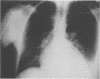
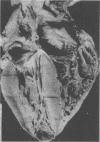
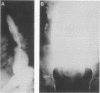
Abstract
Chagas' disease, caused by Trypanosoma cruzi, is an important cause of morbidity in many countries in Latin America. The important modes of transmission are by the bite of the reduviid bug and blood transfusion. The organism exists in three morphological forms: trypomastigotes, amastigotes, and epimastigotes. The mechanism of transformation and differentiation is currently being explored, and signal transduction pathways of the parasites may be involved in this process. Parasite adherence to and invasion of host cells is a complex process involving complement, phospholipase, penetrin, neuraminidase, and hemolysin. Two clinical forms of the disease are recognized, acute and chronic. During the acute stage pathological damage is related to the presence of the parasite, whereas in the chronic stage few parasites are found. In recent years the roles of tumor necrosis factor, gamma interferon, and the interleukins in the pathogenesis of this infection have been reported. The common manifestations of chronic cardiomyopathy are arrhythmias and thromboembolic events. Autoimmune, neurogenic, and microvascular factors may be important in the pathogenesis of the cardiomyopathy. The gastrointestinal tract is another important target, and "mega syndromes" are common manifestations. The diagnosis and treatment of this infection are active areas of investigation. New serological and molecular biological techniques have improved the diagnosis of chronic infection. Exacerbations of T. cruzi infection have been reported for patients receiving immuno-suppressive therapy and for those with AIDS.
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta A. M., Santos-Buch C. A. Autoimmune myocarditis induced by Trypanosoma cruzi. Circulation. 1985 Jun;71(6):1255–1261. doi: 10.1161/01.cir.71.6.1255. [DOI] [PubMed] [Google Scholar]
- Acquatella H., Catalioti F., Gomez-Mancebo J. R., Davalos V., Villalobos L. Long-term control of Chagas disease in Venezuela: effects on serologic findings, electrocardiographic abnormalities, and clinical outcome. Circulation. 1987 Sep;76(3):556–562. doi: 10.1161/01.cir.76.3.556. [DOI] [PubMed] [Google Scholar]
- Acquatella H., Schiller N. B. Echocardiographic recognition of Chagas' disease and endomyocardial fibrosis. J Am Soc Echocardiogr. 1988 Jan-Feb;1(1):60–68. doi: 10.1016/s0894-7317(88)80064-6. [DOI] [PubMed] [Google Scholar]
- Amorim D. S., Olsen E. G. Assessment of heart neurons in dilated (congestive) cardiomyopathy. Br Heart J. 1982 Jan;47(1):11–18. doi: 10.1136/hrt.47.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade S. G., Grimaud J. A. Chronic murine myocarditis due to Trypanosoma cruzi--an ultrastructural study and immunochemical characterization of cardiac interstitial matrix. Mem Inst Oswaldo Cruz. 1986 Jan-Mar;81(1):29–41. doi: 10.1590/s0074-02761986000100004. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A., Andrade S. G., Oliveira G. B., Alonso D. R. Histopathology of the conducting tissue of the heart in Chagas' myocarditis. Am Heart J. 1978 Mar;95(3):316–324. doi: 10.1016/0002-8703(78)90362-9. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A., Andrade S. G., Sadigursky M. Enhancement of chronic Trypanosoma cruzi myocarditis in dogs treated with low doses of cyclophosphamide. Am J Pathol. 1987 Jun;127(3):467–473. [PMC free article] [PubMed] [Google Scholar]
- Andrade Z. A., Andrade S. G., Sadigursky M., Maguire J. H. Experimental Chagas' disease in dogs. A pathologic and ECG study of the chronic indeterminate phase of the infection. Arch Pathol Lab Med. 1981 Sep;105(9):460–464. [PubMed] [Google Scholar]
- Andrews N. W., Abrams C. K., Slatin S. L., Griffiths G. A T. cruzi-secreted protein immunologically related to the complement component C9: evidence for membrane pore-forming activity at low pH. Cell. 1990 Jun 29;61(7):1277–1287. doi: 10.1016/0092-8674(90)90692-8. [DOI] [PubMed] [Google Scholar]
- Andrews N. W., Robbins E. S., Ley V., Hong K. S., Nussenzweig V. Developmentally regulated, phospholipase C-mediated release of the major surface glycoprotein of amastigotes of Trypanosoma cruzi. J Exp Med. 1988 Feb 1;167(2):300–314. doi: 10.1084/jem.167.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Chiari E., Dias J. C. Demonstration of Trypanosoma cruzi antigen in serum from patients with Chagas' disease. Lancet. 1981 Jan 31;1(8214):246–249. doi: 10.1016/s0140-6736(81)92088-2. [DOI] [PubMed] [Google Scholar]
- Arreaza N., Puigbó J. J., Acquatella H., Casal H., Giordano H., Valecillos R., Mendoza I., Pérez J. F., Hirschhaut E., Combellas I. Radionuclide evaluation of left-ventricular function in chronic Chagas' cardiomyopathy. J Nucl Med. 1983 Jul;24(7):563–567. [PubMed] [Google Scholar]
- Avila H. A., Sigman D. S., Cohen L. M., Millikan R. C., Simpson L. Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolated from whole blood lysates: diagnosis of chronic Chagas' disease. Mol Biochem Parasitol. 1991 Oct;48(2):211–221. doi: 10.1016/0166-6851(91)90116-n. [DOI] [PubMed] [Google Scholar]
- Avila J. L., Avila A. Trypanosoma cruzi: allopurinol in the treatment of mice with experimental acute Chagas disease. Exp Parasitol. 1981 Apr;51(2):204–208. doi: 10.1016/0014-4894(81)90109-0. [DOI] [PubMed] [Google Scholar]
- Azogue E., La Fuente C., Darras C. Congenital Chagas' disease in Bolivia: epidemiological aspects and pathological findings. Trans R Soc Trop Med Hyg. 1985;79(2):176–180. doi: 10.1016/0035-9203(85)90328-1. [DOI] [PubMed] [Google Scholar]
- Barousse A. P., Costa J. A., Eposto M., Laplume H., Segura E. L. Enfermedad de Chagas e inmunosupresión. Medicina (B Aires) 1980;40 (Suppl 1):17–26. [PubMed] [Google Scholar]
- Barr S. C., Schmidt S. P., Brown C. C., Klei T. R. Pathologic features of dogs inoculated with North American Trypanosoma cruzi isolates. Am J Vet Res. 1991 Dec;52(12):2033–2039. [PubMed] [Google Scholar]
- Beard C. B., Young D. G., Butler J. F., Evans D. A. First isolation of Trypanosoma cruzi from a wild-caught Triatoma sanguisuga (LeConte) (Hemiptera: Triatominae) in Florida, U.S.A. J Parasitol. 1988 Apr;74(2):343–344. [PubMed] [Google Scholar]
- Ben Younès-Chennoufi A., Said G., Eisen H., Durand A., Hontebeyrie-Joskowicz M. Cellular immunity to Trypanosoma cruzi is mediated by helper T cells (CD4+). Trans R Soc Trop Med Hyg. 1988;82(1):84–89. doi: 10.1016/0035-9203(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Bestetti R. B., Oliveira J. S. A hitherto neglected cause of myocardial infarction associated with normal coronary arteries: chronic Chagas' heart disease. Am J Cardiol. 1989 Mar 15;63(11):766–766. doi: 10.1016/0002-9149(89)90276-2. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt A. L. Congenital Chagas disease. Am J Dis Child. 1976 Jan;130(1):97–103. doi: 10.1001/archpedi.1976.02120020099020. [DOI] [PubMed] [Google Scholar]
- Bittencourt A. L., Vieira G. O., Tavares H. C., Mota E., Maguire J. Esophageal involvement in congenital Chagas' disease. Report of a case with megaesophagus. Am J Trop Med Hyg. 1984 Jan;33(1):30–33. doi: 10.4269/ajtmh.1984.33.30. [DOI] [PubMed] [Google Scholar]
- Bonaldo M. C., Souto-Padron T., de Souza W., Goldenberg S. Cell-substrate adhesion during Trypanosoma cruzi differentiation. J Cell Biol. 1988 Apr;106(4):1349–1358. doi: 10.1083/jcb.106.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Brennessel D. J., Wittner M., Braunstein V., Tanowitz H. B. Acetylcholinesterase levels in skeletal muscle of mice infected with Trypanosoma cruzi. Am J Trop Med Hyg. 1985 May;34(3):460–464. doi: 10.4269/ajtmh.1985.34.460. [DOI] [PubMed] [Google Scholar]
- Burns J. M., Jr, Shreffler W. G., Rosman D. E., Sleath P. R., March C. J., Reed S. G. Identification and synthesis of a major conserved antigenic epitope of Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1239–1243. doi: 10.1073/pnas.89.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPADEIRO E., LOPES E. R., DE MESQUITA P. M., PEREIRA F. E. INCID ENCIA DE "MEGAS" ASSOCIADOS 'A CARDIOPATIA CHAG'ASICA. Rev Inst Med Trop Sao Paulo. 1964 Nov-Dec;6:287–291. [PubMed] [Google Scholar]
- Caeiro T., Amuchastegui L. M., Moreyra E., Gibson D. G. Abnormal left ventricular diastolic function in chronic Chagas' disease: an echocardiographic study. Int J Cardiol. 1985 Dec;9(4):417–424. doi: 10.1016/0167-5273(85)90237-2. [DOI] [PubMed] [Google Scholar]
- Camara E. J., Lima J. A., Oliveira G. B., Machado A. S. Pulmonary findings in patients with chagasic megaesophagus. Study of autopsied cases. Chest. 1983 Jan;83(1):87–91. doi: 10.1378/chest.83.1.87. [DOI] [PubMed] [Google Scholar]
- Campos J. V., Tafuri W. L. Chagas enteropathy. Gut. 1973 Nov;14(11):910–919. doi: 10.1136/gut.14.11.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco Guerra H. A., Palacios-Prü E., Dagert de Scorza C., Molina C., Inglessis G., Mendoza R. V. Clinical, histochemical, and ultrastructural correlation in septal endomyocardial biopsies from chronic chagasic patients: detection of early myocardial damage. Am Heart J. 1987 Mar;113(3):716–724. doi: 10.1016/0002-8703(87)90712-5. [DOI] [PubMed] [Google Scholar]
- Carrasco H. A., Barboza J. S., Inglessis G., Fuenmayor A., Molina C. Left ventricular cineangiography in Chagas' disease: detection of early myocardial damage. Am Heart J. 1982 Sep;104(3):595–602. doi: 10.1016/0002-8703(82)90232-0. [DOI] [PubMed] [Google Scholar]
- Casado J., Davila D. F., Donis J. H., Torres A., Payares A., Colmenares R., Gottberg C. F. Electrocardiographic abnormalities and left ventricular systolic function in Chagas' heart disease. Int J Cardiol. 1990 Apr;27(1):55–62. doi: 10.1016/0167-5273(90)90191-7. [DOI] [PubMed] [Google Scholar]
- Cazzulo J. J., Couso R., Raimondi A., Wernstedt C., Hellman U. Further characterization and partial amino acid sequence of a cysteine proteinase from Trypanosoma cruzi. Mol Biochem Parasitol. 1989 Feb;33(1):33–41. doi: 10.1016/0166-6851(89)90039-x. [DOI] [PubMed] [Google Scholar]
- Cohen S., Fisher R., Tuch A. The site of denervation in achalasia. Gut. 1972 Jul;13(7):556–558. doi: 10.1136/gut.13.7.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Lipshutz W., Hughes W. Role of gastrin supersensitivity in the pathogenesis of lower esophageal sphincter hypertension in achalasia. J Clin Invest. 1971 Jun;50(6):1241–1247. doi: 10.1172/JCI106601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combellas I., Puigbo J. J., Acquatella H., Tortoledo F., Gomez J. R. Echocardiographic features of impaired left ventricular diastolic function in Chagas's heart disease. Br Heart J. 1985 Mar;53(3):298–309. doi: 10.1136/hrt.53.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell A. M., Logan C. J. The role of gastrin in gastroileocolic responses. Am J Dig Dis. 1967 Mar;12(3):277–284. doi: 10.1007/BF02233645. [DOI] [PubMed] [Google Scholar]
- Connelly M. C., Kierszenbaum F. Modulation of macrophage interaction with Trypanosoma cruzi by phospholipase A2-sensitive components of the parasite membrane. Biochem Biophys Res Commun. 1984 Jun 29;121(3):931–939. doi: 10.1016/0006-291x(84)90766-6. [DOI] [PubMed] [Google Scholar]
- Conte V. P. Aspectos anátomo-funcionais da vesícula biliar em pacientes com megaesôfago chagásico. Rev Hosp Clin Fac Med Sao Paulo. 1981 Apr;36(2):69–77. [PubMed] [Google Scholar]
- Contreras V. T., Salles J. M., Thomas N., Morel C. M., Goldenberg S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol Biochem Parasitol. 1985 Sep;16(3):315–327. doi: 10.1016/0166-6851(85)90073-8. [DOI] [PubMed] [Google Scholar]
- Cornette J., Capron A., Ouaissi M. A. Trypanosoma cruzi: fibronectin promotes uptake of epimastigote culture forms by human neutrophils and monocytes. Int Arch Allergy Appl Immunol. 1988;86(2):139–146. doi: 10.1159/000234563. [DOI] [PubMed] [Google Scholar]
- Cossio P. M., Diez C., Szarfman A., Kreutzer E., Candiolo B., Arana R. M. Chagasic cardiopathy. Demonstration of a serum gamma globulin factor which reacts with endocardium and vascular structures. Circulation. 1974 Jan;49(1):13–21. doi: 10.1161/01.cir.49.1.13. [DOI] [PubMed] [Google Scholar]
- Costa R. de B., de Alcântara F. G. Gastropatia chagásica crônica. Rev Bras Med. 1965 Nov;22(11):667–671. [PubMed] [Google Scholar]
- Cotrim P. C., Paranhos G. S., Mortara R. A., Wanderley J., Rassi A., Camargo M. E., da Silveira J. F. Expression in Escherichia coli of a dominant immunogen of Trypanosoma cruzi recognized by human chagasic sera. J Clin Microbiol. 1990 Mar;28(3):519–524. doi: 10.1128/jcm.28.3.519-524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csendes A., Strauszer T., Uribe P. Alterations in normal esophageal motility in patients with Chagas' disease. Am J Dig Dis. 1975 May;20(5):437–442. doi: 10.1007/BF01070788. [DOI] [PubMed] [Google Scholar]
- DIAS E., LARANJA F. S., MIRANDA A., NOBREGA G. Chagas' disease; a clinical, epidemiologic, and pathologic study. Circulation. 1956 Dec;14(6):1035–1060. doi: 10.1161/01.cir.14.6.1035. [DOI] [PubMed] [Google Scholar]
- Da Silveira G. M. Chagas' disease of the colon. Br J Surg. 1976 Oct;63(10):831–835. doi: 10.1002/bjs.1800631025. [DOI] [PubMed] [Google Scholar]
- Dantas R. O., Godoy R. A., Oliveira R. B., Villanova M. G., Meneghelli U. G., Troncon L. E. Effect of nifedipine on the lower esophageal sphincter pressure in chagasic patients. Braz J Med Biol Res. 1986;19(2):205–209. [PubMed] [Google Scholar]
- Dantas R. O. Idiopathic achalasia and Chagasic megaesophagus. J Clin Gastroenterol. 1988 Feb;10(1):13–15. doi: 10.1097/00004836-198802000-00005. [DOI] [PubMed] [Google Scholar]
- Dantas R. O., Rezende Filho J., de Oliveira R. B., de Godoy R. A. Efeito do dinitrato de isosorbitol na pressão do esfíncter inferior do esôfago de pacientes com doença de Chagas. Arq Gastroenterol. 1987 Apr-Jun;24(2):84–87. [PubMed] [Google Scholar]
- Davila D. F., Rossell R. O., Donis J. H. Cardiac parasympathetic abnormalities: Cause or consequence of chagas heart disease? Parasitol Today. 1989 Oct;5(10):327–329. doi: 10.1016/0169-4758(89)90127-0. [DOI] [PubMed] [Google Scholar]
- De Morais C. F., Higuchi M. L., Lage S. Chagas' heart disease and myocardial infarct. Incidence and report of four necropsy cases. Ann Trop Med Parasitol. 1989 Jun;83(3):207–214. doi: 10.1080/00034983.1989.11812334. [DOI] [PubMed] [Google Scholar]
- Dedman J. R., Brinkley B. R., Means A. R. Regulation of microfilaments and microtubules by calcium and cyclic AMP. Adv Cyclic Nucleotide Res. 1979;11:131–174. [PubMed] [Google Scholar]
- Del Castillo M., Mendoza G., Oviedo J., Perez Bianco R. P., Anselmo A. E., Silva M. AIDS and Chagas' disease with central nervous system tumor-like lesion. Am J Med. 1990 Jun;88(6):693–694. doi: 10.1016/0002-9343(90)90544-n. [DOI] [PubMed] [Google Scholar]
- Dias J. C. Control of Chagas disease in Brazil. Parasitol Today. 1987 Nov;3(11):336–341. doi: 10.1016/0169-4758(87)90117-7. [DOI] [PubMed] [Google Scholar]
- Dinoso V. P., Jr, Meshkinpour H., Lorber S. H., Gutierrez J. G., Chey W. Y. Motor responses of the sigmoid colon and rectum to exogenous cholecystokinin and secretin. Gastroenterology. 1973 Sep;65(3):438–444. [PubMed] [Google Scholar]
- Docampo R., Pignataro O. P. The inositol phosphate/diacylglycerol signalling pathway in Trypanosoma cruzi. Biochem J. 1991 Apr 15;275(Pt 2):407–411. doi: 10.1042/bj2750407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds W. J., Dent J., Hogan W. J., Patel G. K., Toouli J., Arndorfer R. C. Paradoxical lower esophageal sphincter contraction induced by cholecystokinin-octapeptide in patients with achalasia. Gastroenterology. 1981 Feb;80(2):327–333. [PubMed] [Google Scholar]
- Earlam R. J. Ganglion cell changes in experimental stenosis of the gut. Gut. 1971 May;12(5):393–398. doi: 10.1136/gut.12.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earlam R. J. Gastrointestinal aspects of Chagas' disease. Am J Dig Dis. 1972 Jun;17(6):559–571. doi: 10.1007/BF02231217. [DOI] [PubMed] [Google Scholar]
- Eisenschlos C. D., Paladini A. A., Molina y Vedia L., Torres H. N., Flawiá M. M. Evidence for the existence of an Ns-type regulatory protein in Trypanosoma cruzi membranes. Biochem J. 1986 Aug 1;237(3):913–917. doi: 10.1042/bj2370913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I. The polymerase chain reaction. A new method of using molecular genetics for medical diagnosis. N Engl J Med. 1990 Jan 18;322(3):178–183. doi: 10.1056/NEJM199001183220307. [DOI] [PubMed] [Google Scholar]
- Espinosa R., Carrasco H. A., Belandria F., Fuenmayor A. M., Molina C., González R., Martínez O. Life expectancy analysis in patients with Chagas' disease: prognosis after one decade (1973-1983). Int J Cardiol. 1985 May;8(1):45–56. doi: 10.1016/0167-5273(85)90262-1. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Cho S., Wittner M., Tanowitz H. Abnormalities of the coronary microcirculation in acute murine Chagas' disease. Am J Trop Med Hyg. 1985 Mar;34(2):246–253. doi: 10.4269/ajtmh.1985.34.246. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Minase T., Cho S., Dominitz R., Sonnenblick E. H. Microvascular spasm in the cardiomyopathic Syrian hamster: a preventable cause of focal myocardial necrosis. Circulation. 1982 Aug;66(2):342–354. doi: 10.1161/01.cir.66.2.342. [DOI] [PubMed] [Google Scholar]
- Ferrans V. J., Milei J., Tomita Y., Storino R. A. Basement membrane thickening in cardiac myocytes and capillaries in chronic Chagas' disease. Am J Cardiol. 1988 May 1;61(13):1137–1140. doi: 10.1016/0002-9149(88)90148-8. [DOI] [PubMed] [Google Scholar]
- Ferreira M. S., Nishioka S. de A., Rocha A., Silva A. M., Ferreira R. G., Olivier W., Tostes Júnior S. Acute fatal Trypanosoma cruzi meningoencephalitis in a human immunodeficiency virus-positive hemophiliac patient. Am J Trop Med Hyg. 1991 Dec;45(6):723–727. doi: 10.4269/ajtmh.1991.45.723. [DOI] [PubMed] [Google Scholar]
- Figuerêdo-Silva J., Coutinho-Netto J., Bestetti R. B., Oliveira J. S. [3H]-acetylcholine release from rat atria in chronic chagasic cardiopathy. Braz J Med Biol Res. 1989;22(6):737–740. [PubMed] [Google Scholar]
- Frank J. S., Langer G. A., Nudd L. M., Seraydarian K. The myocardial cell surface, its histochemistry, and the effect of sialic acid and calcium removal on its stucture and cellular ionic exchange. Circ Res. 1977 Nov;41(5):702–714. doi: 10.1161/01.res.41.5.702. [DOI] [PubMed] [Google Scholar]
- Frasch A. C., Cazzulo J. J., Aslund L., Pettersson U. Comparison of genes encoding Trypanosoma cruzi antigens. Parasitol Today. 1991 Jun;7(6):148–151. doi: 10.1016/0169-4758(91)90284-u. [DOI] [PubMed] [Google Scholar]
- Frasch A. C., Reyes M. B. Diagnosis of Chagas disease using recombinant DNA technology. Parasitol Today. 1990 Apr;6(4):137–139. doi: 10.1016/0169-4758(90)90234-u. [DOI] [PubMed] [Google Scholar]
- Freilij H. L., Corral R. S., Katzin A. M., Grinstein S. Antigenuria in infants with acute and congenital Chagas' disease. J Clin Microbiol. 1987 Jan;25(1):133–137. doi: 10.1128/jcm.25.1.133-137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallerano R. H., Marr J. J., Sosa R. R. Therapeutic efficacy of allopurinol in patients with chronic Chagas' disease. Am J Trop Med Hyg. 1990 Aug;43(2):159–166. doi: 10.4269/ajtmh.1990.43.159. [DOI] [PubMed] [Google Scholar]
- Gallo Júnior L., Morelo Filho J., Maciel B. C., Marin Neto J. A., Martins L. E., Lima Filho E. C. Functional evaluation of sympathetic and parasympathetic system in Chagas' disease using dynamic exercise. Cardiovasc Res. 1987 Dec;21(12):922–927. doi: 10.1093/cvr/21.12.922. [DOI] [PubMed] [Google Scholar]
- Golden J. M., Tarleton R. L. Trypanosoma cruzi: cytokine effects on macrophage trypanocidal activity. Exp Parasitol. 1991 May;72(4):391–402. doi: 10.1016/0014-4894(91)90085-b. [DOI] [PubMed] [Google Scholar]
- Gonzales-Perdomo M., Romero P., Goldenberg S. Cyclic AMP and adenylate cyclase activators stimulate Trypanosoma cruzi differentiation. Exp Parasitol. 1988 Aug;66(2):205–212. doi: 10.1016/0014-4894(88)90092-6. [DOI] [PubMed] [Google Scholar]
- Gonzales-Perdomo M., de Castro S. L., Meirelles M. N., Goldenberg S. Trypanosoma cruzi proliferation and differentiation are blocked by topoisomerase II inhibitors. Antimicrob Agents Chemother. 1990 Sep;34(9):1707–1714. doi: 10.1128/aac.34.9.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I. H., Gold J. W., Wittner M., Tanowitz H. B., Nathan C., Mayer K., Reich L., Wollner N., Steinherz L., Ghavimi F. Transfusion-associated acute Chagas disease acquired in the United States. Ann Intern Med. 1989 Nov 15;111(10):849–851. doi: 10.7326/0003-4819-111-10-849. [DOI] [PubMed] [Google Scholar]
- Greig S., Ashall F. Electrophoretic detection of Trypanosoma cruzi peptidases. Mol Biochem Parasitol. 1990 Feb;39(1):31–37. doi: 10.1016/0166-6851(90)90005-7. [DOI] [PubMed] [Google Scholar]
- Guelrud M., Bettarello A., Cecconello I., Pinotti W., Mantelmacher H., Velasquez H. Sphincter of Oddi pressure in chagasic patients with megaesophagus. Gastroenterology. 1983 Sep;85(3):584–588. [PubMed] [Google Scholar]
- Guzzetti S., Iosa D., Pecis M., Bonura L., Prosdocimi M., Malliani A. Impaired heart rate variability in patients with chronic Chagas' disease. Am Heart J. 1991 Jun;121(6 Pt 1):1727–1734. doi: 10.1016/0002-8703(91)90019-e. [DOI] [PubMed] [Google Scholar]
- Hagar J. M., Rahimtoola S. H. Chagas' heart disease in the United States. N Engl J Med. 1991 Sep 12;325(11):763–768. doi: 10.1056/NEJM199109123251103. [DOI] [PubMed] [Google Scholar]
- Hall B. F., Joiner K. A. Strategies of obligate intracellular parasites for evading host defences. Immunol Today. 1991 Mar;12(3):A22–A27. doi: 10.1016/S0167-5699(05)80007-6. [DOI] [PubMed] [Google Scholar]
- Hammermeister K. E., Caeiro T., Crespo E., Palmero H., Gibson D. G. Left ventricular wall motion in patients with Chagas's disease. Br Heart J. 1984 Jan;51(1):70–76. doi: 10.1136/hrt.51.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath S., Hieny S., Sher A. A cyclic AMP inducible gene expressed during the development of infective stages of Trypanosoma cruzi. Mol Biochem Parasitol. 1990 Nov;43(1):133–141. doi: 10.1016/0166-6851(90)90138-c. [DOI] [PubMed] [Google Scholar]
- Heitmann P., Espinoza J. Oesophageal manometric studies in patients with chronic Chagas disease and megacolon. Gut. 1969 Oct;10(10):848–851. doi: 10.1136/gut.10.10.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Munaín C., Fernández M. A., Alcina A., Fresno M. Characterization of a glycosyl-phosphatidylinositol-anchored membrane protein from Trypanosoma cruzi. Infect Immun. 1991 Apr;59(4):1409–1416. doi: 10.1128/iai.59.4.1409-1416.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff R., Teixeira R. S., Carvalho J. S., Mott K. E. Trypanosoma cruzi in the cerebrospinal fluid during the acute stage of Chagas' disease. N Engl J Med. 1978 Mar 16;298(11):604–606. doi: 10.1056/NEJM197803162981106. [DOI] [PubMed] [Google Scholar]
- Hofflin J. M., Sadler R. H., Araujo F. G., Page W. E., Remington J. S. Laboratory-acquired Chagas disease. Trans R Soc Trop Med Hyg. 1987;81(3):437–440. doi: 10.1016/0035-9203(87)90162-3. [DOI] [PubMed] [Google Scholar]
- Iosa D., DeQuattro V., Lee D. D., Elkayam U., Palmero H. Plasma norepinephrine in Chagas' cardioneuromyopathy: a marker of progressive dysautonomia. Am Heart J. 1989 Apr;117(4):882–887. doi: 10.1016/0002-8703(89)90627-3. [DOI] [PubMed] [Google Scholar]
- Jennewein H. M., Waldeck F., Siewert R., Weiser F., Thimm R. The interaction of glucagon and pentagastrin on the lower oesophageal sphincter in man and dog. Gut. 1973 Nov;14(11):861–864. doi: 10.1136/gut.14.11.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K., Hieny S., Kirchhoff L. V., Sher A. gp72, the 72 kilodalton glycoprotein, is the membrane acceptor site for C3 on Trypanosoma cruzi epimastigotes. J Exp Med. 1985 May 1;161(5):1196–1212. doi: 10.1084/jem.161.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K., Sher A., Gaither T., Hammer C. Evasion of alternative complement pathway by Trypanosoma cruzi results from inefficient binding of factor B. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6593–6597. doi: 10.1073/pnas.83.17.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBERLE F. Die Chagaskrankheit: eine Erkrankung der neurovegetativen Peripherie. Wien Klin Wochenschr. 1956 Apr 27;68(17):333–339. [PubMed] [Google Scholar]
- Kerndt P. R., Waskin H. A., Kirchhoff L. V., Steurer F., Waterman S. H., Nelson J. M., Gellert G. A., Shulman I. A. Prevalence of antibody to Trypanosoma cruzi among blood donors in Los Angeles, California. Transfusion. 1991 Nov-Dec;31(9):814–818. doi: 10.1046/j.1537-2995.1991.31992094668.x. [DOI] [PubMed] [Google Scholar]
- Khoury E. L., Diez C., Cossio P. M., Arana R. M. Heterophil nature of EVI antibody in Trypanosoma cruzi infection. Clin Immunol Immunopathol. 1983 May;27(2):283–288. doi: 10.1016/0090-1229(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Khoury E. L., Ritacco V., Cossio P. M., Laguens R. P., Szarfman A., Diez C., Arana R. M. Circulating antibodies to peripheral nerve in American trypanosomiasis (Chagas' disease). Clin Exp Immunol. 1979 Apr;36(1):8–15. [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F. Autoimmunity in Chagas' disease. J Parasitol. 1986 Apr;72(2):201–211. [PubMed] [Google Scholar]
- Kierszenbaum F., Sztein M. B. Mechanisms underlying immunosuppression induced by Trypanosoma cruzi. Parasitol Today. 1990 Aug;6(8):261–264. doi: 10.1016/0169-4758(90)90187-9. [DOI] [PubMed] [Google Scholar]
- Kirchhoff L. V., Gam A. A., Gilliam F. C. American trypanosomiasis (Chagas' disease) in Central American immigrants. Am J Med. 1987 May;82(5):915–920. doi: 10.1016/0002-9343(87)90152-5. [DOI] [PubMed] [Google Scholar]
- Kirchhoff L. V., Gam A. A., Gusmao R. A., Goldsmith R. S., Rezende J. M., Rassi A. Increased specificity of serodiagnosis of Chagas' disease by detection of antibody to the 72- and 90-kilodalton glycoproteins of Trypanosoma cruzi. J Infect Dis. 1987 Mar;155(3):561–564. doi: 10.1093/infdis/155.3.561. [DOI] [PubMed] [Google Scholar]
- Kirchhoff L. V. Is Trypanosoma cruzi a new threat to our blood supply? Ann Intern Med. 1989 Nov 15;111(10):773–775. doi: 10.7326/0003-4819-111-10-773. [DOI] [PubMed] [Google Scholar]
- Kirchhoff L. V., Neva F. A. Chagas' disease in Latin American immigrants. JAMA. 1985 Dec 6;254(21):3058–3060. [PubMed] [Google Scholar]
- Kohl S., Pickering L. K., Frankel L. S., Yaeger R. G. Reactivation of Chagas' disease during therapy of acute lymphocytic leukemia. Cancer. 1982 Sep 1;50(5):827–828. doi: 10.1002/1097-0142(19820901)50:5<827::aid-cncr2820500503>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Krieger M. A., Almeida E., Oelemann W., Lafaille J. J., Pereira J. B., Krieger H., Carvalho M. R., Goldenberg S. Use of recombinant antigens for the accurate immunodiagnosis of Chagas' disease. Am J Trop Med Hyg. 1992 Apr;46(4):427–434. doi: 10.4269/ajtmh.1992.46.427. [DOI] [PubMed] [Google Scholar]
- Köberle F. Chagas' disease and Chagas' syndromes: the pathology of American trypanosomiasis. Adv Parasitol. 1968;6:63–116. doi: 10.1016/s0065-308x(08)60472-8. [DOI] [PubMed] [Google Scholar]
- Leiguarda R., Roncoroni A., Taratuto A. L., Jost L., Berthier M., Nogues M., Freilij H. Acute CNS infection by Trypanosoma cruzi (Chagas' disease) in immunosuppressed patients. Neurology. 1990 May;40(5):850–851. doi: 10.1212/wnl.40.5.850. [DOI] [PubMed] [Google Scholar]
- Libby P., Alroy J., Pereira M. E. A neuraminidase from Trypanosoma cruzi removes sialic acid from the surface of mammalian myocardial and endothelial cells. J Clin Invest. 1986 Jan;77(1):127–135. doi: 10.1172/JCI112266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Warner S. J., Friedman G. B. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libow L. F., Beltrani V. P., Silvers D. N., Grossman M. E. Post-cardiac transplant reactivation of Chagas' disease diagnosed by skin biopsy. Cutis. 1991 Jul;48(1):37–40. [PubMed] [Google Scholar]
- Long R. G., Bishop A. E., Barnes A. J., Albuquerque R. H., O'Shaughnessy D. J., McGregor G. P., Bannister R., Polak J. M., Bloom S. R. Neural and hormonal peptides in rectal biopsy specimens from patients with Chagas' disease and chronic autonomic failure. Lancet. 1980 Mar 15;1(8168 Pt 1):559–562. doi: 10.1016/s0140-6736(80)91054-5. [DOI] [PubMed] [Google Scholar]
- Lopes E. R., Tafuri W. L., Chapadeiro E. Estudo morfológico e quantitativo dos núcleos dorsal do vato e hipoglosso em chagásicos crónicos com e sem megaesôfago. Rev Inst Med Trop Sao Paulo. 1969 Mar-Apr;11(2):123–129. [PubMed] [Google Scholar]
- Losavio A., Jones M. C., Sanz O. P., Mirkin G., Gonzalez Cappa S. M., Muchnik S., Sica R. E. A sequential study of the peripheral nervous system involvement in experimental Chagas' disease. Am J Trop Med Hyg. 1989 Nov;41(5):539–547. doi: 10.4269/ajtmh.1989.41.539. [DOI] [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo J. F., Meneghelli U. G., Oliveira R. B., Godoy R. A., Troncon L. E., Dantas R. O. Effect of CCK-OP and intraduodenal administration of essential amino acids on intraluminal pressures of sigmoid and rectum in patients with Chagasic megacolon. Dig Dis Sci. 1986 Feb;31(2):145–150. doi: 10.1007/BF01300699. [DOI] [PubMed] [Google Scholar]
- Machado A. B., Machado C. R., Gomes C. B. Depletion of heart norepinephrine in experimental acute myocarditis caused by Trypanosoma cruzi. Experientia. 1975 Oct 15;31(10):1202–1203. doi: 10.1007/BF02326794. [DOI] [PubMed] [Google Scholar]
- Machado A. B., Machado C. R., Gomez M. V. Trypanosoma cruzi: acetylcholine content and cholinergic innervation of the heart in rats. Exp Parasitol. 1979 Feb;47(1):107–115. doi: 10.1016/0014-4894(79)90012-2. [DOI] [PubMed] [Google Scholar]
- Maguire J. H., Hoff R., Sherlock I., Guimarães A. C., Sleigh A. C., Ramos N. B., Mott K. E., Weller T. H. Cardiac morbidity and mortality due to Chagas' disease: prospective electrocardiographic study of a Brazilian community. Circulation. 1987 Jun;75(6):1140–1145. doi: 10.1161/01.cir.75.6.1140. [DOI] [PubMed] [Google Scholar]
- Marr J. J., Docampo R. Chemotherapy for Chagas' disease: a perspective of current therapy and considerations for future research. Rev Infect Dis. 1986 Nov-Dec;8(6):884–903. doi: 10.1093/clinids/8.6.884. [DOI] [PubMed] [Google Scholar]
- McCabe R. E., Mullins B. T. Failure of Trypanosoma cruzi to trigger the respiratory burst of activated macrophages. Mechanism for immune evasion and importance of oxygen-independent killing. J Immunol. 1990 Mar 15;144(6):2384–2388. [PubMed] [Google Scholar]
- McCormick T. S., Rowland E. C. Trypanosoma cruzi: cross-reactive anti-heart autoantibodies produced during infection in mice. Exp Parasitol. 1989 Nov;69(4):393–401. doi: 10.1016/0014-4894(89)90088-x. [DOI] [PubMed] [Google Scholar]
- Meneghelli U. G. Chagas' disease: a model of denervation in the study of digestive tract motility. Braz J Med Biol Res. 1985;18(3):255–264. [PubMed] [Google Scholar]
- Meneghelli U. G., Godoy R. A., Oliveira R. B., Santos J. C., Jr, Dantas R. O., Troncon L. E. Effect of pentagastrin on the motor activity of the dilated and nondilated sigmoid and rectum in Chagas' disease. Digestion. 1983;27(3):152–158. doi: 10.1159/000198945. [DOI] [PubMed] [Google Scholar]
- Mesri E. A., Levitus G., Hontebeyrie-Joskowicz M., Dighiero G., Van Regenmortel M. H., Levin M. J. Major Trypanosoma cruzi antigenic determinant in Chagas' heart disease shares homology with the systemic lupus erythematosus ribosomal P protein epitope. J Clin Microbiol. 1990 Jun;28(6):1219–1224. doi: 10.1128/jcm.28.6.1219-1224.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina H. A., Kierszenbaum F. Eosinophil activation in acute and chronic chagasic myocardial lesions and deposition of toxic eosinophil granule proteins on heart myofibers. J Parasitol. 1989 Feb;75(1):129–133. [PubMed] [Google Scholar]
- Moncayo A., Luquetti A. O. Multicentre double blind study for evaluation of Trypanosoma cruzi defined antigens as diagnostic reagents. Mem Inst Oswaldo Cruz. 1990 Oct-Dec;85(4):489–495. doi: 10.1590/s0074-02761990000400020. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Barr S., Weiss L., Tanowitz H., Wittner M., Bilezikian J. P. Myocardial beta-adrenergic adenylate cyclase complex in a canine model of chagasic cardiomyopathy. Circ Res. 1991 Jul;69(1):185–195. doi: 10.1161/01.res.69.1.185. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Bilezikian J. P., Hatcher V., Weiss L. M., Tanowitz H. B., Wittner M. Trypanosoma cruzi: infection of cultured human endothelial cells alters inositol phosphate synthesis. Exp Parasitol. 1989 Nov;69(4):330–339. doi: 10.1016/0014-4894(89)90082-9. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Bilezikian J. P., Tanowitz H., Wittner M. Infection of L6E9 myoblasts with Trypanosoma cruzi alters adenylate cyclase activity and guanine nucleotide binding proteins. J Cell Physiol. 1987 Oct;133(1):64–71. doi: 10.1002/jcp.1041330108. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Tanowitz H., Factor S. M., Bilezikian J. P., Wittner M. Myocardial adenylate cyclase activity in acute murine Chagas' disease. Circ Res. 1988 Apr;62(4):800–810. doi: 10.1161/01.res.62.4.800. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Tanowitz H., Hatcher V., Bilezikian J. P., Wittner M. Alterations in intracellular calcium following infection of human endothelial cells with Trypanosoma cruzi. Mol Biochem Parasitol. 1988 Jun;29(2-3):213–221. doi: 10.1016/0166-6851(88)90076-x. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Tanowitz H., Makman M., Hatcher V. B., Bilezikian J. P., Wittner M. Trypanosoma cruzi: alteration of cAMP metabolism following infection of human endothelial cells. Exp Parasitol. 1992 Feb;74(1):69–76. doi: 10.1016/0014-4894(92)90140-6. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Weiss L. M., Factor S., Bilezikian J. P., Tanowitz H., Wittner M. Verapamil ameliorates clinical, pathologic and biochemical manifestations of experimental chagasic cardiomyopathy in mice. J Am Coll Cardiol. 1989 Sep;14(3):782–789. doi: 10.1016/0735-1097(89)90126-5. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Wittner M., Weiss L., Hatcher V. B., Tanowitz H. B., Bilezikian J. P., Gordon P. B. Extracellular matrix derived from Trypanosoma cruzi infected endothelial cells directs phenotypic expression. J Cell Physiol. 1990 Nov;145(2):340–346. doi: 10.1002/jcp.1041450220. [DOI] [PubMed] [Google Scholar]
- Mortatti R. C., Maia L. C., de Oliveira A. V., Munk M. E. Immunopathology of experimental Chagas' disease: binding of T cells to Trypanosoma cruzi-infected heart tissue. Infect Immun. 1990 Nov;58(11):3588–3593. doi: 10.1128/iai.58.11.3588-3593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Fernández M. A., Fernández M. A., Fresno M. Synergism between tumor necrosis factor-alpha and interferon-gamma on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur J Immunol. 1992 Feb;22(2):301–307. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Hajjar K. A., Silverstein R. L., Dinarello C. A. Interleukin 1 induces endothelial cell synthesis of plasminogen activator inhibitor. J Exp Med. 1986 Jun 1;163(6):1595–1600. doi: 10.1084/jem.163.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navin T. R., Roberto R. R., Juranek D. D., Limpakarnjanarat K., Mortenson E. W., Clover J. R., Yescott R. E., Taclindo C., Steurer F., Allain D. Human and sylvatic Trypanosoma cruzi infection in California. Am J Public Health. 1985 Apr;75(4):366–369. doi: 10.2105/ajph.75.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson P., Orr P., Schroeder M. L., Sekla L., Johnston J. B. Transfusion-associated Trypanosoma cruzi infection in a non-endemic area. Ann Intern Med. 1989 Nov 15;111(10):851–853. doi: 10.7326/0003-4819-111-10-851. [DOI] [PubMed] [Google Scholar]
- Oliveira J. S. A natural human model of intrinsic heart nervous system denervation: Chagas' cardiopathy. Am Heart J. 1985 Nov;110(5):1092–1098. doi: 10.1016/0002-8703(85)90222-4. [DOI] [PubMed] [Google Scholar]
- Oliveira J. S., Mello De Oliveira J. A., Frederigue U., Jr, Lima Filho E. C. Apical aneurysm of Chagas's heart disease. Br Heart J. 1981 Oct;46(4):432–437. doi: 10.1136/hrt.46.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira J. S., dos Santos J. C., Muccillo G., Ferreira A. L. Increased capacity of the coronary arteries in chronic Chagas' heart disease: further support for the neurogenic pathogenesis concept. Am Heart J. 1985 Feb;109(2):304–308. doi: 10.1016/0002-8703(85)90598-8. [DOI] [PubMed] [Google Scholar]
- Oliveira R. B., Meneghelli U. G., de Godoy R. A., Dantas R. O., Padovan W. Abnormalities of interdigestive motility of the small intestine in patients with Chagas' disease. Dig Dis Sci. 1983 Apr;28(4):294–299. doi: 10.1007/BF01324944. [DOI] [PubMed] [Google Scholar]
- Orr G. A., Tanowitz H. B., Wittner M. Trypanosoma cruzi: stage expression of calmodulin-binding proteins. Exp Parasitol. 1992 Mar;74(2):127–133. doi: 10.1016/0014-4894(92)90039-d. [DOI] [PubMed] [Google Scholar]
- Ortega-Barria E., Pereira M. E. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991 Oct 18;67(2):411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- Ouaissi M. A., Taibi A., Cornette J., Velge P., Marty B., Loyens M., Esteva M., Rizvi F. S., Capron A. Characterization of major surface and excretory-secretory immunogens of Trypanosoma cruzi trypomastigotes and identification of potential protective antigen. Parasitology. 1990 Feb;100(Pt 1):115–124. doi: 10.1017/s0031182000060182. [DOI] [PubMed] [Google Scholar]
- Oz H. S., Wittner M., Tanowitz H. B., Bilezikian J. P., Saxon M., Morris S. A. Trypanosoma cruzi: mechanisms of intracellular calcium homeostasis. Exp Parasitol. 1992 Jun;74(4):390–399. doi: 10.1016/0014-4894(92)90201-k. [DOI] [PubMed] [Google Scholar]
- Padovan W., Godoy R. A., Dantas R. O., Meneghelli U. G., Oliveira R. B., Troncon L. E. Lower oesophageal sphincter response to pentagastrin in chagasic patients with megaoesophagus and megacolon. Gut. 1980 Feb;21(2):85–90. doi: 10.1136/gut.21.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovan W., Godoy R. A., Meneghelli U. G., Dantas R. O., Oliveira R. B., Troncon L. E. Acid and pepsin secretion in chronic Chagas' disease patients in response to graded doses of pentagastrin and pentagastrin plus bethanecol. Digestion. 1982;23(1):48–56. doi: 10.1159/000198709. [DOI] [PubMed] [Google Scholar]
- Pereira Barretto A. C., Mady C., Arteaga-Fernandez E., Stolf N., Lopes E. A., Higuchi M. L., Bellotti G., Pileggi F. Right ventricular endomyocardial biopsy in chronic Chagas' disease. Am Heart J. 1986 Feb;111(2):307–312. doi: 10.1016/0002-8703(86)90144-4. [DOI] [PubMed] [Google Scholar]
- Pereira M. E. A developmentally regulated neuraminidase activity in Trypanosoma cruzi. Science. 1983 Mar 25;219(4591):1444–1446. doi: 10.1126/science.6338592. [DOI] [PubMed] [Google Scholar]
- Pessoa J., de Mesquita C. O comportamento da cárdia na esofagopatia chagásica. Fisiopatologia da esofagopatia chagásica. II. Rev Bras Med. 1967 Jun;24(6):428–441. [PubMed] [Google Scholar]
- Petry K., Eisen H. Chagas disease: a model for the study of autoimmune diseases. Parasitol Today. 1989 Apr;5(4):111–116. doi: 10.1016/0169-4758(89)90052-5. [DOI] [PubMed] [Google Scholar]
- Petry K., Nudelman E., Eisen H., Hakomori S. Sulfated lipids represent common antigens on the surface of Trypanosoma cruzi and mammalian tissues. Mol Biochem Parasitol. 1988 Aug;30(2):113–121. doi: 10.1016/0166-6851(88)90104-1. [DOI] [PubMed] [Google Scholar]
- Petry K., Voisin P., Baltz T., Labouesse J. Epitopes common to trypanosomes (T. cruzi, T. dionisii and T. vespertilionis (Schizotrypanum)): astrocytes and neurons. J Neuroimmunol. 1987 Oct;16(2):237–252. doi: 10.1016/0165-5728(87)90078-6. [DOI] [PubMed] [Google Scholar]
- Pimenta J., Miranda M., Pereira C. B. Electrophysiologic findings in long-term asymptomatic chagasic individuals. Am Heart J. 1983 Aug;106(2):374–380. doi: 10.1016/0002-8703(83)90206-5. [DOI] [PubMed] [Google Scholar]
- Prata A., Porto G. Biopsia de músculo na doença de Chagas. Rev Inst Med Trop Sao Paulo. 1966 Sep-Oct;8(5):193–196. [PubMed] [Google Scholar]
- Prioli R. P., Mejia J. S., Pereira M. E. On the interaction of Trypanosoma cruzi neuraminidase and human lipoproteins. Eur J Epidemiol. 1991 Jul;7(4):344–348. doi: 10.1007/BF00144998. [DOI] [PubMed] [Google Scholar]
- Prioli R. P., Rosenberg I., Pereira M. E. High- and low-density lipoproteins enhance infection of Trypanosoma cruzi in vitro. Mol Biochem Parasitol. 1990 Jan 15;38(2):191–198. doi: 10.1016/0166-6851(90)90022-e. [DOI] [PubMed] [Google Scholar]
- ROSENBAUM M. B. CHAGASIC MYOCARDIOPATHY. Prog Cardiovasc Dis. 1964 Nov;7:199–225. doi: 10.1016/s0033-0620(64)80020-7. [DOI] [PubMed] [Google Scholar]
- Raizman R. E., De Rezende J. M., Neva F. A. A clinical trial with pre- and post-treatment manometry comparing pneumatic dilation with bouginage for treatment of Chagas' megaesophagus. Am J Gastroenterol. 1980 Nov;74(5):405–409. [PubMed] [Google Scholar]
- Rangel-Aldao R., Allende O., Triana F., Piras R., Henriquez D., Piras M. Possible role of cAMP in the differentiation of Trypanosoma cruzi. Mol Biochem Parasitol. 1987 Jan 2;22(1):39–43. doi: 10.1016/0166-6851(87)90067-3. [DOI] [PubMed] [Google Scholar]
- Reed S. G., Grabstein K. H., Pihl D. L., Morrissey P. J. Recombinant granulocyte-macrophage colony-stimulating factor restores deficient immune responses in mice with chronic Trypanosoma cruzi infections. J Immunol. 1990 Sep 1;145(5):1564–1570. [PubMed] [Google Scholar]
- Reed S. G. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988 Jun 15;140(12):4342–4347. [PubMed] [Google Scholar]
- Renny A., Snape W. J., Jr, Sun E. A., London R., Cohen S. Role of cholecystokinin in the gastrocolonic response to a fat meal. Gastroenterology. 1983 Jul;85(1):17–21. [PubMed] [Google Scholar]
- Reyes M. B., Lorca M., Muñoz P., Frasch A. C. Fetal IgG specificities against Trypanosoma cruzi antigens in infected newborns. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2846–2850. doi: 10.1073/pnas.87.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro dos Santos, Hudson L. Denervation and the immune response in mice infected with Trypanosoma cruzi. Clin Exp Immunol. 1981 May;44(2):349–354. [PMC free article] [PubMed] [Google Scholar]
- Rimoldi M. T., Tenner A. J., Bobak D. A., Joiner K. A. Complement component C1q enhances invasion of human mononuclear phagocytes and fibroblasts by Trypanosoma cruzi trypomastigotes. J Clin Invest. 1989 Dec;84(6):1982–1989. doi: 10.1172/JCI114388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero I., Moravenik M., Morales J., Gomez M., de Rosas J. M. Letter: Chagas's disease--another hazard in acute leukemia. N Engl J Med. 1974 Jan 31;290(5):285–285. doi: 10.1056/nejm197401312900517. [DOI] [PubMed] [Google Scholar]
- Rizzo L. V., Cunha-Neto E., Teixeira A. R. Autoimmunity in Chagas' disease: specific inhibition of reactivity of CD4+ T cells against myosin in mice chronically infected with Trypanosoma cruzi. Infect Immun. 1989 Sep;57(9):2640–2644. doi: 10.1128/iai.57.9.2640-2644.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A., Almeida H. de O., Teixeira V. de P., da Silva A. M. Prevalência da colelitíase em chagásicos crônicos necropsiados no triângulo mineiro--correlaço com o megaesôfago, o megacólon e a insuficiência cardíaca. Arq Gastroenterol. 1985 Jan-Mar;22(1):3–6. [PubMed] [Google Scholar]
- Rosemberg S., Chaves C. J., Higuchi M. L., Lopes M. B., Castro L. H., Machado L. R. Fatal meningoencephalitis caused by reactivation of Trypanosoma cruzi infection in a patient with AIDS. Neurology. 1992 Mar;42(3 Pt 1):640–642. doi: 10.1212/wnl.42.3.640. [DOI] [PubMed] [Google Scholar]
- Rosenberg I. A., Prioli R. P., Mejia J. S., Pereira M. E. Differential expression of Trypanosoma cruzi neuraminidase in intra- and extracellular trypomastigotes. Infect Immun. 1991 Jan;59(1):464–466. doi: 10.1128/iai.59.1.464-466.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M. A. Microvascular changes as a cause of chronic cardiomyopathy in Chagas' disease. Am Heart J. 1990 Jul;120(1):233–236. doi: 10.1016/0002-8703(90)90191-y. [DOI] [PubMed] [Google Scholar]
- Rowin K. S., Tanowitz H. B., Wittner M., Nguyen H. T., Nadal-Ginard B. Inhibition of muscle differentiation by trypanosoma cruzi. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6390–6394. doi: 10.1073/pnas.80.20.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOERGEL K. H., ZBORALSKE F. F., AMBERG J. R. PRESBYESOPHAGUS: ESOPHAGEAL MOTILITY IN NONAGENARIANS. J Clin Invest. 1964 Jul;43:1472–1479. doi: 10.1172/JCI105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadigursky M., Acosta A. M., Santos-Buch C. A. Muscle sarcoplasmic reticulum antigen shared by a Trypanosoma cruzi clone. Am J Trop Med Hyg. 1982 Sep;31(5):934–941. doi: 10.4269/ajtmh.1982.31.934. [DOI] [PubMed] [Google Scholar]
- Said G., Joskowicz M., Barreira A. A., Eisen H. Neuropathy associated with experimental Chagas' disease. Ann Neurol. 1985 Dec;18(6):676–683. doi: 10.1002/ana.410180609. [DOI] [PubMed] [Google Scholar]
- Samuel J., Oliveira M., Correa De Araujo R. R., Navarro M. A., Muccillo G. Cardiac thrombosis and thromboembolism in chronic Chagas' heart disease. Am J Cardiol. 1983 Jul;52(1):147–151. doi: 10.1016/0002-9149(83)90085-1. [DOI] [PubMed] [Google Scholar]
- Santos-Buch C. A., Acosta A. M., Zweerink H. J., Sadigursky M., Andersen O. F., von Kreuter B. F., Brodskyn C. I., Sadigursky C., Cody R. J. Primary muscle disease: definition of a 25-kDa polypeptide myopathic specific chagas antigen. Clin Immunol Immunopathol. 1985 Dec;37(3):334–350. doi: 10.1016/0090-1229(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Andrews N. W., Nussenzweig V., Robbins E. S. Trypanosoma cruzi invade a mammalian epithelial cell in a polarized manner. Cell. 1988 Oct 7;55(1):157–165. doi: 10.1016/0092-8674(88)90018-9. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Jiang M. S., Hart G. W., Nussenzweig V. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell. 1991 Jun 28;65(7):1117–1125. doi: 10.1016/0092-8674(91)90008-m. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Pontes de Carvalho L., Nussenzweig V. Trypanosoma cruzi trans-sialidase and neuraminidase activities can be mediated by the same enzymes. J Exp Med. 1992 Feb 1;175(2):567–575. doi: 10.1084/jem.175.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S., Robbins E. S., Nussenzweig V. Attachment of Trypanosoma cruzi to mammalian cells requires parasite energy, and invasion can be independent of the target cell cytoskeleton. Infect Immun. 1991 Feb;59(2):645–654. doi: 10.1128/iai.59.2.645-654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S., Yoshida N., Cardoso de Almeida M. L. Glycophosphatidylinositol-anchored proteins in metacyclic trypomastigotes of Trypanosoma cruzi. Mol Biochem Parasitol. 1988 Jun;29(2-3):141–151. doi: 10.1016/0166-6851(88)90069-2. [DOI] [PubMed] [Google Scholar]
- Schmuñis G. A. Trypanosoma cruzi, the etiologic agent of Chagas' disease: status in the blood supply in endemic and nonendemic countries. Transfusion. 1991 Jul-Aug;31(6):547–557. doi: 10.1046/j.1537-2995.1991.31691306255.x. [DOI] [PubMed] [Google Scholar]
- Shadowen R. D., Sciortino C. V. Improved growth of Campylobacter pylori in a biphasic system. J Clin Microbiol. 1989 Aug;27(8):1744–1747. doi: 10.1128/jcm.27.8.1744-1747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Hieny S., Joiner K. Evasion of the alternative complement pathway by metacyclic trypomastigotes of Trypanosoma cruzi: dependence on the developmentally regulated synthesis of surface protein and N-linked carbohydrate. J Immunol. 1986 Nov 1;137(9):2961–2967. [PubMed] [Google Scholar]
- Sher A., Snary D. Specific inhibition of the morphogenesis of Trypanosoma cruzi by a monoclonal antibody. Nature. 1982 Dec 16;300(5893):639–640. doi: 10.1038/300639a0. [DOI] [PubMed] [Google Scholar]
- Shikanai-Yasuda M. A., Lopes M. H., Tolezano J. E., Umezawa E., Amato Neto V., Barreto A. C., Higaki Y., Moreira A. A., Funayama G., Barone A. A. Doença de Chagas aguda: vias de transmissão, aspectos clínicos e resposta à terapêutica específica em casos diagnosticados em um centro urbano. Rev Inst Med Trop Sao Paulo. 1990 Jan-Feb;32(1):16–27. doi: 10.1590/s0036-46651990000100004. [DOI] [PubMed] [Google Scholar]
- Silva J. S., Morrissey P. J., Grabstein K. H., Mohler K. M., Anderson D., Reed S. G. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992 Jan 1;175(1):169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snape W. J., Jr, Carlson G. M., Cohen S. Human colonic myoelectric activity in response to prostigmin and the gastrointestinal hormones. Am J Dig Dis. 1977 Oct;22(10):881–887. doi: 10.1007/BF01076164. [DOI] [PubMed] [Google Scholar]
- Sterin-Borda L., Gorelik G., Genaro A., Goin J. C., Borda E. S. Human chagasic IgG interacting with lymphocyte neurotransmitter receptors triggers intracellular signal transduction. FASEB J. 1990 Apr 1;4(6):1661–1667. doi: 10.1096/fasebj.4.6.2156743. [DOI] [PubMed] [Google Scholar]
- Stolf N. A., Higushi L., Bocchi E., Bellotti G., Auler J. O., Uip D., Amato Neto V., Pileggi F., Jatene A. D. Heart transplantation in patients with Chagas' disease cardiomyopathy. J Heart Transplant. 1987 Sep-Oct;6(5):307–312. [PubMed] [Google Scholar]
- Sturm N. R., Degrave W., Morel C., Simpson L. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol Biochem Parasitol. 1989 Mar 15;33(3):205–214. doi: 10.1016/0166-6851(89)90082-0. [DOI] [PubMed] [Google Scholar]
- Szarfman A., Terranova V. P., Rennard S. I., Foidart J. M., de Fatima Lima M., Scheinman J. I., Martin G. R. Antibodies to laminin in Chagas' disease. J Exp Med. 1982 Apr 1;155(4):1161–1171. doi: 10.1084/jem.155.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRES C. M. Arterioloscerose das finas ramificaçes arterials do miocárdic (coronarite chagásica) e miocitolise focal do miocárdio na cardiopatia chagásica cronica. Hospital (Rio J) 1958 Nov;54(5):597–610. [PubMed] [Google Scholar]
- Tanaka Y., Kiyotaki C., Tanowitz H., Bloom B. R. Reconstitution of a variant macrophage cell line defective in oxygen metabolism with a H2O2-generating system. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2584–2588. doi: 10.1073/pnas.79.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz H. B., Brosnan C., Guastamacchio D., Baron G., Raventos-Suarez C., Bornstein M., Wittner M. Infection of organotypic cultures of spinal cord and dorsal root ganglia with Trypanosoma cruzi. Am J Trop Med Hyg. 1982 Nov;31(6):1090–1097. doi: 10.4269/ajtmh.1982.31.1090. [DOI] [PubMed] [Google Scholar]
- Tanowitz H. B., Burns E. R., Sinha A. K., Kahn N. N., Morris S. A., Factor S. M., Hatcher V. B., Bilezikian J. P., Baum S. G., Wittner M. Enhanced platelet adherence and aggregation in Chagas' disease: a potential pathogenic mechanism for cardiomyopathy. Am J Trop Med Hyg. 1990 Sep;43(3):274–281. doi: 10.4269/ajtmh.1990.43.274. [DOI] [PubMed] [Google Scholar]
- Tanowitz H. B., Davies P., Factor S. M., Minase T., Herskowitz A., Wittner M. Trypanosoma cruzi: choline acetyltransferase activity in tissues of susceptible and resistant mice infected with the Brazil strain. Exp Parasitol. 1981 Apr;51(2):269–278. doi: 10.1016/0014-4894(81)90115-6. [DOI] [PubMed] [Google Scholar]
- Tanowitz H. B., Davies P., Wittner M. Alterations in acetylcholine receptors in experimental chagas' disease. J Infect Dis. 1983 Mar;147(3):460–466. doi: 10.1093/infdis/147.3.460. [DOI] [PubMed] [Google Scholar]
- Tanowitz H. B., Gumprecht J. P., Spurr D., Calderon T. M., Ventura M. C., Raventos-Suarez C., Kellie S., Factor S. M., Hatcher V. B., Wittner M. Cytokine gene expression of endothelial cells infected with Trypanosoma cruzi. J Infect Dis. 1992 Sep;166(3):598–603. doi: 10.1093/infdis/166.3.598. [DOI] [PubMed] [Google Scholar]
- Tanowitz H. B., Morris S. A., Weiss L. M., Bilezikian J. P., Factor S. M., Wittner M. Effect of verapamil on the development of chronic experimental Chagas' disease. Am J Trop Med Hyg. 1989 Dec;41(6):643–649. doi: 10.4269/ajtmh.1989.41.643. [DOI] [PubMed] [Google Scholar]
- Tanowitz H., Wittner M., Kress Y., Bloom B. Studies of in vitro infection by Trypanosoma cruzi. I. Ultrastructural studies on the invasion of macrophages and L-cells. Am J Trop Med Hyg. 1975 Jan;24(1):25–33. doi: 10.4269/ajtmh.1975.24.25. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J Immunol. 1990 Jan 15;144(2):717–724. [PubMed] [Google Scholar]
- Tarleton R. L., Koller B. H., Latour A., Postan M. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992 Mar 26;356(6367):338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L., Kuhn R. E. Restoration of in vitro immune responses of spleen cells from mice infected with Trypanosoma cruzi by supernatants containing interleukin 2. J Immunol. 1984 Sep;133(3):1570–1575. [PubMed] [Google Scholar]
- Tarleton R. L. Regulation of immunity in Trypanosoma cruzi infection. Exp Parasitol. 1991 Jul;73(1):106–109. doi: 10.1016/0014-4894(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L. Trypanosoma cruzi-induced suppression of IL-2 production. I. Evidence for the presence of IL-2-producing cells. J Immunol. 1988 Apr 15;140(8):2763–2768. [PubMed] [Google Scholar]
- Tarleton R. L. Tumour necrosis factor (cachectin) production during experimental Chagas' disease. Clin Exp Immunol. 1988 Aug;73(2):186–190. [PMC free article] [PubMed] [Google Scholar]
- Teixeira A. R., Córdoba J. C., Souto Maior I., Solórzano E. Chagas' disease: lymphoma growth in rabbits treated with Benznidazole. Am J Trop Med Hyg. 1990 Aug;43(2):146–158. doi: 10.4269/ajtmh.1990.43.146. [DOI] [PubMed] [Google Scholar]
- Teixeira A. R., Silva R., Cunha Neto E., Santana J. M., Rizzo L. V. Malignant, non-Hodgkin's lymphomas in Trypanosoma cruzi-infected rabbits treated with nitroarenes. J Comp Pathol. 1990 Jul;103(1):37–48. doi: 10.1016/s0021-9975(08)80133-8. [DOI] [PubMed] [Google Scholar]
- Teixeira A. R., Teixeira G., Macêdo V., Prata A. Trypanosoma cruzi-sensitized T-lymphocyte mediated 51CR release from human heart cells in Chagas' disease. Am J Trop Med Hyg. 1978 Nov;27(6):1097–1107. doi: 10.4269/ajtmh.1978.27.1097. [DOI] [PubMed] [Google Scholar]
- Teixeira A. R., Teixeira M. L., Santos-Buch C. A. The immunology of experimental Chagas' disease. IV. Production of lesions in rabbits similar to those of chronic Chagas' disease in man. Am J Pathol. 1975 Jul;80(1):163–180. [PMC free article] [PubMed] [Google Scholar]
- Ternynck T., Bleux C., Gregoire J., Avrameas S., Kanellopoulos-Langevin C. Comparison between autoantibodies arising during Trypanosoma cruzi infection in mice and natural autoantibodies. J Immunol. 1990 Feb 15;144(4):1504–1511. [PubMed] [Google Scholar]
- Troncon L. E., Oliveira R. B., Meneghelli U. G., Dantas R. O., Godoy R. A. Fasting and food-stimulated plasma gastrin levels in chronic Chagas' disease. Digestion. 1984;29(3):171–176. doi: 10.1159/000199031. [DOI] [PubMed] [Google Scholar]
- Troncon L. E., Rezende Filho J., Iazigi N. Duodenogastric reflux in Chagas' disease. Dig Dis Sci. 1988 Oct;33(10):1260–1264. doi: 10.1007/BF01536676. [DOI] [PubMed] [Google Scholar]
- Téllez-Iñn M. T., Ulloa R. M., Torruella M., Torres H. N. Calmodulin and Ca2+-dependent cyclic AMP phosphodiesterase activity in Trypanosoma cruzi. Mol Biochem Parasitol. 1985 Nov;17(2):143–153. doi: 10.1016/0166-6851(85)90013-1. [DOI] [PubMed] [Google Scholar]
- VIEIRA C. B., DE GODOY R. A., CARRIL C. F. HYPERSENSITIVITY OF THE LARGE INTESTINE TO CHOLINERGIC AGENTS IN PATIENTS WITH CHAGAS' DISEASE AND MEGACOLON. Rev Bras Gastroenterol. 1964 Mar-Apr;16:41–48. [PubMed] [Google Scholar]
- Van Voorhis W. C. Coculture of human peripheral blood mononuclear cells with Trypanosoma cruzi leads to proliferation of lymphocytes and cytokine production. J Immunol. 1992 Jan 1;148(1):239–248. [PubMed] [Google Scholar]
- Van Voorhis W. C., Schlekewy L., Trong H. L. Molecular mimicry by Trypanosoma cruzi: the F1-160 epitope that mimics mammalian nerve can be mapped to a 12-amino acid peptide. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5993–5997. doi: 10.1073/pnas.88.14.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercesi A. E., Hoffmann M. E., Bernardes C. F., Docampo R. Regulation of intracellular calcium homeostasis in Trypanosoma cruzi. Effects of calmidazolium and trifluoperazine. Cell Calcium. 1991 May;12(5):361–369. doi: 10.1016/0143-4160(91)90052-g. [DOI] [PubMed] [Google Scholar]
- Villanova M. G., Meneghelli U. G., Dantas R. O. Gallbladder motor function in chagasic patients with megacolon and/or megaesophagus. Digestion. 1987;36(4):189–194. doi: 10.1159/000199418. [DOI] [PubMed] [Google Scholar]
- Weinstein C., Fenoglio J. J. Myocarditis. Hum Pathol. 1987 Jun;18(6):613–618. doi: 10.1016/s0046-8177(87)80362-3. [DOI] [PubMed] [Google Scholar]
- Williams G. D., Adams L. G., Yaeger R. G., McGrath R. K., Read W. K., Bilderback W. R. Naturally occurring trypanosomiasis (Chagas' disease) in dogs. J Am Vet Med Assoc. 1977 Jul 15;171(2):171–177. [PubMed] [Google Scholar]
- Wood J. N., Hudson L., Jessell T. M., Yamamoto M. A monoclonal antibody defining antigenic determinants on subpopulations of mammalian neurones and Trypanosoma cruzi parasites. Nature. 1982 Mar 4;296(5852):34–38. doi: 10.1038/296034a0. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Libby P., Prakash S., Prioli R. P., Pereira M. E. Elaboration by mammalian mesenchymal cells infected with Trypanosoma cruzi of a fibroblast-stimulating factor that may contribute to chagasic cardiomyopathy. Infect Immun. 1987 Dec;55(12):3188–3191. doi: 10.1128/iai.55.12.3188-3191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger R. G. The prevalence of Trypanosoma cruzi infection in armadillos collected at a site near New Orleans, Louisiana. Am J Trop Med Hyg. 1988 Mar;38(2):323–326. doi: 10.4269/ajtmh.1988.38.323. [DOI] [PubMed] [Google Scholar]
- Zicker F., Netto J. C., Zicker E. M., Oliveira R. M., Smith P. G. Trypanosoma cruzi infection and electrocardiographic findings among active manual workers. A population-based study in central Brazil. Int J Epidemiol. 1990 Mar;19(1):182–186. doi: 10.1093/ije/19.1.182. [DOI] [PubMed] [Google Scholar]
- de Alcantara F. G., Costa R. de B. Jejunopatia chagásica. Rev Bras Med. 1966 May;23(5):316–317. [PubMed] [Google Scholar]
- de Carvalho A. C., Tanowitz H. B., Wittner M., Dermietzel R., Roy C., Hertzberg E. L., Spray D. C. Gap junction distribution is altered between cardiac myocytes infected with Trypanosoma cruzi. Circ Res. 1992 Apr;70(4):733–742. doi: 10.1161/01.res.70.4.733. [DOI] [PubMed] [Google Scholar]
- de Moraes-Filho J. P., Kohatsu O. S., Bettarello A. Pressão basal do esfíncter inferior do esôfago na doença de Chagas: megaesôfago e forma indeterminada. AMB Rev Assoc Med Bras. 1986 Mar-Apr;32(3-4):51–53. [PubMed] [Google Scholar]
- de Paola A. A., Horowitz L. N., Miyamoto M. H., Pinheiro R., Ferreira D. F., Terzian A. B., Cirenza C., Guiguer N., Jr, Portugal O. P. Angiographic and electrophysiologic substrates of ventricular tachycardia in chronic Chagasic myocarditis. Am J Cardiol. 1990 Feb 1;65(5):360–363. doi: 10.1016/0002-9149(90)90302-h. [DOI] [PubMed] [Google Scholar]
- de Paula-Costa M. D., de Rezende J. M. Pressão basal do esfíncter inferior do esôfago no megaesôfago chagásico. AMB Rev Assoc Med Bras. 1978 Aug;24(8):269–272. [PubMed] [Google Scholar]
- de Rezende J. M., Rosa H., Vaz M. da G., Andrade-Sá N., Porto J. D., Neves Neto J., Ximenes J. A. Endoscopia no megaesôfago. Estudo prospectivo de 600 casos. Arq Gastroenterol. 1985 Apr-Jun;22(2):53–62. [PubMed] [Google Scholar]
- dos Santos R. R., Rossi M. A., Laus J. L., Silva J. S., Savino W., Mengel J. Anti-CD4 abrogates rejection and reestablishes long-term tolerance to syngeneic newborn hearts grafted in mice chronically infected with Trypanosoma cruzi. J Exp Med. 1992 Jan 1;175(1):29–39. doi: 10.1084/jem.175.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]