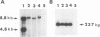Abstract
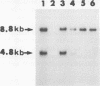
Maternal transmission of a murine leukemia virus (MuLV) mixture named LP-BM5 MuLV, which is knwon to induce murine AIDS (MAIDS), was investigated. Adult female C57BL/10 mice were inoculated intraperitoneally with LP-BM5 MuLV. When the virus-inoculated female mice developed splenomegaly or lymphadenopathy, they were mated with normal C57BL/10 male mice. Of 56 offspring born to MAIDS mothers, 14 appeared to develop MAIDS, as assessed by the occurrence of splenomegaly or lymphadenopathy as well as the mitogen response of spleen cells. The occurrence of MAIDS in offspring was found to be accompanied by the maternal transmission and expansion of a defective virus genome from which almost the entire pol and env regions are deleted. On the other hand, the ecotropic helper virus genome was detected in all offspring regardless of the occurrence of MAIDS. To examine the mode of maternal transmission of LP-BM5 MuLV, foster-nursing experiments were conducted. The ecotropic helper viruses were found in all normal offspring nursed by a MAIDS mother, and some of them developed MAIDS. In contrast, none of offspring born to a MAIDS mother that were nursed by an uninfected foster mother either carried the LP-BM5 MuLV or developed MAIDS. Finally, both the defective and the ecotropic helper viruses were detected in LP-BM5 MuLV-infected mother's milk. These results indicated that maternal transmission of LP-BM5 MuLV occurs with a high frequency and is mediated by mother's milk.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz D. C., Hanna Z., Jolicoeur P. Severe immunodeficiency disease induced by a defective murine leukaemia virus. Nature. 1989 Apr 6;338(6215):505–508. doi: 10.1038/338505a0. [DOI] [PubMed] [Google Scholar]
- Bendinelli M., Matteucci D., Friedman H. Retrovirus-induced acquired immunodeficiencies. Adv Cancer Res. 1985;45:125–181. doi: 10.1016/s0065-230x(08)60268-7. [DOI] [PubMed] [Google Scholar]
- Blanche S., Rouzioux C., Moscato M. L., Veber F., Mayaux M. J., Jacomet C., Tricoire J., Deville A., Vial M., Firtion G. A prospective study of infants born to women seropositive for human immunodeficiency virus type 1. HIV Infection in Newborns French Collaborative Study Group. N Engl J Med. 1989 Jun 22;320(25):1643–1648. doi: 10.1056/NEJM198906223202502. [DOI] [PubMed] [Google Scholar]
- Buffett R. F., Grace J. T., Jr, DiBerardino L. A., Mirand E. A. Vertical transmission of murine leukemia virus through successive generations. Cancer Res. 1969 Mar;29(3):596–602. [PubMed] [Google Scholar]
- Buller R. M., Yetter R. A., Fredrickson T. N., Morse H. C., 3rd Abrogation of resistance to severe mousepox in C57BL/6 mice infected with LP-BM5 murine leukemia viruses. J Virol. 1987 Feb;61(2):383–387. doi: 10.1128/jvi.61.2.383-387.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Morse H. C., 3rd, Makino M., Ruscetti S. K., Hartley J. W. Defective virus is associated with induction of murine retrovirus-induced immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1989 May;86(10):3862–3866. doi: 10.1073/pnas.86.10.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnst A., Lindgren S., Dictor M., Johansson B., Sönnerborg A., Czajkowski J., Sundin G., Bohlin A. B. HIV in pregnant women and their offspring: evidence for late transmission. Lancet. 1991 Jul 27;338(8761):203–207. doi: 10.1016/0140-6736(91)90347-r. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gardner M. B. Type C viruses of wild mice: characterization and natural history of amphotropic, ecotropic, and xenotropic MuLv. Curr Top Microbiol Immunol. 1978;79:215–259. doi: 10.1007/978-3-642-66853-1_5. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huang M., Simard C., Jolicoeur P. Immunodeficiency and clonal growth of target cells induced by helper-free defective retrovirus. Science. 1989 Dec 22;246(4937):1614–1617. doi: 10.1126/science.2480643. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Retroviruses and embryogenesis: microinjection of Moloney leukemia virus into midgestation mouse embryos. Cell. 1980 Jan;19(1):181–188. doi: 10.1016/0092-8674(80)90399-2. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P. Murine acquired immunodeficiency syndrome (MAIDS): an animal model to study the AIDS pathogenesis. FASEB J. 1991 Jul;5(10):2398–2405. doi: 10.1096/fasebj.5.10.2065888. [DOI] [PubMed] [Google Scholar]
- KRISCHKE W., GRAFFI A. The transmission of the virus of myeloid leukaemia of mice by the milk. Acta Unio Int Contra Cancrum. 1963;19:360–361. [PubMed] [Google Scholar]
- Kawashima K., Ikeda H., Hartley J. W., Stockert E., Rowe W. P., Old L. J. Changes in expression of murine leukemia virus antigens and production of xenotropic virus in the late preleukemic period in AKR mice. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4680–4684. doi: 10.1073/pnas.73.12.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATARJET R., DUPLAN J. F. Experiment and discussion on leukaemogenesis by cell-free extracts of radiation-induced leukaemia in mice. Int J Radiat Biol Relat Stud Phys Chem Med. 1962 Aug;5:339–344. doi: 10.1080/09553006214550911. [DOI] [PubMed] [Google Scholar]
- Law L. W. Transmission studies of a leukemogenic virus, MLV, in mice. Natl Cancer Inst Monogr. 1966 Sep;22:267–285. [PubMed] [Google Scholar]
- Mizuochi T., Mizuguchi J., Uchida T., Ohnishi K., Nakanishi M., Asano Y., Kakiuchi T., Fukushima Y., Okuyama K., Morse H. C., 3rd A selective signaling defect in helper T cells induced by antigen-presenting cells from mice with murine acquired immunodeficiency syndrome. J Immunol. 1990 Jan 1;144(1):313–316. [PubMed] [Google Scholar]
- Mosier D. E. Animal models for retrovirus-induced immunodeficiency disease. Immunol Invest. 1986 May;15(3):233–261. doi: 10.3109/08820138609026687. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Yetter R. A., Morse H. C., 3rd Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J Exp Med. 1985 Apr 1;161(4):766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S., Ando Y., Saito K., Moriyama I., Ichijo M., Toyama T., Sugamura K., Imai J., Hinuma Y. Primary infection of Japanese infants with adult T-cell leukaemia-associated retrovirus (ATLV): evidence for viral transmission from mothers to children. J Infect. 1986 May;12(3):205–212. doi: 10.1016/s0163-4453(86)94086-7. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P., Moschese V. Mother-to-child transmission of human immunodeficiency virus. FASEB J. 1991 Jul;5(10):2419–2426. doi: 10.1096/fasebj.5.10.2065890. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Hunter J. J., Ruprecht R. M., Jaenisch R. Maternal transmission of retroviral disease and strategies for preventing infection of the neonate. J Virol. 1989 Mar;63(3):1049–1053. doi: 10.1128/jvi.63.3.1049-1053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker C., Goff S., Gilboa E., Paskind M., Mitra S. W., Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3932–3936. doi: 10.1073/pnas.77.7.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Perre P., Simonon A., Msellati P., Hitimana D. G., Vaira D., Bazubagira A., Van Goethem C., Stevens A. M., Karita E., Sondag-Thull D. Postnatal transmission of human immunodeficiency virus type 1 from mother to infant. A prospective cohort study in Kigali, Rwanda. N Engl J Med. 1991 Aug 29;325(9):593–598. doi: 10.1056/NEJM199108293250901. [DOI] [PubMed] [Google Scholar]
- Via C. S., Morse H. C., 3rd, Shearer G. M. Altered immunoregulation and autoimmune aspects of HIV infection: relevant murine models. Immunol Today. 1990 Jul;11(7):250–255. doi: 10.1016/0167-5699(90)90099-u. [DOI] [PubMed] [Google Scholar]