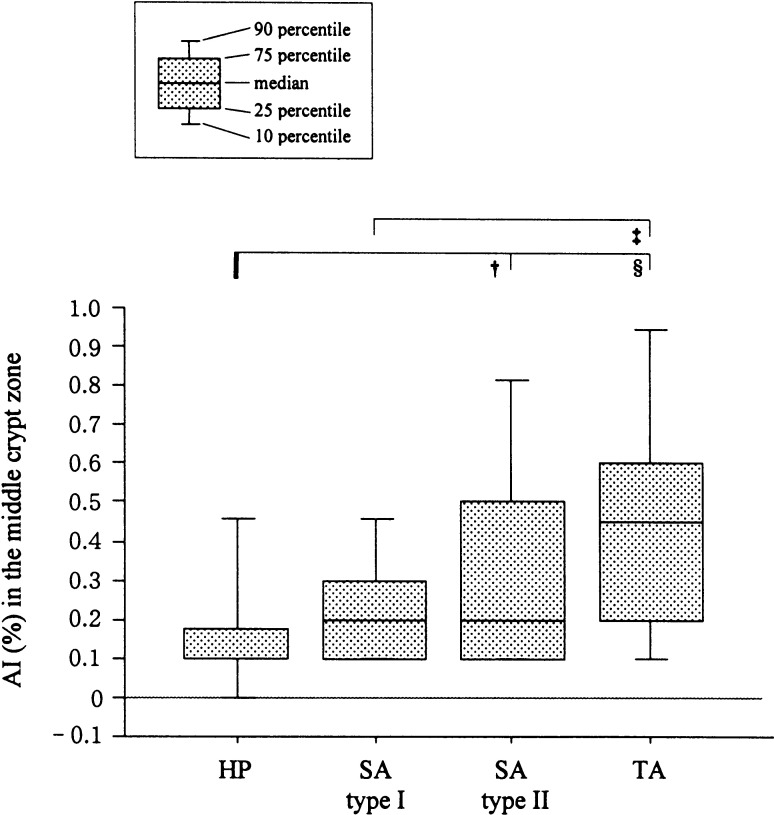
Among the groups studied, differences in apoptotic indices (AIs) were apparent in the middle, representing the post-proliferative and early differentiative compartment, but not the upper or lower crypt zones. As shown in Fig. 4, the AIs in the middle zone for type II SAs with LGD (median 0.2%, IQRs 0.1–0.5%) were significantly higher than those for HPs (0.1%, 0.1–0.18%, P<0.01) whereas those for type I SAs (0.2%, 0.1–0.3%) were lower than those for TAs with LGD (0.45%, 0.2–0.6%; P<0.001). No significant differences were observed between type I and II SAs with LGD.
Fig. 4.
Comparison of AIs for the middle crypt zones of SAs, HPs, and TAs. †, P<0.01; ‡, P<0.001; §, P<0.0001
In all types of SAs and HPs, apoptotic bodies were more frequent in the upper crypt zones than in the middle or lower zones. On the other hand, in TAs, apoptosis was observed equally in each crypt zone (data not shown).
p21WAF1/CIP1 labeling indices
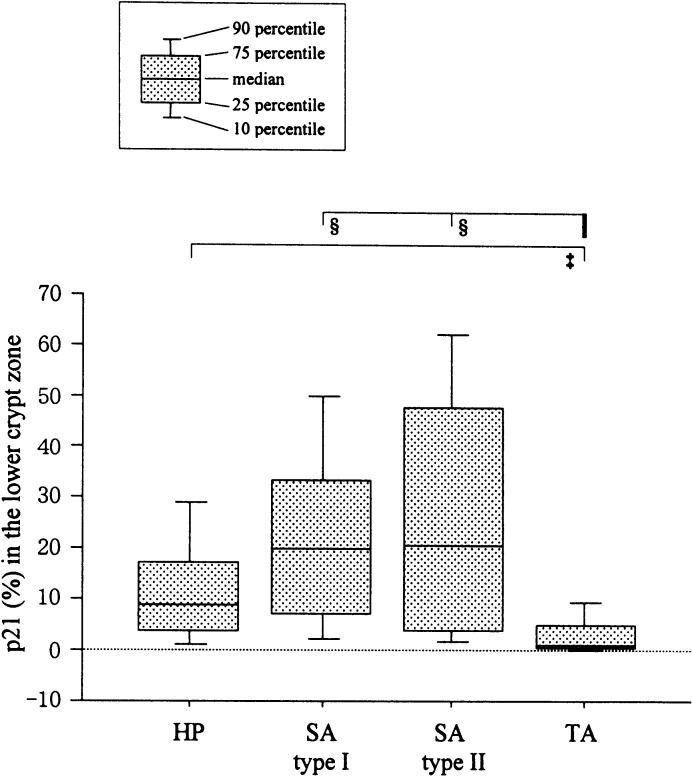
For the p21WAF1/CIP1 labeling indices (LIs), differences among the groups were observed in each crypt zone, but were largest in the lower crypt zone, representing the proliferative compartment (Fig. 5). The p21 LIs in the lower zone for type I SAs (median 19.8%, IQRs 7.0–33.2%) and type II SAs (20.4%, 3.9–47.8%) were significantly higher than those for TAs (1.2%, 0.6–5.2%, P<0.0001). A similar tendency was observed in the middle crypt zone. The values for HPs (8.9%, 3.9–17.2%) tended to be lower than those for SAs, but there was no significant difference.
Fig. 5.
Comparison of p21WAF1/CIP1 labeling indices (p21 LIs) for the lower crypt zones of SAs, HPs, and TAs. ‡, P<0.001; §, P<0.0001
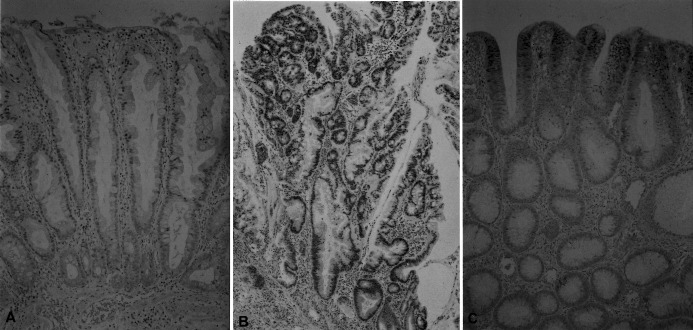
In SAs, p21WAF1/CIP1 positive cells in the lower crypt seemed to be equally dense as those in the upper and middle crypts. In TAs and HPs, however, the p21WAF1/CIP1 immunoreactivities gradually decreased from the upper crypt, through the middle to the lower zone (Fig. 6). No significant correlations between AIs and p21 LIs were demonstrable (data not shown).
Fig. 6A–C.
Immunohistochemical analysis of p21WAF1/CIP1 expression in an HP, an SA, and a TA. A HP demonstrating immunoreactive cells mostly in the upper and middle thirds of crypts (immunoperoxidase; original magnification ×160). B SA (type II) displaying p21WAF1/CIP1 staining extending from the upper to the lower zone of the crypts (immunoperoxidase; original magnification ×80). C p21WAF1/CIP1-positve nuclei in the TA are localized to the superficial region (immunoperoxidase; original magnification ×80)
p27Kip1 labeling indices
No differences in the p27Kip1 LIs were apparent among lesion types and values were almost the same for the upper, middle, and lower crypt zones. There was no correlation with AIs among the compared categories (data not shown).
Discussion
The present study revealed elevated expression of p21WAF1/CIP1 in SAs as compared to TAs and HPs, with an intermediate level of apoptosis. Earlier studies have suggested that among SAs, type I mimics HPs, and type II closely mimics adenomatous polyps (Longacre and Fenoglio-Preiser 1990; Rubio and Jaramillo 1996; Ban 1999). Ban (1999) presented two atypical HPs in which Ki-67-positive cells were extended to the upper portion of the crypt and cytokeratin 20-positive cells were distributed in a mosaiclike fashion, similar to patterns of TA. These findings suggest that such polyps reveal a neoplastic nature and fall into the entity of SA. Ajioka et al. (1998) mentioned that atypical HP may belong within the spectrum of SA, and that further histological subclassification of SA may be needed. Thus, in the present study, we classified the atypical hyperplastic polyp, possibly a distinct neoplastic entity, as type I SA.
In our preliminary study of 127 SAs (Sada et al., unpublished work), 53 (41.7%) were type I and 52 (41.0%) were type II, the remaining 22 (17.3%) being admixes of types I and II. From our careful histologic evaluation of this admixed type (I+II), it would appear that the replacement of serrated epithelium by dysplastic cells gradually takes place from the base of the crypts towards the superficial aspect of the mucosa. Type I SAs were smaller and were more often found in the ascending colon and cecum in comparison with type II lesions. Although type II SAs were frequently sessile and reddish polyps with a cerebriform surface pattern, some type I lesions were flat-elevated in morphology with no reddish color and a speckled surface pattern, resembling otherwise typical HPs, in line with previous reports (Jaramillo et al. 1996; Matsumoto et al. 1999b). In addition, our findings showed that almost all (98%) type I SAs exhibited LGD whereas 26% of type II SAs featured HGD. Furthermore, invasive carcinomas (1.6%) were encountered and were derived only from type II SAs with HGD. In the present study, the apoptotic activity of type I SAs tended to be nearly equal to that of HPs whereas the value of type II lesions was close to that of TAs. No significant differences, however, were evident between the two types of SA. SAs have significantly more frequent p53 gene mutations compared with hyperplastic lesions or tubular adenomas (Hiyama et al. 1998). In addition, the frequency of K-ras codon 12 mutation was significantly lower than that of tubular adenoma (Ajioka et al. 1998). Accordingly, further studies such as genetic analyses may be required to distinguish the two subtypes of SA.
In our earlier report, the Ki-67 positive rate in SAs was documented to be significantly higher than in HPs but lower than in TAs (Kang et al. 1997), in agreement with other authors (Ban 1999; Rubio and Rodensjö 1995a; Fujishima 1996). In the present study, the apoptotic activity of SAs was situated between that of HPs and TAs, similar to proliferative activity. Furthermore, apoptotic bodies were most frequently detected in the upper crypt zone, representing the maturation compartment, in SAs as well as HPs, but not in TAs. In normal colonic mucosa, apoptotic cells are frequently observed in the upper third of the crypt (Strater et al. 1995; Tateyama et al. 2002). Thus, the epithelial cells of SAs and HPs may undergo apoptosis depending on similar regulation in normal mucosa. Tateyama et al. (2002) reported that apoptotic activity, particularly in the upper and middle crypt zones, of SAs and HPs was significantly lower than in TAs. They also mentioned that the reduced apoptotic activity may be related to the characteristic saw-toothed appearance of SAs and HPs.
In normal colorectal mucosa, p21WAF1/CIP1 expression is seen in terminally differentiated cells in the uppermost third of crypts, and cells in the proliferative compartment are negative (Sasaki et al. 1996; Doglioni et al. 1996; Viale et al. 1999). In addition, p21WAF1/CIP1 is known to be expressed in fully differentiated columnar epithelium of the mouse gastrointestinal tract (Parker et al. 1995). In contrast, p27Kip1 is expressed in cells along the entire length of the normal crypts (Ciaparrone et al. 1998). In our immunohistochemical study, p21WAF1/CIP1 immunoreactivities in the lower crypt seem to be equally dense as those in the upper and middle crypts in SAs, but not HPs. Interestingly, p21WAF1/CIP1 expression was found to be higher in SAs than in TAs, suggesting that the SA reflects neoplastic transformation of a more differentiated cell in the crypt, in line with an earlier description (Longacre and Fenoglio-Preiser 1990). p21WAF1/CIP1 and p27Kip1 proteins are negative regulators of the cell cycle (Cordon-Cardo 1995), but in our series, neither protein was correlated with apoptotic activity in SAs or other lesions. The reason is unclear, but it could be that other pathways are more important for cell cycle control in these tumors. Sinicrope et al. (1998) also mentioned a lack of any clear relationship between p21WAF1/CIP1 and apoptotic rates in colorectal adenomas and carcinomas. Indeed, p21WAF1/CIP1 induction alone is reported to be insufficient to promote apoptosis (Kobayashi et al. 1995).
In conclusion, differences in apoptotic activity and cell cycle regulator protein p21WAF1/CIP1 expression between SAs and TAs or HPs indicate that the SA should be considered as a distinct subtype of colorectal epithelial neoplasm. Although the concept of SA has been generally accepted, its diagnosis may not be straightforward (Ban 1999). Thus, we would like to stress the role of morphologic inspection of apoptosis and p21WAF1/CIP1 immunostaining in distinguishing SA from the more common colorectal polyp, especially the HP; p21WAF1/CIP1-positive cells in the lower crypt seem to be equally dense as those in the upper crypt in SAs, but not HPs. Further studies such as genetic analyses may be required to distinguish type I SAs from type II lesions because no significant differences were evident between subtypes in the present study.
Acknowledgments
We thank H. Shinoda, K. Yamashita, M. Yokozawa, K. Kubokawa, I. Yokoyama, T. Tatebayashi, T. Kuba, S. Yoshida, and T. Takahashi, Kitasato University East Hospital, for their expert technical assistance.
This work is supported in part by Health and Labour Sciences Research Grants for Research on Allergic Diseases and Immunology (A.M.).
Footnotes
Drs. Mitomi and Sada contributed equally to this study.
References
- Ajioka Y, Watanabe H, Jass JR, Yokota Y, Kobayashi M, Nishikura K (1998) Infrequent K-ras codon 12 mutation in serrated adenomas of human colorectum. Gut 42:680–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akino F, Mitomi H, Nakamura T, Ohtani Y, Ichinoe M, Okayasu I (2002) High apoptotic activity and low epithelial cell proliferation with underexpression of p21WAF1/CIP1 and p27Kip1 of mucinous carcinomas of the colorectum. Comparison with well-differentiated type. Am J Clin Pathol 117:908–915 [DOI] [PubMed] [Google Scholar]
- Ansari B, Coates PJ, Greenstein BD, Hall PA (1993) In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol 170:1–8 [DOI] [PubMed] [Google Scholar]
- Ban S (1999) Small hyperplastic polyps of the colorectum showing deranged cell organization: a lesion considered to be a serrated adenoma? Am J Surg Pathol 23:1158–1160 [DOI] [PubMed] [Google Scholar]
- Ciaparrone M, Yamamoto H, Yao Y, Sgambato A, Cattoretti G, Tomita N, Monden T, Rotterdam H, Weinstein IB (1998) Localization and expression of p27KIP1 in multistage colorectal carcinogenesis. Cancer Res 58:114–122 [PubMed] [Google Scholar]
- Cordon-Cardo C (1995) Mutation of cell cycle regulators. Biological and clinical implications for human neoplasia. Am J Pathol 147:545–560 [PMC free article] [PubMed] [Google Scholar]
- Doglioni C, Pelosio P, Laurino L, Macri E, Meggiolaro E, Favretti F, Barbareschi M (1996) p21/waf1/cip1 expression in normal mucosa and in adenomas and adenocarcinomas of the colon: its relationship with differentiation. J Pathol 179:248–253 [DOI] [PubMed] [Google Scholar]
- Frankfurt OS, Robb JA, Sugarbaker EV, Villa L (1996) Monoclonal antibody to single-stranded DNA is a specific and sensitive cellular marker of apoptosis. Exp Cell Res 226:387–397 [DOI] [PubMed] [Google Scholar]
- Fujishima N (1996) Proliferative activity of mixed hyperplastic adenomatous polyp/serrated adenoma in the large intestine, measured by PCNA (proliferating cell nuclear antigen). J Gastroenterol 31:207–213 [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama T, Yokozaki H, Shimamoto F, Haruma K, Yasui W, Kajiyama G, Tahara E (1998) Frequent p53 gene mutations in serrated adenomas of the colorectum. J Pathol 186:131–139 [DOI] [PubMed] [Google Scholar]
- Jaramillo E, Watanabe M, Rubio C, Slezak P (1996) Small colorectal serrated adenomas: endoscopic findings. Endoscopy 28:1–3 [DOI] [PubMed] [Google Scholar]
- Jass JR, Sobin LH (1989). WHO histological typing of intestinal tumours, 2nd edn. Springer, Berlin Heidelberg New York
- Kang M, Mitomi H, Sada M, Tokumitsu Y, Takahashi Y, Igarashi M, Katsumata T, Okayasu I (1997) Ki-67, p53, and Bcl-2 expression of serrated adenomas of the colon. Am J Surg Pathol 21:417–423 [DOI] [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Consoli U, Andreeff M, Shiku H, Deisseroth AB, Zhang W (1995) Activation of p21WAF1/Cip1 expression by a temperature-sensitive mutant of human p53 does not lead to apoptosis. Oncogene 11:2311–2316 [PubMed] [Google Scholar]
- Levine DS, Haggitt RC (1992) Colon. In: Sternberg SS, ed. Histology for pathologists. Raven Press, New York
- Longacre TA, Fenoglio-Preiser CM (1990) Mixed hyperplastic adenomatous polyp/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol 14:524–537 [DOI] [PubMed] [Google Scholar]
- Macleod KF, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T (1995) p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev 9:935–944 [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Mizuno M, Shimizu M, Manabe T, Iida M (1999a) Clinicopathological features of serrated adenoma of the colorectum: comparison with traditional adenoma. J Clin Pathol 52:513–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Mizuno M, Shimizu M, Manabe T, Iida M, Fujishima M (1999b) Serrated adenoma of the colorectum: colonoscopic and histologic features. Gastrointest Endosc 49:736–742 [DOI] [PubMed] [Google Scholar]
- Michieli P, Chedid M, Lin D, Pierce JH, Mercer WE, Givol D (1994) Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res 54:3391–3395 [PubMed] [Google Scholar]
- Parker SB, Eichele G, Zhang P, Rawls A, Sands AT, Bradley A, Olson EN, Harper JW, Elledge SJ (1995) p53-independent expression of p21cip1 in muscle and other terminally differentiating cells. Science 267:1024–1027 [DOI] [PubMed] [Google Scholar]
- Polyak K, Kato J, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A (1994) p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev 8:9–22. [DOI] [PubMed] [Google Scholar]
- Rubio CA, Jaramillo E (1996) Flat serrated adenomas of the colorectal mucosa. Jpn J Cancer Res 87:305–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio CA, Rodensjö M (1995a) Flat serrated adenomas and flat tubular adenomas of the colorectal mucosa: differences in the pattern of cell proliferation. Jpn J Cancer Res 86:756–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio CA, Rodensjö M (1995b) p53 overexpression in flat serrated adenomas and flat tubular adenomas of the colorectal mucosa. J Cancer Res Clin Oncol 121:571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Sato T, Kurose A, Ikeda E (1996) Immunohistochemical detection of p21waf1/cip1/sdi1 and p53 proteins in formalin-fixed, paraffin-embedded tissue sections of colorectal carcinoma. Hum Pathol 27:912–916 [DOI] [PubMed] [Google Scholar]
- Sinicrope FA, Roddey G, Lemoine M, Ruan S, Stephens LC, Frazier ML, Shen Y, Zhang W (1998) Loss of p21WAF1/Cip1 protein expression accompanies progression of sporadic colorectal neoplasms but not hereditary nonpolyposis colorectal cancers. Clin Cancer Res 4:1251–1261 [PubMed] [Google Scholar]
- Strater J, Koretz K, Gunthert AR, Moller P (1995) In situ detection of enterocytic apoptosis in normal colonic mucosa and in familial adenomatous polyposis. Gut 37:819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateyama H, Li W, Takahashi E, Miura Y, Sugiura H, Eimoto T (2002) Apoptosis index and apoptosis-related antigen expression in serrated adenoma of the colorectum. The saw-toothed structure may be related to inhibition of apoptosis. Am J Surg Pathol 26:249–256 [DOI] [PubMed] [Google Scholar]
- Torlakovic E, Snover DC (1996) Serrated adenomatous polyposis in humans. Gastroenterology 110:748–755 [DOI] [PubMed] [Google Scholar]
- Viale G, Pellegrini C, Mazzarol G, Maisonneuve P, Silverman ML, Bosari S (1999) p21WAF1/CIP1 expression in colorectal carcinoma correlates with advanced disease stage and p53 mutations. J Pathol 187:302–307 [DOI] [PubMed] [Google Scholar]
- Yao T, Kouzuki T, Kajiwara M, Matsui N, Oya M, Tsuneyoshi M (1999) 'Serrated adenoma' of the colorectum, with reference to its gastric differentiation and its malignant potential. J Pathol 187:511–517 [DOI] [PubMed] [Google Scholar]