Abstract
Our primary goal was to generate an accurate estimate of the daily environmental loading rate of Cryptosporidium parvum oocysts for adult beef cattle, using immunomagnetic separation coupled with direct immunofluorescence microscopy for a highly sensitive diagnostic assay. An additional goal was to measure the prevalence and intensity of fecal shedding of C. parvum oocysts in pre- and postparturient cows as an indicator of their potential to infect young calves. This diagnostic method could detect with a ≥90% probability oocyst concentrations as low as 3.2 oocysts g of feces−1, with a 54% probability of detecting just one oocyst g of feces−1. Using this diagnostic method, the overall apparent prevalence of adult beef cattle testing positive for C. parvum was 7.1% (17 of 240), with 8.3 and 5.8% of cattle shedding oocysts during the pre- and postcalving periods, respectively. The mean intensity of oocyst shedding for test-positive cattle was 3.38 oocysts g of feces−1. The estimated environmental loading rate of C. parvum ranged from 3,900 to 9,200 oocysts cow−1 day−1, which is substantially less than a previous estimate of 1.7 × 105 oocysts cow−1 day−1 (range of 7.7 × 104 to 2.3 × 105 oocysts cow−1 day−1) (B. Hoar, E. R. Atwill, and T. B. Farver, Quant. Microbiol. 2:21-36, 2000). Use of this highly sensitive assay functioned to detect a greater proportion of low-intensity shedders in our population of cattle, which reduced the estimated mean intensity of shedding and thereby reduced the associated environmental loading rate compared to those of previous studies.
Cryptosporidium parvum has emerged as a ubiquitous waterborne microbial pathogen, with specific genotypes readily transmitted ambidirectionally between livestock and humans (7, 9, 12, 25, 28, 36). One of the first steps in designing watershed management programs for minimizing the occurrence of C. parvum in drinking water supplies is to identify significant quantitative sources of this parasite. Adult cattle are often considered potential nonpoint sources of environmental contamination for C. parvum, but there is some disagreement over the relative importance that adult cattle have in loading watersheds with significant amounts of C. parvum oocysts. In particular, there is a wide range of reported prevalences of fecal shedding of C. parvum for adult beef and dairy cattle. Numerous investigators have reported mean prevalences of fecal shedding from ∼20 to ∼70% in groups of clinically healthy adult cattle (23, 31, 34), yet several large cross-sectional epidemiologic surveys have observed prevalences of only 2% or less in asymptomatic adult cattle populations (4, 20, 41). Some of this variation in the observed prevalence of fecal shedding can be explained by different investigators using diagnostic assays of differing sensitivity and specificity (11, 13, 15), but much of the variation is the result of studying different populations of cattle (e.g., beef versus dairy), different age distributions within those populations, and groups of cattle under different management practices, especially when only a single farm or a small sample is examined. For example, we found in two different studies that calving duration for beef herds was associated with a three- to sixfold difference in the proportion of cattle shedding C. parvum (5, 20), making interstudy comparisons of the shedding prevalence for beef cattle potentially confounded if not adjusted for calving duration.
Determining the importance that adult cattle have in loading watersheds with significant amounts of C. parvum oocysts also requires an estimate of mean shedding intensity from populations of cattle. Recent estimates have ranged from 70 to 900 oocysts g of feces−1 for infected noncalf populations (13, 16, 19, 34), but these values are highly conditional on the underlying age distribution of the population (16, 39, 41, 44) and the sensitivity of the diagnostic test being employed (11, 15, 19, 21, 42, 43). Increasing the sensitivity of an assay will function to increasingly detect individuals shedding at low intensities, thereby lowering the overall estimated mean intensity of shedding for the infected cohort of a population.
Reducing the incidence or intensity of fecal shedding of C. parvum oocysts by livestock (5, 18, 20, 24, 29) will reduce the likelihood that animal agricultural operations contaminate surface water with infective C. parvum oocysts. Minimizing infection in calves is paramount for this strategy to work due to the fact that calves have been shown to be the primary producer of C. parvum oocysts for a herd of beef or dairy cattle (3, 4, 15, 16, 26, 35, 41). What remains unresolved is whether pre- or postparturient cows are the primary biological reservoir for calfhood exposure to C. parvum (3, 13, 15, 34) or, as in the case of beef cattle grazing open range, whether resident wildlife function to contaminate pastures before or after calving. We have found high levels of fecal shedding for California ground squirrels that coinhabit rangeland with beef cattle herds, along with isolates of C. parvum that are indistinguishable from those obtained from infected calves (6).
We previously generated a range of estimates of the maximum possible environmental loading rate of C. parvum oocysts by California beef cows, 7.7 × 104 to 2.3 × 105 oocysts cow−1 day−1 (19). Our primary goal for this project was to generate a more accurate estimate of this daily loading rate of C. parvum oocysts for adult beef cattle based on a highly sensitive diagnostic assay for C. parvum oocysts in cattle feces (11). This improved method for detecting and enumerating C. parvum oocysts is highly quantitative, can detect one oocyst per gram of feces with a probability of ∼50%, and has a 90% detection threshold (DT90) of approximately four oocysts g of feces−1. An additional goal was to measure the prevalence and intensity of fecal shedding of C. parvum oocysts in pre- and postparturient cows as an indicator of their potential to infect young calves. Such data would help guide watershed and herd health programs aimed at minimizing bovine cryptosporidiosis and the associated environmental loading with this amphixenotic pathogen.
MATERIALS AND METHODS
Sampling of herds.
Three beef cattle herds were selected from three different geographical regions of California that were representative of the typical oak woodland-annual grassland environment commonly utilized for cow-calf production in this state. From each herd, fecal samples were collected from the rectum from a random sample of 40 adult cows approximately 6 weeks prior to the expected start of calving season, and the same animals were resampled approximately 6 weeks after the start of calving. This provided a diverse set of fecal samples collected from dams ranging from 6 weeks to just a few days both prior to and after calving. All samples were collected in separate disposable containers, diluted with an equal volume of 10% formalin, refrigerated, and forwarded to the Veterinary Medical Teaching and Research Center, Tulare, Calif., for enumeration of C. parvum oocysts.
Enumeration of C. parvum oocysts.
Immunomagnetic separation of oocysts coupled with direct immunofluorescence microscopy (IMS-DFA) was used to enumerate C. parvum oocysts as described previously by das Graças C. Pereira et al. (11). Briefly, 4 g of the 1:1 feces-formalin mixture (2 g of feces) was resuspended in 40 ml of phosphate-buffered saline, strained through folded two-ply gauze, and centrifuged for 10 min at 1,000 × g. The supernatant was discarded, the pellet was resuspended in 10 ml of sterile, distilled water, and transferred to Leighton tubes for immunomagnetic separation using the anti-Cryptosporidium Dynabeads assay as described by the manufacturer (Dynal, Inc., Lake Success, N.Y.), with oocysts labeled using a fluorescein isothiocyanate-labeled anti-Cryptosporidium monoclonal antibody assay (Waterborne, Inc., New Orleans, La.).
Estimating diagnostic test sensitivity.
Naturally infected dairy calves from local commercial dairies were the source of wild-type C. parvum oocysts. We have determined previously that these oocysts are classified as bovine genotype A, using the genotyping scheme of Xiao et al. (45). Using an acid-fast procedure to detect oocysts (18), samples having more than 25 oocysts per microscopic field at a magnification of ×400 were washed through a series of 40-, 100-, 200-, and 270-mesh sieves. The resulting suspension was decanted off and centrifuged at 1,000 × g for 10 min. The supernatant was discarded, and the pellet was washed in Tween water (0.01% Tween 80 in deionized water [vol/vol]). A discontinuous sucrose gradient was used to purify C. parvum oocysts from fecal suspensions (2). The concentration of purified oocysts was determined as the arithmetic mean of six separate counts using a phase-contrast hemacytometer (Bright-Line hemacytometer; Hausser Scientific, Horsham, Pa.).
Fecal samples were collected from the rectum from three cows from each of the three herds enrolled in the study and determined to be free of Cryptosporidium oocysts by IMS-DFA. Aliquots (4.5 g) of negative fecal sample were combined with 500 μl of C. parvum oocyst suspension to yield final concentrations of 10, 50, 100, and 500 oocysts g of adult cow feces−1. The spiked fecal samples were then subjected to the processing method described above (IMS-DFA) for the enumeration of oocysts in periparturient cow fecal samples.
Percent recovery (r) is defined as r = x/k, where x is the observed number of oocysts counted for each fecal sample by IMS-DFA and k is the total number of oocysts added to the sample. The k variable can be further decomposed into cW, where c is the number of oocysts per gram of feces and W is the mass of fecal material examined per assay (e.g., weight of the smear on a slide) (21). In practice, the Poisson distribution tends to underestimate the variance of r for oocyst count data (3, 19, 37, 38). A flexible model to characterize the variance of r that also allows for covariates and clustering effects to enter the model is negative binomial regression, derived as a Poisson gamma mixture distribution (17). With the observed number of oocysts per assay set as the outcome variable, oocyst concentration was modeled as a continuous variable, the number of oocysts added per fecal sample set was modeled as the offset (or exposure) variable, and cow identification (source of feces) was set as a cluster or group variable to adjust the standard errors for intracow dependencies.
Test sensitivity for a randomly selected sample with c oocysts per gram of feces, S(c), was then defined as the probability of detecting one or more oocysts per assay given that oocysts were present. This is equivalent to saying that sensitivity is equal to 1 minus the probability that no oocysts were detected, S(c) = P(X > 0) = 1 − P(X = 0), where X is the number of oocysts observed per assay. For the negative binomial regression model under consideration (Hardin and Hilbe [17]), the sensitivity equation is
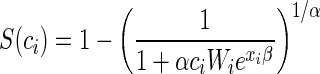 |
(1) |
where the mean of the negative binomial is given by ciWiexiβ, overdispersion is 1 + αciWiexiβ defined above, ci is the number of oocysts per gram of feces, Wi is the mass of fecal material examined, exiβ is the percent recovery of the diagnostic assay as a function of the covariates, and α is an ancillary parameter for modeling dispersion.
Estimating environmental loading of C. parvum.
For this project, we define environmental loading of C. parvum from cattle as the mean number of oocysts produced by an adult cow (age ≥ 24 months) per day, calculated as the arithmetic mean of kilogram of feces produced per animal per day × arithmetic mean intensity of oocyst shedding for test-positive cattle × prevalence of test-positive cattle. Although a crude estimate of environmental loading can be calculated from the cattle classified as positive by our IMS-DFA assay, this ignores the possibility of any false-negative results in the sample (e.g., infected cattle shedding very low concentrations of oocysts that were not detected by the IMS-DFA procedure). A more conservative estimate of the environmental loading rate would be to add these potential false-negative cattle to the calculated load (19, 21). This is accomplished by using the sensitivity function S(c) and the specificity of the assay Sp in conjunction with the binomial distribution to estimate the maximum mean oocyst concentration, c, for a given prevalence, p, of unobserved positive cattle (false-negative results) (3, 19, 21). Specifically, the probability that a randomly selected sample will test positive is pS(c) + (1 − p)(1 − Sp), and the probability that z or fewer of the N fecal samples will be positive is given by the following binomial distribution:
 |
(2) |
To calculate the maximum oocyst load from test-negative cattle, z is zero and equation 2 simplifies to
 |
(3) |
where P is fixed at a threshold of, say 5%, S(c) is defined above, Sp is fixed at a justifiable level, N is the sample size of test-negative cattle, and the right-hand side of the equation is then solved for the maximum oocyst concentration (c) for a given prevalence (p) of false-negative cattle under the given constraints of P, S(c), Sp, and N (3, 19, 21). Values for the specificity Sp tend to be highly constrained when modeling test-negative cattle, given the limited opportunity for false-positive results.
Statistical analysis.
The prevalences of apparent shedding before and after calving for the 120 cows enrolled in the study were compared using McNemar's test, and the intensities of oocyst shedding were compared before and after calving by using linear mixed effects regression, with ln(oocyst counts) as the outcome variable, time when sample was taken (pre- or postparturient) as the covariate, and herd set as the group variable to adjust the standard errors for intraherd dependencies (30).
RESULTS
The overall prevalence of adult beef cattle testing positive for C. parvum oocysts was 7.1% (17 of 240) (exact 95% confidence interval, 4.2 to 11.1%) (Table 1). For the 17 positive cattle, we observed a crude arithmetic mean (unadjusted for percent recovery) of 2.706 oocysts per 2 g of fecal sample. The frequency distribution of the number of oocysts per 2 g of fecal sample follows: 7 of 17 positive samples had one oocyst each, 2 of 17 positive samples had two oocysts each, 3 of 17 positive samples had three oocysts each, 3 samples had four oocysts each, and the last 2 samples had five and nine oocysts, respectively.
TABLE 1.
Observed prevalence and intensity of fecal shedding of C. parvum oocysts by beef cows in California, stratified by herd and calving status
| Herd and calving status | No. of cattlea (%) | Mean no. of oocysts/g of feces (SD)
|
|
|---|---|---|---|
| Positiveb | Totalc | ||
| Herd A | |||
| Preparturition | 3/40 (7.5) | 1.25 (0.00) | 0.09 (0.33) |
| Postparturition | 2/40 (5.0) | 6.25 (7.07) | 0.31 (1.78) |
| Herd B | |||
| Preparturition | 3/40 (7.5) | 4.58 (1.44) | 0.34 (1.27) |
| Postparturition | 4/40 (10.0) | 3.44 (1.88) | 0.34 (1.17) |
| Herd C | |||
| Preparturition | 4/40 (10.5) | 2.19 (1.20) | 0.22 (0.74) |
| Postparturition | 1/40 (2.5) | 5.00 (NAd) | 0.13 (0.79) |
| Overall | 17/240 (7.1) | 3.38 (2.64) | 0.24 (1.11) |
| Preparturition | 10/120 (8.3) | 2.63 (1.71) | 0.22 (0.87) |
| Postparturition | 7/120 (5.8) | 4.46 (3.45) | 0.26 (1.31) |
Number of cattle positive for C. parvum oocytes/number of cattle sampled.
Arithmetic mean for the number of oocysts shed per gram in positive fecal samples from the specified population, adjusted for percent recovery of IMS-DFA.
Arithmetic mean for the number of oocysts shed per gram in all fecal samples collected from the specified population, adjusted for percent recovery of IMS-DFA.
NA, not applicable.
Data from the experiment to estimate percent recovery of the diagnostic assay are presented in Table 2, with the coefficients and associated P values for the negative binomial regression model presented in Table 3. Percent recovery was significantly associated with oocyst concentration (P = 0.005), such that for each additional 100 oocysts per g of feces, percent recovery declined ∼4%. The estimated sensitivity function S(c) (equation 1) generated by the coefficients in Table 3 are shown in Fig. 1. The concentration of oocysts detected with a 90% probability (DT90) occurred at 3.2 oocysts g of feces−1, and the probability of detecting 1 oocyst g of feces−1 was 54% (Fig. 1).
TABLE 2.
Percent recovery of IMS-DFA for enumerating C. parvum oocysts in adult beef cattle fecesa
| No. of oocysts added g of feces−1 | Mean no. of oocysts in 2 g of feces | Range of oocysts in 2 g of feces | % Recovery (95% CI)b |
|---|---|---|---|
| 10 | 7 | 4-10 | 40.1 (34, 48) |
| 50 | 39 | 16-63 | 39.4 (33, 47) |
| 100 | 83 | 49-109 | 38.5 (33, 46) |
| 500 | 320 | 141-479 | 32.3 (27, 39) |
Nine fecal samples were evaluated, with the same three cattle from each of three herds used at each oocyst concentration.
Mean percent recovery was estimated through negative binomial regression, derived as Poisson gamma mixture distribution, with errors adjusted for intracow dependencies on test recovery (17). 95% CI, 95% confidence interval.
TABLE 3.
Estimated maximum likelihood coefficients of the negative binomial regression model fitted to the recovery dataa
| Parameter | Negative binomial regression model
|
||
|---|---|---|---|
| Coefficient | P value | 95% CIb | |
| Intercept | −0.91 | 0.005 | −1.08, −0.73 |
| Concn (no. of oocysts/g of feces) | −0.00044 | <0.001 | −0.0007, −0.00013 |
| α | 0.091 | <0.001 | 0.047, 0.18 |
n = 36. Log likehihood for estimated model, −154.4.
95% CI, 95% confidence interval.
FIG. 1.
Sensitivity of IMS-DFA for detecting C. parvum oocysts in adult beef cattle fecal material. The sensitivity, defined as the probability of detecting one or more oocysts in positive fecal material, was modeled using negative binomial regression.
On the basis of the percent recovery of our diagnostic assay (0.40 at low oocyst concentrations) and an arithmetic mean of 2.706 oocysts per positive slide from 2 g of feces, the mean intensity of oocyst shedding was 3.38 oocysts g of feces−1 for test-positive cattle and 0.24 oocyst g of feces−1 for all cattle in the study (Table 1). A crude estimate of the environmental loading rate of C. parvum per animal can be calculated from these values. For example, using a hypothetical 400-kg adult beef cow producing 16 kg of feces per day (1), we calculate 16 kg of feces day−1 × 240 oocysts kg of feces−1 = 3,840 oocysts cow−1 day−1. This estimate of 3,840 oocysts cow−1 day−1 ignores the possibility of false-negative results among test-negative cattle (i.e., infected cattle shedding very low concentrations of oocysts that were missed by the IMS-DFA procedure). A profile of feasible scenarios for the prevalence of unobserved shedding (p) by the intensity of oocyst shedding (c) is shown in Fig. 2, with p × c scenarios below the threshold of P = 0.05 having a probability of occurrence of >0.05 and scenarios above the threshold having a probability of occurrence of <0.05. On the basis of the constraints for equation 3 [i.e., S(c) constraints described above, Sp = 1.0, N = 223, and P = 0.05] and allowing the prevalence of unobserved shedding to range from 2.5 to 97.5%, the maximum intensity of oocyst shedding from our test-negative cattle would be 0.017 to 0.988 oocyst g of feces−1. Reducing Sp to 99% substantially reduces the range of oocyst shedding to 0.017 to 0.195 oocyst g of feces−1 (Fig. 2). Collectively, these calculations result in a combined range of estimates for the intensity of oocyst shedding of 240 to 263 oocysts kg of feces−1 for all cattle in the study. If we assume that our beef cattle produce between 15 and 35 kg of feces day−1, the environmental loading rate for our study population of California beef cows would range from ∼3,600 to ∼9,200 oocysts cow−1 day−1 (Table 4).
FIG. 2.
Profile of feasible scenarios for the prevalence of unobserved shedding (p) by the intensity of oocyst shedding (c), with p × c scenarios below the threshold of P = 0.05 having a probability of occurrence of >0.05 and scenarios above the threshold having a probability of occurrence of <0.05. The specificity of the diagnostic assay, IMS-DFA, was set at 99% (- - - - ) and 100% (—).
TABLE 4.
Estimated daily environmental loading rate of C. parvum oocysts by adult beef cattle in California
| Prevalence of unobserved fecal shedding of C. parvuma (%) | Daily environmental loading rateb
|
|||||
|---|---|---|---|---|---|---|
| 99% Sp
|
100% Sp
|
|||||
| kg of feces produced animal−1 day−1
|
kg of feces produced animal−1 day−1
|
|||||
| 15 | 25 | 35 | 15 | 25 | 35 | |
| 0 | 3,602 | 6,003 | 8,404 | 3,602 | 6,003 | 8,404 |
| 2.5 | 3,670 | 6,116 | 8,563 | 3,946 | 6,577 | 9,207 |
| 97.5 | 3,832 | 6,386 | 8,940 | 3,836 | 6,393 | 8,951 |
Prevalence of adult cattle that shed undetected C. parvum oocysts (false negatives).
The daily environmental loading rate of C. parvum oocysts (total number of C. parvum oocysts shed in the feces of adult cattle per day) at two different specificities of the diagnostic assay and with different values for fecal production per animal per day.
The overall prevalences of apparent fecal shedding during the pre- and postcalving periods were 8.3% (10 of 120) and 5.8% (7 of 120), respectively (Table 1). This difference of 2.5% in the prevalence of fecal shedding between the pre- and postcalving periods was not significantly different (P = 0.61). None of the cows that tested positive during the precalving period tested positive during the postcalving period. The apparent intensity of oocyst shedding was not significantly different for cows in the precalving versus postcalving period (P = 0.22).
DISCUSSION
We determined that the environmental loading rate of C. parvum oocysts from adult beef cattle in California ranged from ∼3,900 to ∼9,200 oocysts cow−1 day−1, depending on whether an individual animal produces 15 to 35 kg of feces day−1, respectively. This range of values for oocyst loading should be quite robust in that these values are based not only on apparent or observable fecal shedding of oocysts (test-positive cattle) but also include a wide range of assumed prevalences of unobserved fecal shedding in test-negative beef cattle (2.5 to 97.5% of test-negative cattle shedding undetectable levels of oocysts). Furthermore, if we assume that our specificity is less than 100%, then estimates of environmental loading are likewise reduced due to the occurrence of false-positive cattle in the loading estimate (Table 4). To put these values of oocyst loading into a broader perspective, we have calculated that a typical California ground squirrel (Spermophilus beecheyi) weighing 550 g sheds on average 113,000 C. parvum oocysts animal−1 day−1 (6), or approximately 12 to 29 times the amount of an adult beef cow in our study population. Population densities of these squirrels in areas grazed by beef cattle range from 8 to 94 adults ha−1 in California (8, 27, 32), resulting in oocyst loading rates from ground squirrel populations of 9.0 × 105 oocysts ha−1 day−1 for low-density populations to 1.1 × 107 oocysts ha−1 day−1 for high-density populations (6). In contrast, population densities of beef cattle herds, when calculated as the number of adult cattle per total area of access per year, typically vary from 0.1 to 0.25 adult cow ha−1 for the annual grassland-oak woodland complexes of central California. Assuming an individual cow sheds about 6,500 oocysts day−1, this results in oocyst loading rates of 650 oocysts ha−1 day−1 for low-density cow populations to 1,625 oocysts ha−1 day−1 for high-density populations, or ≤0.002 times the oocyst loading rate produced by colonies of ground squirrels. Alternatively, an infected dairy calf can produce far in excess of 106 oocysts animal−1 day−1 (14, 22, 39, 44), or ≥100 times the amount of a mature beef cow in our study population.
We previously estimated that the maximum environmental loading rate of C. parvum per adult beef cow defecating 16 kg of feces day−1 was ∼1.7 × 105 oocysts cow−1 day−1 (range of 7.7 × 104 to 2.3 × 105 oocysts cow−1 day−1), which used a similar statistical approach but relied on a substantially less sensitive assay (DT90 = 755 oocysts g of feces−1) (19). For comparison purposes, we can likewise assume that we have 400-kg beef cows defecating on average 16 kg of feces day−1 (1). Therefore, 16 kg of feces day−1 multiplied by 263 oocysts kg of feces−1 equals 4,208 oocysts cow−1 day−1. This estimate of 4,208 oocysts cow−1 day−1 is 2.5% of our previous estimate, or a ∼1.6 log10 reduction in the calculated loading rate. It is important to note that this reduced estimate for maximum oocyst loading by adult beef cattle can be directly attributed to the highly sensitive assay of IMS-DFA. To our knowledge, this diagnostic method has the lowest DT90 (3.2 oocysts/g of feces) published thus far for detecting and enumerating oocysts in bovine feces. Quantitative estimates for environmental loading of fecal-oral pathogens from host species are often overestimated by using less sensitive tests, not underestimated. While the prevalence of test-positive animals is certainly biased downward by the use of insensitive diagnostic assays, estimates for the intensity of pathogen shedding can be biased upward for insensitive tests, particularly if the population of interest sheds below the DT90 of the assay, as is the case for many asymptomatic infections in healthy adult vertebrate species. The use of highly sensitive assays in such populations will function to increasingly detect low-intensity shedders, reducing the calculated mean intensity of shedding and thereby reducing the associated environmental loading rate.
We found an apparent prevalence of ∼7% for periparturient beef cows shedding on average 3.38 oocysts g of feces−1 (Table 1). This finding of a low prevalence of fecal shedding for C. parvum in beef cattle is consistent with previous work on beef cattle populations in California (4, 20). More generally, the reported prevalence of fecal shedding of C. parvum oocysts in adult beef and dairy cattle varies widely in the literature, ranging from a low of 0 to 10% prevalence (3, 5, 15, 19, 26, 35, 40, 41) to highs of 20 to 70% (16, 23, 34). This variation occurs in part due to different investigators studying different populations of cattle using diagnostic methods of differing sensitivity and specificity, but it is also likely that a systematic difference in the medical ecology of asymptomatic adult shedding of bovine C. parvum exists between these populations with low and high prevalence of fecal shedding of C. parvum oocysts. For example, a hypothesis test for a significant difference in the prevalence of oocyst shedding reported in this study for adult beef cattle in California (7.1% [17 of 240]) to the prevalence reported for adult beef cattle in Scotland (62.4% [345 of 553]) (34) has an exact P value of 0.002 (95% confidence interval for difference in prevalence, 0.49 to 0.61), indicating that the underlying prevalence is likely different in the two populations.
One explanation for not finding a higher prevalence of C. parvum oocysts in periparturient beef cattle is that we used an insensitive method to detect oocysts. This speculation is likely to be incorrect given the fact that the IMS-DFA method is highly sensitive (11), with the probability of detecting just one oocyst g of feces−1 being 54% (Fig. 1). If we assume that our sample of periparturient beef cattle were shedding oocysts similar to the mean 500 oocysts g of feces−1 reported for dairy cattle in Quebec, Canada (13), then the probability that our diagnostic method failed to detect one or more oocysts from such a cow would be P < 10−10. Intensities of fecal shedding have been reported for cows and heifers in Pennsylvania (range of 90 to 371 oocysts g of feces−1) and from 6- to 10-year-old cattle in Scotland (range of 25 to 1.8 ×104 oocysts g of feces−1) (16, 33). The probability of our diagnostic method failing to detect one or more oocysts from similarly infected animals would range from P = 1.2 × 10−5 for cattle shedding 25 oocysts g of feces−1 to P < 1 × 10−10 for cattle shedding 18,000 oocysts g of feces−1. If cattle are shedding moderate to high intensities of oocysts (≥25 oocysts g of feces−1) in our study population, then the cattle are present at a low prevalence.
The statistical methods described herein allow one to calculate the probability of detecting one or more oocysts for specific combinations of prevalence (p) by shedding intensity (number of oocysts per gram of feces) in order to ascertain whether certain p × c scenarios are plausible (3, 19). Setting a threshold of P < 0.05 to classify scenarios of p × c as unlikely, given our sample size of 240 fecal samples, sensitivity function S(c) as described above, and Sp equal to ≥99%, scenarios above the thresholds outlined in Fig. 2 are unlikely (P < 0.05). Scenarios below the threshold of P = 0.05 cannot be excluded from occurring in our population of beef cattle. Hence, given our sample size of 240 fecal samples and given our S(c) as shown in Fig. 1 and Sp of ≥99%, it is highly unlikely that we have more than 1.4% of periparturient beef cattle shedding moderate to high concentrations of undetectable oocysts in our study population. Using a different diagnostic method and a different sample of beef cattle, we calculated a similar value of ≤2% for these potential moderate-to-high intensity yet undetected shedders in California beef cows (19). In contrast, 26% of dairy cows and heifers were found to shed an average of 70 oocysts g of feces−1 in Pennsylvania (16), and 70% (92 of 131) of asymptomatic adult cattle tested positive for C. parvum oocysts in Spain (23). These higher prevalences and intensities of oocyst shedding compared to our data suggest that there are substantial and systematic differences in the medical ecology of genotypes of C. parvum that infect adult cattle in different geographical regions in the United States and the world.
We found no evidence of elevated levels of C. parvum shedding from beef cows in the postparturient period than in the preparturient period. With respect to the biological reservoir of C. parvum oocysts for newborn beef calves, it is noteworthy that 7% of our periparturient cattle were shedding on average 3.4 oocysts g of feces−1. Accurate information on the 50% infective dose of bovine-derived C. parvum for young beef calves of mixed genetic origin is not available at this time, but presumably the daily contact between an infected dam and a susceptible beef calf, such as during nursing behavior in combination with fecally contaminated udders, would cause C. parvum to be transmitted to the calf eventually. Once a set of index calves become infected within the herd, C. parvum could be readily transmitted to the remaining susceptible calves. This pattern of intraherd transmission of C. parvum within beef calf populations (i.e., a propagating epidemic) would also be created by a wildlife reservoir seeding pastures or rangeland with C. parvum. For example, we have found that California ground squirrels (Spermophilus beecheyi) that inhabit the annual grassland-oak woodland complexes of California can shed substantial numbers of oocysts during spring and summer, with genotypes of C. parvum similar to those obtained from beef calves (6). Similar speculations have been made previously for populations of mice and voles at an agricultural site in Warwickshire, United Kingdom (10). A more detailed longitudinal study would need to be conducted in order to resolve which, if either of these hosts of C. parvum, function as the primary source of C. parvum for beef calves located on rangeland.
Acknowledgments
This work was supported in part by grants from the Division of Agriculture and Natural Resources, University of California.
REFERENCES
- 1.American Society of Agricultural Engineers. 1992. Manure production and characteristics, p. 485-487. In ASAE standards D384.1. Agricultural engineering yearbook. American Society of Agricultural Engineers, St. Joseph, Mich.
- 2.Arrowood, M. J., and C. R. Sterling. 1987. Isolation of Cryptosporidium parvum and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J. Parasitol. 73:314-319. [PubMed] [Google Scholar]
- 3.Atwill, E. R., J. A. Harp, T. Jones, P. W. Jardon, S. Checel, and M. Zylstra. 1998. Evaluation of periparturient dairy cows and contact surfaces as a reservoir of Cryptosporidium parvum for calfhood infection. Am. J. Vet. Res. 59:1116-1121. [PubMed] [Google Scholar]
- 4.Atwill, E. R., E. Johnson, D. J. Klingborg, G. M. Veserat, G. Markegard, W. A. Jensen, D. W. Pratt, R. E. Delma, H. A. George, L. C. Forero, R. L. Phillips, S. J. Barry, N. K. McDougald, R. R. Gildersleeve, and W. E. Frost. 1998. Age, geographic, and temporal distribution of fecal shedding of Cryptosporidium parvum oocysts in cow-calf herds. Am. J. Vet. Res. 60:420-425. [PubMed] [Google Scholar]
- 5.Atwill, E. R., E. Johnson, and M. das Graças C. Pereira. 1999. Association of herd composition, stocking rate, and calving duration with fecal shedding of Cryptosporidium parvum oocysts in beef herds. J. Am. Vet. Med. Assoc. 215:1833-1838. [PubMed] [Google Scholar]
- 6.Atwill, E. R., S. Maldonado Camargo, R. Phillips, L. H. Alonso, K. W. Tate, W. A. Jensen, J. Bennet, S. Little, and T. P. Salmon. 2001. Quantitative shedding of two genotypes of Cryptosporidium parvum in California ground squirrels (Spermophilus beecheyi). Appl. Environ. Microbiol. 67:2840-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awad-El-Kariem, F. M., H. A. Robinson, F. Petry, V. McDonald, D. Evans, and D. Casemore. 1998. Differentiation between human and animal isolates of Cryptosporidium parvum using molecular and biological markers. Parasitol. Res. 84:297-301. [DOI] [PubMed] [Google Scholar]
- 8.Boellstorff, D. E., and D. H. Owings. 1995. Home range, population structure, and spatial organization of California ground squirrels. J. Mammol. 76:551-561. [Google Scholar]
- 9.Caccio, S., W. Homan, R. Camill, G. Traldi, T. Kortbeek, and E. Pozio. 2000. A microsatellite marker reveals population heterogeneity within human and animal genotypes of Cryptosporidium parvum. Parasitology 120:237-244. [DOI] [PubMed] [Google Scholar]
- 10.Chalmers, R. M., A. P. Sturdee, S. A. Bull, A. Miller, and S. E. Wright. 1997. The prevalence of Cryptosporidium parvum and C. muris in Mus domesticus, Apodemus sylvaticus and Clethrionomys glareolus in an agricultural system. Parasitol. Res. 83:478-482. [DOI] [PubMed] [Google Scholar]
- 11.das Graças C. Pereira, M., E. R. Atwill, and T. Jones. 1999. Comparison of sensitivity of immunofluorescent microscopy to that of a combination of immunomagnetic separation and immunofluorescent microscopy for detection of Cryptosporidium parvum oocysts in adult bovine feces. Appl. Environ. Microbiol. 65:3236-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enemark, H. L., P. Ahrens, C. D. Juel, E. Petersen, R. F. Petersen, J. S. Andersen, P. Lind, and S. M. Thamsborg. 2002. Molecular characterization of Danish Cryptosporidium parvum isolates. Parasitology 125:331-341. [DOI] [PubMed] [Google Scholar]
- 13.Faubert, G. M., and Y. Litvinsky. 2000. Natural transmission of Cryptosporidium parvum between dams and calves on a dairy farm. J. Parasitol. 86:495-500. [DOI] [PubMed] [Google Scholar]
- 14.Fayer, R., L. Gasbarre, P. Pasquali, A. Canals, S. Almeria, and D. Zarleng. 1998. Cryptosporidium parvum infection in bovine neonates: dynamic clinical, parasitic and immunologic patterns. Int. J. Parasitol. 28:49-56. [DOI] [PubMed] [Google Scholar]
- 15.Fayer, R., J. M. Trout, T. K. Graczyk, and E. J. Lewis. 2000. Prevalence of Cryptosporidium, Giardia, and Eimeria infections in post-weaned and adult cattle on three Maryland farms. Vet. Parasitol. 93:103-112. [DOI] [PubMed] [Google Scholar]
- 16.Grazyck, T. K., B. M. Evans, C. J. Shiff, H. J. Karreman, and J. A. Patz. 2000. Environmental and geographical factors contributing to watershed contamination with Cryptosporidium parvum oocysts. Environ. Res. 82:263-271. [DOI] [PubMed] [Google Scholar]
- 17.Hardin, J., and J. Hilbe. 2001. The negative binomial family, p. 141-158. In Generalized linear models and extensions. Stata Press, College Station, Tex.
- 18.Harp, J. A., P. Jardon, E. R. Atwill, M. Zylstra, S. Checel, J. P. Goff, and C. Desimone. 1996. Field testing of prophylactic measures against Cryptosporidium parvum infection in calves in a California dairy herd. Am. J. Vet. Res. 57:1586-1588. [PubMed] [Google Scholar]
- 19.Hoar, B., E. R. Atwill, and T. B. Farver. 2000. Estimating maximum possible environmental loading amounts of Cryptosporidium parvum attributable to adult beef cattle. Quant. Microbiol. 2:21-36. [Google Scholar]
- 20.Hoar, B., E. R. Atwill, C. Elmi, and T. B. Farver. 2001. An examination of risk factors associated with beef cattle shedding pathogens of potential zoonotic concern. Epidemiol. Infect. 127:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, T., and E. R. Atwill. 1998. Statistical methods for quantifying and comparing loading of Cryptosporidium on watersheds from different livestock sources using epidemiologic survey data, p. 201-207. In Proceedings of the Source Water Assessment and Protection Conference 1998. National Water Research Institute, Dallas, Tex.
- 22.Kuchzynska, E., and D. R. Shelton. 1999. Method for detection and enumeration of Cryptosporidium parvum oocysts in feces, manures, and soils. Appl. Environ. Microbiol. 65:2820-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenzo, M. J., E. Ares-Mazas, and I. Villacorta. 1993. Detection of oocysts and IgG antibodies to Cryptosporidium parvum in asymptomatic adult cattle. Vet. Parasitol. 47:9-15. [DOI] [PubMed] [Google Scholar]
- 24.Mohammed, H. O., S. E. Wade, and S. Schaaf. 1999. Risk factors associated with Cryptosporidium parvum infection of dairy cattle in southeastern New York State. Vet. Parasitol. 83:1-13. [DOI] [PubMed] [Google Scholar]
- 25.Okhuysen, P. C., C. L. Chappell, J. H. Crabb, C. R. Sterling, and H. L. DuPont. 1999. Virulence of three different Cryptosporidium parvum isolates for healthy adults. J. Infect. Dis. 180:1275-1281. [DOI] [PubMed] [Google Scholar]
- 26.Olson, M. E., C. L. Thorlakson, L. D. Deselliers, D. W. Morck, and T. A. McAllister. 1997. Giardia and Cryptosporidium in Canadian farm animals. Vet. Parasitol. 68:375-381. [DOI] [PubMed] [Google Scholar]
- 27.Owings, D. H., M. Borchert, and R. Virginia. 1977. The behaviour of California ground squirrels. Anim. Behav. 25:221-230. [Google Scholar]
- 28.Peng, M. M., L. Xiao, A. R. Freeman, M. J. Arrowood, A. A. Escalante, A. C. Weltman, C. S. L. Ong, W. R. MacKenzie, A. A. Lal, and C. B. Beard. 1997. Genetic polymorphism among Cryptosporidium isolates: evidence of two distinct human transmission cycles. Emerg. Infect. Dis. 3:567-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perryman, L. E., S. J. Kapil, M. L. Jones, and E. L. Hunt. 1999. Protection against calves against cryptosporidiosis with immune bovine colostrum induced by a Cryptosporidium parvum recombinant protein. Vaccine 17:2142-2149. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro, J. C., and D. M. Bates. 2000. Theory and computational methods for LME models, p. 57-96. In Mixed effects model in S and S-Plus. Springer, New York, N.Y.
- 31.Quilez, J., C. Sanchez-Acedo, E. del Cacho, A. Clavel, and A. C. Causape. 1996. Prevalence of Cryptosporidium and Giardia infections in cattle in Aragon (northeastern Spain). Vet. Parasitol. 66:139-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schitoskey, F., Jr., and S. R. Woodmansee. 1978. Energy requirements and diet of the California ground squirrel. J. Wildl. Manage. 42:373-382. [Google Scholar]
- 33.Scott, C. A., H. V. Smith, and H. A. Gibbs. 1994. Excretion of Cryptosporidium parvum oocysts by a herd of beef suckler cows. Vet. Rec. 134:172. [DOI] [PubMed] [Google Scholar]
- 34.Scott, C. A., H. V. Smith, M. M. A. Mtambo, and H. A. Gibbs. 1995. An epidemiological study of Cryptosporidium parvum in two herds of adult beef cattle. Vet. Parasitol. 57:277-288. [DOI] [PubMed] [Google Scholar]
- 35.Sischo, W. M., E. R. Atwill, L. E. Lanyon, and J. George. 2000. Cryptosporidia on dairy farms and the role these farms may have in contaminating surface water supplies in the northeastern United States. Prev. Vet. Med. 43:253-267. [DOI] [PubMed] [Google Scholar]
- 36.Spano, F., L. Putignani, J. McLauchlin, D. P. Casemore, and A. Crisantil. 1997. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrari and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol. Lett. 150:209-217. [DOI] [PubMed] [Google Scholar]
- 37.Teunis, P. F. M., E. G. Evers, and W. Slob. 1999. Analysis of variable fractions resulting from microbial counts. Quant. Microbiol. 1:63-68. [Google Scholar]
- 38.Teunis, P. F. M., G. J. Medema, L. Kruidnier, and A. H. Havelaar. 1997. Assessment of the risk of infection by Cryptosporidium or Giardia in drinking water from a surface water source. Water Res. 31:1333-1346. [Google Scholar]
- 39.Uga, S., J. Matsuo, E. Kono, K. Kimura, M. Inoue, S. K. Rai, and K. Ono. 2000. Prevalence of Cryptosporidium parvum infection and pattern of oocyst shedding in calves in Japan. Vet. Parasitol. 94:27-32. [DOI] [PubMed] [Google Scholar]
- 40.Villacorta, I., E. Ares-Mazas, and M. J. Lorenzo. 1991. Cryptosporidium parvum in cattle, sheep, pigs, in Galicia (N.W. Spain). Vet. Parasitol. 38:249-252. [DOI] [PubMed] [Google Scholar]
- 41.Wade, S. E., H. O. Mohammed, and S. L. Schaaf. 2000. Prevalence of Giardia sp., Cryptosporidium parvum, and Cryptosporidium muris (C. andersoni) in 109 dairy herds in five counties of southeastern New York. Vet. Parasitol. 93:1-11. [DOI] [PubMed] [Google Scholar]
- 42.Weber, R., R. T. Bryan, H. S. Bishop, S. P. Wahlquist, J. J. Sullivan, and D. D. Juranek. 1991. Threshold of detection of Cryptosporidium oocysts in human stool specimens: evidence for low sensitivity of current diagnostic methods. J. Clin. Microbiol. 29:1323-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao, L., and R. Herd. 1993. Quantitation of Giardia cysts and Cryptosporidium oocysts in fecal samples by direct immunofluorescence assay. J. Clin. Microbiol. 31:2944-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao, L., and R. Herd. 1994. Infection patterns of Cryptosporidium and Giardia in calves. Vet. Parasitol. 55:257-262. [DOI] [PubMed] [Google Scholar]
- 45.Xiao, L., U. M. Morgan, J. Limor, A. Escalante, M. Arrowwood, W. Shulaw, R. C. A. Thompson, R. Fayer, and A. A. Lal. 1999. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl. Environ. Microbiol. 65:3386-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]




