Abstract
The Candida albicans cell wall participates in both growth and morphological transitions between yeast and hyphae. Our studies here focus on Dfg5p and Dcw1p, two similar proteins with features of glycosylphosphatidylinositol-linked cell surface proteins. Mutants lacking Dfg5p are defective in alkaline pH-induced hypha formation; mutants lacking Dcw1p have no detected hypha formation defect. Both homozygote-triplication tests and conditional expression strategies indicate that dfg5 and dcw1 mutations are synthetically lethal. Therefore, Dfg5p and Dcw1p share a function required for growth. Epitope-tagged Dfg5p, created through an insertional mutagenesis strategy, is found in cell membrane and cell wall extract fractions, and endoglycosidase H digestion shows that Dfg5p undergoes N-linked mannosylation. Surprisingly, Dfg5p is required for expression of the hypha-specific gene HWP1 in alkaline media. Because Dfg5p is a cell surface protein, it is poised to generate or transmit an external signal required for the program of hypha-specific gene expression.
Candida albicans is an opportunistic fungal pathogen. It inhabits the gastrointestinal and genitourinary tracts in most healthy individuals as a benign commensal organism. However, it can cause diverse infections when host or environmental factors permit tissue invasion or overgrowth.
C. albicans cells are surrounded by a cell wall composed of β-glucan, chitin, and mannoprotein (4, 15). The cell wall is of interest for several reasons. First, it has a role in cell morphogenesis. C. albicans produces several morphologically distinct types of cells, such as yeast and hyphal cells (20), that differ in cell wall architecture and composition (4, 11, 36). Second, it has a role in virulence. The cell wall is the surface of contact between pathogen and host, and several cell wall proteins contribute to adherence, a major virulence trait (27, 36). Third, as an essential pathogen-specific structure, it comprises many targets for drug or vaccine therapy (9, 37).
Several strategies have permitted isolation of C. albicans cell wall protein genes. These strategies include purification and sequencing, expression cloning of surface antigens, functional cloning in Saccharomyces cerevisiae, and identification of C. albicans sequence homologs of characterized S. cerevisiae cell wall protein genes (4, 27). In addition, a recent report described a gene fusion library for cloning of C. albicans gene segments that direct secretion or surface localization in S. cerevisiae (24). Many well-characterized surface proteins have features of glycosylphosphatidylinositol (GPI)-linked surface proteins, including an N-terminal signal sequence and a C-terminal GPI anchor addition signal (15, 36). GPI anchors provide a mechanism for membrane association in many eukaryotes, but in fungi, the GPI moiety can also be used to provide a covalent linkage to cell wall β-glucan (15, 19, 36). Other classes of fungal cell wall proteins lack a GPI anchor (4, 15, 35, 36). A major challenge is to establish the functional relationships between each C. albicans cell wall protein and morphogenesis and virulence and to assess each protein's potential as a target for therapeutic strategies.
We have been interested in the mechanisms by which C. albicans recognizes and responds to its external environment. The response to external pH is of particular interest, because it includes a change in cell morphology: C. albicans grows as yeast cells at pH 4 and as hyphae at pH 8. In addition, many mutants defective in pH-responsive growth or morphogenesis in vitro are also defective in virulence in animal models (1, 6, 27). Many genes that govern C. albicans alkaline pH responses are conserved in S. cerevisiae and act in the Rim101p signal transduction pathway (7, 28, 29). In S. cerevisiae, mutations in these genes cause defects in the ability to invade agar, growth in alkaline media, and other aspects of growth and cell differentiation (16-18).
This report describes our characterization of two putative C. albicans cell wall protein genes, DFG5 and DCW1. Our interest in DFG5 was based on the fact that S. cerevisiae dfg5 mutants are defective in agar invasion (25). More recently, S. cerevisiae DFG5 has been found to promote growth at alkaline pH as well (12). Thus, it has seemed possible that C. albicans DFG5 might govern alkaline pH responses. Our interest in DCW1 was based on the fact that it is similar to DFG5. In the course of our studies, a published report described the characterization of S. cerevisiae DFG5 and DCW1 (14). We report here that in C. albicans, as in S. cerevisiae, DFG5 and DCW1 share a function that is required for growth. In addition, we have used scanning mutagenesis to epitope tag C. albicans Dfg5p and demonstrate its surface localization. The role of S. cerevisiae Dfg5p in invasive growth is not understood. We have found unexpectedly that C. albicans Dfg5p is required for expression of a hypha-specific gene, thus arguing that Dfg5p or its dependent biological process has a regulatory role in hypha development.
MATERIALS AND METHODS
Strains and plasmids.
The C. albicans strains used in this study (Table 1) are derivatives of strain BWP17 (39). All strains have the genotype of BWP17 (ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG) and the additional genetic alterations indicated. PCR product-directed gene disruption methods (38, 39) were used with parent strain BWP17 to construct dfg5Δ/dfg5Δ strains DAY210 and DAY254, using primers DFG5-5DR and DFG5-3DR. (All primer sequences are listed in Table 2.) The dfg5Δ deletion removes nucleotides −150 to +409, which includes residues 1 to 136 of the predicted Dfg5p protein. Correct integration was demonstrated by PCR with the primers DFG5 5 Detect and DFG5 3 Detect, which flank the site of integration. The same methods were used to construct dcw1Δ/dcw1Δ strain DAY229, using primers YKL046-5DR and YKL046-3DR for disruption and primers 5′YKL046 Det and 3′YKL046 Det for detection. The dcw1Δ deletion removes nucleotides +600 to +818, which includes residues 200 to 272 of Dcw1p. The dfg5Δ/dfg5Δ dcw1Δ/DCW1 strain DAY280 was constructed from dfg5Δ/dfg5Δ strain DAY254 by transformation with the UAU1 disruption cassette (10) amplified with primers YKL046-5DR and YKL046-3DR. Integration was demonstrated by PCR with the primers YKL046 5′CL2 and YKL046 3′CL1/2 to detect the DCW1 allele and primers ARG4 and YKL046 s2 to identify the dcw1Δ::UAU1 allele.
TABLE 1.
Genotypes of the C. albicans strains used in this study
| Genotypea |
|---|
| DAY210 |
| dfg5::HIS1 |
| dfg5::ARG4 |
| DAY229 |
| dcw1::HIS1 |
| dcw1::ARG4 |
| DAY254 |
| dfg5::dpl200 |
| dfg5::dpl200 |
| DAY280 |
| dfg5::dp1200dcw1::UAU1 |
| dfg5::dpl200 DCW1 |
| ES1 |
| dfg5::dpl200dcw1::UAU1 pHIS1 |
| dfg5::dpl200 DCW1 |
| ES5 |
| dfg5::dpl200dcw1::UAU1 pHIS1:DFG5 |
| dfg5::dpl200 DCW1 |
| ES51 |
| dfg5::dpl200 pHIS1 |
| dfg5::dpl200 |
| ES55 |
| dfg5::dpl200 pHIS1:DFG5 |
| dfg5::dpl200 |
| ES57 |
| pHIS1 |
| ES59 |
| pHIS1:DFG5 |
| ES187 |
| dfg5::dpl200dcw1::UAU1 pHIS1:DFG5 |
| dfg5::dpl200 dcw1::URA3 |
| ES190 |
| dfg5::dpl200dcw1::UAU1 pHIS1:DFG5-1001-V5 |
| dfg5::dpl200 DCW1 |
| ES193 |
| dfg5::dpl200dcw1::UAU1 pHIS1:DFG5-1001 |
| dfg5::dpl200 DCW1 |
| ES195 |
| dfg5::dpl200dcw1::UAU1 pHIS1:MET3-DFG5 |
| dfg5::dpl200 dcw1::URA3 |
| ES218 |
| dfg5::dpl200dcw1::UAU1 pHIS1:PHR1-DFG5 |
| dfg5::dpl200 dcw1::URA3 |
All strains have the genotype ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG along with the mutations or alterations indicated (underlined) and are derived from strain BWP17. Plasmid integration was targeted to the his1::hisG locus by digestion with NruI.
TABLE 2.
Sequences of the synthetic oligonucleotides used in this study
| Primer name | Sequence (5′→3′) |
|---|---|
| DFG5-5DR | GTATACTGGCTTTGCTGTGTACTTTGGAATCGAAGCATTCTACAAAATTTATATAATTCTTTTCCCAGTCACGACGTT |
| DFG5-3DR | CATTCATGGTATTGAATACGGCTTGGACCATTTCTAACCAACTGTGTGATTCCGGCTCAGGTGGAATTGTGAGCGGATA |
| YKL046-5DR | TACCAATACAGATGTTAACCCAAGAAATCGTGAAAAATATGCTTTAAATGACCGTTGATCTTTCCCAGTCACGACGTT |
| YKL046-3DR | CGGTTTCTAATGGGGCACTTTTCCATTTGGCGCCCAGATTAGCAAGATACACTGGTAATGGTGGAATTGTGAGCGGATA |
| DFG5 5 Detect | ATTGGTGTTCATCCTATTCC |
| DFG5 3 Detect | ATTCCAGGTAAAAATTTGCC |
| 5′ YKL046 Det | GCCTTGAGACCAGTCAGTCC |
| 3′ YKL046 Det | GATGGCAAATTTTCCAATGA |
| ARG4 Det | GGAATTGATCAATTATCTTTTGAAC |
| YKL046 5′CL2 | GGTATAAGAACTTTCTATCATGAAGTT |
| YKL046 3′CL1/2 | AAAGGAATTGGTGATAGTGGTTGTG |
| 5′DFG5-MET3p1,5kb | CTTATTCGCTGTTTATATTTTGTATTAAACTGCACGACTGGATCTTCAATAAAGGAAGCAAGCTACACCCTTCGTATCTATTTTTCC |
| 3′DFG5-MET3 | GATTGCACAGATGCCGTAAAAAGTAGTAATATTGATATTGTTAGTTGTTGTAGTGAGACCATGTTTTCTGGGGAGGGTATTTACTT |
| V5-His6 5′ | GCTGGTAAGCCTATCCCTAACCCTCTCCTCGGTCTCGATTCTACGCGTACCGGTCATCATCACCATCACCATGCT |
| V5-His6 3′ | AGCATGGTGATGGTGATGATGACCGGTACGCGTAGAATCGAGACCGAGGAGAGGGTTAGGGATAGGCTTACCAGC |
| 3′ DFG5 s1 | CTTATTTAAATATATATG |
| 3′ DFG5 s2 | CAGAAAGTGGTGGCTCAA |
| 3′ DFG5 s3 | TTACATTTGTTTTGAGGC |
| 3′ DFG5 s4 | TTGTCCTCTTCAGTTAAA |
| 3′ DFG5 s5 | CTAACCAACTGTGTGATT |
| 3′ DFG5 s6 | GGGTGGAGCAAACATTCC |
| prs/pgemt-5 | GTGAACCATCACCCTAATCAAGTTTTTTGGGGTCGAGGTGCCGTAAAGCATACGACTCACTATAGGGCGA |
| prs/pgemt-3 | CGCGGCGGGTGTGGTGGTTACGCGCAGCGTGACCGCTACACTTGCCAGCGCTCAAGCTATGCATCCAACG |
| DFG5 −759 | GTTACGTAGTGCTTTATTGCG |
| DFG5 +1619 | GCAATGCTTCCTTTCGATCCG |
For the homozygote-triplication (HT) test (10), strain DAY280 was transformed with plasmids pDDB78 (a vector carrying HIS1) and pMM8 (pDDB78 carrying DFG5) with selection for His+ transformants to generate strains ES1 and ES5, respectively. Thirty independent His+ transformants were grown overnight on YPD at 30°C, and one Arg+ Ura+ mitotic segregant was selected from each transformant.
For DFG5 insertion mutant analysis, strain DAY280 was transformed with pDCTx insertion mutant plasmids (described below). Six independent transformants with each plasmid were tested for filamentation ability in pH 8 liquid M199 medium. The experiment included transformants carrying control plasmids pDDB78 (pHIS1) and pMM8 (pHIS1:DFG5).
For analysis of epitope-tagged Dfg5p, strain DAY280 was transformed with plasmid pDCT1001 (pHIS1:DFG5-1001) or pES10 (pHIS1:DFG5-1001-V5), and His+ transformants were selected to generate the strains ES193 and ES190, respectively. Restoration of filamentation ability was verified on pH 8 M199 medium plus uridine.
For DGF5 shutoff experiments, strain DAY280 was transformed with plasmids pMM8 (pHIS1:DFG5), pES18 (pHIS1:MET3-DFG5), and pES16 (pHIS1:PHR1-DFG5) with selection for His+ transformants. The dcw1Δ::UAU1/dcw1Δ::URA3 recombinants were then selected from the transformants after overnight growth in YPD. We used Arg+ Ura+ selection conditions in which we expected the MET3-DFG5 and PHR1-DFG5 hybrid genes to be expressed (2, 33): SD minimal medium (lacking methionine and cysteine) for the MET3-DFG5 and SD medium titrated to pH 7 for PHR1-DFG5. Arg+ Ura+ mitotic segregants were genotyped by PCR with the primers YKL046 5′CL2 and YKL046 3′CL1/2 to detect the DCW1 allele and primers ARG4 and YKL046 s2 to detect the dcw1Δ::UAU1 allele. We characterized the growth properties of three independent Arg+ Ura+ mitotic segregants that had lost the DCW1 allele for each plasmid. These strains were ES187, ES188, and ES189 (dfg5Δ/dfg5Δ dcw1Δ/dcw1Δ pMM8); ES195, ES269, and ES270 (dfg5Δ/dfg5Δ dcw1Δ/dcw1Δ pES18); and ES218, ES219, and ES220 (dfg5Δ/dfg5Δ dcw1Δ/dcw1Δ pES16).
Plasmid pDDB78, a HIS1 vector, was generated by in vivo recombination in S. cerevisiae (23) of NgoMI-linearized pRS314 (34) with a HIS1 PCR fragment amplified from plasmid pGEM-HIS (39) with primers prs/pgemt-5 and prs/pgemt-3. It has a unique NruI site in HIS1 sequences that is used to target integration to the C. albicans HIS1 locus. We refer to this plasmid as “pHIS1” in genotypic designations.
Plasmid pDDB100 was constructed by ligation of a DFG5 PCR product, generated with primers DFG5 −759 and DFG5 +1619, into vector pGEMt-Easy (Promega).
Plasmid pMM8 (pHIS1:DFG5) was constructed as follows. Plasmid pDDB100 was digested with PvuI, plasmid pDDB78 was digested with EcoRI and NotI, and both were agarose gel purified. In vivo recombination of the DFG5-containing fragment into pDDB78 was carried out through S. cerevisiae cotransformation (23) to generate plasmid pMM8.
To construct DFG5 insertion-bearing plasmids, the Tn7 transposon GPS-LS (New England Biolabs) was used to mutagenize the DFG5 insert of plasmid pDDB100 according to the manufacturer's protocol. Each insertion-bearing plasmid was screened through NcoI digestion to identify DFG5 open reading frame (ORF) insertions, which were then sequenced with primer S (New England Biolabs), which primes out from one end of the transposon. Plasmids were then digested with PmeI and religated to remove the bulk of Tn7 sequences, leaving behind a 15-bp insertion. Each plasmid insert was released with PvuI and moved by in vivo recombination into vector pDDB78. The resulting plasmids were designated pDTCx, with “x” representing the DFG5 ORF nucleotide number immediately 5′ to each insertion.
Plasmid pES10 (pHIS1:DFG5-1001-V5), which expresses the V5 epitope-tagged DFG5 allele, was generated as follows. The plasmid pDTC1001 was digested with PmeI, which cleaves within the 15-bp insertion. Then, theV5-His6 5′ and V5-His6 3′ oligonucleotides were annealed and ligated into the PmeI site. Sequence analysis verified the fidelity and orientation of the inserted sequence.
To express DFG5 under the MET3 promoter (2), the primers 5′DFG5-MET3 and 3′DFG5-MET3 were used to amplify the MET3 promoter sequence from −1615 to −1; to express DFG5 under the PHR1 promoter (33), the primers 5′DFG5-PHR1 and 3′DFG5-PHR1 were used to amplify the PHR1 promoter sequence from −1998 to −1. In vivo recombination in S. cerevisiae was used to integrate the PCR products into PmeI-linearized plasmid pDTC14, yielding the plasmids pES18 (pHIS1:MET3-DFG5) and pES16 (pHIS1:PHR1-DFG5).
Media and growth conditions.
C. albicans was routinely grown in YPD + Uri (2% Bacto Peptone, 1% yeast extract, 2% dextrose, and 80 μg of uridine per ml, with 2% Bacto agar for solid media). Selection following transformation was done on SD minimal medium (6.7% yeast nitrogen base plus ammonium sulfate and without amino acids, 2% dextrose, and 2% Bacto agar for solid media) supplemented with amino acids and nucleotides as required. M199 medium (Gibco BRL) was buffered at either pH 4.0 or pH 8.0 with 150 mM HEPES, supplemented if necessary with 80 μg of uridine per ml, and solidified if necessary with 2% agar. (We previously [reference 8] called this medium TC199, but we have found that it is more commonly called M199.) Cell densities were determined by light scattering at 600 nm. For filamentation tests, C. albicans strains were grown overnight in YPD + Uri at 30°C and then subcultured at an optical density at 600 nm (OD600) of 0.05 into buffered M199 medium or 4% serum liquid prewarmed to 37°C. For tests on solid media, 5 μl of the overnight cultures was plated on M199 medium or 4% serum plates (4% [vol/vol] calf serum [Sigma] added to 2% agar) and incubated at 37°C.
For MET3-DFG5 shutoff experiments, C. albicans strains were grown overnight in SD minimal medium and then subcultured in SD medium with or without 5 mM methionine (Met) and 2 mM cysteine (Cys). For PHR1-DFG5 shutoff experiments, the cells were grown overnight in SD medium at pH 7 (buffered with 150 mM HEPES) and subcultured in SD media at pHs of 7 and 4 (each containing 150 mM HEPES).
Preparation of cell wall and membrane protein fractions.
We followed the S. cerevisiae fractionation procedure of Lu et al. (21) with minor modifications. Mid-exponential-phase 30°C YPD cultures were pelleted, washed, and broken in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2% protease inhibitor cocktail [Sigma]) with glass beads as described previously (32). The lysate was cleared by centrifugation at 1,000 × g for 10 min at 4°C. The 1,000 × g pellet (cell wall fraction) was frozen at −80°C, and the supernatant, containing the membrane and the soluble protein fractions, was centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was removed, and the 10,000 × g pellet (membrane fraction) was resuspended in 1 ml of 0.1 M sodium acetate (pH 5.5) containing 2% sodium dodecyl sulfate (SDS) and protease inhibitors and then boiled for 5 min. The SDS membrane extract was separated from the insoluble material by spinning at 10,000 × g for 10 min at 4°C, dialyzed overnight against 2 liters of 0.1 M potassium phosphate buffer (pH 5.5) at 4°C, and lyophilized.
The cell wall fraction was resuspended in lysis buffer containing 2% SDS, 2% β-mercaptoethanol, and protease inhibitors and then boiled for 5 min and centrifuged at 10,000 × g for 5 min. Boiling and centrifugation were repeated twice, and the pellet was washed three times with deionized water containing protease inhibitors. The washed pellet was digested with the recombinant β-1,3-glucanase yeast lytic enzyme (ICN Biomedicals, Aurora, Ohio) (1,500 U g−1 [wet weight of cell walls]) at room temperature overnight. The lytic enzyme-treated cell walls were centrifuged, and the supernatant, containing the glucanase extracts, was lyophilized into a vacuum pump.
The dry pellet from membranes and the cell wall fraction was resuspended in approximately 10 times the pellet volume of 0.1 M sodium acetate (pH 5.5) buffer containing protease inhibitors. Where indicated, samples were treated with endoglycosidase H (8 mU/μg of extract protein [Sigma]) for 2 h at room temperature.
Western blot analysis.
Membrane and cell wall fractions were boiled in 1× Laemmli reducing buffer and centrifuged at 10,000 × g for 5 min. Samples were analyzed by SDS-polyacrylamide gel electrophoresis on 10% polyacrylamide gels and transferred to nitrocellulose. The filter was then incubated with a blocking solution (3% bovine serum albumin in TBST [Tris-buffered saline plus Tween]) for 60 min at room temperature and washed four times with 1× TBST. Thereafter, the membrane was probed for 2 h at room temperature with horseradish peroxidase-coupled anti-V5 antibody (Invitrogen; 1:2,500 dilution in TBST). Immunoreactivity was detected with an enhanced chemiluminescence kit (Amersham) according to the manufacturer's instructions. As a control, each blot was stripped and reprobed with a concanavalin A-horseradish peroxidase conjugate (Sigma), which was again detected by chemiluminescence.
Northern blot analysis.
Preparation of RNA, Northern analysis, and probes have been described previously (8).
RESULTS
Analysis of Dfg5p and Dcw1p function.
A search of the C. albicans genome sequence revealed two genes that specify proteins similar to S. cerevisiae Dfg5p and Dcw1p (Fig. 1). Based on reciprocal Blastp searches, the predicted C. albicans ORF 19.2075 product and the allelic ORF 19.9622 product are more similar to ScDfg5p than to ScDcw1p, and so we designated the C. albicans gene DFG5. The predicted C. albicans ORF 19.1989 product and the identical allelic 19.9540 product are more similar to ScDcw1p, and we designated the C. albicans gene DCW1. The two C. albicans genes lie approximately 150 kbp apart on allelic sequence contigs 19-1019 and 19-2019.
FIG. 1.
Alignments of Dcw1p and Dfg5p and positions of Dfg5p insertions. A Clustal alignment of the C. albicans and S. cerevisiae proteins Dcw1p and Dfg5p (Ca and Sc, respectively) is shown. Identical residues in at least two of the proteins are shaded black; conservative substitutions are shaded gray. The triangles indicate the positions of Dfg5p mutant insertions; under each triangle is the allele designation and the deduced sequence of the mutant protein with inserted residues in italics. The four insertion sequences that are underlined are partially or fully functional. The extent of filamentation promoted by each construct in strain DAY280, after incubation in pH 8 M199 medium for 6 h, is as follows: pHIS1, <0.25%; pHIS1:DFG5, 80%; pHIS1:DFG5-14, 82%; pHIS1:DFG5-296, -389, -670, -703, -761, -888, -1193, and -1196, all <0.25%; pHIS1:DFG5-680, 41%; pHIS1:DFG5-918, 70%; pHIS1:DFG5-1001, 83%. Numbers represent the mean percent filamentation for six transformants, with standard deviations within 10% of the mean.
S. cerevisiae Dfg5p promotes agar invasion, a form of filamentous growth (25). To determine whether C. albicans Dfg5p or Dcw1p may promote filamentous growth, we examined the phenotype of homozygous deletion mutant strains. The dfg5Δ mutation replaces nucleotides −150 to +409 with a marker cassette; the dcw1Δ mutation replaces nucleotides +600 to +818. These deletion endpoints correspond to the regions of greatest sequence certainty from C. albicans sequence assembly 4, which was current when this work was initiated. Each mutation removes a significant portion of the respective ORF and separates putative signal sequences from putative GPI addition sequences (3), so it is likely that these mutations abolish gene function. Filamentation ability was tested after induction either by alkaline conditions or by the presence of serum. We observed that a dfg5Δ/dfg5Δ strain was defective in filamentation when tested in alkaline (pH 8) liquid or solid medium (Fig. 2, strain ES51). Introduction of a functional DFG5 allele restored filamentation of the dfg5Δ/dfg5Δ strain (Fig. 2, strain ES55), thus indicating that the dfg5Δ mutation is recessive and is the cause of the filamentation defect. The dfg5Δ/dfg5Δ strain had no defect in filamentation in response to serum (Fig. 2). A dcw1Δ/dcw1Δ strain had no filamentation defect under several conditions (Fig. 2) (data not shown). Our results indicate that C. albicans Dfg5p is required for filamentation under alkaline conditions.
FIG. 2.
Filamentation defects caused by dfg5 and dcw1 mutations. Strains indicated across the top of the figure were incubated on 4% serum agar (top row), pH 8 M199 agar (middle row), or pH 8 M199 broth culture (bottom row) to induce filamentation (see Materials and Methods). Photographs of colonies or cells are presented.
Because Dfg5p and Dcw1p are highly related (>50% identity), it seemed possible that they contribute to the same function. This model predicts that the phenotype of a dfg5Δ/dfg5Δ strain may be exacerbated by reduction or elimination of Dcw1p activity. To test this prediction, we first created a dfg5Δ/dfg5Δ strain with a deletion of one DCW1 allele. Like other dfg5Δ/dfg5Δ strains, the dfg5Δ/dfg5Δ dcw1Δ/DCW1 strain was defective in filamentation under alkaline conditions. However, this strain was also defective in filamentation on solid serum-containing medium (Fig. 2, strain ES1). Filamentation ability was restored by introduction of a wild-type DFG5 allele (Fig. 2, strain ES5). The fact that a dfg5Δ/dfg5Δ dcw1Δ/DCW1 strain has a more severe filamentation defect than dfg5Δ/dfg5Δ DCW1/DCW1 strains provides one line of evidence that Dfg5p and Dcw1p have overlapping functions.
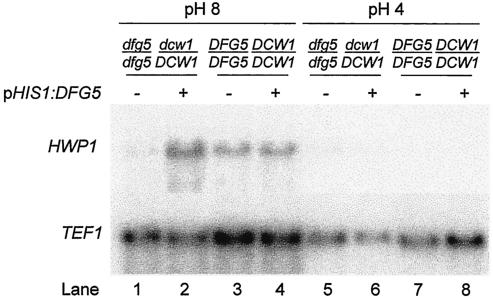
Many gene products are required for C. albicans to produce hyphae in response to alkaline growth conditions. Known mutants that grow but fail to form hyphae in pH 8 medium are defective in signal transduction in some way, as indicated by their failure to express hypha-specific genes (7, 8, 28, 29), such as HWP1 (36). We used Northern analysis to determine whether Dfg5p is also required for HWP1 expression in pH 8 medium. In a DFG5/DFG5 DCW1/DCW1 control strain, the HWP1 transcript was derepressed in pH 8 medium and repressed in pH 4 medium, as expected (Fig. 3, lanes 3 and 7, respectively). In a dfg5Δ/dfg5Δ dcw1Δ/DCW1 mutant, the HWP1 transcript was barely detectable under either growth condition (lanes 1 and 5). Introduction of an ectopic copy of DFG5 restored regulated HWP1 expression in the dfg5Δ/dfg5Δ dcw1Δ/DCW1 mutant (lanes 2 and 6) and did not alter HWP1 expression in a DFG5/DFG5 DCW1/DCW1 control strain (lanes 4 and 8). Hybridization to a control probe for TEF1 RNA verified the integrity of the RNA samples. These results indicate that Dfg5p is required for expression of the hypha-specific HWP1 gene in pH 8 medium.
FIG. 3.
Northern analysis of hypha-specific HWP1 transcript. Strains were grown in pH 8 M199 broth (lanes 1 to 4) and in pH 4 M199 broth (lanes 5 to 8), and RNA was prepared and used for Northern analysis of HWP1 (upper panel) and control TEF1 (lower panel) transcripts. The strains examined were ES1 (dfg5Δ/dfg5Δ dcw1Δ/DCW1 mutant with pHIS1; lanes 1 and 5), ES5 (dfg5Δ/dfg5Δ dcw1Δ/DCW1 mutant with complementing pHIS1:DFG5; lanes 2 and 6), ES57 (control DFG5/DFG5 DCW1/DCW1 strain with pHIS1; lanes 3 and 7), and ES59 (control DFG5/DFG5 DCW1/DCW1 strain with pHIS1:DFG5; lanes 4 and 8).
Synthetic lethality of dfg5 and dcw1 mutations.
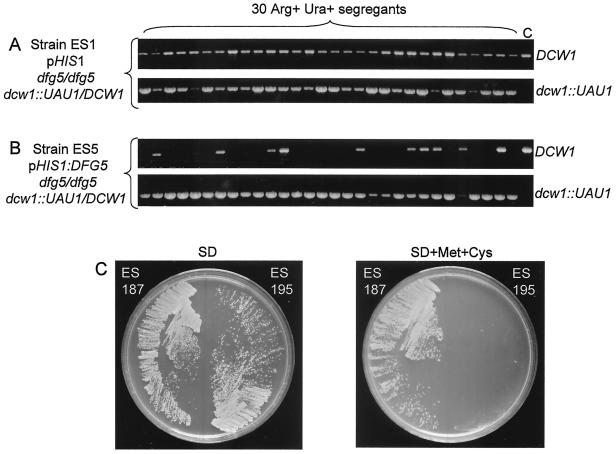
We sought to create a dfg5Δ/dfg5Δ dcw1Δ/dcw1Δ double homozygote to obtain additional evidence for Dfg5p-Dcw1p functional overlap. Attempts to create such a strain through sequential transformation were unsuccessful, which can indicate that the double mutant is inviable. We tested this idea through use of an HT test (10). This test determines whether selection from a heterozygous mutant strain yields homozygous mutant mitotic segregants. The test employs a gene disruption cassette called UAU1, which can express either ARG4 or, after intracassette recombination, URA3. Thus a strain that is heterozygous for a UAU1 insertion is Arg+ Ura− (of the genotype dcw1::UAU1/DCW1, for example) and can undergo recombination to yield mitotic segregants that are Arg− Ura+ (of the genotype dcw1::URA3/DCW1). Rare mitotic segregants arise that are Arg+ Ura+, and these include mitotic recombinants that have become homozygous for the UAU1 insertion mutation and have undergone recombination in one cassette (of the genotype dcw1::UAU1/dcw1::URA3). Other Arg+ Ura+ mitotic segregants have three copies of the relevant locus (dcw1::UAU1/dcw1::URA3/DCW1): such mitotic segregants are the only type found from heterozygous UAU1 insertions in essential genes (10). In practice, we select independent Arg+ Ura+ derivatives of a strain that is heterozygous for a UAU1 insertion after growth in rich medium and assay the presence of the wild-type copy of the locus. In the present case, the analysis was conducted with two strains that were derived by transformation of the same progenitor, strain DAY280, of the genotype dfg5Δ/dfg5Δ dcw1Δ::UAU1/DCW1. The experimental strain, ES1, had no functional copy of DFG5. If loss of both Dfg5p and Dcw1p is lethal, this strain should be unable to yield mitotic segregants that lack a wild-type DCW1 allele. The control strain, ES5, had a copy of DFG5 integrated at the HIS1 locus. This strain should be able to yield mitotic segregants that lack a wild-type DCW1 allele regardless of the Dfg5p-Dcw1p functional relationship. We observed that experimental strain ES1 failed to lose the wild-type DCW1 allele among 30 independent Arg+ Ura+ mitotic segregants (Fig. 4A). In contrast, control strain ES5 lost the wild-type DCW1 allele in 18 of 30 independent Arg+ Ura+ mitotic segregants (Fig. 4B). These observations support the model that Dfg5p and Dcw1p share an essential function, so that cells that lack both gene products are inviable.
FIG. 4.
Tests for synthetic lethality of dfg5 and dcw1 mutations. (A and B) HT tests. Strains ES1 (A) and ES5 (B), both of which are dcw1::UAU1/DCW1, were subjected to Arg+ Ura+ selection to yield homozygous (dcw1::UAU1/dcw1::URA3) and triplication-bearing (dcw1::UAU1/dcw1::URA3/DCW1) mitotic segregants (10). Strain ES1 has no functional copy of DFG5; strain ES5 has a functional copy of DFG5. The presence of a wild-type DCW1 allele and a mutant dcw1::UAU1 allele in each of 30 independent Arg+ Ura+ mitotic segregants was detected by PCR (see Materials and Methods). Lane C shows parallel PCRs using DNA of strain BWP17 (DCW1/DCW1) as a control. (C) Consequences of MET3-DFG5 repression. Strains ES187 and ES195 were streaked on SD (left) and SD + Met + Cys (right) solid media. Growth was assayed by visual inspection after 2 days. Both strains have deletions of the native DFG5 and DCW1 loci. Strain ES187 carries an ectopic copy of the intact DFG5 gene, and strain ES195 carries an ectopic copy of the DFG5 ORF and 3′ region fused to the MET3 promoter, which is repressed in SD + Met + Cys medium.
To test this model directly, we examined the growth of strains homozygous for deletions of the native DFG5 and DCW1 loci that carried an ectopic copy of a conditionally expressed DFG5 gene. For strain ES195, the DFG5 ORF and 3′ region were fused to the MET3 promoter, which is repressed in the presence of methionine and cysteine (2). In the absence of methionine and cysteine, strain ES195 grew as well as control strain ES187, in which an ectopic copy of DFG5 was expressed from its native promoter (Fig. 4C and Table 3). However, strain ES195 failed to grow in the presence of methionine and cysteine, while control strain ES187 grew well. Two independently constructed strains with the same genotype as strain ES195 also displayed a growth defect in the presence of methionine and cysteine (data not shown). We also created strain ES218, in which the DFG5 ORF and 3′ region were fused to the PHR1 promoter, which is expressed in neutral and alkaline media and is repressed in acidic media. Strain ES218 grew as well as control strain ES187 in pH 7 medium, but it displayed a quantitative growth defect in pH 4 medium (Table 3). These results support the model that cells require at least one of the proteins Dfg5p and Dcw1p for growth, so that dfg5 and dcw1 are synthetic lethal mutations.
TABLE 3.
Effects of conditional DFG5 expression on cell growth
| Strain | Relevant genotypea | OD600 at 24 hb
|
|
|---|---|---|---|
| Repressing | Nonrepressing | ||
| ES187 | DFG5 | 7.2 | 7.1 |
| ES195 | MET3-DFG5 | 0.05 | 7.4 |
| ES218 | PHR1-DFG5 | 2.4 | 10.1 |
Strains were of genotype dfg5/dfg5 dcw1/dcw1 and carried the indicated DFG5 construct integrated at the HIS1 locus.
Strains were inoculated at an OD600 of 0.05 and grown for 24 h. Each OD value is the mean of three independent cultures, and standard deviations were within 10% of the mean. For strains ES187 and ES195, the repressing medium is SD + Met + Cys, and the nonrepressing medium is pH7 SD. For strain ES218, the repressing medium is pH4 SD, and the nonrepressing medium is pH7 SD. Cultures of control strain ES187 reached OD600 values of 6.0 and 8.7 in the latter two media, respectively. In all cases, overnight cultures were grown in nonrepressing medium prior to inoculation.
Evidence that Dfg5p is a membrane and cell wall protein.
We sought to determine the localization of Dfg5p through subcellular fractionation. To identify Dfg5p in extracts, we wished to introduce an epitope tag. Dfg5p presents two difficulties for epitope tagging. First, introduction of an epitope at the N or C terminus may alter localization by blocking recognition of the signal sequence or GPI-anchor addition signal. Sequence inspection confirmed presence of these hydrophobic sequences (3) at the Dfg5p N and C termini (Fig. 1). Also, these segments are generally cleaved during maturation of surface proteins, so an appended epitope would be lost. Second, because Dfg5p is highly conserved throughout the length of the protein (Fig. 1), few internal sites are logical choices for insertion of an epitope. Thus, we used insertional mutagenesis to identify tolerant sites in Dfg5p, introduced an epitope tag at a tolerant internal site, and analyzed membrane and cell wall fractions for the presence of the epitope-tagged protein.
Insertion mutants were created and analyzed as follows. Transposon Tn7-GPS-LS was inserted into a DFG5 plasmid through in vitro transposition, and insertions within the ORF were identified through restriction digestion and sequence analysis. The bulk of each transposon was removed via restriction enzyme digestion and ligation to yield a set of 15-bp insertion mutants. Some of these insertions introduced chain-terminating nonsense codons and were not analyzed further. The remaining 12 DFG5 insertion alleles were subjected to functional analysis (Fig. 1). Each allele, carried on a HIS1 vector, was transformed into strain DAY280 (dfg5Δ/dfg5Δ dcw1Δ/DCW1) with integration directed to the HIS1 locus through cleavage within the C. albicans HIS1 vector sequences. Six independent transformants were then tested for filamentation ability in pH 8 liquid medium (Fig. 1). Most of the insertions (8 of 12) interfered with DFG5 function, because the transformants were as defective in filamentation as control transformants carrying the pHIS1 vector. Four insertion alleles retained considerable function: DFG5-14, -680, -918, and -1001 (underlined in Fig. 1). DFG5-14 is within the putative signal sequence; this site is not suitable for epitope introduction, as discussed above. DFG5-680 is at the junction between two sequence blocks that are highly conserved among C. albicans and S. cerevisiae Dfg5p and Dcw1p. Nearby insertions (DFG5-670, -703, and -761) abolish detectable function, thus suggesting that this region has limited tolerance for sequence alteration. Therefore, we considered this region less than optimal for epitope introduction. DFG5-918 lies within a well-conserved sequence block; DFG5-1001 lies at the end of the same sequence block. The fact that these two nearby insertions are both functional suggests that this region may be relatively tolerant of sequence alterations. Therefore, we inserted sequences specifying an in-frame V5-His6 cassette into the DFG5-1001 insertion site. Complementation tests verified that epitope-tagged Dfg5-1001-V5p retained function (data not shown).
To determine the subcellular distribution of Dfg5p, we prepared membrane and cell wall fractions from two strains, one expressing tagged Dfg5-1001-V5p and one expressing untagged Dfg5-1001p. Samples from the two strains were compared on anti-V5 immunoblots (Fig. 5). The anti-V5 antibody detected a 72-kDa protein present in both membrane and cell wall fractions of the strain expressing Dfg5-1001-V5p (lanes 2 and 10) and not the strain expressing Dfg5-1001p (lanes 1 and 9). Some Dfg5-1001-V5p was also detectable as a heterogeneous ∼83-kDa species (lanes 2 and 10). The membrane fractions had cross-reacting proteins of 53 and 45 kDa; the presence of these species in both untagged and tagged extracts (lanes 1 and 2) indicates that they are not derived from Dfg5p. Reprobing of the blots with concanavalin A verified that samples from both strains had comparable amounts of total glycoprotein (lanes 5, 6, 13, and 14). These results support the idea that Dfg5p is a 72-kDa protein present in the cell membrane and cell wall.
FIG. 5.
Analysis of Dfg5p localization and processing. Membrane fractions (lanes 1 to 8) and cell wall fractions (lanes 9 to 16) were analyzed by Western blotting with anti-V5 epitope monoclonal antibody (lanes 1 to 4 and 9 to 12) or control concanavalin A (lanes 5 to 8 and 13 to 16). Samples were analyzed before (lanes 1, 2, 5, 6, 9, 10, 13, and 14) and after (lanes 3, 4, 7, 8, 11, 12, 15, and 16) treatment with endoglycosidase H. Strains ES193 (expressing untagged Dfg5-1001p; odd-numbered lanes) and ES190 (expressing tagged Dfg5-1001-V5p) were compared.
Many cell surface proteins are modified by N-linked glycosylation, and Dfg5p contains seven potential N-glycosylation sites (NXT/T) at amino acid positions 86, 111, 135, 203, 243, 268, and 402. Thus, we determined whether the apparent molecular weight of Dfg5-1001-V5p is altered by digestion with endoglycosidase H, which removes N-linked carbohydrate residues. We observed that the mobility of Dfg5-1001-V5p in both membrane and cell wall fractions was increased after digestion with endoglycosidase H, yielding a 55-kDa protein species (Fig. 5, lanes 4 and 12). Parallel digestion of samples containing untagged Dfg5-1001p (lanes 3 and 11) indicated that the 55-kDa protein is Dfg5-1001-V5p and not a cross-reacting protein. Reprobing with concanavalin A indicated that total glycoprotein mobility was increased by endoglycosidase H treatment (lanes 7, 8, 15, and 16). These results indicate that Dfg5p undergoes N-linked glycosylation. This finding is consistent with the model that Dfg5p is a cell surface protein.
DISCUSSION
The C. albicans cell wall is a participant in dramatic morphological changes, a contributor to virulence, and a source of prospective targets for therapeutic strategies. Our studies here have focused on a pair of closely related proteins, Dfg5p and Dcw1p, with structural features of cell membrane and cell wall proteins. Our findings argue that C. albicans Dfg5p and, by analogy, Dcw1p are localized in the cell membrane and cell wall and that they are together essential for growth. These properties are similar to those of S. cerevisiae Dfg5p and Dcw1p (14). Dfg5p is required for hypha formation under some conditions in C. albicans, a feature that we expected based on the requirement for S. cerevisiae Dfg5p in agar invasion (25). Surprisingly, we find that C. albicans Dfg5p is required for expression of a hypha-specific gene, which suggests that Dfg5p functions in some way as a regulator of hypha development, as discussed below.
Two lines of evidence indicate that Dfg5p is a cell membrane and cell wall protein. First, we find that epitope-tagged Dfg5-1001-V5p is associated with cell membrane and cell wall fractions. The epitope-tagged protein is functional, because it complements the filamentation defect of a dfg5Δ/dfg5Δ mutant. This observation argues that its localization does not arise from misfolding or artifactual channeling of the protein into the secretory system. Second, endoglycosidase H digestion shows that the protein is N glycosylated. This modification occurs during transit of proteins to the cell surface and membrane-bound organelles (15). Most or all detectable Dfg5-1001-V5p migrates at 72 kDa, thus arguing that most or all Dfg5p is N glycosylated. Therefore, we conclude that Dfg5p is largely associated with membranes and the cell wall or en route to those destinations.
We are unaware of other reports of Tn7 mutagenesis for epitope tagging in C. albicans, and aspects of this strategy may prove generally useful. The N and C termini of proteins are simple sites for epitope introduction for technical reasons, but epitope attachment there can disrupt protein function. Such problems are expected in the case of secreted and surface proteins. Therefore, an epitope must be introduced at an internal site. Although suitable internal sites can be deduced from conservation or structural information, our experience has been that epitope introduction at such sites often compromises protein function. Identification of tolerant sites is thus a trial-and-error exercise. Tn7 mutagenesis simplifies sampling of many insertion sites and permits introduction of a variety of epitopes, functional domains, or other sequences. For example, here we used Tn7 insertions both for introduction of an epitope tag and for fusions to regulated promoters.
Our studies indicate that C. albicans Dfg5p and Dcw1p share a function that is required for growth, as indicated by both an HT test and the consequences of Dfg5p depletion. Depletion experiments were conducted with strains lacking native functional DFG5 and DCW1 loci and carrying instead fusions of the DFG5 ORF to either the MET3 or PHR1 promoter. Growth of the strain carrying MET3-DFG5 was blocked on repressing medium. This observation argues that Dfg5p is required for growth in the absence of Dcw1p function. Growth of the strain carrying PHR1-DFG5 was impaired but not blocked on repressing medium. This result is consistent with the conclusion that Dfg5p and Dcw1p are required for growth; we infer that PHR1-DFG5 is expressed to some extent under our repression conditions. MET3-DFG5 strains provide the clearest demonstration of Dfg5p-Dcw1p essentiality, but PHR1-DFG5 strains might be useful in screening for inhibitors of Dfg5p activity.
The insertional mutagenesis of Dfg5p reported here was not extensive, but supports the model that Dfg5p and Dcw1p have similar biochemical functions. First, most mutations (8 of 12) impaired Dfg5p function, as expected given the extensive sequence conservation of Dfg5p and Dcw1p. Second, two of the four functional insertion mutations (DFG5-14 and -680) affected regions of low conservation. Sequence conservation within the Dfg5p-Dcw1p protein family thus appears to be significant for function.
S. cerevisiae Dfg5p and Dcw1p have been proposed to function in cell wall biogenesis, as indicated by several observations (14). First, a dcw1Δ mutant is hypersensitive to β-glucan hydrolysis by zymolyase. Second, the dcw1Δ dfg5Δ genotype is lethal, and depletion of Dcw1p in a strain lacking Dfg5p causes cells to acquire properties typical of cell wall-defective mutants. These properties include an enlarged, rounded appearance, accumulation of delocalized chitin, and release of the GPI-linked cell wall protein Cwp1p into the medium. The parallels between C. albicans and S. cerevisiae Dfg5p and Dcw1p functions and properties are substantial. All four proteins share considerable sequence identity, and S. cerevisiae Dcw1p is an N-glycosylated protein found in the cell membrane and cell wall, as we report here for C. albicans Dfg5p. Moreover, Dfg5p is required for forms of filamentous growth in both S. cerevisiae (25) and C. albicans. Thus, the inference that C. albicans Dfg5p and Dcw1p also function in cell wall biogenesis is reasonable, although we have not addressed this question directly.
We note one difference between observations in S. cerevisiae and C. albicans. Our strains in which Dfg5p is depleted through repression of MET3-DFG5 do not acquire aberrant morphology and remain viable for several days. This observation stands in contrast to the results of Dcw1p depletion in a dcw1Δ dfg5Δ S. cerevisiae strain, which yielded an aberrant cell morphology and cell death. It is possible that C. albicans is more tolerant of cell wall aberrations than S. cerevisiae. A second explanation is that a low repressed level of MET3-DFG5 expression is sufficient to permit stasis but not growth.
If Dfg5p functions in cell wall biogenesis, how can it be required for hypha development? One simple model is that a defect in cell wall integrity may block initiation of hypha formation. Three prior studies support this model. First, a kre9/kre9 mutant, which has reduced β1,6-glucan levels, grows as yeast cells rather than hyphae in serum (22). Second, a gpi7/gpi7 mutant, which has abnormal cell wall structure and composition, grows as yeast cells rather than hyphae in Spider medium (30, 31). Third, the C. albicans mitogen-activated protein kinase Mkc1p is required for both cell wall integrity and filamentation (26). Our study extends this model with the observation that Dfg5p is required for full expression of the hypha-specific gene HWP1. According to this model, the cell wall defect of a dfg5/dfg5 mutant does not simply cause a structural impediment to hyphal growth, but causes a regulatory response that reduces expression of a hypha-specific gene. Thus, there may be a cell wall integrity “checkpoint” that is required for the hyphal growth and gene expression program. A second model is that Dfg5p has a more direct role in sensing the environmental signals that induce hypha formation. Analysis of double mutants has not allowed us to place Dfg5p clearly in either the Rim101p or Mds3p pH-response regulatory pathways (8), but a Dfg5p-dependent signal could act downstream of both pathways (M. Kim and A. P. Mitchell, unpublished results). This model may seem unlikely, because Dfg5p has no obvious transmembrane domain for signal transmission across the plasma membrane. However, there are precedents for transmembrane signaling by GPI-linked cell surface proteins (5, 13). It is also possible that Dfg5p may promote signaling through interaction with or modification of a protein that has a transmembrane domain. Our work here lays the foundation for elucidation of this signaling mechanism.
Acknowledgments
We thank Rafael Ovalle and Peter Lipke for advice concerning preparation of cell wall and cell membrane fractions. We are grateful to John E. Edwards, Jr., Scott G. Filler, Ashraf Ibrahim, Tom Kozel, Jim Cutler, and other members of the Candida Adherence Mycology Research Unit for many helpful discussions. We acknowledge gratefully the availability of the C. albicans genome sequence, provided by the Stanford DNA Sequencing and Technology Center with the support of the NIDR, the NIH and the Burroughs Wellcome Fund. We are grateful to members of the Mitchell lab for very useful input and for comments on this manuscript.
This work was supported by a Mycology Scholar Award from the Burroughs Wellcome Fund (to A.P.M.) and by grants T32 AI07161-21 and PO1 AI377194 from the National Institutes of Health.
REFERENCES
- 1.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 2.Care, R. S., J. Trevethick, K. M. Binley, and P. E. Sudbery. 1999. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol. Microbiol. 34:792-798. [DOI] [PubMed] [Google Scholar]
- 3.Caro, L. H., H. Tettelin, J. H. Vossen, A. F. Ram, H. van den Ende, and F. M. Klis. 1997. In silicio identification of glycosyl-phosphatidylinositol-anchored plasma-membrane and cell wall proteins of Saccharomyces cerevisiae. Yeast 13:1477-1489. [DOI] [PubMed] [Google Scholar]
- 4.Chaffin, W. L., J. L. Lopez-Ribot, M. Casanova, D. Gozalbo, and J. P. Martinez. 1998. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol. Mol. Biol. Rev. 62:130-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi, A., Z. Siddiqui, F. Bayiroglu, and K. V. Rao. 2002. A GPI-linked isoform of the IgD receptor regulates resting B cell activation. Nat. Immunol. 3:951-957. [DOI] [PubMed] [Google Scholar]
- 6.Davis, D., J. E. Edwards, Jr., A. P. Mitchell, and A. S. Ibrahim. 2000. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect. Immun. 68:5953-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, D., R. B. Wilson, and A. P. Mitchell. 2000. RIM101-dependent and -independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, D. A., V. M. Bruno, L. Loza, S. G. Filler, and A. P. Mitchell. 2002. Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics 162:1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon, D. M., A. Casadevall, B. Klein, L. Mendoza, L. Travassos, and G. S. Deepe, Jr. 1998. Development of vaccines and their use in the prevention of fungal infections. Med. Mycol. 36(Suppl. 1):57-67. [PubMed] [Google Scholar]
- 10.Enloe, B., A. Diamond, and A. P. Mitchell. 2000. A single-transformation gene function test in diploid Candida albicans. J. Bacteriol. 182:5730-5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu, Y., A. S. Ibrahim, D. C. Sheppard, Y. C. Chen, S. W. French, J. E. Cutler, S. G. Filler, and J. E. Edwards, Jr. 2002. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol. Microbiol. 44:61-72. [DOI] [PubMed] [Google Scholar]
- 12.Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles, S. Veronneau, S. Dow, A. Lucau-Danila, K. Anderson, B. Andre, A. P. Arkin, A. Astromoff, M. El-Bakkoury, R. Bangham, R. Benito, S. Brachat, S. Campanaro, M. Curtiss, K. Davis, A. Deutschbauer, K. D. Entian, P. Flaherty, F. Foury, D. J. Garfinkel, M. Gerstein, D. Gotte, U. Guldener, J. H. Hegemann, S. Hempel, Z. Herman, D. F. Jaramillo, D. E. Kelly, S. L. Kelly, P. Kotter, D. LaBonte, D. C. Lamb, N. Lan, H. Liang, H. Liao, L. Liu, C. Luo, M. Lussier, R. Mao, P. Menard, S. L. Ooi, J. L. Revuelta, C. J. Roberts, M. Rose, P. Ross-Macdonald, B. Scherens, G. Schimmack, B. Shafer, D. D. Shoemaker, S. Sookhai-Mahadeo, R. K. Storms, J. N. Strathern, G. Valle, M. Voet, G. Volckaert, C. Y. Wang, T. R. Ward, J. Wilhelmy, E. A. Winzeler, Y. Yang, G. Yen, E. Youngman, K. Yu, H. Bussey, J. D. Boeke, M. Snyder, P. Philippsen, R. W. Davis, and M. Johnston. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387-391. [DOI] [PubMed] [Google Scholar]
- 13.Hiscox, S., M. B. Hallett, B. P. Morgan, and C. W. van den Berg. 2002. GPI-anchored GFP signals Ca2+ but is homogeneously distributed on the cell surface. Biochem. Biophys. Res. Commun. 293:714-721. [DOI] [PubMed] [Google Scholar]
- 14.Kitagaki, H., H. Wu, H. Shimoi, and K. Ito. 2002. Two homologous genes, DCW1 (YKL046c) and DFG5, are essential for cell growth and encode glycosylphosphatidylinositol (GPI)-anchored membrane proteins required for cell wall biogenesis in Saccharomyces cerevisiae. Mol. Microbiol. 46:1011-1022. [DOI] [PubMed] [Google Scholar]
- 15.Klis, F. M., P. Mol, K. Hellingwerf, and S. Brul. 2002. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26:239-256. [DOI] [PubMed] [Google Scholar]
- 16.Lamb, T. M., and A. P. Mitchell. 2003. The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 23:677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamb, T. M., W. Xu, A. Diamond, and A. P. Mitchell. 2001. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 276:1850-1856. [DOI] [PubMed] [Google Scholar]
- 18.Li, W., and A. P. Mitchell. 1997. Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics 145:63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipke, P. N., and R. Ovalle. 1998. Cell wall architecture in yeast: new structure and new challenges. J. Bacteriol. 180:3735-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, H. 2001. Transcriptional control of dimorphism in Candida albicans. Curr. Opin. Microbiol. 4:728-735. [DOI] [PubMed] [Google Scholar]
- 21.Lu, C. F., J. Kurjan, and P. N. Lipke. 1994. A pathway for cell wall anchorage of Saccharomyces cerevisiae α-agglutinin. Mol. Cell. Biol. 14:4825-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lussier, M., A. M. Sdicu, S. Shahinian, and H. Bussey. 1998. The Candida albicans KRE9 gene is required for cell wall beta-1, 6-glucan synthesis and is essential for growth on glucose. Proc. Natl. Acad. Sci. USA 95:9825-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma, H., S. Kunes, P. J. Schatz, and D. Botstein. 1987. Plasmid construction by homologous recombination in yeast. Gene 58:201-216. [DOI] [PubMed] [Google Scholar]
- 24.Monteoliva, L., M. L. Matas, C. Gil, C. Nombela, and J. Pla. 2002. Large-scale identification of putative exported proteins in Candida albicans by genetic selection. Eukaryot. Cell 1:514-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosch, H. U., and G. R. Fink. 1997. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics 145:671-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navarro-Garcia, F., R. Alonso-Monge, H. Rico, J. Pla, R. Sentandreu, and C. Nombela. 1998. A role for the MAP kinase gene MKC1 in cell wall construction and morphological transitions in Candida albicans. Microbiology 144:411-424. [DOI] [PubMed] [Google Scholar]
- 27.Navarro-Garcia, F., M. Sanchez, C. Nombela, and J. Pla. 2001. Virulence genes in the pathogenic yeast Candida albicans. FEMS Microbiol. Rev. 25:245-268. [DOI] [PubMed] [Google Scholar]
- 28.Porta, A., A. M. Ramon, and W. A. Fonzi. 1999. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J. Bacteriol. 181:7516-7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramon, A. M., A. Porta, and W. A. Fonzi. 1999. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J. Bacteriol. 181:7524-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard, M., P. De Groot, O. Courtin, D. Poulain, F. Klis, and C. Gaillardin. 2002. GPI7 affects cell-wall protein anchorage in Saccharomyces cerevisiae and Candida albicans. Microbiology 148:2125-2133. [DOI] [PubMed] [Google Scholar]
- 31.Richard, M., S. Ibata-Ombetta, F. Dromer, F. Bordon-Pallier, T. Jouault, and C. Gaillardin. 2002. Complete glycosylphosphatidylinositol anchors are required in Candida albicans for full morphogenesis, virulence and resistance to macrophages. Mol. Microbiol. 44:841-853. [DOI] [PubMed] [Google Scholar]
- 32.Santoni, G., R. Lucciarini, C. Amantini, J. Jacobelli, E. Spreghini, P. Ballarini, M. Piccoli, and A. Gismondi. 2002. Candida albicans expresses a focal adhesion kinase-like protein that undergoes increased tyrosine phosphorylation upon yeast cell adhesion to vitronectin and the EA.hy 926 human endothelial cell line. Infect. Immun. 70:3804-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saporito-Irwin, S. M., C. E. Birse, P. S. Sypherd, and W. A. Fonzi. 1995. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol. Cell. Biol. 15:601-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singleton, D. R., J. Masuoka, and K. C. Hazen. 2001. Cloning and analysis of a Candida albicans gene that affects cell surface hydrophobicity. J. Bacteriol. 183:3582-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundstrom, P. 2002. Adhesion in Candida spp. Cell Microbiol. 4:461-469. [DOI] [PubMed] [Google Scholar]
- 37.Tkacz, J. S., and B. DiDomenico. 2001. Antifungals: what's in the pipeline. Curr. Opin. Microbiol. 4:540-545. [DOI] [PubMed] [Google Scholar]
- 38.Wilson, R. B., D. Davis, B. M. Enloe, and A. P. Mitchell. 2000. A recyclable Candida albicans URA3 cassette for PCR product-directed gene disruptions. Yeast 16:65-70. [DOI] [PubMed] [Google Scholar]
- 39.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]