Abstract
Bacillus subtilis synthesizes pyridoxal 5′-phosphate, the active form of vitamin B6, by a poorly characterized pathway involving the yaaD and yaaE genes. The pdxS (yaaD) mutant was confirmed to be a strict B6 auxotroph, but the pdxT (yaaE) mutant turned out to be a conditional auxotroph depending on the availability of ammonium in the growth medium. The PdxS and PdxT proteins copurified during affinity chromatography and apparently form a complex that has glutaminase activity. PdxS and PdxT appear to encode the synthase and glutaminase subunits, respectively, of a glutamine amidotransferase of as-yet-unknown specificity essential for B6 biosynthesis.
Pyridoxal 5′-phosphate (PLP), the biologically active form of vitamin B6, is an essential cofactor for numerous metabolic enzymes (17). Two pathways of de novo PLP synthesis are known. The PdxA/PdxJ pathway, comprising six dedicated steps, has been extensively characterized in Escherichia coli (9, 15). Genes encoding similar enzymes can be found in the genomes of many other gram-negative bacteria. A second pathway of PLP synthesis, PDX1/PDX2, has been recently discovered in fungi through identification of two proteins (PDX1/SNZ/SOR1/PYROA and PDX2/SNO) that are required for PLP synthesis (2, 10, 11, 27, 31). Genes coding for similar proteins are highly conserved in plants, sponges, plasmodia, archaea, and many bacteria (12, 25, 36, 37).
The adjacent Bacillus subtilis genes, yaaD and yaaE, homologous to PDX1 and PDX2, respectively, have recently been shown to be required for PLP biosynthesis (33). We present evidence here that YaaD (renamed PdxS) and YaaE (renamed PdxT) form a glutamine amidotransferase of as-yet-unknown specificity required for the pathway of de novo PLP synthesis.
Construction and characterization of the pdxS null mutant.
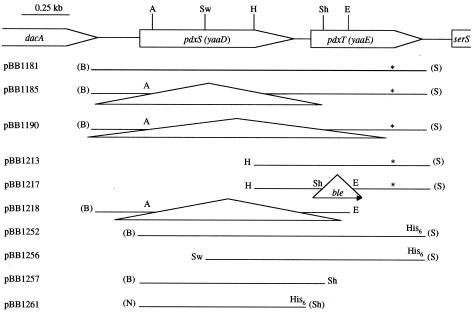
To create a deletion mutation within the pdxS gene, pBB1181 (Fig. 1) was cut with AatII and HindIII and religated after filling in the ends. The actual deletion endpoint in the resulting plasmid, pBB1185 (Fig. 1), as determined by sequencing, turned out to be beyond the HindIII site; the sequence removed was 606 bp long and did not alter the reading frame of pdxS. pBB1218, a truncated version of pBB1185 (Fig. 1), was used to replace the wild-type pdxS gene of B. subtilis strain SMY with the ΔpdxS allele, resulting in strain BB2253. Methods for plasmid isolation, agarose and polyacrylamide gel electrophoresis, use of restriction and DNA modification enzymes, DNA ligation, PCR, and electroporation of E. coli JM107 cells were as described by Sambrook et al. (35). Growth of B. subtilis cells, transformation by plasmid DNA, and procedures for gene replacement were as described previously (4, 5).
FIG. 1.
Genetic map of the pdxST (yaaDE) region and plasmids carrying different parts of this region. The restriction sites are abbreviated as follows: A, AatII; B, BamHI; E, EcoRI; H, HindIII; N, NcoI; S, SalI; Sh, SphI; Sw, SwaI. The sites shown in parentheses were created by PCR. Deletion mutations are shown with triangles. The 1.4-kb ble cassette is not drawn to scale. His6 notations indicate that six histidine-encoding codons were added at the 3′ ends of the corresponding genes. To create pBB1181, a 1.8-kb PCR product containing the pdxS and pdxT genes was synthesized using B. subtilis strain SMY chromosomal DNA as a template and pdxST-specific primers oBB120 (5′-GATTTGGATCCGGAATTTGGGGAAGC) and oBB121 (5′-TTATAGTCGACTATAAGTTTCCACAGC), containing BamHI and SalI sites, respectively (indicated in bold). The PCR fragment was cloned in an integrative vector, pBB544 (3). pBB1181 contained a point mutation, shown by an asterisk, in the penultimate codon of the pdxT gene (GGC to AGC with a corresponding Gly-to-Ser change) and was stable only in the pcnB80 zad::Tn10 derivative of E. coli strain JM107 which reduces the plasmid copy number (23) and apparently alleviates the toxicity of the cloned fragment. To create pBB1252, a 1.54-kb DNA fragment coding for the wild-type version of PdxS and a modified version of PdxT containing a His6 tag at its C terminus was synthesized by PCR, using SMY chromosomal DNA as a template, and oligonucleotides oBB138 (5′-ATAACGGATCCTTGATTAGGGGGACC) and oBB144 (5′-TTTCAGTCGACTTAATGGTGATGGTGATGGTGTACAAGTGCCTTTTGCTTAT) as 5′ and 3′ primers, respectively (the BamHI and SalI sites are indicated in bold, and the histidine codons are underlined). The PCR fragment was first blunt ended, then cut with SalI, and cloned in the expression vector pBAD18 containing the inducible E. coli araBAD promoter (14) and cut with SmaI and SalI. Two derivatives of pBB1252 expressing either PdxT-His6 only (pBB1256) or unmodified PdxS only (pBB1257) were generated by removing either the 0.37-kb Acc65I (the vector site)-SwaI fragment containing the 5′ part of the pdxS gene or the 0.54-kb fragment between the insert and vector SphI sites containing most of the pdxT gene. Plasmid pBB1261 overexpressing the PdxS-His6 protein was constructed by cloning the 0.90-kb NcoI-SphI fragment in the pBAD24 vector containing the E. coli araBAD promoter and an appropriate ribosomal binding site (14). The pdxS fragment was synthesized by PCR using SMY chromosomal DNA as a template and oligonucleotides oBB151 (5′-CCAAGCCATGGCTCAAACAGGTAC) and oBB152 (5′-TGTTCGCATGCTTAATGGTGATGGTGATGGTGCCAGCCGCGTTCTTGCAT) as 5′ and 3′ primers, respectively (the NcoI and SphI sites are indicated in bold, and the histidine codons are underlined).
Strain BB2253 (pdxS) lost the ability to grow in minimal medium, even if the medium was enriched with amino acids; growth was fully restored by addition of 0.2 μM pyridoxal (PL). This result is consistent with the phenotype of the pdxS insertion mutant (33) and the documented role in PLP biosynthesis of fungal pdxS homologs (2, 10, 27, 31). Pyridoxine (PN) was 1,000-fold less effective in restoration of BB2253 growth, indicating that B. subtilis cells utilize exogenous PN for PLP biosynthesis weakly or not at all (growth in the presence of PN may be due to PL contamination or to oxidation of PN to PL). Pyridoxamine was several times less effective than PL (probably due to the specificity of the pyridoxal kinase required for phosphorylation of all simple B6 vitamers [45] or the corresponding transport system).
Construction and characterization of the pdxT null mutant.
A deletion-insertion mutation within the pdxT gene was created by replacing the 135-bp SphI-EcoRI fragment of pBB1213 (Fig. 1) with a 1.4-kb SphI-EcoRI ble cassette determining resistance to phleomycin, excised from pJPM136 (5). pBB1217 was introduced into B. subtilis SMY, and strain BB2256 (ΔpdxT::ble) was isolated as described previously (5).
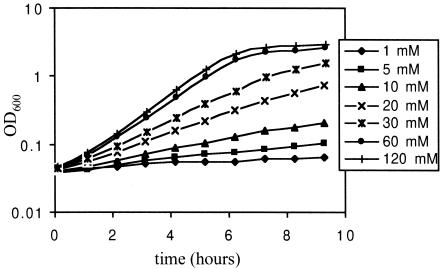
In contrast to strain BB2253 (pdxS), strain BB2256 (pdxT) behaved in minimal medium as a conditional PL auxotroph depending on ammonium availability. Virtually no growth was observed in glucose-glutamate or glucose-1 mM NH4Cl medium, but growth at almost the wild-type rate was observed in the presence of 60 to 120 mM NH4Cl (Fig. 2); intermediate growth was observed in glucose-0.2% (37.4 mM) NH4Cl medium. Growth of strain BB2256 was restored to the wild-type rate by addition of PL. Similarly, less severe effects of fungal PDX2 gene defects on PLP synthesis, compared with what is seen with PDX1 defects, have been described previously (11, 31, 40). In fact, the B6 dependency of the Aspergillus nidulans PYROB (similar to PDX2; GenBank accession number AAK50016) mutant was also rescued by a high concentration of ammonium (1). The partial B6 auxotrophy of pdxT mutants and the poor capacity of B. subtilis cells to utilize PN explain the ability of 50 μM PN to relieve the growth defect of the pdxT mutant but not that of the pdxS mutant (33).
FIG. 2.
Growth of the yaaE (pdxT) mutant with different ammonium concentrations. Cells of strain BB2256 were grown overnight in TSS-glucose minimal medium with 30 mM NH4Cl and diluted 100-fold into fresh medium with the indicated NH4Cl concentrations. OD600, optical density at 600 nm.
A Bacillus circulans mutant defective in the yaaE (pdxT) gene has been described recently. Though the function of the gene was not determined, a conditional growth defect dependent on ammonium availability was also observed for this mutant (41). The authors of this earlier study tried to relieve the growth defect by adding PN but failed in accord with the very poor ability of B. subtilis cells to utilize PN.
A strain (BB2254) carrying a deletion of parts of both pdxS and pdxT was constructed as described above by using pBB1190, which lacks the 898-bp fragment of pBB1181 starting at the AatII site (Fig. 1). BB2254 had the same strict PL auxotrophy as a single pdxS null mutant. No pseudorevertants of such mutants have been found. Because both pdxS and pdxST deletions cause a strict requirement for B6 vitamers, we conclude that only one pathway of de novo PLP synthesis, which cannot be easily bypassed due to mutations in other genes, operates in B. subtilis.
B. subtilis mutants requiring PN, PL, or pyridoxamine for growth have been isolated previously (30) but have not been characterized genetically. The B6 requirement of most such mutants was partially or completely alleviated by isoleucine or isoleucine plus low concentrations of PN (30). In our work, no effect on the growth of the pdxS or pdxT mutants was observed when isoleucine (500 μg/ml), with or without a suboptimal amount of PN, was added (data not shown), suggesting that our mutants are different from the ones isolated previously.
The fact that PL is utilized preferentially and PN only very poorly correlates well with an apparent lack in B. subtilis of the E. coli pdxH-like gene and the corresponding pyridoxine 5′-phosphate oxidase activity (29, 30) required for conversion of PN to PLP. No genes likely to encode pyridoxine 5′-phosphate oxidase can be found in most bacteria harboring the pdxS- and pdxT-like genes (data not shown). It is likely that all such bacteria will prove to depend on PL if their de novo pathway of PLP synthesis becomes inactivated. In accord with this conclusion, several lactic acid bacteria whose genomes lack pdxH and the genes for de novo PLP synthesis were shown to use PL preferentially (38).
A mixture of PdxS and PdxT has glutaminase activity.
PdxT and other PDX2-like proteins are similar to some glutamine amidotransferases (47), consistent with the observation that in some fungi and bacteria the nitrogen group of PLP originates in glutamine (42). In E. coli and some other bacteria, the nitrogen group of PLP comes from glutamate (42) through the SerC-catalyzed transamination step (21); in B. subtilis, SerC aminotransferase is not involved in B6 synthesis (34).
The sequence of pdxT suggests that this gene is unlikely to encode a full glutamine amidotransferase by itself. It is more likely to encode a glutaminase subunit of a heteromultimeric glutamine amidotransferase responsible for hydrolysis of glutamine to glutamate and ammonia and for channeling the latter to the corresponding synthase subunit. The location of pdxS adjacent to pdxT makes pdxS the most likely candidate to encode the synthase subunit of this glutamine amidotransferase of a novel, as-yet-unknown specificity. The phenotypes of pdxS and pdxT null mutants (strict and conditional requirements for B6 vitamers, respectively) are entirely consistent with this suggestion as (i) most glutamine amidotransferases are able to utilize ammonium, though with low efficiency, as a substitute for glutamine, (ii) the glutaminase subunit is completely dispensable when ammonium is the substrate, and (iii) inactivation of glutaminase subunits of other glutamine amidotransferases often leads to conditional, ammonium-dependent phenotypes of corresponding mutants (46).
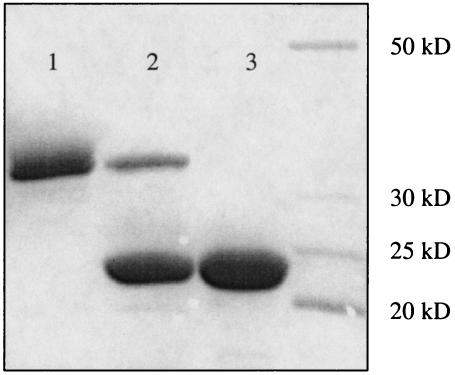
In the absence of precise knowledge of the reaction catalyzed by the putative PdxST complex, we tested a known partial activity of glutamine amidotransferases, the glutaminase reaction which frequently occurs in the absence of other substrates (46). A His6-tagged version of B. subtilis PdxS (the predicted molecular mass of the unmodified protein is 31.6 kDa) was overproduced in E. coli LMG194 (ara) cells (14) containing pBB1261 (Fig. 1) after addition of 0.2% l-arabinose to Luria broth cultures at an A600 of 0.25 to 0.4 and incubation for 4 h. The cells were pelleted, washed in 50 mM Tris-Cl (pH 8.0)-5% glycerol, and disrupted by sonication in 20 mM Tris-Cl (pH 7.9)-500 mM NaCl-5 mM imidazole-5% glycerol-1 mM phenylmethylsulfonyl fluoride-0.1% Nonidet P-40. The supernatant was clarified by low-speed centrifugation, and PdxS was purified to virtual homogeneity on a Ni2+ affinity column (His•Bind resin; Novagen) as described by the manufacturer, using 485 mM imidazole for elution (Fig. 3, lane 1). A His6-tagged version of B. subtilis PdxT (the predicted molecular mass of the unmodified protein is 21.4 kDa) was purified from E. coli (pBB1256) cells in a similar manner using 385 mM imidazole for elution (Fig. 3, lane 3). The modified proteins, when expressed in B. subtilis cells, were active and could complement null mutations in the corresponding chromosomal genes (data not shown).
FIG. 3.
Purification of PdxS, PdxT, and the PdxST complex. The proteins were purified by Ni2+ affinity chromatography and subjected to sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis. Lane 1, purified PdxS-His6; lane 2, PdxS-(PdxT-His6) complex; lane 3, purified PdxT-His6.
Glutaminase activity was detected in a coupled reaction with bovine glutamate dehydrogenase as the increase in optical density at 363 nm due to reduction of 3-acetylpyridine adenine dinucleotide (APAD), which accompanies conversion of glutamine-derived glutamate to 2-ketoglutarate. Samples were assayed at room temperature in 1 ml of 50 mM Tris-Cl (pH 8.2)-10 mM glutamine-0.6 mM APAD containing 1 U of bovine glutamate dehydrogenase (Sigma). The use of APAD, an analog of NAD, shifts the unfavorable equilibrium of the glutamate dehydrogenase reaction (44). The data were corrected for very low reduction of APAD in reactions lacking Pdx proteins. Protein concentration was determined using a Bio-Rad protein assay with bovine serum albumin as a standard.
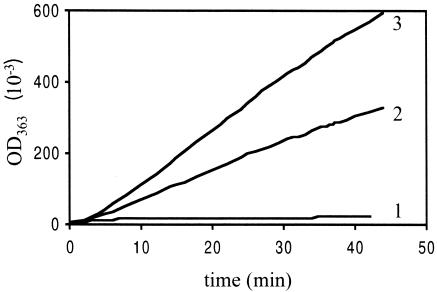
Neither PdxT (Fig. 4, curve 1) nor PdxS (data not shown) alone had glutaminase activity when glutamine was provided as the only substrate. When PdxS and PdxT were both present, significant glutaminase activity was observed. Higher glutaminase activity was obtained when the ratio of PdxS to PdxT was increased (Fig. 4, curves 2 and 3).
FIG. 4.
Glutaminase activity of the PdxT protein and PdxST complex. Purified PdxT-His6 and PdxS-His6 were assayed for glutaminase activity singly or in combination after 15 min of preincubation at room temperature. Curve 1, 40 μM PdxT; curve 2, 5 μM PdxT plus 2.5 μM PdxS; curve 3, 5 μM PdxT plus 5 μM PdxS. OD363, optical density at 363 nm.
Glutaminase subunits of some other glutamine amidotransferases are also activated by the presence of the corresponding synthase subunits (18, 46). This result strongly confirms our hypothesis that PdxS and PdxT are components of the same enzyme. Additionally, we conclude that the conditional growth phenotype of the pdxT-like mutants reflects the ammonium-dependent activity of PdxS in the absence of its cognate glutaminase subunit.
PdxS and PdxT interact physically.
An E. coli strain containing pBB1252 (Fig. 1) and overproducing the wild-type version of the B. subtilis PdxS protein and a His6-tagged version of PdxT was used for purification of the PdxT-His6 protein by Ni2+ affinity chromatography. Wild-type PdxS, which by itself had no affinity for the Ni2+ affinity column, copurified with PdxT-His6 (though not in the stoichiometric amount), indicating that the two proteins form a complex that does not completely dissociate even under the high salt conditions (0.5 M NaCl, 0.4 M imidazole) used for purification (Fig. 3, lane 2). This result is consistent with the postulated interaction of yeast PDX1 and PDX2 homologs, as deduced from experiments with two-hybrid systems (28). No protein copurified with PdxT-His6 if part of the pdxS gene was deleted from pBB1252, as in pBB1256 (Fig. 1 and 3, lane 3).
Analysis of the PdxST pathway.
All gram-positive bacteria have either the PdxST pathway or no PLP synthetic pathway; most other bacteria have the PdxA/PdxJ pathway (data not shown; published genome sequence data were searched by using the National Center for Biotechnology Information Blast server available at http://www.ncbi.nlm.nih.gov/BLAST; preliminary sequence data were searched at http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi and http://www.sanger.ac.uk/DataSearch). Some lactic acid bacteria and Clostridium spp. are known to be B6 auxotrophs (6, 19, 26, 38), consistent with the absence of the genes of de novo PLP synthesis in their genomes. No single bacterial genome has genes coding for all components of both pathways. Some bacteria with the PdxST pathway have a PdxA homolog; it is likely that these orphan PdxA-like proteins, as well as additional PdxA paralogs in some gram-negative bacteria (data not shown), have a dehydrogenase activity unrelated to PLP synthesis. The pdxS- and pdxT-like genes are linked in all bacterial genomes except that of Mycobacterium leprae (8).
In Saccharomyces cerevisiae, a glucose-derived five-carbon-containing compound and a triose are utilized for PLP synthesis (13, 48). No other components of the PDX1/PDX2 pathway, other than glutamine, are known, and it is unclear whether all steps of the PDX1/PDX2 pathway are common in different organisms. In our work, we demonstrated that the PdxST complex is likely to catalyze the corresponding glutamine amidotransferase reaction though neither the additional substrate(s) nor the products of the reaction have been identified. PdxS may have a phosphate-binding site (12), implicating a phosphorylated compound as a substrate; another possible role for this site is binding of flavin mononucleotide (FMN; the IPR003009 motif available at www.ebi.ac.uk/interpro).The latter designation may be misleading, however. Many enzymes containing the IPR003009 motif are not known to bind FMN; instead, they have pent(ul)ose 5-phosphate derivatives as their substrates. Since FMN contains a ribityl-5-phosphate group, we suggest that the IPR003009 motif reflects binding of a five-carbon unit phosphorylated at position 5. In that case, one of the PdxST substrates, in addition to glutamine, is a phosphorylated five-carbon carbohydrate derivative, as has been suggested by tracer experiments for yeast (13). Guanylylation of a protein that turned out to be PdxS by extracts of B. subtilis cells has been reported (24). The physiological role of this modification remains unknown.
In yeast grown anaerobically, glutamine does not serve as B6 precursor (16). It is possible that under these conditions the yeast PdxS-like SNZ protein uses ammonium as its substrate. Interestingly, the genomes of several bacteria, such as Fusobacterium nucleatum, Corynebacterium efficiens, Clostridium botulinum, and Treponema denticola, include pdxS-like genes but no pdxT-like gene (data not shown). PdxS proteins of these organisms may operate as ammonium-dependent synthases of the PLP pathway, may use other types of glutaminase subunits, or may not synthesize PLP in vivo. The tight B6 requirement of the pdxT null mutant in the absence of ammonium indicates that no other B. subtilis glutaminase can substitute for PdxT.
1-Deoxy-d-xylulose-5-phosphate, whose formation is catalyzed by the dxs gene product (22, 39), is required for PLP synthesis in E. coli (7, 20). Most bacteria that have pdxS- and pdxT-like genes have dxs orthologs, but no orthologs of dxs can be found in the genomes of archaea, Staphylococcus aureus, or Streptococcus pneumoniae, all of which apparently utilize the PdxST pathway (data not shown). Moreover, the B. subtilis conditional dxs mutant was not impaired in B6 synthesis (32). Additionally, the five-carbon-containing compound utilized for PLP synthesis in yeast is derived from glucose as an intact unit (13); in contrast, 1-deoxy-d-xylulose-5-phosphate originates from condensation of pyruvate and glyceraldehyde 3-phosphate (9, 15). Therefore, Dxs-produced 1-deoxy-d-xylulose-5-phosphate is an unlikely intermediate in the PdxST-dependent pathway of PLP synthesis. Though an alternative route of 1-deoxy-d-xylulose-5-phosphate synthesis was described in E. coli, its physiological role is unknown (43).
Exogenous 2′-hydroxypyridoxine can be converted to PLP in yeast (49). It is possible that 2′-hydroxypyridoxine 5′-phosphate is an immediate precursor of PLP in B. subtilis. If so, the putative five-carbon intermediate of the PdxST pathway should have a hydroxyl group at its 1 position.
ADDENDUM IN PROOF
The crystal structure of the B. subtilis PdxT (YaaE) protein was determined recently (J. A. Bauer, E. M. Bennett, T. P. Begley, and S. E. Ealick, J. Biol. Chem., in press).
Acknowledgments
I am grateful to A. L. Sonenshein and J. Smith for many helpful discussions and for careful reading of the manuscript.
This work was supported by a grant from the National Science Foundation (MCB-0110651) to B.R.B.
REFERENCES
- 1.Arst, H. N., Jr., A. G. Brownlee, and S. A. Cousen. 1982. Nitrogen metabolite repression in Aspergillus nidulans: a farewell to tamA? Curr. Genet. 6:245-257. [DOI] [PubMed] [Google Scholar]
- 2.Bean, L. E., W. H. Dvorachek, Jr., E. L. Braun, A. Errett, G. S. Saenz, M. D. Giles, M. Werner-Washburne, M. A. Nelson, and D. O. Natvig. 2001. Analysis of the pdx-1 (snz-1/sno-1) region of the Neurospora crassa genome: correlation of pyridoxine-requiring phenotypes with mutations in two structural genes. Genetics 157:1067-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belitsky, B. R., M. C. Gustafsson, A. L. Sonenshein, and C. Von Wachenfeldt. 1997. An lrp-like gene of Bacillus subtilis involved in branched-chain amino acid transport. J. Bacteriol. 179:5448-5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belitsky, B. R., and A. L. Sonenshein. 2002. GabR, a member of a novel protein family, regulates utilization of γ-aminobutyrate in Bacillus subtilis. Mol. Microbiol. 45:569-583. [DOI] [PubMed] [Google Scholar]
- 5.Belitsky, B. R., and A. L. Sonenshein. 1998. Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J. Bacteriol. 180:6298-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd, J. M., M. A. Logan, and A. A. Tytell. 1948. The growth requirements of Clostridium perfringens (welchii) BP6K. J. Biol. Chem. 174:1013-1025. [PubMed] [Google Scholar]
- 7.Cane, D. E., S. Du, J. K. Robinson, Y. Hsiung, and I. D. Spenser. 1999. Biosynthesis of vitamin B6: enzymatic conversion of 1-deoxy-d-xylulose-5-phosphate to pyridoxol phosphate. J. Am. Chem. Soc. 121:7222-7223. [Google Scholar]
- 8.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 9.Drewke, C., and E. Leistner. 2001. Biosynthesis of vitamin B6 and structurally related derivatives. Vitam. Horm. 61:121-155. [DOI] [PubMed] [Google Scholar]
- 10.Ehrenshaft, M., P. Bilski, M. Y. Li, C. F. Chignell, and M. E. Daub. 1999. A highly conserved sequence is a novel gene involved in de novo vitamin B6 biosynthesis. Proc. Natl. Acad. Sci. USA 96:9374-9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrenshaft, M., and M. E. Daub. 2001. Isolation of PDX2, a second novel gene in the pyridoxine biosynthesis pathway of eukaryotes, archaebacteria, and a subset of eubacteria. J. Bacteriol. 183:3383-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galperin, M. Y., and E. V. Koonin. 1997. Sequence analysis of an exceptionally conserved operon suggests enzymes for a new link between histidine and purine biosynthesis. Mol. Microbiol. 24:443-445. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, R. N., T. Hemscheidt, B. G. Sayer, and I. D. Spenser. 2001. Biosynthesis of vitamin B6 in yeast: incorporation pattern of glucose. J. Am. Chem. Soc. 123:11353-11359. [DOI] [PubMed] [Google Scholar]
- 14.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill, R. E., and I. D. Spenser. 1996. Biosynthesis of vitamin B6, p. 695-703. In F. C. Neidhardt, R. I. Curtiss, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 16.Ishida, S., and K. Yamada. 2002. Biosynthesis of pyridoxine in Saccharomyces cerevisiae—origin of the pyridoxine nitrogen atom differs under anaerobic and aerobic conditions. J. Nutr. Sci. Vitaminol. 48:448-452. [DOI] [PubMed] [Google Scholar]
- 17.John, R. A. 1995. Pyridoxal phosphate-dependent enzymes. Biochim. Biophys. Acta 1248:81-96. [DOI] [PubMed] [Google Scholar]
- 18.Klem, T. J., and V. J. Davisson. 1993. Imidazole glycerol phosphate synthase: the glutamine amidotransferase in histidine biosynthesis. Biochemistry 32:5177-5186. [DOI] [PubMed] [Google Scholar]
- 19.Koser, S. A. 1968. Vitamin requirements of bacteria and yeasts. Charles C. Thomas Publisher, Springfield, Ill.
- 20.Laber, B., W. Maurer, S. Scharf, K. Stepusin, and F. S. Schmidt. 1999. Vitamin B6 biosynthesis: formation of pyridoxine 5′-phosphate from 4-(phosphohydroxy)-l-threonine and 1-deoxy-d-xylulose-5-phosphate by PdxA and PdxJ protein. FEBS Lett. 449:45-48. [DOI] [PubMed] [Google Scholar]
- 21.Lam, H. M., and M. E. Winkler. 1990. Metabolic relationships between pyridoxine (vitamin B6) and serine biosynthesis in Escherichia coli K-12. J. Bacteriol. 172:6518-6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lois, L. M., N. Campos, S. R. Putra, K. Danielsen, M. Rohmer, and A. Boronat. 1998. Cloning and characterization of a gene from Escherichia coli encoding a transketolase-like enzyme that catalyzes the synthesis of d-1-deoxyxylulose 5-phosphate, a common precursor for isoprenoid, thiamin, and pyridoxol biosynthesis. Proc. Natl. Acad. Sci. USA 95:2105-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopilato, J., S. Bortner, and J. Beckwith. 1986. Mutations in a new chromosomal gene of Escherichia coli K-12, pcnB, reduce plasmid copy number of pBR322 and its derivatives. Mol. Gen. Genet. 205:285-290. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell, C., P. W. Morris, and J. C. Vary. 1992. Amino acid sequences of several Bacillus subtilis proteins modified by apparent guanylylation. Mol. Microbiol. 6:1579-1581. [DOI] [PubMed] [Google Scholar]
- 25.Mittenhuber, G. 2001. Phylogenetic analyses and comparative genomics of vitamin B6 (pyridoxine) and pyridoxal phosphate biosynthesis pathways. J. Mol. Microbiol. Biotechnol. 3:1-20. [PubMed] [Google Scholar]
- 26.Mulligan, J. H., and E. E. Snell. 1977. Transport and metabolism of vitamin B6 in lactic acid bacteria. J. Biol. Chem. 252:835-839. [PubMed] [Google Scholar]
- 27.Osmani, A. H., G. S. May, and S. A. Osmani. 1999. The extremely conserved pyroA gene of Aspergillus nidulans is required for pyridoxine synthesis and is required indirectly for resistance to photosensitizers. J. Biol. Chem. 274:23565-23569. [DOI] [PubMed] [Google Scholar]
- 28.Padilla, P. A., E. K. Fuge, M. E. Crawford, A. Errett, and M. Werner-Washburne. 1998. The highly conserved, coregulated SNO and SNZ gene families in Saccharomyces cerevisiae respond to nutrient limitation. J. Bacteriol. 180:5718-5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pflug, W., and F. Lingens. 1983. Occurrence of pyridoxamine-phosphate oxidase and pyridoxal kinase in gram-negative and gram-positive bacteria. Hoppe-Seyler's Z. Physiol. Chem. 364:1627-1630. [DOI] [PubMed] [Google Scholar]
- 30.Pflug, W., and F. Lingens. 1978. Vitamin-B6-biosynthesis in Bacillus subtilis. Hoppe-Seyler's Z. Physiol. Chem. 359:559-570. [PubMed] [Google Scholar]
- 31.Rodriguez-Navarro, S., B. Llorente, M. T. Rodriguez-Manzaneque, A. Ramne, G. Uber, D. Marchesan, B. Dujon, E. Herrero, P. Sunnerhagen, and J. E. Perez-Ortin. 2002. Functional analysis of yeast gene families involved in metabolism of vitamins B1 and B6. Yeast 19:1261-1276. [DOI] [PubMed] [Google Scholar]
- 32.Sakai, A., N. Kinoshita, M. Kita, T. Katsuragi, and Y. Tani. 2003. Investigation of 1-deoxy-d-xylulose 5-phosphate synthase and transketolase of Bacillus subtilis in relation to vitamin B-6 biosynthesis. J. Nutr. Sci. Vitaminol. 49:73-75. [DOI] [PubMed] [Google Scholar]
- 33.Sakai, A., M. Kita, T. Katsuragi, N. Ogasawara, and Y. Tani. 2002. yaaD and yaaE are involved in vitamin B6 biosynthesis in Bacillus subtilis. J. Biosci. Bioeng. 93:309-312. [DOI] [PubMed] [Google Scholar]
- 34.Sakai, A., M. Kita, T. Katsuragi, and Y. Tani. 2002. serC is involved in vitamin B-6 biosynthesis in Escherichia coli but not in Bacillus subtilis. J. Biosci. Bioeng. 93:334-337. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. J. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Seack, J., S. Perovic, V. Gamulin, H. C. Schroder, P. Beutelmann, I. M. Muller, and W. E. Muller. 2001. Identification of highly conserved genes: SNZ and SNO in the marine sponge Suberites domuncula: their gene structure and promoter activity in mammalian cells. Biochim. Biophys. Acta 1520:21-34. [DOI] [PubMed] [Google Scholar]
- 37.Sivasubramaniam, S., V. M. Vanniasingham, C. T. Tan, and N. H. Chua. 1995. Characterisation of HEVER, a novel stress-induced gene from Hevea brasiliensis. Plant Mol. Biol. 29:173-178. [DOI] [PubMed] [Google Scholar]
- 38.Snell, E. E. 1989. Nutrition research with lactic acid bacteria: a retrospective view. Annu. Rev. Nutr. 9:1-19. [DOI] [PubMed] [Google Scholar]
- 39.Sprenger, G. A., U. Schorken, T. Wiegert, S. Grolle, A. A. de Graaf, S. V. Taylor, T. P. Begley, S. Bringer-Meyer, and H. Sahm. 1997. Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-d-xylulose 5-phosphate precursor to isoprenoids, thiamin, and pyridoxol. Proc. Natl. Acad. Sci. USA 94:12857-12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stolz, J., and M. Vielreicher. 2003. Tpn1p, the plasma membrane vitamin B6 transporter of Saccharomyces cerevisiae. J. Biol. Chem. 278:18990-18996. [DOI] [PubMed] [Google Scholar]
- 41.Tamegai, H., E. Nango, A. Koike-Takeshita, F. Kudo, and K. Kakinuma. 2002. Significance of the 20-kDa subunit of heterodimeric 2-deoxy-scyllo-inosose synthase for the biosynthesis of butirosin antibiotics in Bacillus circulans. Biosci. Biotechnol. Biochem. 66:1538-1545. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka, K., K. Tazuya, K. Yamada, and H. Kumaoka. 2000. Biosynthesis of pyridoxine: origin of the nitrogen atom of pyridoxine in microorganisms. J. Nutr. Sci. Vitaminol. 46:55-57. [DOI] [PubMed] [Google Scholar]
- 43.Wungsintaweekul, J., S. Herz, S. Hecht, W. Eisenreich, R. Feicht, F. Rohdich, A. Bacher, and M. H. Zenk. 2001. Phosphorylation of 1-deoxy-d-xylulose by d-xylulokinase of Escherichia coli. Eur. J. Biochem. 268:310-316. [DOI] [PubMed] [Google Scholar]
- 44.Wyngaarden, J. B., and D. M. Ashton. 1959. The regulation of activity of phosphoribosylpyrophosphate amidotransferase by purine ribonucleotides: a potential feedback control of purine biosynthesis. J. Biol. Chem. 234:1492-1496. [PubMed] [Google Scholar]
- 45.Yang, Y., H. C. Tsui, T. K. Man, and M. E. Winkler. 1998. Identification and function of the pdxY gene, which encodes a novel pyridoxal kinase involved in the salvage pathway of pyridoxal 5′-phosphate biosynthesis in Escherichia coli K-12. J. Bacteriol. 180:1814-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zalkin, H. 1993. The amidotransferases. Adv. Enzymol. Relat. Areas Mol. Biol. 66:203-309. [DOI] [PubMed] [Google Scholar]
- 47.Zalkin, H., and J. L. Smith. 1998. Enzymes utilizing glutamine as an amide donor. Adv. Enzymol. Relat. Areas Mol. Biol. 72:87-144. [DOI] [PubMed] [Google Scholar]
- 48.Zeidler, J., R. N. Gupta, B. G. Sayer, and I. D. Spenser. 2003. Biosynthesis of vitamin B6 in yeast. Incorporation pattern of trioses. J. Org. Chem. 68:3486-3493. [DOI] [PubMed] [Google Scholar]
- 49.Zeidler, J., N. Ullah, R. N. Gupta, R. M. Pauloski, B. G. Sayer, and I. D. Spenser. 2002. 2′-Hydroxypyridoxol, a biosynthetic precursor of vitamins B6 and B1 in yeast. J. Am. Chem. Soc. 124:4542-4543. [DOI] [PubMed] [Google Scholar]