Abstract
Enterohemorrhagic Escherichia coli (EHEC) is a prominent, food-borne cause of diarrhea, bloody diarrhea, and the hemolytic uremic syndrome in industrialized countries. Most strains of EHEC carry the locus for enterocyte effacement (LEE) pathogenicity island, but a proportion of isolates from patients with severe disease do not carry LEE and very little is known about virulence factors in these organisms. LEE-negative strains of EHEC typically express Shiga toxin 2 and carry a large plasmid that encodes the production of EHEC hemolysin. In this study, we determined the nucleotide sequence of the transfer region of pO113, the large hemolysin plasmid from LEE-negative EHEC O113:H21 (EH41). This 63.9-kb region showed a high degree of similarity with the transfer region of R64, and pO113 was capable of self-transmission at low frequencies. Unlike R64 and the related dot/icm system of Legionella pneumophila, however, pO113 was unable to mobilize RSF1010. In addition, the pO113 transfer region encoded a novel high-molecular-weight serine protease autotransporter of Enterobacteriaceae (SPATE) protein, termed EpeA. Like other SPATEs, EpeA exhibited protease activity and mucinase activity, but expression was not associated with a cytopathic effect on epithelial cells. Analysis of a second high-molecular-weight secreted protein revealed that pO113 also encodes EspP, a cytopathic SPATE identified previously in EHEC O157:H7. The nucleotide sequences encoding the predicted β-domains of espP and epeA were identical and also shared significant homology with a third SPATE protein, EspI. Both espP and epeA were detected in several LEE-negative clinical isolates of EHEC and thus may contribute to the pathogenesis of this subset of EHEC.
Strains of enterohemorrhagic Escherichia coli (EHEC) comprise a subset of Shiga toxin (Stx)-producing E. coli (STEC) and are a common cause of hemorrhagic colitis (HC) and the hemolytic uremic syndrome (HUS). Although the expression of Stx is a defining feature of virulence, strains of EHEC also harbor a large plasmid encoding EHEC hemolysin (Ehx) and/or a chromosomal pathogenicity island, termed the locus for enterocyte effacement (LEE) (5, 13). Strains of EHEC carrying LEE belong to a group of bacterial pathogens that use a distinctive mechanism of colonization characterized by intimate bacterial adherence and the formation of attaching and effacing (A/E) lesions on the host intestinal mucosa. The most common serotype of EHEC associated with outbreaks and sporadic disease worldwide is O157:H7 (29). EHEC O157:H7 carries LEE and a large hemolysin plasmid, pO157, that is highly conserved among O157:H7 isolates (3, 36). The complete nucleotide sequence of pO157 has revealed an F-like plasmid with 100 predicted open reading frames (ORFs), many encoding putative virulence determinants (5). One of these is a cytopathic, secreted protease, EspP, which is a member of the serine protease autotransporter of Enterobacteriaceae (SPATE) family of exported proteins (4). Functional analysis has shown that EspP cleaves pepsin A and human coagulation factor V and that the protein induces a cytopathic effect on Vero cells.
Although the majority of EHEC strains isolated from patients are LEE-positive A/E pathogens, some serotypes of EHEC do not carry LEE and are not A/E pathogens. These strains have been termed LEE-negative EHEC or STEC and have been regularly associated with sporadic cases and small outbreaks of severe disease, namely HC and HUS (10, 12, 33). Although LEE-negative EHEC serotypes were represented in the original description of EHEC pathogens, the virulence mechanisms of this subset of EHEC have been largely overlooked (11, 23). In the absence of LEE, little is known about the way in which these strains colonize the human intestine, although full adherence undoubtedly requires both plasmid-borne and chromosomal factors (10, 32). Clinical isolates of LEE-negative EHEC typically express Shiga toxin type 2 and also harbor a large plasmid that encodes EHEC hemolysin (12). However, restriction fragment length polymorphism analysis of the ehxA gene from LEE-negative EHEC strains has shown that these plasmids comprise an evolutionarily distinct group when compared with the similarly sized plasmids of LEE-positive EHEC (2). Recently, two novel regions of pO113 from LEE-negative EHEC O113:H21 were characterized. One of these regions encoded an autoagglutinating adhesin, Saa, that was associated with the ability of EHEC O113:H21 (98NK2) to adhere to epithelial cells (32). The second region of pO113 comprised a novel type IV pilus gene cluster with predicted amino acid homology to the thin pilus encoded by the self-transmissible IncI plasmid, R64 (25). The type IV pilus of R64 is required for efficient plasmid transfer in liquid matings, where the pilus promotes donor-recipient contact. Recipient specificity is determined by the minor pilin subunit, PilV, which is under the control of a site-specific shufflon (22). A site-specific recombinase encoded by the shufflon, Rci, rearranges the C-terminal segment of PilV to yield seven alternative adhesins (17). The pil genes encoding the thin pilus of R64 are located within a 54-kb tra region that encodes one of the most complex DNA transfer systems described to date (26). This region comprises at least 49 genes, 10 of which share similarity with the dot/icm secretion system of Legionella pneumophila (26).
In this study, we present the complete nucleotide sequence of the transfer region of pO113 and show that components of this region share a high degree of amino acid homology with R64 tra gene products. pO113 was self-transmissible at low frequencies when compared with R64 but, unlike R64, pO113 could not mobilize RSF1010 (38). In addition, we found that the pO113 transfer region encoded a novel SPATE protein, EpeA, that exhibited in vitro mucinolytic activity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Wild-type strains of pathogenic E. coli used to determine the prevalence of epeA and espP were obtained from the culture collection of Roy Robins-Browne (University of Melbourne) (30). EHEC O113:H21 (EH41) was isolated from a child with HUS in New South Wales, Australia, and expresses Stx2 and EHEC hemolysin (10). R64 was obtained from Derek Pickard (Imperial College London). E. coli strains were grown in either Luria-Bertani (LB) broth or serum-free tissue culture medium (Dulbecco's modified Eagle's medium [DMEM]; GIBCO Invitrogen). Broth cultures were incubated aerobically at 37°C to logarithmic phase or overnight. E. coli carrying the thermosensitive suicide vector pCACTUS was cultured at 30°C (32, 40). For the induction of λ Red recombinase from plasmid pKD46, 10 mM arabinose was added to LB broth and electrocompetent cells were prepared by growth at 30°C (6). Where necessary, the growth medium was supplemented with 100 μg of ampicillin/ml, 100 μg of kanamycin/ml, 300 μg of rifampin/ml, or 30 μg of chloramphenicol/ml (Sigma Aldrich, St. Louis, Mo.).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristic(s) | Source or reference |
|---|---|---|
| E. coli strains | ||
| EH41 | Wild-type EHEC O113:H21 HUS isolate | 10 |
| EH41c | pO113-cured strain of EH41 | This study |
| EDL933 | Wild-type EHEC O157:H7 | 34 |
| LT101 | Rifampin-resistant derivative of E. coli HB101 | 31 |
| XL-1 Blue | supE44 hsdR17 recA1 endA1 gyrA46 thi relA1 lac- F′ [p roAB+ lacIq lacZ ΔM15 Tn10] (Tetr) | Stratagene |
| Plasmids | ||
| pO113 | Large hemolysin plasmid from EHEC O113:H21 | This study; 32 |
| R64 | Self-transmissible plasmid from Salmonella enterica serovar Typhimurium | 26 |
| pMMB67EH | Ampicillin-resistant derivative of RSF1010 | 15 |
| pCR-Script | High-copy-number cloning vector (Ampr) | Stratagene |
| pKD46 | Expression vector for phage λ Red recombinase (γ, β, exo) induced by l-arabinose, thermosensitive Ampr | 6 |
| p13g | pCR-Script carrying 9.1-kb fragment of pO113 containing epeA | This study |
| p13gXho | XhoI fragment deletion derivative of p13g lacking epeA | This study |
| pCACTUS | Temperature-sensitive suicide plasmid which encodes chloramphenicol resistance and carries sacB | 40 |
| pCC1FOS | Copy control cosmid cloning vector | Epicentre |
DNA and electrophoresis techniques.
Standard techniques were employed for all recombinant DNA manipulations, Southern blotting, and electrophoresis procedures, including sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (35). DNA-modifying enzymes (Promega, Madison, Wis.) were used according to the manufacturer's instructions. Chromosomal DNA was isolated using the DNeasy tissue kit (Qiagen, Hilden, Germany), and plasmid DNA was isolated using the Qiaprep Spin miniprep kit (Qiagen).
To prepare a pO113 plasmid library, plasmid DNA from EHEC strain O113:H21 (EH41) was extracted and digested with BamHI. The resulting fragments were cloned randomly into the BamHI site of pCR-Script, and the nucleotide sequences of both strands were determined using M13-based sequencing primers or custom-made oligonucleotides. The plasmid clone, p13g, derived from this library carries a 9.1-kb fragment of pO113 that includes the entire epeA gene. p13gXho is a derivative of p13g where a XhoI fragment containing epeA has been deleted by restriction enzyme digestion and religation.
To obtain larger fragments of pO113 for sequence analysis, a random genomic library was created in the Copy Control pCC1FOS cosmid vector according to the manufacturer's instructions (Epicentre, Madison, Wis.). pO113 tra clones were then selected by dot blot hybridization with digoxigenin (DIG)-labeled probes generated by PCR (Roche Molecular Biochemicals, Basel, Switzerland), and nucleotide sequencing was performed directly on purified pCC1FOS DNA.
Nucleotide sequencing and analysis.
Plasmid or cosmid DNA was prepared for sequence analysis using a PRISM Ready Reaction DyeDeoxy Terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.). The nucleotide sequence of samples was determined by automated DNA sequencing using an Applied Biosystems model 373A DNA sequencing system. DNA sequences were assembled using Sequencher 3.1.1 (Gene Codes Corp., Ann Arbor, Mich.). GeneMark.hmm for prokaryotes (http://opal.biology.gatech.edu/GeneMark) was used to identify putative genes, and BLAST programs (http://www.ncbi.nlm.nih.gov:80/BLAST) were used to determine nucleotide and amino acid homologies with sequences in GenBank. The cellular localizations of putative proteins were predicted using the PSORT programs (http://psort.ims.u-tokyo.ac.jp).
Transfer of pO113 and RSF1010.
Liquid and surface bacterial conjugations were performed as described previously (24). For pO113 transfer experiments, EH41 carrying pO113ehx:km was used as the donor strain. For mobilization experiments, the donor strains EHEC O113:H21 (EH41) and E. coli carrying R64 were transformed with an ampicillin-resistant derivative of RSF1010, pMMB67EH (15). EH41c and E. coli LT101 (31) acted as rifampin-resistant recipient strains. Log-phase cultures of donor cells were mixed with overnight cultures of recipient cells, and the mixtures were incubated at 37°C for 90 min before plating onto LB agar containing kanamycin and rifampin for selection of pO113ehx:km transfer or ampicillin and rifampin for selection of pMMB67EH mobilization. Transfer frequencies were expressed as the ratio of transconjugants to donor cells.
Construction of a pO113-cured derivative and epeA and ehx mutants of EHEC O113:H21 (EH41).
Curing of pO113 was achieved by recombination of the thermosensitive vector pCACTUS into pO113 (26). Briefly, a 1.3-kb segment of the ehx operon was cloned into pCACTUS, which was then electroporated into EHEC O113:H21 (EH41). Recombination of pCACTUS:ehx with pO113 was selected by growth at 37°C on Luria agar supplemented with chloramphenicol. The resulting colonies were EHEC hemolysin negative on EHEC hemolysin agar plates, confirming the interruption of ehx. Curing of the recombined pO113:CACTUS plasmid was achieved by overnight growth of this strain at 43°C in LB broth supplemented with 6% sucrose. Bacteria were then diluted and spread onto EHEC hemolysin agar. The vast majority of colonies were EHEC hemolysin positive, indicating the excision of pCACTUS:ehx. However, 12 EHEC hemolysin-negative colonies were identified and tested for the presence of pO113 by PCR using primers designed for the pO113-encoded genes ehx, pilS, traB, and epeA. The resulting pO113-cured strain was designated EH41c and confirmed to be a derivative of EHEC O113:H21 (EH41) by PCR using O113 lipopolysaccharide-specific primers.
To construct an epeA mutant of EHEC O113:H21 (EH41), we used the λ Red recombinase system to introduce a kanamycin resistance gene into epeA located on pO113. Briefly, the temperature-sensitive replicon pKD46 was introduced into EH41, and electrocompetent cells were prepared from bacteria grown at 30°C with 10 mM arabinose to induce Red recombinase expression as described previously (6). A kanamycin cassette was cloned into the PstI site located 1.3 kb downstream of the putative start codon of epeA, and the interrupted epeA gene fragment was amplified by PCR and introduced into EH41 containing pKD46 by electroporation. Successful interruption of epeA was confirmed by PCR analysis and was evident by an increase in molecular weight of 1,185 bp corresponding to the insertion of the kanamycin cassette (data not shown) and loss of EpeA from the supernatant of EH41 cultures. Using a similar approach, we ligated the kanamycin cassette into a PstI site 310 bp from the start codon of ehxA and introduced the mutation onto pO113 using Red recombinase. Successful integration of the kanamycin resistance marker was confirmed by PCR and the absence of hemolysis on EHEC hemolysin agar.
PCR procedures and generation of DNA probes.
The PCR primer pairs used in this study are listed in Table 2. PCR amplification was performed on 500 ng of template DNA with approximately 1 μg of each primer PCR per 100-μl reaction mixture. Amplification of epeA, the β-domain of epeA/espP (beta), ehx, traB, and pilS, was performed under the following conditions: 2 min at 94°C and 30 cycles of 1 min at 94°C, 50 s at appropriate annealing temperature (Table 2), and 1 min at 72°C, followed by 5 min at 72°C. PCR amplification of espP was performed under the following conditions: 5 min at 94°C and 30 cycles of 30 s at 94°C, 1 min at 56°C, and 2.5 min at 72°C, followed by 5 min at 72°C as described previously (4), using the primers EspPF and EspPR. PCR-generated DNA products were examined by agarose gel electrophoresis, and where necessary DIG-dUTP was incorporated into the PCR according to the manufacturer's instructions (Roche) to generate DIG-labeled DNA probes.
TABLE 2.
Sequence of PCR primers used in this study
| Gene | Primer sequences | Annealing temp (°C) | Reference |
|---|---|---|---|
| epeA | EpeAF: 5′-CACCCTGTAGAATCTTA-3′ | 46 | This study |
| EpeAR: 5′-CTGAATAAATCCAGCCC-3′ | |||
| espP | EspPF: 5′-AAACAGCAGGCACTTGAACG-3′ | 56 | 4 |
| EspPR: 5′-GGAGTCGTCAGTCAGTAGAT-3′ | |||
| beta | BetaF: 5′-GGCTCTGCCAGTGGTGG-3′ | 42 | This study |
| BetaR: 5′-GTTGTACTTACCAAAGG-3′ | |||
| ehx | EhxF: 5′-GGAACCGCTGAAAATGTAGG-3′ | 50 | This study |
| EhxR: 5′-ACTGGTCGTCTCCCTGTCC-3′ | |||
| pilS | PilSF: 5′-TCCAGTGACAACCAGCGC-3′ | 52 | This study |
| PilSR: 5′-TTAGCTGTTGGTTTCCAG-3′ | |||
| tra | TraF: 5′-AAACAGCAGGCACTTGAACG-3′ | 50 | This study |
| TraR: 5′-AAACAGCAGGCACTTGAACG-3′ | |||
| O113lps | O113F: 5′-AGCGTTTCTGACATATGGAGTG-3′ | 50 | 33 |
| O113R: 5′-GTGTTAGTATCAAAAGAGGCTCC-3′ |
Preparation of culture supernatant proteins.
For SDS-PAGE, secreted proteins were precipitated from bacterial culture supernatants. Briefly, overnight bacterial broth cultures were diluted 1:20 in 10 ml of prewarmed DMEM and grown to mid-log phase. The bacteria were pelleted by centrifugation (10,000 × g, 4°C, 10 min), and the supernatants were filtered through 0.22-μm-pore-size filters (Millipore, Bedford, Mass.). A 10% (wt/vol) final concentration of trichloroacetic acid (TCA; BDH Laboratory Supplies, Poole, Dorst, England) was used to precipitate the proteins, which were then pelleted by centrifugation (10,000 × g, 4°C, 45 min). The protein pellets were washed twice with 10 ml of 100% (vol/vol) methanol (BDH Laboratory Supplies), pelleted as before, dried for 1 h at 37°C, and finally resuspended in 2× SDS sample buffer.
For functional assays, proteins were concentrated from culture supernatants. Overnight broth cultures were diluted 1:20 into 800 ml of prewarmed LB and grown to mid-log phase. Bacteria were then pelleted by centrifugation (10,000 × g, 4°C, 10 min), and supernatants were filtered through 0.22-μm-pore-size filters (Millipore). The clarified supernatants were then concentrated 400-fold by ultrafiltration through PM10, PM30, or XM50A membranes (Amicon Corp., Lexington, Mass.). All manipulations were performed at 4°C.
N-terminal amino acid sequencing.
Automated Edman degradation was used to determine the N-terminal amino acid sequences of secreted proteins. The proteins were separated by SDS-10% PAGE and blotted onto a polyvinylidene difluoride sequencing-grade membrane (Perkin-Elmer Inc., Wellesley, Mass.). After blotting, proteins were visualized by staining with Coomassie brilliant blue (Bio-Rad Laboratories), and the appropriate bands were excised and sequenced using an Applied Biosystems 476A amino acid sequencer.
Assays for protease and mucinase activity.
Assays for protease activity with swine pepsin A (Roche) were performed as described previously with a minor variation (37). Briefly, 5 μg of concentrated supernatant was mixed with 3 μg of pepsin A, resuspended in phosphate-buffered saline (PBS) to a final volume of 20 μl, and incubated overnight at 37°C. Protein mixtures were then separated by SDS-PAGE, transferred to nitrocellulose, and detected with rabbit antipepsin antibodies (provided by I. van Driel). Protease inhibition assays were performed in the presence of 10 μg of phenylmethylsulfonyl fluoride (PMSF)/ml. Zymogram analysis of gelatine proteolysis was adapted from a previous method (41). In this assay, 8 μg of the preparation of secreted proteins was electrophoresed into a 10% nondenaturing PAGE gel containing 2 μg of gelatine (BDH Biochemical)/ml. After electrophoresis, the gel was incubated for 24 h in 40 mM Tris, 2 mM CaCl2 (pH 8.0) at 37°C, and the gel was then stained with 0.125% (wt/vol) Coomassie brilliant blue and destained in 40% (vol/vol) methanol-10% (vol/vol) acetic acid. Proteases from Dichelbacter nodosus were used as a positive control (28).
The lysis of bovine submaxillary mucin was assessed as described previously (18). Briefly, equivalent preparations of concentrated supernatant proteins were added to wells bored into agar containing 1.5% (wt/vol) agarose, 1.0% (wt/vol) glucose, and 0.5% (wt/vol) mucin in Luria broth. Following incubation at 37°C for 24 h, the agar was stained with 0.1% (wt/vol) amido black in 3.5 M acetic acid. Lysis of mucin was observed as a halo of clearing around the inoculation point.
Assays for cytotoxicity.
Assays for cytotoxicity were carried out as described by Guyer et al. (16) using cultured HeLa cells and CHO-K1 cells. Cells were stained with Giemsa (Sigma) and observed for morphological changes by bright-field microscopy using a Leica DM-RB HC fluorescence phase-contrast microscope.
Immunodetection of EpeA.
To obtain antibodies specific for EpeA, a region encoding the C-terminal 300 amino acids of the passenger domain of EpeA was amplified by PCR and cloned into pET28a to generate a His6-tagged fusion, His-EpasC. Rabbits were immunized four times with 100 μg of purified recombinant His-EpasC at 3-week intervals. The resulting antiserum was then absorbed three times with the epeA mutant of EH41. To determine the effect of temperature and pH on EpeA secretion, strain EH41 was grown in 25 ml of LB broth to an optical density at 600 nm (OD600) of 1.0 at differing pH and temperatures, and supernatant proteins were precipitated with 10% (wt/vol) TCA. Supernatant proteins were then separated by SDS-PAGE, and EpeA was detected by immunoblotting with antibodies raised against His-EpasC.
Nucleotide sequence accession number.
The nucleotide sequence presented in this study has been assigned GenBank accession no. AY258503.
RESULTS
Genetic organization of the pO113 transfer region.
The nucleotide sequence of the 63.9-kb pO113 transfer region revealed 65 putative ORFs, including 41 homologues of R64 transfer genes (Table 3). The overall genetic organization of the pO113 transfer region and comparison with R64 are shown in Fig. 1. Overall, the G+C content of the pO113 transfer region was 51%, which is similar to that of the E. coli K-12 genome (50.8%). Amino acid identity between pO113 products and their R64 homologues varied from 25 to 82% (Table 3). Major differences between the R64 and pO113 transfer regions were the absence of traA, traD, and traG in pO113 and a frameshift in traF. However, these tra genes are not required for R64 transfer (26). In addition, pO113 lacked nuc, pilJ, and pilK and the pilV shufflon. Although pilV was present, there were no inverted repeat regions characteristic of the R64 shufflon and, importantly, the site-specific recombinase rci, which is responsible for pilV rearrangements in R64, was missing. These sequence data agreed with a previous pilO113 sequence determined from a different strain of EHEC O113:H21, which has been shown to code for type IV pili (38). Instead of the R64 nuc gene, pO113 carried yigB, a gene encoding a 15.6-kDa putative nuclease similar to YigB from R100 (8). The pO113 transfer region also encoded several ORFs not present in R64. Two of these, ygiJ and B3051, were located within the pil region and encoded a putative oxidoreductase and outer membrane protein, respectively. ygiJ and B3051 are present in EHEC O157:H7 and E. coli K-12 but are located on the chromosome in these organisms. The additional 10.7-kb region located between pndC and trbA that was not present in R64 comprised 11 predicted ORFs, including a gene encoding a putative SPATE protein that we termed EpeA for EHEC plasmid-encoded autotransporter (Fig. 2; Table 3). Analysis of the region immediately upstream of the 4.1-kb epeA gene revealed a homologue of Z1195 (Orf9), a hypothetical protein with unknown function in EHEC O157:H7 EDL933, and investigation of the region immediately downstream from epeA revealed a small ORF identical to L7021 encoded by pO157 (5). The 35-amino-acid predicted product of L7021 has no known homologues but is located adjacent to espP on pO157.
TABLE 3.
Properties of putative pO113 tra gene products
| Gene | No. of amino acids | Mass (kDa) | pI | Predicted locationa | Homologue | Property | Origin | % Identity/similarity |
|---|---|---|---|---|---|---|---|---|
| traB | 213 | 24.6 | 9.5 | C | TraB | Regulator of transfer | R64 | 65/79 |
| traC | 221 | 25.0 | 7.8 | C | TraC | Regulator of transfer | R64 | 36/51 |
| ygiJ | 206 | 22.4 | 5.3 | IM | YgiJ | Oxidoreductase | E. coli K-12 | 57/72 |
| B3051 | 564 | 62.2 | 5.0 | OM | B3051 | Membrane protein | E. coli K-12 | 81/92 |
| pilI | 80 | 9.1 | 9.2 | C | PilI | R64 | 32/51 | |
| pilL | 356 | 38.8 | 10.5 | IM/OM | PilL | Lipoprotein | R64 | 42/55 |
| pilM | 145 | 16.3 | 7.0 | IM | PilM | Pilus biogenesis | R64 | 29/53 |
| pilN | 539 | 57.5 | 7.7 | OM | PilN | Secretin | R64 | 39/60 |
| pilO | 431 | 47.7 | 6.0 | IM | PilO | Pilus biogenesis | R64 | 22/38 |
| pilP | 150 | 16.3 | 6.3 | P/OM | PilP | Pilus biogenesis | R64 | 27/42 |
| pilQ | 502 | 56.0 | 6.4 | C | PilQ | Nucleotide-binding protein | R64 | 43/58 |
| pilR | 366 | 41.0 | 9.4 | IM | PilR | Pilus biogenesis | R64 | 28/49 |
| pilS | 147 | 15.9 | 9.1 | OM | PilS | Type IV prepilin | R64 | 25/45 |
| pilT | 157 | 17.8 | 9.9 | OM | PilT | Transglycosylase | R64 | 52/66 |
| pilU | 211 | 23.1 | 9.6 | IM | PilU | Prepilin peptidase | R64 | 25/42 |
| pilV | 454 | 49.0 | 8.4 | OM | PilV | Type IV prepilin | R64 | 41/56 |
| orf1 | 38 | 4.2 | 12.3 | OM | None | |||
| orf2 | 55 | 6.2 | 5.7 | C | None | |||
| artA | 106 | 12.1 | 9.3 | IM | ArtA | F-plasmid | 34/60 | |
| traE | 273 | 31.2 | 8.9 | C | TraE | R64 | 56/72 | |
| traF | 184 | 19.6 | 5.5 | P/OM | TraF (first 165 aa)b | R64 | 71/81 | |
| traF′ | 221 | 24.1 | 5.1 | C | TraF (last 220 aa) | R64 | 78/91 | |
| traH | 150 | 16.5 | 8.9 | IM/OM | TraH | R64 | 64/77 | |
| traI | 278 | 31.7 | 10.1 | IM/OM | TraI | Lipoprotein | R64 | 78/86 |
| traJ | 382 | 42.5 | 6.4 | IM | TraJ | Nucleotide-binding protein | R64 | 78/89 |
| traK | 96 | 11.5 | 9.8 | IM | TraK | R64 | 71/86 | |
| sogL | 1,338 | 146.1 | 4.8 | C | SogL | DNA primase | R64 | 52/65 |
| traL | 116 | 12.6 | 6.6 | P/OM | TraL | R64 | 73/84 | |
| traM | 231 | 25.5 | 9.0 | IM | TraM | R64 | 70/83 | |
| traN | 324 | 34.2 | 5.6 | P/OM | TraN | R64 | 76/85 | |
| traO | 443 | 47.1 | 7.2 | IM | TraO | R64 | 58/71 | |
| traP | 237 | 26.2 | 7.8 | IM | TraP | R64 | 47/61 | |
| traQ | 175 | 18.1 | 8.0 | IM | TraQ | R64 | 76/86 | |
| traR | 132 | 7.1 | 9.6 | IM/OM | TraR | R64 | 54/74 | |
| traS | 83 | 9.1 | 6.3 | IM | TraS | R64 | 67/79 | |
| traT | 272 | 31.1 | 9.6 | C | TraT | R64 | 60/78 | |
| orf3 | 49 | 5.5 | 4.7 | C | None | |||
| orf4 | 127 | 14.8 | 10.5 | P/OM | Psyr0721 | P. aeruginosa | 56/76 | |
| traU | 1,014 | 114.0 | 6.5 | C | TraU | Nucleotide-binding protein | R64 | 77/89 |
| traV | 205 | 23.4 | 9.5 | C | TraV | R64 | 66/81 | |
| traW | 401 | 42.9 | 5.9 | IM/OM | TraW | Lipoprotein | R64 | 67/78 |
| traX | 189 | 22.0 | 10.5 | IM | TraX | R64 | 40/58 | |
| traY | 721 | 77.3 | 6.7 | IM | TraY | Integral membrane protein | R64 | 65/78 |
| excA | 216 | 25.1 | 9.1 | IM | ExcA | Surface exclusion | R64 | 48/66 |
| orf5 | 320 | 36.3 | 9.9 | C | RpoS | R. solanacearum | 31/57 | |
| orf6 | 224 | 26.2 | 8.9 | C | Gmet1519 | G. metallireducens | 28/45 | |
| pndC | 94 | 10.5 | 9.0 | C | PndC | R64 | 82/86 | |
| orf7 | 120 | 13.4 | 7.1 | C | C3606 | UPEC | 68/78 | |
| orf8 | 411 | 46.6 | 6.0 | P/OM | YjgX | UPEC | 52/67 | |
| orf9 | 207 | 23.7 | 9.6 | C | Z1195 | EHEC O157:H7 | 64/74 | |
| yigB | 103 | 11.6 | 9.8 | P/OM | YigB | R100 | 56/68 | |
| epeA | 1,359 | 147.3 | 6.4 | OM | EspI | STEC | 58/71 | |
| L7021 | 36 | 3.9 | 5.3 | C | L7021 | pO157 | 96/100 | |
| orf10 | 63 | 7.3 | 9.9 | C | C3605 | UPEC | 71/82 | |
| orf11 | 119 | 13.3 | 6.6 | C | C3604 | UPEC | 40/59 | |
| orf12 | 121 | 14.1 | 6.5 | C | C3603 | UPEC | 68/75 | |
| orf13 | 63 | 7.0 | 7.8 | IM | C3602 | UPEC | 85/91 | |
| orf14 | 103 | 12.0 | 9.9 | C | R8 | Neurotensin receptor | UPEC | 57/65 |
| trbA | 423 | 48.6 | 9.2 | IM | TrbA | R64 | 67/78 | |
| trbB | 372 | 40.3 | 5.8 | IM | TrbB | R64 | 50/63 | |
| trbC | 767 | 87.1 | 7.6 | IM | TrbC | R64 | 75/84 | |
| orf15 | 100 | 11.5 | 7.8 | C | C5174 | Iron-regulated protein | UPEC | 66/81 |
| L7092 | 149 | 16.7 | 9.0 | IM/OM | L7092 | pO157 | 98/100 | |
| nikB | 903 | 104.5 | 7.2 | C | NikB | Relaxosome complex | pO157 | 94/94 |
| nikA | 139 | 16.1 | 9.4 | C | NikA | Relaxosome complex | R64 | 60/75 |
C, cytoplasm; IM, innermembrane; OM, outer membrane; P, periplasm.
aa, amino acids.
FIG. 1.
Schematic representation of the 63.9-kb transfer region of pO113 and comparison with R64. Grey arrows represent ORFs shared by pO113 and R64. White arrows represent ORFs unique to pO113, and black arrows indicate ORFs present in R64 and not pO113. Arrows indicate the direction of gene transcription. The scale shown is for pO113, and the R64 gene names are provided below the corresponding ORFs. The dotted line represents the absence of an equivalent R64 DNA sequence.
FIG. 2.
Schematic representation of a 9.1-kb BamHI fragment of pO113 containing epeA. The white arrow represents epeA (4,080 bp), and the dark arrows represent other putative ORFs encoded by this fragment. Cleavage positions for KpnI, SacI, BamHI, and XhoI are indicated, and the direction of lacZ transcription from pCR-Script is shown. Structure of EpeA is indicated below the 9.1-kb fragment and shows the N- and C-terminal cleavage sites, serine protease, and P-loop motifs and their corresponding amino acid sequences and positions. The predicted molecular mass of each domain is indicated.
pO113 transfer and mobilization of RSF1010 by pO113 and R64.
Previous work has suggested that pO113 is self-transmissible (38). In this study we confirmed that pO113 is capable of conjugal transfer but at a much lower frequency (∼0.00005%) than that reported for R64 (∼4.6%) (Table 4) (26). In addition, there was no significant difference in the transfer frequency of pO113 using E. coli LT101 or EH41c as recipients or between liquid or filter conjugations. Due to the high degree of similarity between pO113 and R64, we also tested the ability of pO113 to mobilize RSF1010; however, we were unable to detect mobilization of an RSF1010 derivative, pMMB67EH, by pO113 in this study (Table 4).
TABLE 4.
Conjugal transfer of pO113ehx:km and mobilization of RSF1010(pMMB67EH) by pO113 and R64
| Plasmid transferred | Transfer or mobilization frequency (%)a
|
|||
|---|---|---|---|---|
| Filter matings
|
Liquid matings
|
|||
| LT101b | EH41c | LT101 | EH41c | |
| pO113ehx:km | 0.00006 | 0.00009 | 0.00004 | 0.0001 |
| pMMB67EH | ||||
| by R64c | 14.4 | 0.066 | 3.4 | 0.0088 |
| by pO113 | <0.00001 | <0.00001 | <0.00001 | <0.00001 |
Transfer or mobilization frequency of pO113ehx:km and pMMB67EH from donor cells. Results are expressed as the percentage of transconjugants relative to donor cells.
Recipient strains E. coli LT101 or pO113-cured EHEC O113:H21 (EH41c).
Donor plasmids R64 or pO113 for mobilization of pMMB67EH.
Identification of epeA and homology with other SPATE proteins.
The epeA gene located in the pO113 transfer region was predicted to encode a 1,359-amino-acid precursor protein with a molecular mass of 147 kDa. EpeA was comparable in length and exhibited a significant degree of amino acid similarity to several SPATE proteins, including Pic, Tsh, SepA, and EspI (Table 5) (1, 18, 37, 39). Sequence analysis showed that like other SPATE proteins, EpeA comprised an extended N-terminal leader peptide signal sequence, a passenger or functional α-domain, and a C-terminal β-domain for which the cleavage site was predicted to lie between residues N1082 and N1083 (Fig. 2). The C-terminal β-domain also exhibited a conserved 3-amino-acid motif, YSF, essential for outer membrane localization of Hap, a SPATE protein from Haemophilus influenzae (20, 21). In addition, the β-domain contained an ATP/GTP-binding site motif (P-loop), A1152DVFSGKT, which is carried by many nucleotide-binding proteins (19, 20). Importantly, the putative passenger domain of EpeA possessed a potential serine protease motif, G254DSGSP, which was conserved in sequence and location in comparison to other SPATE proteins (Fig. 2) (19). Interestingly, while the nucleotide sequence of the passenger domain was unique and showed no homology to other nucleotide sequences present in GenBank, the β-domain exhibited 99% nucleotide homology to the β-domain of espP (GenBank accession no. AF074613) and 91% nucleotide homology to the β-domain of espI (accession no. AJ278144). Other genetic homologues that share this β-domain include eaaA from prophage P-EibA (87% homology; accession no. AF151091) and also pet from enteroaggregative E. coli and sat from uropathogenic E. coli (83% homology; accession numbers AF056581 and AF289092, respectively). Overall, the G+C content of the epeA gene was 41.9%, markedly lower than that of the entire pO113 transfer region and the E. coli K-12 chromosome (50.8%).
TABLE 5.
Amino acid sequence identity and similarity of EpeA with its closest homologues
| Protein | Accession no. | Major reported function | No. of amino acids | % Identity (% similarity) with:
|
|||
|---|---|---|---|---|---|---|---|
| Entire protein | Leader sequence | Passenger domain | β-Domain | ||||
| EspI | CAC39286 | Protease | 1,363 | 58 (71) | 91 (96) | 47 (63) | 96 (97) |
| SepA | CAC05786 | Tissue invasion | 1,364 | 50 (65) | 58 (75) | 43 (60) | 79 (91) |
| Pic | AAK00464 | Mucinase | 1,373 | 49 (64) | 64 (84) | 41 (57) | 80 (90) |
| Tsh | I54632 | Hemagglutinin | 1,377 | 42 (58) | 52 (75) | 37 (54) | 62 (78) |
Expression of EpeA by EHEC O113:H21.
To determine if epeA was functional, we examined the secreted protein profile of EHEC O113:H21 (EH41) for the presence of a high-molecular-weight secreted protein. Analysis of the secreted protein fraction revealed two dominant high-molecular-weight proteins of 104 and 112 kDa which appeared to be common to several strains of EHEC O113:H21 and other serotypes of LEE-negative EHEC (Fig. 3). N-terminal amino acid sequencing was performed on both proteins. The amino acid sequence, (Y/S/H/K)TVDAEIP, obtained for the 112-kDa protein corresponded to the first 8 amino acids predicted for the secreted form of EpeA and was located 52 amino acids from the predicted start of the precursor protein. The second sequence obtained for the 104-kDa protein, (S/K)QMDISNF, exhibited 100% identity with the first 8 amino acids of the mature, secreted form of EspP, a product of the large hemolysin plasmid, pO157, carried by EHEC O157:H7.
FIG. 3.
SDS-PAGE gel showing the dominant high-molecular-mass proteins, EpeA and EspP, in secreted protein fractions from LEE-negative strains of EHEC. Lane 1, EHEC O157:H7 (EDL933) (LEE positive); lane 2, EHEC O113:H21 (EH41); lane 3, EHEC O113:H21 (EH53); lane 4, EHEC O113:H21 (EH71); lane 5, EHEC O116:H21 (EH42); lane 6, EHEC O130:H11 (EH43); lane 7, EHEC O1:H7 (EH69). Bacteria were grown to mid-log phase, and supernatant proteins were precipitated with TCA and resolved by an SDS-12.5% PAGE gel. Arrows indicate protein bands corresponding to EpeA and EspP. Arrowheads indicate LEE-encoded proteins.
Functional analysis of EpeA.
Based on the presence of a conserved serine protease motif and homology to a number of SPATE proteins, all of which exhibit protease activity, we postulated that EpeA also possessed proteolytic activity. To test the protease activity of EpeA, we concentrated supernatant proteins from two E. coli strains expressing EpeA, namely wild-type EHEC O113:H21 (EH41) and E. coli XL1-Blue harboring the plasmid p13g, which carries a 9.1-kb fragment of pO113 including a complete copy of epeA (Fig. 2 and 4). As a negative control, we constructed an epeA mutant of EHEC O113:H21 (EH41) by the insertion of a kanamycin resistance marker into a unique PstI site within the coding region of epeA. This mutant was designated EH41epeA, and analysis of the secreted protein profile of the mutant showed that interruption of epeA resulted in loss of the 112-kDa protein from the culture supernatant (Fig. 4). In addition, we constructed a control for p13g by the deletion of a XhoI fragment containing epeA, and this plasmid was termed p13gXho. E. coli XL-1 Blue (p13gXho) also failed to secrete a 112-kDa protein into the culture supernatant (Fig. 4).
FIG. 4.
Secreted proteins isolated from EHEC O113:H21 (EH41) (lane 1), EH41epeA (lane 2), E. coli XL1-Blue(p13g) (lane 3), and E. coli XL1-Blue(p13gXho) (lane 4). Bacterial strains were grown to mid-log phase, and supernatant proteins were precipitated with TCA and resolved on an SDS-12.5% PAGE gel. The open arrowhead indicates EpeA, and the closed arrowhead indicates EspP. The asterisk indicates a breakdown product of EpeA.
Concentrated supernatant proteins prepared from all four strains were used to assay protease activity in a pepsin A-based assay as described previously (37). Complete degradation of pepsin A was observed when concentrated supernatant proteins derived from EH41 and E. coli XL1-Blue(p13g) were incubated with the substrate (Fig. 5A, lanes 2 and 4). Conversely, EH41epeA and E. coli XL1-Blue(p13gXho) supernatant proteins did not cleave pepsin A (Fig. 5A, lanes 3 and 5). In addition, preincubation for 1 h at 37°C with 10 μg of PMSF/ml abolished the proteolytic activity of the concentrated supernatants from EH41 and E. coli XL1-blue(p13g) cells (data not shown), indicating that protease activity was responsible for the cleavage of pepsin A in this assay. In addition to cleavage of pepsin A, we tested the ability of EpeA to digest gelatine in a zymogram assay. In this system, concentrated supernatant proteins from EH41 and E. coli XL1-Blue(p13g) produced extensive zones of clearance indicating the proteolysis of gelatine (Fig. 5B, lanes 2 and 4), whereas protein samples derived from EH41epeA and E. coli XL1-Blue(p13gXho) showed no protease activity (Fig. 5B, lanes 3 and 5).
FIG. 5.
(A) Immunoblot demonstrating cleavage of swine pepsin A by concentrated supernatant proteins derived from EH41 (lane 2), EH41epeA (lane 3), E. coli XL1-Blue(p13g) (lane 4), and E. coli XL1-Blue(p13gXho) (lane 5). The arrow indicates uncleaved pepsin, which is also shown for reference in lane 1. (B) Gelatinase zymogram analysis of concentrated supernatant proteins derived from EH41 (lane 2), EH41epeA (lane 3), E. coli XL1-Blue(p13g) (lane 4), and E. coli XL1-Blue(p13gXho) (lane 5). Lane 1 shows activity of secreted proteases from D. nodosus as a positive control. (C) Mucinolytic activity of EpeA. Agar containing 0.5% bovine submaxillary mucin was inoculated with 5 μg of supernatant proteins derived from E. coli XL1-Blue(p13g) (i) and E. coli XL1-Blue(p13gXho) (ii) per ml. Agar was stained with 0.1% amido black.
Mucinase activity has been reported for the Shigella flexneri SPATE protein, Pic, which is closely related to EpeA by amino acid similarity (18). Here we also tested the ability of EpeA to cleave bovine submaxillary mucin. Concentrated supernatant proteins derived from E. coli XL1-Blue(p13g) and E. coli XL1-Blue(p13gXho) were added to agar containing 0.5% (wt/vol) bovine submaxillary mucin. Incubation with supernatant proteins from E. coli XL1-Blue(p13g) containing EpeA produced a clear zone of lysis around the inoculation site (Fig. 5C, well i), whereas incubation with supernatant proteins from E. coli XL1-Blue(p13gXho) failed to produce a zone of clearing in the agar containing mucin (Fig. 5C, well ii). These results indicated that, similar to Pic, EpeA can lyse bovine submaxillary mucin (18).
Although EpeA is less related by homology to the cytopathic group of SPATE proteins, we nevertheless tested the cytotoxic activity of EpeA against HeLa cells. We were unable, however, to attribute a cytopathic effect to EpeA at a concentration of 25 μg/ml, which was shown previously to be sufficient for induction of cytotoxicity by other SPATE proteins such as EspP, Sat, and Pet (4, 14, 16).
Effect of temperature and pH on EpeA secretion.
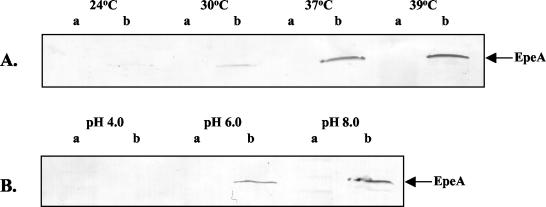
To ascertain if temperature and/or pH regulates EpeA secretion as it does for Pic, we monitored the amount of EpeA released into the culture supernatant from EH41 cells grown under different conditions to the same optical density (OD600 = 1.0). EpeA was detected by immunoblotting using anti-EpeA antibodies raised in rabbits. The results showed that EpeA was expressed maximally at 37 to 39°C and at alkaline pH (pH ≥ 8) as described for Pic (Fig. 6) (18). Interestingly, EpeA was not detected in whole-cell protein extracts, suggesting that the majority of the protein is released into the culture supernatant (Fig. 6).
FIG. 6.
Effect of temperature and pH on EpeA secretion. Western blot analysis of EpeA in whole-cell protein extracts (a lanes) and precipitated supernatant proteins (b lanes) from EH41 cultures grown to an OD600 of 1.0 at 24, 30, 37, and 39°C (A) and pH 4.0, 6.0, and 8.0 (B).
epeA and espP share a common β-domain.
A BLASTN comparison of espP and epeA suggested that these two genes share a near-identical β-domain. As this level of nucleotide homology has not been reported among the β-domains of SPATE genes previously, we performed a Southern blot analysis on EH41 and the pO113-cured derivative of EH41 (EH41c) to confirm that two copies of the β-domain were present on pO113. Firstly, specific PCR analysis of whole-cell DNA from EH41c showed that epeA and espP were missing from the cured strain, thus confirming that both genes are located on pO113 (data not shown). For Southern analysis, genomic DNA from EH41 and EH41c was digested with EcoRI, which does not cut within the β-domain, and hybridized with a DIG-labeled probe, beta, that was specific for the β-domain of epeA. The beta probe was generated by PCR using p13g as a template and the primers BetaF and BetaR. Unexpectedly, the Southern blotting showed that EH41 carried three copies of the β-domain and that EH41c carried one copy (Fig. 7). This result confirmed that two β-domains were present on pO113, and we hypothesized that the third copy of the β-domain carried on the chromosome of EH41 was part of a large genomic island described recently in a serogroup O91:H- LEE-negative strain of STEC which encodes EspI (37). Further PCR analysis confirmed that EH41 carried this genomic island inserted at the selC locus and suggested that our beta probe had hybridized to the β-domain region of an espI homologue (data not shown).
FIG. 7.
Southern blot analysis of EcoRI-digested genomic DNA derived from EH41c (lane 1) and EH41 (lane 2). The membrane was hybridized with DIG-labeled beta probe specific for the β-domain of epeA.
Distribution of epeA and espP among LEE-negative and LEE-positive strains of EHEC.
N-terminal amino acid sequencing of two high-molecular-weight secreted proteins identified in this study revealed that EH41 secreted a homologue of EspP. As the presence of EspP in LEE-negative strains of EHEC has not been described previously, we examined the distribution of espP and the novel gene epeA among strains of EHEC and EPEC using PCR primers specific for their respective passenger domains (Table 6). The bacterial strains collected for testing have been described previously in other studies (10, 12, 30, 32). The majority of LEE-negative EHEC strains (15 of 24 strains tested) possessed the epeA gene, while none of the LEE-positive EHEC strains tested carried epeA (Table 6). As epeA is a novel gene, we also tested its prevalence among isolates of EPEC and other E. coli pathogens, including nine strains of EPEC and five isolates each of enteroaggregative E. coli, enteroinvasive E. coli, enterotoxigenic E. coli, and S. flexneri; however, all were negative (data not shown). Thus, epeA appeared to be specifically associated with LEE-negative isolates of EHEC. Interestingly, although epeA was not found in every LEE-negative EHEC serotype tested, it was found in 85% of strains associated with severe disease, i.e., isolates derived from patients with HUS and thrombotic thrombocytopenic purpura and/or HC (12). In contrast to epeA, espP was found in both LEE-positive strains of EHEC, as reported previously (3), and in the majority of LEE-negative EHEC strains (18 of 24 strains tested) (Table 6). Interestingly, 97% of the EHEC strains tested (32 of 33) were positive for the β-domain (beta) of espP and epeA, while only 1 of 15 EPEC isolates tested (7%) was positive. This serogroup O142:H6 EPEC strain was also positive for espP. In addition, beta was not detected by PCR in the other E. coli pathogens described above, suggesting that a beta-specific PCR may be a useful molecular diagnostic test for the detection of EHEC in combination with detection methods for stx and/or ehx and eae.
TABLE 6.
Prevalence of epeA and espP among different serotypes of EHEC
| Serotype | eaea | No. of positive strains/no. tested
|
||
|---|---|---|---|---|
| espP | epeA | beta | ||
| O157:H7 | + | 2/2 | 0/2 | 2/2 |
| O111:H- | + | 1/4 | 0/4 | 3/4 |
| O15:H- | + | 1/1 | 0/1 | 1/1 |
| O26:H11 | + | 2/2 | 0/2 | 2/2 |
| O113:H21 | − | 11/11 | 9/11 | 11/11 |
| O116:H21 | − | 1/1 | 1/1 | 1/1 |
| O130:H11 | − | 1/1 | 1/1 | 1/1 |
| O5:H- | − | 0/2 | 1/2 | 2/2 |
| O1:H7 | − | 1/1 | 1/1 | 1/1 |
| NT:H7 | − | 1/1 | 1/1 | 1/1 |
| O48:H21 | − | 1/1 | 1/1 | 1/1 |
| O76:H7 | − | 0/1 | 0/1 | 1/1 |
| O128:H2 | − | 0/3 | 0/3 | 3/3 |
| O91:H- | − | 2/2 | 0/2 | 2/2 |
Marker for LEE pathogenicity island.
DISCUSSION
LEE-negative strains of EHEC are a significant cause of HUS and HC in many parts of the world (33). In Australia, EHEC O113:H21 is the second most common serotype isolated from patients with HUS after O111:H- (12). To date, the virulence factors possessed by LEE-negative strains of EHEC remain poorly characterized, and in particular the determinants that mediate adherence to the gastrointestinal tract in the absence of LEE are unknown. Recently, we described a novel chromosomal fimbrial gene cluster in EHEC O113:H21 that shares predicted similarity to long polar fimbrial components (10). This locus was required for full adherence of EHEC O113:H21 to epithelial cells and thus may play a role in colonization of the host intestine.
Like EHEC O157:H7, LEE-negative strains of EHEC carry a large plasmid that encodes EHEC hemolysin and EspP, although apart from these two factors, the LEE-negative EHEC plasmids appear to be unrelated to pO157. In this study we determined the nucleotide sequence of the transfer region of pO113, the large hemolysin plasmid from EHEC O113:H21 (32). This region shared a high degree of similarity with components of the tra region from R64, a self-transmissible plasmid identified originally in Salmonella enterica serovar Typhimurium (26). Although in this study we confirmed that pO113 was self-transmissible, the transfer frequency of pO113 was around 105-fold lower than that reported for R64 (26). In addition, unlike R64 and the related dot/icm system of L. pneumophila, pO113 was unable to mobilize the IncQ plasmid RSF1010. This suggests that the two systems are functionally different and may indicate that some components of the pO113 transfer region are not expressed or functional.
Although gene orientation and order among homologues of the R64 and pO113 transfer regions were largely conserved, we observed several differences, including the presence of an additional 10.7-kb region in pO113 located between pndC and trbA. Several putative ORFs were present in this region that shared similarity with hypothetical proteins from uropathogenic E. coli and EHEC O157:H7. The function and origin of these hypothetical genes in either pathogen is unclear, but together they represent a combination of plasmid-borne and chromosomal factors, suggesting that, like pO157, pO113 has a mosaic structure and has evolved through the acquisition of determinants from a number of sources. In addition, we identified a novel SPATE protein in the additional 10.7-kb region, termed EpeA. EpeA was one of two dominant high-molecular-weight proteins secreted by LEE-negative strains of EHEC that was also identified by N-terminal amino acid sequencing. By amino acid similarity, EpeA was most closely related to EspI, a SPATE protein described recently in a serogroup O91:H- strain of LEE-negative STEC. However, EpeA was also highly related to SPATEs from other pathogens, namely SepA, Pic, and Tsh (1, 18, 37, 39). EpeA possessed protease and mucinase activity but was not able to induce a cytopathic effect on HeLa cells. Like its close relative Pic, EpeA was maximally expressed at body temperature under alkaline conditions, suggesting that the protein may be produced as the pathogen enters the small intestine (18). Although the contribution of EpeA to pathogenesis is unclear, the protein may aid colonization and adherence to the host intestine through mucinolytic activity.
The second high-molecular-weight secreted protein identified in EHEC O113:H21 (EH41) was EspP. EspP is a cytopathic SPATE protein which is encoded by pO157 in EHEC O157:H7 (4, 9). In EHEC O113:H21 (EH41), both epeA and espP were encoded by pO113, although espP was not located within the 63.9-kb transfer region. We identified epeA and espP in the majority of LEE-negative EHEC isolates tested, and espP was also prevalent in LEE-positive strains of EHEC as reported previously (4). Importantly we have shown that, together with ehx and stx, espP is common to both LEE-negative and LEE-positive clinical isolates of EHEC and thus may represent a good molecular marker for the detection of EHEC in general. In addition, a PCR specific for the β-domain nucleotide sequence common to both epeA and espP was able to detect 97% of EHEC strains tested in this study (32 of 33), including 7 strains that were PCR negative for the espP and epeA passenger domains. This may indicate that these isolates carry additional autotransporters, such as EspI, that utilize the β-domain of epeA and espP but have a unique passenger domain. Indeed, autotransporters are believed to have evolved by domain shuffling where a new passenger domain is linked with a generic β-domain (7, 27).
The nucleotide sequence of pO157 contains remnants of a transfer system that indicate this plasmid was once conjugative. Through evolution, however, the F-plasmid-like transfer region of pO157 has been interrupted and replaced by insertion sequence elements and putative virulence genes (5). Although pO113 has retained much of its transfer region, several additional genes are present within this region that are not found in R64 or other closely related transfer systems. The acquisition of these additional genes and the loss of other components such as the shufflon may reflect the changing role of pO113 from a conjugative plasmid to a stable virulence-associated plasmid like pO157. Overall, the results of this study suggest that the megaplasmids of LEE-negative EHEC have a different evolutionary origin than the plasmids of LEE-positive EHEC strains but nevertheless share at least some virulence determinants, including EHEC hemolysin and EspP. The complete nucleotide sequence of pO113 will help to resolve the genetic relationship between pO113 and pO157 and will aid our understanding of the role of these plasmids in the pathogenesis of EHEC infections.
Acknowledgments
We gratefully acknowledge the Australian Paediatric Surveillance Unit and Roy Robins-Browne for the provision of EHEC and other E. coli strains. R64 was kindly provided by Derek Pickard. We are also indebted to Ian van Driel for the provision of pepsin antibodies used in this study and to Dane Parker for providing D. nodosus proteases.
This work was supported in part by funding from the Australian National Health and Medical Research Council, ANZ Charitable Trusts, Ramaciotti Foundations for Biomedical Research, and Monash University. D.L.L. is the recipient of an Australian Postgraduate Award.
Editor: A. D. O'Brien
REFERENCES
- 1.Benjelloun-Touimi, Z., P. J. Sansonetti, and C. Parsot. 1995. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 17:123-135. [DOI] [PubMed] [Google Scholar]
- 2.Boerlin, P., S. Chen, J. K. Colbourne, R. Johnson, S. De Grandis, and C. Gyles. 1998. Evolution of enterohemorrhagic Escherichia coli hemolysin plasmids and the locus for enterocyte effacement in Shiga toxin-producing E. coli. Infect. Immun. 66:2553-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunder, W., H. Schmidt, M. Frosch, and H. Karch. 1999. The large plasmids of Shiga-toxin-producing Escherichia coli (STEC) are highly variable genetic elements. Microbiology 145:1005-1014. [DOI] [PubMed] [Google Scholar]
- 4.Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. [DOI] [PubMed] [Google Scholar]
- 5.Burland, V., Y. Shao, N. T. Perna, G. Plunkett, H. J. Sofia, and F. R. Blattner. 1998. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Res. 26:4196-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, J., A. L. Smith, W. R. Hughes, and M. Golomb. 2001. Evolution of an autotransporter: domain shuffling and lateral transfer from pathogenic Haemophilus to Neisseria. J. Bacteriol. 183:4626-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dempsey, W. B. 1994. Regulation of R100 conjugation requires traM in cis to traJ. Mol. Microbiol. 13:987-1000. [DOI] [PubMed] [Google Scholar]
- 9.Djafari, S., F. Ebel, C. Deibel, S. Kramer, M. Hudel, and T. Chakraborty. 1997. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol. Microbiol. 25:771-784. [DOI] [PubMed] [Google Scholar]
- 10.Doughty, S., J. Sloan, V. Bennett-Wood, M. Robertson, R. M. Robins-Browne, and E. L. Hartland. 2002. Identification of a novel fimbrial gene cluster related to long polar fimbriae in locus of enterocyte effacement-negative strains of enterohemorrhagic Escherichia coli. Infect. Immun. 70:6761-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dytoc, M. T., A. Ismaili, D. J. Philpott, R. Soni, J. L. Brunton, and P. M. Sherman. 1994. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect. Immun. 62:3494-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott, E. J., R. M. Robins-Browne, E. V. O'Loughlin P. Henning, G. G. Hogg, J. Knight, H. Powell, D. Redmond, and V. Bennett-Wood. 2001. Nationwide surveillance of haemolytic uraemic syndrome in Australia: a comparison of endemic cases with those in a single epidemic. Arch. Dis. Child. 85:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 14.Eslava, C., F. Navarro-Garcia, J. R. Czeczulin, I. R. Henderson, A. Cravioto, and J. P. Nataro. 1998. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 66:3155-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furste, J. P., W. Pansegrau, R. Frank, H. Blocker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 16.Guyer, D. M., I. R. Henderson, J. P. Nataro, and H. L. Mobley. 2000. Identification of Sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 38:53-66. [DOI] [PubMed] [Google Scholar]
- 17.Gyohda, A., N. Furuya, N. Kogure, and T. Komano. 2002. Sequence-specific and non-specific binding of the Rci protein to the asymmetric recombination sites of the R64 shufflon. J. Mol. Biol. 318:975-983. [DOI] [PubMed] [Google Scholar]
- 18.Henderson, I. R., J. Czeczulin, C. Eslava, F. Noriega, and J. P. Nataro. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 20.Hendrixson, D. R., M. L. de la Morena, C. Stathopoulos, and J. W. St. Geme III. 1997. Structural determinants of processing and secretion of the Haemophilus influenzae Hap protein. Mol. Microbiol 26:505-518. [DOI] [PubMed] [Google Scholar]
- 21.Hendrixson, D. R., and J. W. St. Geme III. 1998. The Haemophilus influenzae Hap serine protease promotes adherence and microcolony formation, potentiated by a soluble host protein. Mol. Cell 2:841-850. [DOI] [PubMed] [Google Scholar]
- 22.Ishiwa, A., and T. Komano. 2000. The lipopolysaccharide of recipient cells is a specific receptor for PilV proteins, selected by shufflon DNA rearrangement, in liquid matings with donors bearing the R64 plasmid. Mol. Gen. Genet. 263:159-164. [DOI] [PubMed] [Google Scholar]
- 23.Karmali, M. A., M. Petric, C. Lim, P. C. Fleming, G. S. Arbus, and H. Lior. 1985. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 151:775-782. [DOI] [PubMed] [Google Scholar]
- 24.Kim, S. R., N. Funayama, and T. Komano. 1993. Nucleotide sequence and characterization of the traABCD region of IncI1 plasmid R64. J. Bacteriol. 175:5035-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, S. R., and T. Komano. 1997. The plasmid R64 thin pilus identified as a type IV pilus. J. Bacteriol. 179:3594-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komano, T., T. Yoshida, K. Narahara, and N. Furuya. 2000. The transfer region of IncI1 plasmid R64: similarities between R64 tra and Legionella icm/dot genes. Mol. Microbiol. 35:1348-1359. [DOI] [PubMed] [Google Scholar]
- 27.Loveless, B. J., and M. H. Saier, Jr. 1997. A novel family of channel-forming, autotransporting, bacterial virulence factors. Mol. Membr. Biol. 14:113-123. [DOI] [PubMed] [Google Scholar]
- 28.Moses, E. K., J. I. Rood, W. K. Yong, and G. G. Riffkin. 1989. Molecular analysis of one of multiple protease-encoding genes from the prototype virulent strain of Bacteroides nodosus. Gene 77:219-228. [DOI] [PubMed] [Google Scholar]
- 29.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholls, L., T. H. Grant, and R. M. Robins-Browne. 2000. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35:275-288. [DOI] [PubMed] [Google Scholar]
- 31.Palombo, E. A., K. Yusoff, V. A. Stanisich, V. Krishnapillai, and N. S. Willetts. 1989. Cloning and genetic analysis of tra cistrons of the Tra 2/Tra 3 region of plasmid RP1. Plasmid 22:59-69. [DOI] [PubMed] [Google Scholar]
- 32.Paton, A. W., P. Srimanote, M. C. Woodrow, and J. C. Paton. 2001. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect. Immun. 69:6999-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paton, A. W., M. C. Woodrow, R. M. Doyle, J. A. Lanser, and J. C. Paton. 1999. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3357-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 36.Schmidt, H., and H. Karch. 1996. Enterohemolytic phenotypes and genotypes of Shiga toxin-producing Escherichia coli O111 strains from patients with diarrhea and hemolytic-uremic syndrome. J. Clin. Microbiol. 34:2364-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt, H., W. L. Zhang, U. Hemmrich, S. Jelacic, W. Brunder, P. I. Tarr, U. Dobrindt, J. Hacker, and H. Karch. 2001. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect. Immun. 69:6863-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srimanote, P., A. W. Paton, and J. C. Paton. 2002. Characterization of a novel type IV pilus locus encoded on the large plasmid of locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect. Immun. 70:3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stathopoulos, C., D. L. Provence, and R. Curtiss III. 1999. Characterization of the avian pathogenic Escherichia coli hemagglutinin Tsh, a member of the immunoglobulin A protease-type family of autotransporters. Infect. Immun. 67:772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van den Bosch, L., P. A. Manning, and R. Morona. 1997. Regulation of O-antigen chain length is required for Shigella flexneri virulence. Mol. Microbiol. 23:765-775. [DOI] [PubMed] [Google Scholar]
- 41.Young, A. R., N. Mancuso, E. N. Meeusen, and V. M. Bowles. 2000. Characterization of proteases involved in egg hatching of the sheep blowfly, Lucilia cuprina. Int. J. Parasitol. 30:925-932. [DOI] [PubMed] [Google Scholar]