Abstract
An ideal vaccine delivery system would elicit persistent protection following a single administration, preferably by a noninvasive route, and be safe even in the face of immunosuppression, either inherited or acquired, of the recipient. We have exploited the unique life cycle of the autonomous parvoviruses to develop a nonproliferating vaccine platform that appears to both induce priming and continually boost a protective immune response following a single inoculation. A crippled parvovirus vector was constructed, based on a chimera between minute virus of mice (MVM) and LuIII, which expresses Borrelia burgdorferi outer surface protein A (OspA) instead of its coat protein. The vector was packaged into an MVM lymphotropic capsid and inoculated into naive C3H/HeNcr mice. Vaccination with a single vector dose, either intravenously or intranasally, elicited high-titer anti-OspA-specific antibody that provided protection from live spirochete challenge and was sustained over the lifetime of the animal. Both humoral and cell-mediated Th1 immunity was induced, as shown by anti-OspA immunoglobulin G2a antibody and preferential gamma interferon production by OspA-specific CD4+ T cells.
Despite their potential for safety and specificity, recombinant subunit vaccines have proven difficult to deliver in a way that provokes an appropriate, vigorous and persistent immune response. Approaches to solving these problems include the use of new adjuvants, direct plasmid DNA inoculation, and virus vectors, either alone or in combination. Although virus vectors give the advantage of introducing antigen to the immune system in the context of an infected cell, their use is complicated by immune response to the vector itself, which limits the efficacy of subsequent administration of the same vector. This drawback is circumvented in priming-boosting strategies (38, 44), in which antigen is first introduced by inoculation of a nonviral source, such as recombinant protein or DNA delivered by gene gun. The initial immunization is then boosted by inoculation with a virus vector expressing the same antigen, often an avipoxvirus (41) or modified vaccinia virus (2, 36).
While priming-boosting strategies show great promise, they have several drawbacks for large-scale immunization programs. They require two separate and somewhat sophisticated technologies, and the use of replication-competent virus vectors may pose significant problems for vaccinees who are immunocompromised. In an attempt to circumvent these problems, we set out to test whether a single inoculation of a persistent but nonproliferating parvovirus vector could safely provide both prime and boost. In this scenario, we speculated that the boost could be provided by antigen-expressing, lytically infected cells that would arise continually over time from a pool of cells harboring the vector in a silent state. For this we exploited unique aspects of the autonomous parvovirus life cycle. These viruses, and vectors based on them, are exquisitely dependent on the host cell cycle program, as they are unable to initiate cell proliferation but require their host to enter S phase to support replication and transcription of the virus's linear, single-stranded DNA genome (40, 45). Thus, in resting cells such as peripheral blood lymphocytes or quiescent fibroblasts, infectious virus has been observed to replicate only after addition of phytomitogens (42) or serum (53), respectively, indicating that infected resting cells harbor virus in a cryptic state (55). In vivo, when a cryptically infected host cell enters S phase in response to an exogenous signal, its resident virus or vector would be expected to initiate lytic replication, express its encoded antigens, and subsequently die.
Parvovirus genes are arranged in two expression cassettes. The initiating viral promoter (P4) drives nonstructural gene expression, which strongly transactivates the otherwise quiescent P38 promoter, leading to massive upregulation of capsid protein synthesis. For the vector described here, we used a capsid replacement strategy (40), where the vector contains the nonstructural genes of minute virus of mice (MVM), and the transgene, in this case OspA, substituted for sequences encoding the capsid protein VP2. The vector was packaged in capsids of the lymphotropic strain MVMi, with the intention that, at low input multiplicities, it would target a very small fraction of lymphocytes in the host. Initially, a limited burst of antigen should be expressed from infected cells that are cycling at the time of inoculation. Since relatively few lymphocytes will be cycling, infection will predominantly be cryptic. However, individual resting, cryptically infected cells will be activated stochastically during an extended period following inoculation and, as a population, should represent a continual source of cells expressing antigen. Since these would be seen by the immune system as cells dying as a result of an infectious process, we postulated that this process would continually boost the response against the antigen rather than inducing tolerance to it.
MATERIALS AND METHODS
Eukaryotic and prokaryotic cells.
Monkey CMT4 and human NB324K cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen, Rockville, Md.) supplemented with 5% fetal bovine serum at 37°C in 5% CO2. Low in vitro passage of Borrelia burgdorferi clone N40, with previously proven infectivity and pathogenicity in C3H mice (6), was used for challenges. Cultures were grown in modified Barbour-Stoenner-Kelly (BSK II) medium at 28°C, under which conditions the spirochete expresses high levels of OspA on its surface (5).
Mice.
Six-week-old, female C3H/HeNcr (C3H) mice were obtained from the National Cancer Institute (Frederick, Md.). Upon receipt, all animals were confirmed to be MVM antibody free by enzyme-linked immunosorbent assay (ELISA).
Recombinant vector construction.
The vector genome was constructed as a chimera between MVM and the related rodent parvovirus LuIII. An amber translation termination codon was engineered just downstream of the LuIII VP1 start by PCR-based mutagenesis to prevent expression of aberrant forms of the transgene from the minor alternative transcript normally encoding VP1. The OspA coding sequence was amplified by PCR from DNA isolated from B. burgdorferi strain cN40. The forward primer created an ApaI site at the 5′ end, placing the authentic MVM VP2 start codon immediately upstream of codon 15 of OspA, thus removing its hydrophobic leader peptide. Overlapping primers were used to fuse the C terminus and UAA termination codon of OspA to the MVM VP2 coding sequence position, so as to restore the vector to exactly the viral genome length, after an outside MVM-specific reverse primer amplified across an MscI site already in the vector sequence.
The forward primer sequence was 5′-G A G G G C C C A G A C A A T G G T G A A G C A A A A T G T T A G C A G C C -3′; the overlapping primer sequences were 5′-c t c a a c t c t a g c a g c t g T T A T T T T A A A G C G T T T T T A A T-3′ and 5′-A T T A A A A A C G C T T T A A A A T A A c a g c t g c t a g a g t t g a g c-3′; and the reverse primer sequence was 5′-G T A A A A A A T T G G G A G T T C A G G C C A C -3′ (the ApaI site is in italic and AUG start is indicated in bold; in the overlapping primer pair, lowercase indicates sequences unique to either the MVM vector or OspA, whereas uppercase indicates the sequence overlap). The amplified fragment was then double-digested with ApaI and MscI and cloned between these sites in the vector backbone. The fusion of OspA coding sequences with the VP2 start codon results in a shunting down of the 5′ coding sequence of VP2, with a compensatory deletion in the 3′ end of the coat protein gene. This increases the number of recombination events between vector and helper plasmid required to generate intact capsid genes within the vector.
Vector production and purification.
Packaged vector was produced by CaPO4 cotransfection of vector and helper plasmids into CMT4 monolayers, and crude vector stocks were harvested 72 h later by lysing pelleted cells in TE 8.7 (10 mM Tris, 1 mM EDTA, pH 8.7). Lysates were frozen and thawed three times and purified by sedimentation to equilibrium in CsCl through a 60% sucrose cushion. The packaged virion fraction was identified by hemagglutination, dialyzed against TE 8.7, and concentrated on Centricon filters (Millipore, Bedford, Mass.) with a 30-kDa cutoff.
Titers of purified vector stocks were determined by enumerating transduced centers on Southern blots of NB324K monolayers adsorbed with paired, serially diluted aliquots. A second set of vector-transduced monolayers were routinely passaged three times in NB324K cells and tested for the presence or absence of replication-competent virus by plaque assay, as previously described (54). Only stocks showing no detectable replication-competent virus by this assay were used for immunizing mice.
Infection or transduction of primary splenocytes.
Primary splenocyte cultures were prepared by centrifugation through lymphocyte separation medium (ICN Bio, Santa Ana, Calif.). Infection with MVMi was performed in bulk culture by adding MVMi (100 μl at 2 × 108 PFU/ml) to 107 splenocytes in 1.0 ml of Bruff's medium containing 1% fetal bovine serum, to give a multiplicity of infection of 2 PFU/cell, and incubating for 1 h at 37°C before plating. Cells were adjusted to 5 × 106 cells/ml by the addition of 1.0 ml of Bruff's medium containing 20% fetal bovine serum, and 50-μl aliquots of this suspension were added per well in flat-bottomed 96-well Falcon plates. Proliferative responses were elicited by plating in wells that had been coated with monoclonal antibody 145-2C11 (32, 47), a hamster anti-murine CD3 monoclonal antibody, at 10 μg/ml in phosphate-buffered saline (PBS) adjusted to pH 9.6, for 3 h at 37°C, and subsequently washed three times with neutral PBS. For unstimulated controls, uninfected or MVMi-infected cells were plated at 2.5 × 105 cells/well in uncoated wells.
Western blots.
Four wells per experimental group were harvested at the times indicated in the figure legends, washed in PBS, pelleted, denatured in Laemmli buffer, electrophoresed on 10% polyacrylamide gel, and transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, Calif.). Immunostaining was performed as previously described (21), with rabbit antibodies raised against the common N termini of MVM NS1 and NS2, against denatured capsid protein, or against B. burgdorferi cN10 OspA protein. For detection of NS1 alone, murine anti-NS1 monoclonal antibody (Centricon 5X concentrate from hybridoma supernatant) was used at a dilution of 1:100.
Vaccination and challenge of mice.
Mice were randomly caged in groups of five. Mice were vaccinated by injection into the tail vein or subcutaneously with a single 0.1-ml dose (1.2 × 106 infectious equivalents) of purified vector stock. For intranasal inoculation, 50 μl/nostril was delivered dropwise to methoxyfluorane-anaesthetized mice. Live spirochete challenge was performed by intradermal injection of 0.1 ml of saline containing 105 live B. burgdorferi strain cN40 per ml, as described by Barthold et al. (8). All protocols for the mouse studies were approved by the Institutional Animal Care and Use Committee at Yale University.
Antibodies and ELISA.
For ELISA, 96-well plates (ICN Biomedicals, Santa Ana, Calif.) were coated overnight at 4°C with 500 ng of purified recombinant OspA per ml in PBS, blocked for 2 h at 37°C with 1% bovine serum albumin (Sigma, St. Louis, Mo.) in PBS, and then washed twice with PBS-Tween 20 (0.1% vol/vol). Dilutions of mouse serum (spanning 1:100 to 1:12,800) were added to the wells, and the plates were incubated at 37°C for 1 h. Plates were washed three times with PBS-Tween and alkaline phosphatase-conjugated goat anti-mouse IgG (all samples) or goat anti-mouse IgM (for weeks 1, 2, 3, and all samples following challenge with live B. burgdorferi), was applied at 1:1,000 dilution at 37°C for 1 h. Alkaline phosphatase-conjugated secondary antibodies were obtained from Jackson ImmunoResearch, Inc., West Grove, Pa.
For isotype determination, plates were incubated for 1 h at 37°C with either biotinylated-rat anti-mouse IgG1 (Biosource International, Camarillo, Calif.) or biotinylated-rat anti-mouse IgG2a (BD-PharMingen, San Diego, Calif.) as secondary antibodies. After three washes with PBS-Tween, isotyping ELISA plates were incubated with alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch, West Grove, Pa.) for 1 h at 37°C. Quantitation was performed, after three further washes with PBS-Tween, by applying p-nitrophenylphosphate substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) for 30 min at room temperature. Color development was stopped by 0.05 M EDTA, and absorbance was read at 450 nm on a Bio-Tek Instruments (Winooski, Vt.) EL340 microplate reader, and 630 nm background absorbance was subtracted from each. Naïve mouse serum was used as the negative control. Antibody titers are expressed as the reciprocal of the highest dilution still greater than the maximum mean optical density at 450 nm + 2 standard deviations for naive mouse serum. Samples with high antibody titers were retested at dilutions between 1:500 and 1:64,000. Control sera from mice immunized with lipidated OspA vaccine were generously provided by Venetta Thomas and Erol Fikrig, Section of Rheumatology, Department of Internal Medicine, at Yale University School of Medicine.
Histology and reisolation of spirochetes.
CO2-euthanized mice were dissected under sterile conditions. Hearts and tibiotarsi were fixed in formalin, joints were decalcified, and tissues were paraffin embedded, sectioned, stained with hematoxylin-eosin, coded blindly, and examined microscopically for inflammation. Arthritis severity was estimated according to published criteria (26). Active carditis was characterized by aortic and coronary endarteritis, with inflammation in surrounding tissues (3). Skin from the site of inoculation, spleen, and bladder was incubated in BSK II medium at 28°C for 14 days and examined by dark-field microscopy for the presence of spirochetes.
Measurement of IFN-γ and IL-4.
CD4+ T cells (2 × 105), prepared as described by Levin et al. (33) from OspA- or enhanced green fluorescent protein (EGFP)-immunized mice were stimulated with 4 × 105 T-depleted splenocytes from naïve C3H mice and lipidated OspA for 96 h. Supernatants were analyzed for gamma interferon (IFN-γ) and interleukin-4 (IL-4) with Quantikine murine IFN-γ and IL-4 ELISA minikits, respectively (R&D Systems, Minneapolis, Minn.).
RESULTS
MVMi infects primary murine T lymphocytes in vitro.
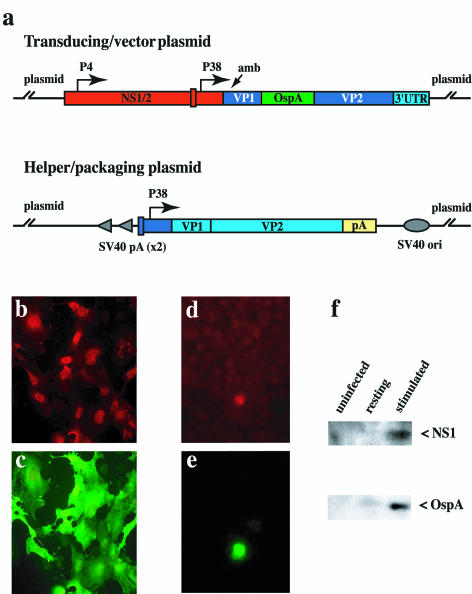
In order to establish that primary mouse T lymphocytes could be cryptically infected with a lymphotropic parvovirus, we inoculated freshly isolated, unstimulated splenocytes with MVMi at a multiplicity of 2 PFU per cell, plated them either in uncoated wells or on surfaces carrying anti-CD3 antibody, and then monitored them for 72 h by Western blotting for the expression of nonstructural and structural proteins. As shown in Fig. 1, unstimulated splenocytes showed no significant viral gene expression compared to the uninfected controls throughout the period examined. In contrast, infected splenocyte cultures exposed to anti-CD3 antibody started to express both viral nonstructural proteins by 48 h after infection and stimulation, and by 72 h were expressing abundant structural polypeptides in addition to NS1 and NS2.
FIG. 1.
(a) Time course of induction of viral NS1 (83 kDa) and NS2 (24 kDa) expression following infection by MVMi of primary C3H/HeNcr splenocytes either unstimulated, or stimulated by solid-phase cross-linking of murine CD3e (T-cell receptor), as described in the text. Proteins were detected by Western blot with an antibody specific for the N-terminal region common between NS1 and NS2. (b) Time course of induction of VP1 (83 kDa) and VP2 (64 kDa) expression in splenocyte cultures treated as in panel a. Proteins were detected by Western blot with an antibody specific for denatured capsid protein.
We reasoned that a vector based on MVMi, designed to express a polypeptide antigen of choice, could establish such a cryptic infection in a small fraction of resting lymphocytes when delivered in vivo. As many of these cryptically infected cells would be activated for proliferation stochastically over time, they should represent a continuously replenished reservoir of antigen-expressing cells, dying as a result of viral infection, and result in recurrent boosting of the immune response to the encoded antigen.
Construction of the lymphotropic vaccine vector.
For the vector used here, we employed the capsid replacement strategy (23, 40, 46) outlined in Fig. 2a, where the vector itself contains the viral nonstructural genes, and the transgene is substituted for sequences encoding VP2. Packaging was achieved by cotransfection with another plasmid, in this case one containing the MVMi coat protein genes under the control of the viral P38 promoter. Initially (23, 46), this type of vector and packaging strategy were plagued by significant contamination with replication-competent virus, believed to arise predominantly by reciprocal recombination between viral sequences in the two plasmids. Recent improvements in vector and packaging plasmid design (12, 17, 22) have been achieved by reducing the overlap between viral sequences within the two components and, where overlap has been deemed necessary, by changing the nucleotide sequence in one without changing the sequence of the protein it encodes (22).
FIG. 2.
(a) Sequence organization of transducing/vector and helper/packaging plasmids. OspA transgene insertion is shown in green, MVM sequences are in red (NS) or blue (VP), and LuIII is in purple. The positions of the viral early (P4) and late (P38) promoters are indicated. Amb indicates the position of a translation termination codon engineered into the VP1 coding sequence in order to abolish its expression. Simian virus 40 origin of replication and simian virus 40 polyadenylation sites in the packaging construct are shown as gray ovals and arrowheads, respectively. The rabbit β-globin polyadenylation site is shown in yellow. Panels b through f show immunofluorescent staining for NS1 (b and d) and OspA (c and e) in human NB324K cells (b and c) and anti-CD3-stimulated murine splenocytes (d and e) transduced with MVMi-OspA vector, stained at 48 h postinoculation Panel f shows a Western blot for NS1 (83 kDa) and OspA (30 kDa) expressed in vector-transduced murine splenocytes 48 h after stimulation by CD3 cross-linking.
We have found, as has also recently been reported by Wrzesinski and colleagues (57), that replication-competent virus formation was completely suppressed by creating reciprocal chimeras between two related parvoviruses in the sequences of overlap between vector and helper plasmids. For this we chose to use sequences derived from parvovirus LuIII to replace overlapping regions in either plasmid, as shown in Fig. 2a. While LuIII and MVM are highly related at the protein sequence level, they differ by 15% in nucleotide sequence through the central core of their genomes, and these differences are fairly uniformly distributed, in a manner we believed would suppress recombination between them. Throughout the capsid coding region the similarity drops further, to around 65%, allowing us to use capsid sequences from LuIII as “stuffer” in the vector to adjust the total length of the vector to equal that of the wild-type virus. This maintains the parvovirus character of all of the vector sequences other than the transgene itself and suppresses interplasmid recombination to the extent that we could produce vector devoid of replication-competent virus in amounts suitable for use in animals. Further details of the construction and characterization of this vector and packaging system will be reported elsewhere (G. A. Palmer and P. Tattersall, unpublished data).
As shown by immunofluorescence staining, simian virus 40-transformed human fibroblasts transduced with purified vector, packaged in the MVMi coat, express abundant OspA antigen, which correlates with expression of NS1 (Fig. 2b and c). Vector-transduced primary murine splenocytes expressed OspA and NS1 polypeptides simultaneously when stimulated to proliferate by cross-linking CD3 complex on their surface (Fig. 2d and e). Likewise, both NS1 and OspA expression could be detected by Western blot in stimulated populations of transduced primary T lymphocytes, while unstimulated naïve cells remained negative for expression of either protein (Fig. 2f). A more detailed analysis of the cell types within this heterogeneous splenocyte population that are capable of acting as hosts for the vector will be reported elsewhere (G. A. Palmer and P. Tattersall, unpublished data).
A single intravenous dose of vector elicits a sustained and protective anti-OspA antibody response.
Packaged, purified vector was used to vaccinate C3H mice because this strain has been used for the development of a small-animal Lyme disease model (7). Initially, five mice were inoculated intravenously, with the idea that the vector would encounter resting lymphocytes most efficiently in the circulation. All animals vaccinated with a single injection of OspA vector responded with significant titers of anti-OspA-specific antibody by 3 weeks postinoculation, while control mice that received an equal dose of an equivalently packaged vector transducing enhanced green fluorescent protein (EGFP) produced no detectable anti-OspA antibody (Fig. 3).
FIG. 3.
ELISAs of anti-OspA serum antibody for individual C3H mice (•, ✶, ♦, and □) following a single intravenous vaccination with 106 transducing units of MVMi-OspA vector per mouse, obtained at the indicated times postinoculation. MVMi-EGFP vector-immunized animals were followed as a control (▪).
In order to assess the efficacy of the single-dose vector approach relative to immunization with recombinant OspA vaccine, peak anti-OspA antisera from MVMi-OspA vaccinated mice were compared with sera obtained 3 weeks after the last boost from C3H/HeNcr and C3H/HeJ mice injected intraperitoneally with 2 μg of lipidated OspA vaccine every 14 days for 2 months, as described by Alexopoulou and colleagues (1). Anti-OspA antibody titers were twofold higher in sera obtained from these recombinant, lipidated OspA-vaccinated mice compared to those for vector-immunized animals when assayed in parallel by ELISA. Thus, the single dose of vector elicits antibody titers that should be well within the protective range induced by repeated immunization with recombinant antigen vaccine.
In order to determine directly whether these vector-induced antibodies were protective, three of the immune mice and three of the EGFP-vaccinated control group were challenged at 7 weeks postinoculation with 104 OspA-expressing B. burgdorferi cN40 (virulent strain) spirochetes injected intradermally. Attempts to reisolate spirochetes from various body sites at 14 days postchallenge, combined with histological examination of joint tissue, showed that two of three MVMi-OspA-vaccinated animals were completely protected from spirochete challenge. Spirochetes were not found in the spleens of any of the immunized mice, although live spirochetes could be isolated from the site of inoculation and bladder of one of the immune mice. This mouse also showed histological evidence of infection at two sites examined, but in each case the joint inflammation was significantly less severe than that seen in the nonimmune, control animals. The tendonitis with moderate to severe inflammation of the tibiotarsus, swelling of the tendon sheath, and inflammatory infiltration coupled with bone erosion (arrows) observed in EGFP-vaccinated mice (Fig. 4a) were completely absent from the OspA-immunized animals (Fig. 4b). Likewise, OspA-vaccinated mice did not develop the active carditis (Fig. 4c) seen in the control animals (Fig. 4d). In this initial experiment, the remaining animals were monitored over a 2-year period, during which their anti-OspA antibody titers remained stably elevated (Fig. 3).
FIG. 4.
Photomicrographs of hematoxylin- and eosin-stained tissue sections of tibiotarsus (a and b) and heart (c and d) taken from MVMi-EGFP vector-immunized control mice (a and c) and from MVMi-OspA vector-immunized animals (b and d). The mice were challenged 7 weeks after immunization and tissues were prepared 2 weeks later. Arrows indicate regions of bone erosion. Insets are at 10-fold-higher magnification.
Intranasal inoculation of OspA vector is as effective as the intravenous route.
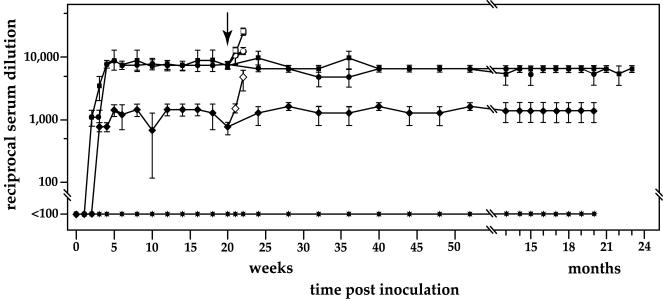
To confirm and expand these observations, intranasal, subcutaneous, and intravenous routes of inoculation were compared, again with the EGFP vector inoculated intravenously as a negative control. Significant titers of anti-OspA-specific antibody were established as soon as 2 weeks following a single dose of vector by each of the three routes tested (Fig. 5). Intranasal inoculation elicited peak antibody titers by 4 weeks postinoculation, 1 week earlier than intravenously, whereas subcutaneous inoculation produced only moderate titers of anti-OspA antibody. Titers were sustained at 1:8,000 for up to 2 years following a single vaccination in both the intranasal and intravenous groups, whereas subcutaneous inoculation never induced titers above 1:1,200. In this experiment, the data represent the geometric mean titers for five animals per group and showed extremely little variation between animals within a group. The anti-OspA-specific antibody was found to be predominantly of IgG2a isotype rather than IgG1 for each regimen at all times tested postinoculation (Fig. 6a).
FIG. 5.
Results from ELISAs of serum antibodies obtained at the indicated times from C3H/HeNcr mice following a single vaccination with the MVMi-OspA vector by the intravenous (○), intranasal (▪), and subcutaneous (♦) routes and with MVMi-EGFP vector administered intravenously (✶). Each point represents the geometric mean of titers for five mice assayed individually.
FIG. 6.
(a) Histogram of anti-OspA immunoglobulin isotypes induced by different inoculation routes. Sera were obtained from five individuals at 3 weeks following inoculation. (b) Scattergram of IL-4 and IFN-γ secretion by CD4+ cells purified at 9 or 12 months after inoculation from individual spleens of MVMi-OspA vector-immunized or EGFP-vaccinated control mice and stimulated by coculture with naive splenocytes presenting lipidated OspA.
Long-lived antigen-specific memory CD4+ T cells are induced by single intranasal inoculation of vector.
Consistent with an immunoglobulin class switch to IgG2a, we observed elevated levels of IFN-γ secretion in CD4+ T-cell preparations cultured with antigen-presenting cells from OspA-immune animals pulsed with optimal concentrations of OspA compared with T cells derived from the EGFP-vaccinated control animals (Fig. 6b). While the IFN-γ secretion was modest, these results are for animals sacrificed 9 and 12 months after a single intranasal administration of vector and likely represent the relatively low frequency of antigen-specific memory T cells present in such aged animals. We were unable to detect any IL-4 secreted by OspA-stimulated T cells from either OspA-immune or EGFP-vaccinated control mice (Fig. 6b). Taken together, these data support the conclusion that the parvovirus vector induces a Th1-driven immune response specific for OspA.
Intranasal inoculation of OspA vector affords protection against live spirochete challenge.
At 20 weeks postinoculation, we randomly chose three mice from each group for challenge with live spirochetes in order to measure the protection afforded by the antibody induced by each inoculation procedure. Interestingly, antibody titers increased to over 1:12,000 in both the intranasal and intravenous groups within 1 week of intradermal injection of live spirochetes, in contrast to companion animals that were not challenged, whose titers remained stable at prechallenge titers (as shown in Fig. 5). By 2 weeks postchallenge, when the experiment was terminated and animals were sacrificed for pathological examination, the intranasal group had experienced a further doubling of antibody titers compared with the intravenous group. Mice initially given the vector by the subcutaneous route also responded with an anamnestic response, but the increase only reached prechallenge antibody titers seen in the other two groups.
The results of pathological examination in animal tissues for reisolation of spirochetes and for inflammation in joint and heart tissues are summarized in Table 1 and show that complete protection was afforded all animals given a single inoculation of OspA vector by either the intranasal or intravenous route. Although one mouse of the group inoculated by the subcutaneous route appears to have been protected, spirochetes could be isolated from the other two. Recovery was positive from all tissues cultured for one mouse in this group, which also showed inflammation in the heart. However, even the relatively low antibody elicited by subcutaneous inoculation provided some protection from live spirochete challenge, since only the control animals showed arthritic inflammatory disease of the tibiotarsus. The intranasal and intravenous MVMi-OspA immunizations have been repeated several times in C3H mice and in other mouse strains, always resulting in sustained, high-titer anti-OspA antibody induction. Further studies exploring the responses of inbred and outbred mouse strains to immunization with this vector will be reported elsewhere (G. A. Palmer and P. Tattersall, unpublished data).
TABLE 1.
Histopathologya
| Inoculation route | No. of animals (n = 3/group)
|
||||
|---|---|---|---|---|---|
| Reisolation of spirochetes
|
Histopathology
|
||||
| Inoculation site | Bladder | Spleen | Tibiotarsus | Heart | |
| Intranasal | 0 | 0 | 0 | 0 | 0 |
| Intravenous | 0 | 0 | 0 | 0 | 0 |
| Subcutaneous | 2 | 1 | 1 | 0 | 0 |
| EGFP construct, intravenous | 2 | 3 | 2 | 2 | 3 |
Numbers of animals testing positive per group of three animals for each inoculation route. Spirochetes were detected by dark-field microscopy after 72 h of incubation in BSKII medium at 28°C, as described by Barthold and colleagues (6, 8). Histopathology was scored for inflammatory processes as described in references 3 and 8.
DISCUSSION
Here we describe the development of a novel parvovirus-based vector system for the delivery of subunit vaccines. The efficacy and ultimate success of a vaccine are predicated on safety, effectiveness, stability, and effective concentration — the vaccine must be amenable to manufacturing in sufficient quantity and quality to be of therapeutic value. While many reagents and techniques have evolved for the introduction of genetic material into cells, few can approach the efficiency of viruses for meeting all the criteria required for effective gene transfer. Virus vectors expressing subunit polypeptide antigens for vaccine purposes present the attractive property of amplifying the antigen without the need for repeated immunizations.
The parvovirus vector described here meets the major desirable criteria for delivery of a subunit vaccine antigen: it elicits a protective humoral immune response against a clinically important pathogen with a single, noninvasive dose of a nonpathogenic, nonproliferating recombinant virus. Moreover, the apparent induction of a Th1-driven immune response, marked by sustained titers of antigen-specific protective IgG2a, not only provide an ideal vaccine against Lyme disease, but offer an attractive new candidate vaccine approach for any infectious disease where long-lived, opsonizing antibody is desirable or required for protection and an effective protein antigen target can be identified (4, 9, 10). Significantly, the parvovirus vector is able to elict this response without itself being able to proliferate, thus providing an additional layer of protection for immunocompromised vaccinees that is not enjoyed by most other effective viral vaccine vectors.
We describe here the first successful use, to our knowledge, of an autonomous parvovirus vector for the induction of a humoral immune response to a heterologous, nonviral antigen. This vaccine strategy parallels, to some extent, the adeno-associated virus systems, currently generating considerable excitement as gene therapy vectors (15, 30, 37, 39, 52, 56), in that they both exploit similar genomic termini and capsids for packaging and delivery of the transgene, and thus share positive features such as physical stability, genetic simplicity and the additional safety feature that comes from not being based on tumor viruses. However, some crucial differences between the MVM-based system and those derived from adeno-associated virus should be noted. In almost all adeno-associated virus vector strategies described to date, the vector contains no viral genes and induces constitutive expression of the encoded transgene, driven by strong, heterologous promoters, in every successfully transduced cell, whether or not it is mitotically cycling, without intrinsically harming the cell. In the MVM-based system described here, transgene expression is under viral control, and noncycling cells do not express the transgene until they start to proliferate, following which they rapidly die, since even the crippled form of the vector is cytolytic once activated.
In many studies of adeno-associated virus-mediated gene transfer in which the immune response to the transgene was examined, this gene delivery system elicited low or undetectable innate or adaptive immune responses to the expressed transgene, in some cases demonstrating a very significant advantage in this respect over other vector systems (19, 25, 37, 49, 60). However, in other adeno-associated virus vector studies, humoral or cell-mediated immune responses have been observed, and this gene delivery system has also been developed as a vaccine strategy (24, 34, 35, 43, 59). The reasons underlying this dichotomy of immunological sequelae are not well established but appear to involve differences in species of host, type of cell targeted, route of delivery, and dose of vector (11, 28, 29, 48, 58).
In one case where adeno-associated virus-induced humoral response in mice to human factor IX was followed over an extended period, a switch from antibody production to tolerance was observed, which resulted in the restoration of therapeutic circulating levels of human factor IX (16). The possibility of eventual induction of tolerance, a counterproductive outcome in any vaccine strategy, has not been extensively examined for adeno-associated virus-based vaccine vectors, but was not observed in the murine lifetime studies of responses to the MVMi-OspA vector reported here. If these two systems do differ in their abilities to elicit persistent immunity versus tolerance, this may reflect alternative long-term consequences of stimulation of the immune system by constitutive expression from otherwise healthy cells rather than by intermittent expression from cells undergoing virus-induced cytolysis.
The observation that a single intranasal inoculation of MVMi-OspA can prime a sustained memory antibody response raises important questions about mechanisms of antigen presentation. While we know that the vector, like its parent, MVMi, targets T-cell lines in culture, the target cell repertoire in vivo remains unknown. Studies on the pathogenesis of wild-type MVMi indicate that infection of other tissue types in vivo is likely, including target cells within endothelium and erythropoietic precursors (13, 14, 31, 50). Recent experiments have shown that the humoral response to OspA delivered in the fibrotropic rather than lymphotropic coat (27) is 10- to 20-fold lower antibody titers, suggesting that the ability to target lymphocytes, and therefore a population of naturally resting cells that maintain proliferative potential, may be important. In addition, adoptive transfer of purified splenocyte subpopulations has shown that B cells as well as CD4+ and CD8+ T cells, transduced ex vivo, can induce substantial antibody titers (G. A. Palmer and P. Tattersall, unpublished data).
Since vector-targeted cells of T lymphoid lineage do not express major histocompatibility complex class II, they are unlikely to function directly as antigen-presenting cells for CD4+ T cells, but could function as a repository for vector maintained in a quiescent state until the cells become activated. In studies with major histocompatibility complex class I and class II knockouts on the C57BL/6 background, class II expression was necessary for vector to induce anti-OspA antibody (data not shown). Antibody specific for OspA is predominantly IgG2a, indicating an IFN-γ-driven Th1 response (18, 51), typically primed by association of CD4+ T cells with dendritic cells expressing abundant antigen-derived peptides on class II (20).
Taken together, these observations suggest that cryptically infected lymphocytes become activated in a stochastic manner, presumably as a result of homeostatic maintenance or antigen-specific T-cell receptor engagement. These activated cells enter S phase and upregulate NS1 expression, which in turn transactivates the viral P38 promoter, driving high-level expression of OspA. Transduced cells would thus simultaneously accumulate antigen and become apoptotic due to the cytotoxic effects of NS1. Apoptotic cells would be engulfed by professional antigen-presenting cells on which antigen-derived peptides are presented by class II, thereby inducing a sustained memory antibody response against the antigen. Current experimental approaches are aimed at testing each of these possible elements in the induction of persistent immunity by parvovirus vaccine vectors.
Acknowledgments
We are indebted to Erol Fikrig, Juan Anguita, Venetta Thomas, and Utpal Pal for kindly providing OspA protein and anti-OspA antisera, to Steven Barthold for histological examination of joint tissues, and to Kim Bottomly for helpful discussions. We also thank Deborah Beck and Gordon Terwilliger for technical assistance with the challenge studies and preparation of tissues. We are grateful to Robert Homer for generously reviewing histological analyses.
This work was supported by U.S. Public Health Service grants AI47027 and CA29303 (to P.T.) and AI39158 (to S.L.C.) from the National Institutes of Health. G.A.P. was supported, in part, by a postdoctoral training grant, T32 CA09159, from the National Cancer Institute.
REFERENCES
- 1.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2002. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Vaccine 20:1949-1955. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong, A. L., S. W. Barthold, D. H. Persing, and D. S. Beck. 1992. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 47:249-258. [DOI] [PubMed] [Google Scholar]
- 4.Arulanandam, B. P., J. M. Lynch, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 7.Barthold, S. W., M. S. de Souza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959-971. [PMC free article] [PubMed] [Google Scholar]
- 8.Barthold, S. W., D. H. Persing, A. L. Armstrong, and R. A. Peeples. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 139:263-273. [PMC free article] [PubMed] [Google Scholar]
- 9.Beenhouwer, D. O., S. Shapiro, M. Feldmesser, A. Casadevall, and M. D. Scharff. 2001. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infect. Immun. 69:6445-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan, F. R., T. D. Jones, L. B. Gilleland, T. Bellaby, F. Xu, P. C. North, J. Staczek, T. Lin, W. D. Hamilton, and H. E. J. Gilleland. 1999. Pseudomonas aeruginosa outer-membrane protein F epitopes are highly immunogenic in mice when expressed on a plant virus. Microbiology 145:211-220. [DOI] [PubMed] [Google Scholar]
- 11.Brockstedt, D. G., G. M. Podsakoff, L. Fong, G. Kurtzman, W. Mueller-Ruchholtz, and E. G. Engleman. 1999. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin. Immunol. 92:67-75. [DOI] [PubMed] [Google Scholar]
- 12.Brown, C. S., F. M. DiSumma, J. Rommelaere, A. Y. Dege, J. J. Cornelis, C. Dinsart, and W. J. Spaan. 2002. Production of recombinant H1 parvovirus stocks devoid of replication-competent viruses. Hum. Gene Ther. 13:2135-2145. [DOI] [PubMed] [Google Scholar]
- 13.Brownstein, D. G., A. L. Smith, R. O. Jacoby, E. A. Johnson, G. Hansen, and P. Tattersall. 1991. Pathogenesis of infection with a virulent allotropic variant of minute virus of mice and regulation by host genotype. Lab. Investig. 65:357-364. [PubMed] [Google Scholar]
- 14.Bueren, J. A., J. C. Segovia, and J. M. Almendral. 1991. Cytotoxic infection of hematopoietic stem and committed progenitor cells by the parvovirus minute virus of mice. Propagation of an acute myelosuppression in culture. Ann. N. Y. Acad. Sci. 628:262-272. [DOI] [PubMed] [Google Scholar]
- 15.Buning, H., M. U. Ried, L. Perabo, F. M. Gerner, N. A. Huttner, J. Enssle, and M. Hallek. 2003. Receptor targeting of adeno-associated virus vectors. Gene Ther. 10:1142-1151. [DOI] [PubMed] [Google Scholar]
- 16.Chao, H., and C. E. Walsh. 2001. Induction of tolerance to human factor VIII in mice. Blood 97:3311-3312. [DOI] [PubMed] [Google Scholar]
- 17.Clement, N., T. Velu, and A. Brandenburger. 2002. Construction and production of oncotropic vectors, derived from MVM(p), that share reduced sequence homology with helper plasmids. Cancer Gene Ther. 9:762-770. [DOI] [PubMed] [Google Scholar]
- 18.Coffman, R. L., B. W. Seymour, D. A. Lebman, D. D. Hiraki, J. A. Christiansen, B. Shrader, H. M. Cherwinski, H. F. J. Savelkoul, F. D. Finkelman, M. W. Bond, and T. R. Mosmann. 1988. The role of helper T-cell products in mouse B cell differentiation and isotype regulation. Immunol. Rev. 102:5-28. [DOI] [PubMed] [Google Scholar]
- 19.Conrad, C. K., S. S. Allen, S. A. Afione, T. C. Reynolds, S. E. Beck, M. Fee-Maki, X. Barrazza-Ortiz, R. Adams, F. B. Askin, B. J. Carter, W. B. Guggino, and T. R. Flotte. 1996. Safety of single-dose administration of an adeno-associated virus (adeno-associated virus)-CFTR vector in the primate lung. Gene Ther. 3:658-668. [PubMed] [Google Scholar]
- 20.Constant, S. L., and K. Bottomly. 1997. Induction of Th1 and Th2 CD4+ T-cell responses: the alternative approaches. Annu. Rev. Immunol. 15:297-322. [DOI] [PubMed] [Google Scholar]
- 21.Cotmore, S. F., M. D'Abramo, A., Jr., C. M. Ticknor, and P. Tattersall. 1999. Controlled conformational transitions in the MVM virion expose the VP1 N terminus and viral genome without particle disassembly. Virology 254:169-181. [DOI] [PubMed] [Google Scholar]
- 22.Dupont, F., A. Karim, J. C. Dumon, N. Mine, and B. Avalosse. 2001. A novel MVMp-based vector system specifically designed to reduce the risk of replication-competent virus generation by homologous recombination. Gene Ther. 8:921-929. [DOI] [PubMed] [Google Scholar]
- 23.Dupont, F., L. Tenenbaum, L. P. Guo, P. Spegelaere, M. Zeicher, and J. Rommelaere. 1994. Use of an autonomous parvovirus vector for selective transfer of a foreign gene into transformed human cells of different tissue origins and its expression therein. J. Virol. 68:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.During, M. J., C. W. Symes, P. A. Lawlor, J. Lin, J. Dunning, H. L. Fitzsimons, D. Poulsen, P. Leone, R. Xu, B. L. Dicker, J. Lipski, and D. Young. 2000. An oral vaccine against NMDAR1 with efficacy in experimental stroke and epilepsy. Science 287:1453-1460. [DOI] [PubMed] [Google Scholar]
- 25.Fields, P. A., D. W. Kowalczyk, V. R. Arruda, E. Armstrong, M. L. McCleland, J. N. Hagstrom, K. J. Pasi, H. C. Ertl, R. W. Herzog, and K. A. High. 2000. Role of vector in activation of T-cell subsets in immune responses against the secreted transgene product factor IX. Mol. Ther. 1:225-235. [DOI] [PubMed] [Google Scholar]
- 26.Fikrig, E., S. W. Barthold, and R. A. Flavell. 1993. OspA vaccination of mice with established Borrelia burgdorferi infection alters disease but not infection. Infect. Immun. 61:2553-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardiner, E. M., and P. Tattersall. 1988. Mapping of the fibrotropic and lymphotropic host range determinants of the parvovirus minute virus of mice. J. Virol. 62:2605-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge, Y., S. Powell, M. Van Roey, and J. G. McArthur. 2001. Factors influencing the development of an anti-factor IX (FIX) immune response following administration of adeno-associated virus-FIX. Blood 97:3733-3737. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez, Y. J., J. Wang, W. G. Kearns, S. Loiler, A. Poirier, and T. R. Flotte. 1999. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J. Virol. 73:8549-8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.High, K. 2002. adeno-associated virus-mediated gene transfer for hemophilia. Genet. Med. 4:56S-61S. [DOI] [PubMed] [Google Scholar]
- 31.Kimsey, P. B., H. D. Engers, B. Hirt, and C. V. Jongeneel. 1986. Pathogenicity of fibroblast- and lymphocyte-specific variants of minute virus of mice. J. Virol. 59:8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leo, O., M. Foo, D. H. Sachs, L. E. Samelson, and J. A. Bluestone. 1987. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc. Natl. Acad. Sci. 84:1374-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin, D., S. Constant, T. Pasqualini, R. Flavell, and K. Bottomly. 1993. Role of dendritic cells in the priming of CD4+ T lymphocytes to peptide antigen in vivo. J. Immunol. 151:6742-6750. [PubMed] [Google Scholar]
- 34.Liu, D. W., Y. P. Tsao, J. T. Kung, Y. A. Ding, H. K. Sytwu, X. Xiao, and S. L. Chen. 2000. Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 peptide DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer. J. Virol. 74:2888-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, Y., M. Chiriva-Internati, F. Grizzi, E. Salati, J. J. Roman, S. Lim, and P. L. Hermonat. 2001. Rapid induction of cytotoxic T-cell response against cervical cancer cells by human papillomavirus type 16 E6 antigen gene delivery into human dendritic cells by an adeno-associated virus vector. Cancer Gene Ther. 8:948-957. [DOI] [PubMed] [Google Scholar]
- 36.McShane, H., R. Brookes, S. C. Gilbert, and A. V. Hill. 2001. Enhanced immunogenicity of CD4+ T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect. Immun. 69:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monahan, P. E., and R. J. Samulski. 2000. Adeno-associated virus vectors for gene therapy: more pros than cons? Mol. Med. Today 6:433-440. [DOI] [PubMed] [Google Scholar]
- 38.Newman, M. J. 2002. Heterologous prime-boost vaccination strategies for HIV-1: augmenting cellular immune responses. Curr. Opin. Investig. Drugs 3:374-378. [PubMed] [Google Scholar]
- 39.Nicklin, S. A., and A. H. Baker. 2002. Tropism-modified adenoviral and adeno-associated virus vectors for gene therapy. Curr. Gene Ther. 2:273-293. [DOI] [PubMed] [Google Scholar]
- 40.Palmer, G. A., and P. Tattersall. 2000. Autonomous parvoviruses as gene transfer vectors, p. 178-200. In S. Faisst and J. Rommelaere (ed.), Contributions to microbiology, vol. 4: parvoviruses. Karger Press, Basel, Switzerland. [DOI] [PubMed]
- 41.Pancholi, P., D. H. Lee, Q. Liu, C. Tackney, P. Taylor, M. Perkus, L. Andrus, B. Brotman, and A. M. Prince. 2001. DNA prime/canarypox boost-based immunotherapy of chronic hepatitis B virus infection in a chimpanzee. Hepatology 33:448-454. [DOI] [PubMed] [Google Scholar]
- 42.Paul, P. S., W. L. Mengeling, and T. T. Brown, Jr. 1979. Replication of porcine parvovirus in peripheral blood lymphocytes, monocytes, and peritoneal macrophages. Infect. Immun. 25:1003-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ponnazhagan, S., G. Mahendra, D. T. Curiel, and D. R. Shaw. 2001. Adeno-associated virus type 2-mediated transduction of human monocyte-derived dendritic cells: implications for ex vivo immunotherapy. J. Virol. 75:9493-9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramshaw, I. A., and A. J. Ramsay. 2000. The prime-boost strategy: exciting prospects for improved vaccination. Immunol. Today 21:163-165. [DOI] [PubMed] [Google Scholar]
- 45.Rommelaere, J., and P. Tattersall. 1990. Oncosuppression by parvoviruses, p. 41-58. In P. Tjissen (ed.), Handbook of parvoviruses, vol. II. CRC Press, Boca Raton, Fla.
- 46.Russell, S. J., A. Brandenburger, C. L. Flemming, M. K. Collins, and J. Rommelaere. 1992. Transformation-dependent expression of interleukin genes delivered by a recombinant parvovirus. J. Virol. 66:2821-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samelson, L. E., J. J. O'Shea, H. Luong, P. Ross, K. B. Urdahl, R. D. Klausner, and J. Bluestone. 1987. T-cell antigen receptor phosphorylation induced by an anti-receptor antibody. J. Immunol. 139:2708-2714. [PubMed] [Google Scholar]
- 48.Sarukhan, A., S. Camugli, B. Gjata, H. von Boehmer, O. Danos, and K. Jooss. 2001. Successful interference with cellular immune responses to immunogenic proteins encoded by recombinant virus vectors. J. Virol. 75:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider, H., C. Muhle, A. M. Douar, S. Waddington, Q. J. Jiang, K. von der Mark, C. Coutelle, and W. Rascher. 2002. Sustained delivery of therapeutic concentrations of human clotting factor IX-a comparison of adenoviral and adeno-associated virus vectors administered in utero. J. Gene Med. 4:46-53. [DOI] [PubMed] [Google Scholar]
- 50.Segovia, J. C., J. A. Bueren, and J. M. Almendral. 1995. Myeloid depression follows infection of susceptible newborn mice with the parvovirus minute virus of mice (strain i). J. Virol. 69:3229-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snapper, C. M., and W. E. Paul. 1987. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236:944-947. [DOI] [PubMed] [Google Scholar]
- 52.Stilwell, J. L., and R. J. Samulski. 2003. Adeno-associated virus vectors for therapeutic gene transfer. BioTechniques 50:152-154. [DOI] [PubMed] [Google Scholar]
- 53.Tattersall, P. 1972. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J. Virol. 10:586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tattersall, P., and J. Bratton. 1983. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J. Virol. 46:944-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tattersall, P., and E. M. Gardiner. 1990. Autonomous parvovirus-host interactions, p. 111-122. In P. Tjissen (ed.), Handbook of parvoviruses, vol. I. CRC Press, Boca Raton, Fla.
- 56.Wright, J. F., G. Qu, C. Tang, and J. M. Sommer. 2003. Recombinant adeno-associated virus: formulation challenges and strategies for a gene therapy vector. Curr. Opin. Drug Discov. Dev. 6:174-178. [PubMed] [Google Scholar]
- 57.Wrzesinski, C., L. Tesfay, N. Salome, J. C. Jauniaux, J. Rommelaere, J. Cornelis, and C. Dinsart. 2003. Chimeric and pseudotyped parvoviruses minimize the contamination of recombinant stocks with replication-competent viruses and identify a DNA sequence that restricts parvovirus H-1 in mouse cells. J. Virol. 77:3851-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao, W., N. Chirmule, M. A. Schnell, J. Tazelaar, J. V. Hughes, and J. M. Wilson. 2000. Route of administration determines induction of T-cell-independent humoral responses to adeno-associated virus vectors. Mol. Ther. 1:323-329. [DOI] [PubMed] [Google Scholar]
- 59.Xin, K. Q., T. Ooki, H. Mizukami, K. Hamajima, K. Okudela, K. Hashimoto, Y. Kojima, N. Jounai, Y. Kumamoto, S. Sasaki, D. Klinman, K. Ozawa, and K. Okuda. 2002. Oral administration of recombinant adeno-associated virus elicits human immunodeficiency virus-specific immune responses. Hum. Gene Ther. 13:1571-1581. [DOI] [PubMed] [Google Scholar]
- 60.Zaiss, A. K., Q. Liu, G. P. Bowen, N. C. Wong, J. S. Bartlett, and D. A. Muruve. 2002. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J. Virol. 76:4580-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]