Abstract
The purpose of this study was to collect initial data to determine the potential clinical usefulness of a 13C-aminopyrine demethylation blood test, and whether additional clinical investigation is warranted. Six dogs, initially suspected of having hepatic disease based on their history, physical examination, imaging studies, general laboratory parameters, or any combination of the above, were enrolled in the study. A baseline blood sample was collected, 2 mg/kg 13C-aminopyrine was administered intravenously, and another blood sample was collected 45 min afterwards. Carbon dioxide was extracted from the blood samples and analyzed using fractional mass spectrometry. Results from the 13C-aminopyrine demethylation blood test were compared to clinical data and histologic findings. Intravenous administration of 13C-aminopyrine leads to a decrease in the percent dose of 13C recovered from dogs with histologically confirmed liver disease. Based on our results, a full-scale investigation of the potential clinical usefulness of a 13C-aminopyrine demethylation blood test for assessment of hepatic function in dogs is warranted.
Introduction
Primary or secondary hepatic disorders are common in dogs. Conclusive diagnosis and staging of disease severity are difficult. Histologic examination, which requires an invasive procedure to obtain a biopsy specimen, has been considered the gold standard for the diagnosis of hepatic disease. However, evaluation of hepatic histopathology is highly subjective and different diagnoses are often given for the same specimen by different pathologists (1). Serum chemistry profile includes several parameters that can be used to evaluate the liver, but none of these parameters are sensitive or specific to assess hepatic function. Abdominal palpation and radiography can reveal hepatomegaly or micro hepatica (2,3). However, hepatomegaly may or may not be associated with altered hepatic function, and several extra-hepatic disorders cause hepatomegaly without decreasing hepatic function. Serum activities of hepatic enzymes are analyzed as markers for hepatocellular damage (2,4). Unfortunately, some of these enzymes are also synthesized by cells of other tissues. Non-hepatic disorders, such as hyperadrenocorticism, can cause hepatomegaly and increases in hepatic enzymes without the loss of hepatic function (3). Abdominal ultrasonography is an excellent diagnostic tool for non-invasive evaluation of hepatic morphology, but does not allow for the determination of hepatic function (5). Finally, end stage disease may be manifested by decreased serum albumin, blood urea nitrogen, and cholesterol concentrations, or may be associated with coagulopathies. However, these parameters are rather insensitive as the liver has an enormous functional reserve and only end-stage disease will cause significant changes. Also, there are many other causes of hypoalbuminemia. In fact most dogs with hypoalbuminemia do not have hepatic failure, but rather protein-losing enteropathy, blood loss, protein-losing nephropathy, or systemic inflammation (2).
Currently, only one clinically significant hepatic function test is available to determine pre- and postprandial serum bile acid concentrations. Historically, this test has been considered sensitive and specific for loss of hepatic function (4). But clinically, bile acids lack specificity, as they cannot be used to distinguish loss of hepatic function from cholestasis (4). Dogs with hepatic or posthepatic biliary obstruction have increased serum bile acid concentrations, yet may have unaltered hepatic function (6,7,8). Also, recently clinically healthy research beagles with serum folate concentrations indicative of small intestinal bacterial overgrowth have been shown to have increased serum unconjugated cholic acid concentrations that are high enough to lead to a significant increase in serum total bile acid concentrations (9). Other quantitative hepatic function tests have been investigated, but have little use clinically because they lack sensitivity (10,11). For example, production of clotting factors is decreased in dogs with altered hepatic function. However, this change occurs very late in the course of hepatic disease. Early diagnosis of hepatic dysfunction is essential to allow successful intervention. A 13C-aminopyrine demethylation breath test has been shown to be both sensitive and specific for identifying hepatic dysfunction in human beings and laboratory animals, and could be very useful in assessing hepatic dysfunction in dogs and cats in a clinical setting (12,13,14,15,16).
Several studies have shown that the 13C-aminopyrine breath test (ABT) is a useful indicator of hepatic function in human patients with chronic hepatitis or cirrhosis (12,13,14,17,18). One study showed that ABT correlated well with disease severity in 21 patients with histologically confirmed alcoholic hepatitis (19). Microsomal enzymes in the liver demethylate aminopyrine, a compound chemically similar to the nonsteroidal anti-inflammatory drugs phenylbutazone and antipyrine. The liberated methyl groups are oxidized to CO2, diffuse into the blood stream, reach the pulmonary alveoli, and are released into the expiratory air (15,19,20). The administration of aminopyrine labeled with a 13C isotope allows for the specific measurement of CO2 derived from hepatic microsomal aminopyrine demethylation by detection of the fraction of the 13CO2 in expiratory air (21). The amount of 13CO2 measured is compared to the amount of 13C administered as 13C-aminopyrine and expressed as percent dose (PCD) (22). Initially, aminopyrine was administered orally for the ABT. However, some studies indicate that gastric emptying might significantly influence the peak time of the labeled CO2 fraction in the expiratory air (23,24). In order to eliminate this possible error, other investigators administered aminopyrine by intravenous injection (25,26,27). The administration of 13C-aminopyrine to clinically healthy dogs is considered to be safe (25,26,27). The purpose of this study was to collect initial data to determine the potential clinical usefulness of a 13C-aminopyrine demethylation blood test in dogs with suspected hepatic disease, to further determine if a full scale clinical evaluation is warranted.
Materials and methods
The protocol was reviewed and approved by the Institutional Animal Care and Use Committee of The Caspary Institute, The Bobst Hospital, The Animal Medical Center, New York, USA. Dogs were prospectively included in the study if they had a medical workup consistent with possible hepatic disease based on history, physical examination, imaging studies, and/or clinical pathology, and a hepatic biopsy was indicated and approved by the owner to confirm the presence of significant hepatic disease. Dogs were not included if only a fine needle aspirate was obtained. All dogs had a complete blood count, biochemical profile, and coagulation panel. Urine was analyzed when available. Prior to enrolling their dog, owners received comprehensive information regarding the study and were required to sign an informed consent document.
Hepatic tissue was obtained by ultrasound guided needle biopsy (16 or 18 gauge), during exploratory surgery, or at necropsy. The hepatic tissue was fixed in buffered formalin, embedded in paraffin, stained with hematoxylin and eosin, and read under routine light microscopy. Histological changes in the liver were interpreted independent of physical exam, laboratory evaluation, or history. Hepatic changes were classified into 6 categories: biliary proliferation, cholestasis, necrosis, inflammation, fibrosis, and neoplasia. Medical history was evaluated to determine if dogs were treated with enzyme inducing drugs (such as glucocorticoids or phenobarbital) in the immediate pre-test period, in order to evaluate any effect the enzyme would have on the hepatic demethylating capacity of 13C-aminopyrine. Pre- and postprandial serum bile acid concentrations were measured by spectrophotometric assay. A 13C-aminopyrine demethylation blood test was performed on each dog.
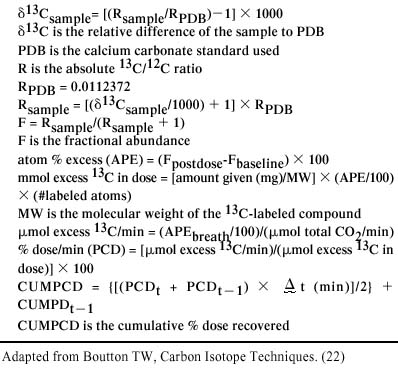
Briefly an intravenous catheter was placed aseptically and a 1 mL base-line blood sample was collected. A 10 mL sodium heparin tube (VWR Scientific Products, West Chester, Pennsylvania, USA) containing 1 mL of 6-N hydrochloric acid was vortexed while the sample was added. 13C-aminopyrine (4-dimethyl-13C2-aminoantipyrine; Isotech, Miamisburg, Ohio, USA) was administered intravenously at 2 mg/kg over 1 min and the 2nd blood sample was collected 45 min after 13C-aminopyrine administration, based on previous data. The dogs were monitored for any adverse effects throughout the time period during which the samples were obtained and for several hours following the testing procedure. The samples were placed on ice and shipped overnight to The Gastrointestinal Laboratory at Texas A&M University, Texas, USA. Fractional 13C concentration was analyzed by fractional mass spectrometry using an automated breath carbon analyzer (ABCA; Europa House, Crewe, United Kingdom). A series of calculations were performed to calculate the PCD. Calculations are detailed in Figure 1. All biopsies were reviewed by a single pathologist (KB). Hepatic lesions were characterized and scored. Each biopsy was examined blindly and evaluated subjectively for 6 characteristics: cholestasis, inflammation, biliary proliferation, fibrosis, necrosis, and neoplasia. On the basis of severity of the lesion, each characteristic was evaluated by a scoring system (0 = normal, 1 = mild, 2 = moderate, 3 = severe).
Figure 1. Formula for calculation of percent dose (PCD). Percent dose belemnitella (PDB) is the calcium carbonate standard used. The standard was a limestone fossil of Belemnitella americana from the Cretaceous Pee Dee formation in South Carolina. The standard has been assigned a δ13C-value of 0% and the absolute 13C/12C-ratio (R) has been reported to be 0.0112372. The δ13Csample is the relative difference of the sample to PDB. The 13Csample indicates whether the sample is higher or lower than PDB, with a negative indicating that the sample is lower than PDB. The fractional abundance (F) is useful in isotopic mass calculations. Atom % is also used and is the expression of the isotopic enrichment in samples highly enriched with 13C. Atom % excess (APE), which is used in these calculations, is the enrichment level of a sample following administration of a 13C tracer in excess of the 13C background or baseline level prior to administration of the tracer. These values were used to calculate both mmol excess 13C in dose and μmol excess 13C/min. The PCD is calculated directly from these 2 values, as indicated in the formula for PCD (24).
Results
Table I shows the demographic data, laboratory values, imaging, diagnosis, and a measurable increase of PCD in the gas extracted from all of the dogs. None of the dogs showed any side effects after intravenous injection of 13C-aminopyrine. Also, the owners did not report any problems during the week following the study. Histopathology was obtained from the livers of all 6 dogs and graded (Table II). Four dogs had biopsies obtained percutaneously, 1 had liver tissue obtained at abdominal exploratory surgery, and the final dog's biopsy was taken at necropsy. All dogs had a normal complete blood cell count or showed a stress response. Five of the 6 dogs had urinalyses; the results of which did not contribute any additional information to this study.
Table I.
Table II.
Discussion
This is the first study involving a 13C-aminopyrine demethylation blood test in dogs with suspected hepatic dysfunction. Previous studies involved only clinically healthy dogs (25,26,27). As in the previous studies, no side effects were observed after administration of 13C-aminopyrine. This, however, does not allow a conclusive statement about the safety of intravenous 13C-aminopyrine administration. Such a statement could only be made after extensive studies of functional and morphologic parameters in patients after administration of 13C-aminopyrine. However, the study reported here does not suggest that intravenous 13C-aminopyrine causes any gross side effects in dogs with suspected hepatic dysfunction. For ABT testing in human patients with hepatic cirrhosis, a dose of 2 mg/kg 13C-aminopyrine was used without any reported side effects (19).
The use of 13C labeled compounds has been successful in the diagnosis of Helicobacter infection in both human beings and dogs (28). In human beings, the 13C-urea breath test is a non-invasive test that has high specificity and sensitivity in the diagnosis of Helicobacter pylori. A similar test has been evaluated in the dog and involves oral administration of a test meal containing 13C urea. 13CO2 is produced from urea by the action of Helicobacter urease. The 13CO2 diffuses into the blood and is measured in expired air, just as 13CO2 is released by demethylation of 13C-aminopyrine in the liver and is measured in the blood (23,25,26,27,29,30,31). A blood test eliminates the need for breath collection, as breath samples are more difficult to obtain in dogs than in human beings. Dogs may be anxious about a mask being placed over their muzzle, which can cause tachypnea, thus influencing test results. Breed and anatomical differences between dogs complicates the collection of the expired air due to leaks, which can occur at the commisures of the mouth. Consequently, in dogs, a 13C-aminopyrine demethylation blood test is likely to be more accurate than a breath test to assess hepatic function.
13C-aminopyrine is demethylated in the liver and the 13C is transferred to 13CO2. In the bloodstream, the 13CO2 is transported by 3 mechanisms, as bicarbonate, bound to hemoglobin, or dissolved in the blood. When it reaches the pulmonary circulation, it is released as gaseous CO2 in the expiratory air (32). The CO2 transported in the blood can be extracted from blood samples by adding a strong acid. This shifts the equilibrium reaction, HCO3- + H+ · H2O + CO2, to the right (33). Since an excess of hydrogen ions (strong acid) is added to the blood sample, the equilibrium shifts far to the right, releasing all of the CO2 that is dissolved (32). This reaction works equally well for 13CO2 and makes assessment of hepatic function by determination of 13CO2 in gas extracted from blood possible.
Dogs enrolled in this study showed increased, normal, or decreased 13C-aminopyrine demethylation. Cases 1 and 2 showed enhanced demethylation, cases 3 and 4 had normal demethylation, and cases 5 and 6 showed decreased demethylation.
Case 1 was a dog undergoing phenobarbital loading (2.2 mg/kg IV TID) for the treatment of intense, acute onset seizures and was included in the study because the abdominal ultrasound revealed a mottled liver. Hepatic enzyme induction has been reported to develop following anticonvulsant therapy (2). For example, during a 30-day trial, administration of phenobarbital (4.4 mg/kg TID) to normal dogs produced serum enzyme activities that reached a peak of 30-fold by day 24, which then declined (2). Case 1 received 2.2 mg/kg IV TID of phenobarbital for 72 h prior to 13C-aminopyrine demethylation testing, which is a relatively low dose and short duration for phenobarbital administration than described for the dogs in the study cited. Despite the short duration of phenobarbital therapy, we were able to demonstrate an increased aminopyrine demethylation capacity, which we believe to be indicative of hepatic enzyme induction. Hepatic imaging showed a mottled appearance consistent with chronic active hepatitis. Hepatic histopathology demonstrated hepatocellular vacuolization, a nonspecific finding, which could be associated with phenobarbital administration and may be due to phenobarbital toxicity (34). This case showed minimal increases in serum hepatic enzyme activities (serum alkaline phosphatase was only slightly increased) and normal serum bile acid concentrations.
Our results suggest that the microsomal enzymes involved in hepatic aminopyrine demethylation are induced by phenobarbital administration. This would suggest that 13C-aminopyrine demethylation is not beneficial to assess hepatic function in dogs on phenobarbital therapy. However, it is speculated that 13C-aminopyrine demethylation could be useful in assessing hepatic function in dogs on chronic phenobarbital therapy if hepatic demethylation capacity is followed longitudinally. Detection of phenobarbitol-induced hepatotoxicity is complicated by the fact that phenobarbitol also induces serum alkaline phosphatase and serum alanine aminotransferase activities, but the increase is not necessarily associated with hepatotoxicity (35,36,37). Hepatic failure due to phenobarbital toxicity will only be recognized when decreases in albumin, cholesterol, and blood urea nitrogen are seen (35,36,37). However, at that point hepatic damage is usually extensive and recuperation of the patient may not be possible. Further studies are necessary in order to further assess 13C-aminopyrine demethylation capacity in dogs with chronic phenobarbital therapy.
Case 2 was also diagnosed with hepatocellular vacuolization, suspected to be due to high levels of endogenous glucocorticoids. Development of glucocorticoid hepatopathy can impair hepatic function (2). The proposed mechanism of hepatic dysfunction is impaired sinusoidal perfusion and diminished perisinusoidal space related to cellular distension. Daily dexamethasone phosphate therapy, 3 mg/kg intravenously, caused mean serum bile acid concentrations to increase from a normal of 1.0 μg/dL (± 0.0) to 19.2 μg/dL (± 23.8), within 12 d (38). The elevations of serum bile acid concentrations in case 2 are consistent with a diagnosis of vacuolar hepatopathy as well. The elevated PCD in this dog would suggest that, similar to serum activities of hepatic enzymes, 13C-aminopyrine is being induced by glucocorticoids. Further studies are needed in order to arrive at a final conclusion. However, these data would suggest that glucocorticoid therapy, cushing's disease, or both have to be excluded prior to using a 13C-aminopyrine demethylation blood test to evaluate hepatic function.
Cases 3 and 4 showed normal hepatic demethylation. Case 3 had a diagnosis of mild hepatocellular vacuolization along with normal serum bile acid concentrations, suggesting minimal to no hepatic dysfunction despite elevations in liver enzymes.
Microvascular dysplasia in case 4 was caused by a portosystemic vascular anomaly (PSVA), confirmed by a splenoportogram and again during abdominal exploratory surgery for ameroid ring placement. Portosystemic vascular anomaly would be expected to be associated with normal 13C-aminopyrine demethylation since it is not associated with decreased hepatic function but rather altered splanchnic blood flow to the liver.
The final 2 dogs in this series demonstrated a decreased 13C-aminopyrine demethylation. These dogs had the most abnormal liver biopsies found in any of the dogs enrolled in this study based on the histopathologic grading scale used (Table II). Case 5 was diagnosed with severe granulomatous hepatitis and case 6 with microvascular dysplasia with generalized hepatocellular cholestasis. Even though the serum bile acid concentrations were normal in both dogs, the 13C-aminopyrine demethylation blood test indicated that the demethylating capacity was decreased by over 60% compared to that of normal dogs. Measurement of serum bile acid concentrations is excellent to identify hepatoportal perfusion abnormalities typical for PSVA or cirrhosis (7). However, serum bile acid concentrations were normal in cases 5 and 6. The 13C-aminopyrine demethylation blood test measures the metabolic activity of hepatic cells and, thus, would be expected to be abnormal in cases where the metabolic activity of the hepatocytes was compromised by the underlying hepatic disease, as it was in cases 5 and 6.
Current laboratory tests available for the evaluation of the liver have limitations, which may be overcome by using a 13C-aminopyrine demethylation blood test. Elevations in serum alkaline phosphatase, alanine aminotransferase, and alanine aminoaspartate represent leakage or induction and not necessarily altered function. These serum enzyme activities can increase with trauma or hypoxia, but elevations indicate hepatic damage rather than the degree of hepatic function. There are many advantages to a 13C-aminopyrine demethylation blood test. The principle of the test is based on known reactions and the methodology is simple and clear. The test can be performed in a matter of hours. The ingredients are easy to obtain and the protocol requires only a few items that every veterinarian has access to in a typical veterinary hospital.
The information obtained from this study should be interpreted with caution due to the small sample size and the limited number of hepatic diseases studied, none of which had elevations of total bilirubin. Additionally, not all dogs had liver biopsies obtained during exploratory surgery and inaccuracies of percutaneous needle biopsies are well known in cases of patchy and lobar hepatic disease. A diffuse pattern of hepatic disease would be expected to yield a representative sample when a percutaneous biopsy is obtained (1).
In conclusion, we have shown that intravenous administration of 13C-aminopyrine leads to a decrease in the PCD in dogs with histologically confirmed liver disease. Based on our results, a full-scale investigation of 13C-aminopyrine demethylation as a test of hepatic function is warranted.
Footnotes
This study was supported by a grant from the Iams Company, Dayton, Ohio, USA.
Address all correspondence and reprint requests to Dr. Deirdre Chiaramonte; telephone: (212) 838-8100 ext. 8644; fax: (212) 753-9820; e-mail: Deirdre.Chiaramonte@amcny.org
Received November 4, 2002. Accepted February 19, 2003.
References
- 1.Cole TL, Center SA, Flood SN, et al. Diagnostic comparison of needle and wedge biopsy specimens of the liver in dogs and cats. J Am Vet Med Assoc 2002;220:1483–1489. [DOI] [PubMed]
- 2.Center SA. Diagnostic procedures for evaluation of hepatic disease. In: Guilford WG, Center SA, Strombeck DR, Williams DA, Myer DJ, eds. Strombeck's Small Animal Gastroenterology. 1996;3:130–188,782–788.
- 3.Feldman EC, Nelson RW. Hyperadrenocorticism. In: Feldman EC, Nelson RW, eds. Canine and Feline Endocrinology and Reproduction. 1996;2:187–265.
- 4.Center SA. Pathophysiology, laboratory diagnosis, and diseases of the liver. In: Ettinger SJ, Feldman EC, eds. Textbooks of Veterinary Internal Medicine 1995;4:1261–1312.
- 5.Cartee RE. Diagnostic real time ultrasonography of the liver of the dog and cat. J Am Anim Hosp Assoc 1981;17:731–737.
- 6.Center SA, Leveille CR, Baldwin BH, Tennant BC. Direct spectrometric determination of serum bile acids in the dog and cat. Am J Vet Res 1984;45:2043–2050. [PubMed]
- 7.Center SA. Serum bile acids in companion animal medicine. Veterinary Clinics of North Am: Small Anim Pract 1993;23:625–653. [DOI] [PubMed]
- 8.Rutgers HC, Stradley RP, Johnson SE. Serum bile acid analysis in dogs with experimentally induced cholestatic jaundice. Am J Vet Res 1988;49:317–320. [PubMed]
- 9.Williams DA, Ruaux CG, Steiner JM, Meyer D. Small intestinal bacterial overgrowth leading to high fasting serum total and unconjugated bile acids in beagle dogs with no evidence of hepatic disease. Proceedings of the 10th Congress of the International Society of Animal Clinical Biochemistry, Gainesville, Florida, 2002, pp 195.
- 10.Bircher J. Quantitive assessment of deranged hepatic function: a missed opportunity? Sem Liver Dis 1983;3:275–284. [DOI] [PubMed]
- 11.Center SA, Bunch SE, Baldwin BH, Hornbuckle WE, Tennant BC. Comparison of sulfobromophthalein and indocyanine green clearances in the dog. Am J Vet Res 1983;44:722–726. [PubMed]
- 12.Beyeler C, Reichen J, Thomann SR, Lauterburg BH, Gerber NJ. Quantitative liver function in patients with rheumatoid arthritis treated with low-dose methotrexate: a longitudinal study. Br J Rheum 1997;36:338–344. [DOI] [PubMed]
- 13.Miotti T, Bircher J, Preisig R. The 30-minute aminopyrine breath test: optimization of sampling times after intravenous administration of C-aminopyrine. Digestion 1988;39:241–250. [DOI] [PubMed]
- 14.Baker AL, Krager PS, Kotake AN, Schoeller DA. The aminopyrine breath test does not correlate with histologic disease severity in patients with cholestasis. Hepatology 1987;7:464–467. [DOI] [PubMed]
- 15.Henry DA, Kitchingman G, Langman MJ. Aminopyrine breath analysis and conventional biochemical tests as predictors of survival in cirrhosis. Digest Dis Sci 1985;30:813–818. [DOI] [PubMed]
- 16.Gikalov I, Bircher J. Dose dependence of the C-aminopyrine breath test. Intrasubject comparison of tracer and pharmacological doses. Eur J Clin Pharm 1977;12:229–233. [DOI] [PubMed]
- 17.Schneider JF, Baker AL, Haines NW, Hatfield G, Boyer JL. Aminopyrine N-demethylation: a prognostic test of liver function in patients with alcoholic liver disease. Gastroenterology 1980;79:1145–1150. [PubMed]
- 18.Baker AL, Kotake AN, Schoeller DA. Clinical utility of breath tests for the assessment of hepatic function. Sem Liv Dis 1983;3:318–329. [DOI] [PubMed]
- 19.Villeneuve JP, Rivard CI, Ampelas M, Layrargues GP, Huet PM, Marleau D. Prognostic value of the aminopyrine breath test in cirrhotic patients. Hepatology 1986;6:928–931. [DOI] [PubMed]
- 20.Schoeller DA, Kotake AN, Lambert GH, Krager PS, Baker AL. Comparison of the phenacetin and aminopyrine breath tests: effect of liver disease, inducers and cobaltous chloride. Hepatology 1985;5:276–281. [DOI] [PubMed]
- 21.Hoffmann WE, Renegar WE, Dorner JL. Serum half-life of intravenously injected intestinal and hepatic alkaline phoshphatase isoenzymes in the cat. Am J Vet Res 1977;38:1637–1638. [PubMed]
- 22.Boutton T. Tracer studies with 13C-enriched substrates: humans and large animals. In: Coleman AC, Fry B, eds. Carbon Isotope Techniques. New York: Academic Press 1991:219–242.
- 23.Cutler AF, Toskes P. Comparison of urea blood test to urea breath test for the diagnosis of Helicobacter pylori. Am J Gast 1999;94:959–961. [DOI] [PubMed]
- 24.Cutler A, Kretzschmer K. Confirmation of H. pylori eradication using the urea blood test. Gastroenterology 1999;116:142–142.
- 25.Steiner JM, Moeller EM, Klein P, et al. Kinetics of 13C-aminopyrine (AP) demethylation in healthy dogs. J Vet Int Med 2000;14:346.
- 26.Moeller EM, Steiner JM, Williams DA, Tetrick M, Burr J. Comparison of different doses of 13C-aminopyrine for a 13C-aminopyrine demethylation blood test in clinically healthy dogs. J Vet Int Med 2001;15:314.
- 27.Moeller EM, Steiner LA, Williams DA, Klein PD. Preliminary studies of a canine 13C-aminopyrine demethylation blood test. Can J Vet Res 2001;65:45–49. [PMC free article] [PubMed]
- 28.Cornetta AM, Simpson KW, Strauss-Ayali D, McDonough PL, Gleed RD. Use of a C-urea breath test for detection of gastric infection with Helicobacter spp. in dogs. Am J Vet Res 1998;59:1364–1369. [PubMed]
- 29.Moore LE. Recent advances in diagnostic tests for gastrointestinal disease. ACVIM Proceedings 2002;20:531–532.
- 30.Chey WD, Murthy U, Toskes P, Carpenter S, Laine L. The C-urea blood test accurately detects active Helicobacter pylori infection: a United States, multicenter trial. Am Med J Gast 1999;94:1522–1524. [DOI] [PubMed]
- 31.Monkemuller KE, Hortin G, Li T, Hirschowitz BI. Comparison of 13C-urea breath and blood tests for H. pylori. Gastroenterology 1998;114:232.
- 32.Sherwood L. The respiratory system. In: Human Physiology: From Cells to Systems. 1997;3:417–545.
- 33.Zumdahl S. Chemical equilibrium. In: Heath DC, ed. Chemical Principals. 1992:179–210.
- 34.Müller PB, Taboada J, Hosgood G, et al. Effects pf long-term phenobarbital treatment on the liver in dogs. J Vet Int Med 2000;14:165–171. [DOI] [PMC free article] [PubMed]
- 35.Boothe DM. Anticonvulsants and other neurologic therapies in small animals. In: Booth DM, ed. Small Animal Clinical Pharmacology and Therapeutics. New York: WB Saunders Company, 2001:439–441.
- 36.Trepanier LA. Hepatic drug metabolism in the dog and cat: what's relevant for the internist? ACVIM Proceedings 2002;20:666–668.
- 37.Trepanier LA. Mechanisms of drug-associated hepatotoxicity in the dog and cat. ACVIM Proceedings 2002;20:669–671.
- 38.DeNovo RC, Prasse KW. Comparison of serum biochemical and hepatic functional alterations in dogs treated with corticosteroids and hepatic duct ligation. Am J Vet Res 1983;44:1703–1709. [PubMed]