Abstract
We have identified a new steroid-inducible gene (designated teiR [testosterone-inducible regulator]) in Comamonas testosteroni that is required for testosterone degradation. Nucleotide sequence analysis of teiR predicts a 391-amino-acid protein which shows homology between residues 327 and 380 (C-terminal domain) to the LuxR helix-turn-helix DNA binding domain and between residues 192 and 227 to the PAS sensor domain. This domain distribution resembles that described for TraR, a specific transcriptional regulator involved in quorum sensing in Agrobacterium tumefaciens. Analysis of the gene expression indicated that teiR is tightly controlled at the transcriptional level by the presence of testosterone in the culture medium. A teiR-disrupted mutant strain was completely unable to use testosterone as the sole carbon and energy source. In addition, the expression of several steroid-inducible genes was abolished in this mutant. Northern blot assays revealed that teiR is required for full expression of sip48-β-HSD gene mRNA (encoding a steroid-inducible protein of 48 kDa and 3β-17β-hydroxysteroid dehydrogenase) and also of other steroid degradation genes, including those encoding 3α-hydroxysteroid dehydrogenase, Δ5-3-ketoisomerase, 3-oxo-steroid Δ1-dehydrogenase, and 3-oxo-steroid Δ4-(5α)-dehydrogenase enzymes. Moreover, when teiR was provided to the teiR-disrupted strain in trans, the transcription level of these genes was restored. These results indicate that TeiR positively regulates the transcription of genes involved in the initial enzymatic steps of steroid degradation in C. testosteroni.
Steroids, phenylalkanoic acids, resin acids, and different polycyclic aromatic hydrocarbons represent a group of molecules that are widespread in the environment as breakdown products of lignin or other plant-derived molecules (7, 22, 31). These compounds (known collectively as endocrine disruptors) interfere with the normal endocrine system physiology of vertebrates, particularly in the mechanisms governing reproductive development and function (10), and constitute an important group of bioactive environmental pollutants.
Comamonas testosteroni is a gram-negative bacterium able to use steroids (as well as many other aromatic compounds) as a sole carbon source; it is an attractive model for the study of the mechanisms involved in the mineralization of these bioactive compounds for their removal from the environment (3, 5, 6, 11, 19, 26, 30, 37, 38). C. testosteroni metabolizes certain steroids through a complex metabolic pathway involving many steps catalyzed by steroid-inducible enzymes (11, 26, 38, 42). Interestingly, recent works revealed that testosterone simultaneously induced both steroid- and PAH-metabolizing enzymes in this bacterium (29, 36). For this reason, the study of the mechanisms regulating the steroid-inducible gene transcription is concerned with understanding both catabolic pathways.
While the genes encoding some of the enzymes catalyzing the oxidoreduction at different positions of the steroid nucleus and the ring opening of the steroid molecule have been identified (1, 2, 8, 9, 12, 16, 18, 20, 21, 25, 29, 32, 36, 44, 45), only limited information is available about the mechanisms governing steroid-inducible gene expression. The following items have been reported to date. Horinouchi et al. (20) suggest that an intermediate compound produced in the course of testosterone degradation induces expression of tesB, encoding a meta-cleavage steroid-inducible enzyme. The induction of 3α-hydroxysteroid dehydrogenase-carbonyl reductase (α-HSD), in contrast, appears to represent a derepression in which the steroidal inducer prevents the binding of two repressor proteins (RepA and RepB) to the α-HSD gene promoter and mRNA, respectively (44, 45).
For this study we report the identification and characterization of the teiR gene, which is essential for testosterone degradation in C. testosteroni. teiR encodes a LuxR-type transcription factor required for the expression of several steroid-inducible genes, suggesting that a quorum-sensing mechanism is involved in its regulation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli was grown at 37°C in Luria-Bertani (LB) medium (34). C. testosteroni was grown at 30°C in LB medium or in M9 minimal medium (34) plus acetate (0.2% [wt/vol]) or testosterone (0.25 mg ml−1) or both, as indicated in the text. Overnight cultures were diluted 1/100 in fresh medium and incubated for 2 h in LB medium or for 12 h in M9 medium, and cells were washed, diluted 1/50 in fresh medium, and incubated as indicated for each experiment.
TABLE 1.
Bacterial strains, plasmids, and cosmids used
| Strains, plasmids, and cosmids | Relevant genotype | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli DH5α | F−recA1 endA1 gyr96 thi-1 hsdR17 (rK− mK+) supE44 relA1 ΔlacZYA-argF U169 φ80dlacZΔM15; host strain for DNA manipulation | Stratagene |
| C. testosteroni | ||
| Wild type | ATCC 11996 | |
| UT2.5 | Cmr; C. testosteroni bearing a chromosomal insertion of miniTn5 Cm/sip48-β-HSD::lacZ | This study |
| UT2.5teiR-3′URΩ | Gmr, Spr; mutant of C. testosteroni ATCC 11996 carrying SmaI 2-kb cassette from pHP45Ω inserted in teiR 3′ untranslated region | This study |
| UT2.5Mut50 | Cmr, Tcr; C. testosteroni bearing chromosomal insertions of mini-Tn5 Cm/sip48-β-HSD::lacZ and mini-Tn5 Tc | This study |
| Plasmids and cosmids | ||
| pSL9 | pGEM3 containing 3.2-kb HindIII fragment of β-HSD gene and contiguous genes; Apr | 18 |
| pRK2013 | Kmr, Mob+, Tra+; donor of transfer functions | 15 |
| pBIISK | Apr; multipurpose cloning vector | |
| pGEMT easy | Apr; PCR fragment cloning vector | Promega |
| pUTmini-Tn5 Tc | Apr, Tcr; R6KoriV, RP4oriT, mini-Tn5 Tc transposon vector delivery plasmid | 13 |
| pBBR1MCS2 | Kmr; broad-host-range plasmid | 24 |
| pVK102 | Tcr, Kmr, RP432 (IncP-1) | 23 |
| pHP45:Ω | Apr, Smr,a Spr | 33 |
| pVK102teiR | Tcr, containing HindIII partially digested fragment DNA of C. testosteroni | This study |
| pAK1370 | pUC19 containing-1,370-bp PstI fragment encoding the C-terminal sequences of α-HSD and Δ5-KSI genes | 25 |
| pTEK21 | pUC19 containing 2.2 KpnI fragment encoding Δ1-DH and the N end of Δ4-DH genes | 32 |
| pUT 2.5 | Apr, Cmr; HindIII-EcoV (from pSL9)-lacZ transcriptional fusion inserted as a NotI fragment in pUTminiTn5 | This study |
| pGteiR | pGEMT easy containing 1.2-kb teiR DNA fragment from PCR | This study |
| pBteiR-3′URΩ | pBIISKMut50 containing Ω cassette insertion into the NdeI site located in teiR 3′ untranslated region | This study |
| pBIISKMut50 | Apr; pBIISK containing 2.5-kb NotI fragment from genomic DNA of C. testosteroni UT2.5Mut50 | This study |
| pBBteiR | Apr, Kmr; pBBR1MCS2 containing 1.2-kb teiR gene insertion into the EcoRI site | This study |
| pBB20H | Apr, Kmr; pBBR1MCS2 containing 20-kb HindIII fragment from pVK102 cosmid library of C. testosteroni | This study |
Smr, streptomycin resistance.
Growth of C. testosteroni was monitored by measuring optical density at 600 nm (OD600). Alternatively, growth in M9 medium plus testosterone was monitored by counting colonies that appeared on LB plates (on which appropriately diluted cultures have been spread) after incubation at 30°C. When needed, antibiotics were added at the following concentrations (in micrograms per milliliter): ampicillin (Ap), 100; chloramphenicol (Cm), 20; gentamicin (Gm), 10; kanamycin (Km), 20; spectinomycin (Sp), 600; and tetracycline (Tc), 10.
DNA manipulations and sequence determinations.
Standard protocols or manufacturers' instructions were followed for DNA isolation and recombinant DNA procedures (34). DNA sequencing was performed on double-stranded templates derived (using the dideoxy chain termination method) (35) from pBIISKMut50 and pBB20H. For TaqDNA polymerase-initiated cycle sequencing reactions with fluorescently labeled dideoxynucleotide terminators (Applied Biosystems Inc.), standard protocols of the manufacturer were used. The sequencing reactions were analyzed using a model 377 automated DNA sequencer (Applied Biosystems Inc.). Blast software was used to screen DNA and protein databases for similar proteins (4). Multiple sequence alignments were made with ClustalW software (version 1.7) (40).
Construction of plasmids and allele replacement.
Plasmid pBIISKMut50 was constructed by the ligation of NotI DNA fragments from C. testosteroni UT2.5Mut50 into pBIISK. After transformation into E. coli DH5α, Tc-resistant (Tcr) strains were isolated.
For the construction of pBB20H, a pVK102 C. testosteroni cosmid library was screened using a 0.5-kb PstI/NotI fragment from pBIISKMut50 as a probe (see Southern blot analysis results) and a cosmid containing a teiR gene was isolated (pVK102teiR). Finally, a 20-kb HindIII fragment from pVK102teiR (containing teiR) was subcloned into the HindIII site of pBBR1MCS2.
Plasmid pGteiR was constructed by PCR amplification of teiR coding sequence with the primers teiR-Fw (5′-ggaagcttgctagcATGTGCCCATATTTCGACAC-3′) and teiR-Rv (5′-cccggggctagcaagcttTCACTTGTTCCCCAGCCA-3′). The amplification product was ligated into the pGEMT easy vector.
Plasmid pBteiR-3′URΩ was constructed by insertion of a 2-kb SmaI fragment (obtained from pHP45::Ω) (33) into the NdeI site located in the teiR 3′ untranslated region of pBIISKMut50. The recombinant plasmid was transferred (using the mobilizing plasmid pRK2013) into C. testosteroni UT2.5 by triparental mating. Donor, helper, and recipient cells were grown overnight in LB medium. Cell suspensions (0.2 ml each) were mixed, filtered on a 0.4-μm-pore-size nitrocellulose membrane filter, and incubated at 30°C on an LB agar plate for 24 h. Cells were suspended in sterile 1% (wt/vol) NaCl and plated onto LB agar containing Gm (10 μg ml−1) and Sp (600 μg ml−1) to select transconjugants. Southern hybridization was performed to confirm the genomic structure of the mutant strain (C. testosteroni UT2.5teiR-3′URΩ).
β-Galactosidase assays.
The standard procedures described by Miller (27) were used for quantitative measurements of β-galactosidase activity. Samples were collected after 12 h (LB medium) or 17 h (M9 medium) of incubation. The values given throughout this paper represent the averages of the results of three independent experiments, each of which was conducted with duplicate samples.
Southern blot analysis.
Genomic DNAs were prepared essentially as described by Sambrook et al. (34). Southern blot analysis was performed as described previously (8). DNA fragments were transferred from agarose gels or from bacterial colonies to nylon membranes after alkali denaturation. A 650-bp EcoRV-HindIII fragment from pSL9 (complementary to the 3′ end of the gene encoding 3β-17β-hydroxysteroid dehydrogenase [β-HSD]) and a 540-bp PstI-NotI fragment from pBIISKMut50 (complementary to the teiR 3′ untranslated region) were labeled with [α32P]dATP (3,000 Ci mmol−1) by a random priming method (14) and used as probes as indicated in the text.
Testosterone degradation.
Testosterone degradation was performed as described previously (18). Briefly, bacterial cells (grown in LB medium plus testosterone during 12 h of culture) were harvested by centrifugation at 4°C. Aliquots of culture supernatants were extracted three times with 5 vol of ethyl ether and submitted (using benzene-ethanol [95:5 {vol/vol}] as a solvent system) to thin-layer chromatography on silica gel GF254 plates. The pattern of testosterone degradation was visualized using 254-nm UV light. Testosterone, 4-androstene-3,17-dione, and 1,4-androstadiene-3,17-dione were used as standards.
RNA isolation and Northern blot analysis.
C. testosteroni was grown in LB medium or M9 medium plus acetate during the indicated periods of culture growth in the absence or presence of testosterone. Total RNA was extracted as described previously (8). RNA samples (20 μg per lane) were electrophoresed on a 1.2% (wt/vol) agarose gel containing 18% (vol/vol) formaldehyde and transferred to nitrocellulose membranes (8). Equal levels of loading and transfer were assessed by methylene blue staining of membranes. Prehybridization and hybridization reactions were performed as described previously (8). A 600-bp HincII-PstI restriction fragment from pSL9 (complementary to the sip48 gene), a 650-bp EcoV-HindIII restriction fragment from pSL9 (complementary to the β-HSD gene), a 1,400-bp PstI restriction fragment from pAK1370 (complementary to the α-HSD and Δ5-KSI genes), a 2,200-bp KpnI restriction fragment from pTEK21 (complementary to the 3-oxo-steroid Δ1-dehydrogenase [Δ1-DH] and 3-oxo-steroid Δ4-(5α)-dehydrogenase [Δ4-DH] genes), and a 1,200-bp EcoRI restriction fragment from pGteiR (complementary to teiR) were labeled with [α32P]dATP (3,000 Ci mmol−1) by a random priming method (14) and used as probes as indicated in the text.
Genetic complementation of teiR mutant.
The complete coding sequence of teiR was obtained as a 1.2-kb EcoRI fragment from pGteiR and then subcloned into the EcoRI site of pBBR1MCS2 to generate pBBteiR. Plasmids pBBR1MCS2 (negative control) and pBBteiR were mobilized (using the mobilizing plasmid pRK2013) from E. coli to C. testosteroni UT2.5Mut50 by triparental mating. Donor, helper, and recipient cells were grown overnight in LB medium. Cell suspensions (0.2 ml each) were mixed, filtered on a 0.4-μm-pore-size nitrocellulose membrane filter, and incubated on an LB agar plate for 8 h at 30°C. Cells were suspended in sterile 1% NaCl, and transformants were selected on LB agar plates containing Gm (10 μg ml−1) and Km (500 μg ml−1).
Nucleotide sequence accession number.
The nucleotide sequences reported in this paper have been deposited in the GenBank database under accession number AY363220. The 3.1-kb HindIII fragment of pSL9 bearing stdC, sip48, and the β-HSD gene has the following accession number: U41265.
RESULTS
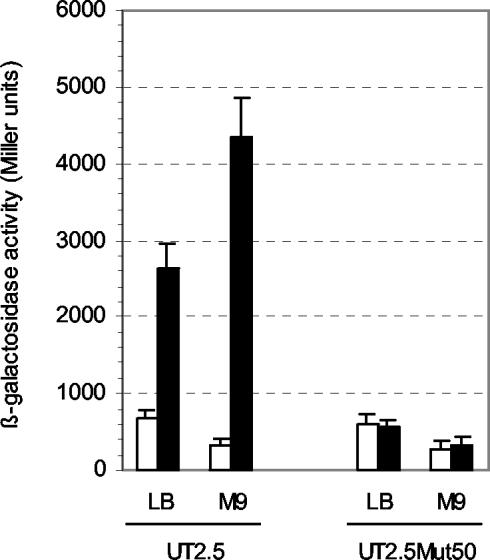
Identification of a gene required for sip48-β-HSD gene steroid-inducible expression. Previously, we characterized two steroid-inducible genes encoding a protein of unknown function (Sip48) and β-HSD (which are transcribed as a polycistronic message). We localized the promoter activity responsible for sip48-β-HSD gene steroid-inducible transcription in the sip48 5′ untranslated region (unpublished data). A lacZ transcriptional fusion containing the promoter region inserted into the chromosome of C. testosteroni (C. testosteroni UT2.5) (Fig. 1) allowed us to measure promoter activity by quantifying the β-galactosidase activity produced by this strain in different experimental conditions. High levels of β-galactosidase activity (3,000 to 4,000 Miller units) were found when C. testosteroni UT2.5 was grown in presence of testosterone irrespective of whether LB medium or M9 medium was used (Fig. 2). In addition, this strain showed the same growth rate as wild-type C. testosteroni cells in LB medium and M9 minimal medium supplemented with acetate or testosterone (data not shown).
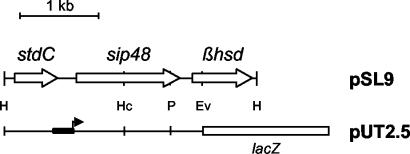
FIG. 1.
Restriction map of the 3.2-kb HindIII fragment cloned into pSL9 plasmid. The regions carrying stdC and sip48 and the β-HSD gene are indicated by boxes. H, HindIII; Hc, HincII; P, PstI; Ev, EcoRV. A schematic representation of pUT2.5 carrying the transcriptional fusion of the β-HSD gene upstream region (thick line) to a promoterless lacZ gene (open box) is shown. The black box and arrow indicate the sip48-β-HSD gene steroid-inducible promoter.
FIG. 2.
Activity of sip48-β-HSD gene promoter in C. testosteroni UT2.5 and UT2.5Mut50 strains. The levels of β-galactosidase activity of a sip48-β-HSD gene promoter-lacZ transcriptional fusion in C. testosteroni UT2.5 and UT2.5Mut50 strains growing in LB medium and M9 minimal medium supplemented with acetate (M9) in the presence (black bars) and the absence (white bars) of testosterone are shown. β-Galactosidase activities were measured with permeabilized cells as described in Materials and Methods. Each value is the average of the results from three independent experiments (error bars indicate standard deviations).
To characterize genes involved in the regulation of sip48-β-HSD gene steroid-inducible expression, a mini-Tn5 insertional mutagenesis procedure was carried out by the transference of a Tc minitransposon element (pUTminiTn5) into C. testosteroni UT2.5. A number of mutant strains that exhibited resistance to Tc were isolated. Screening of the resulting transconjugants revealed the presence of white-colored colonies grown on induction medium containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and testosterone. One of these colonies (designated UT2.5Mut50) was isolated and further characterized. The effect of the minitransposon insertion on sip48-β-HSD gene promoter activity in this mutant bacterium was confirmed by comparing the β-galactosidase activity produced by this strain to that produced by C. testosteroni UT2.5 when the strains were grown in LB medium and M9 minimal medium supplemented with acetate in the presence and the absence of testosterone (Fig. 2). The results clearly demonstrate that the testosterone-inducible expression of the reporter gene controlled by the sip48-β-HSD gene promoter is absent from the C. testosteroni UT2.5Mut50 mutant strain. To assess whether the β-galactosidase levels determined for the UT2.5Mut50 strain reflected the transcriptional state of the corresponding sip48-β-HSD gene transcript, their mRNA levels were analyzed by Northern blot analysis using sip48 and β-HSD gene probes as described in Materials and Methods. Strong signals were revealed in the lanes corresponding to mRNAs from C. testosteroni wild-type and UT2.5 strains growing in the presence of testosterone. In contrast, no sip48-β-HSD gene transcript was observed with mRNAs from C. testosteroni UT2.5Mut50 grown under the same experimental conditions (data not shown), thus confirming the results obtained with the β-galactosidase reporter fusion.
Characterization of the identified gene.
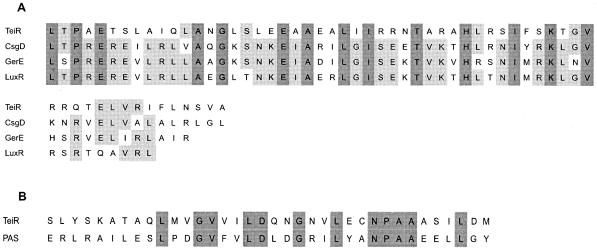
Chromosomal DNA of the C. testosteroni UT2.5Mut50 strain was isolated and digested with a NotI enzyme, which does not cut within the minitransposon. The digested mixture was ligated into the NotI site of pBIISK plasmid, and transformants able to grow on Tc were selected. The resulting plasmid (pBIISKMut50) was found to contain a 2.5-kb insert (including the 1.8-kb Tcr fragment). Complete analysis of the 0.7-kb fragment sequence located downstream of the Tcr gene revealed an incomplete open reading frame (3′ end). To obtain the 5′ coding sequence of the interrupted gene, a DNA pVK102 C. testosteroni cosmid library was screened with a probe complementary to the 3′ end of the disrupted gene. A positive-testing clone was isolated and characterized by Southern blot analysis. A 20-kb HindIII fragment was subcloned into pBBR1MCS2 (pBB20H) and partially sequenced, and the complete sequence of the disrupted open reading frame was obtained. The deduced amino acid sequence of this gene predicts a 391-amino-acid protein with a molecular mass of 43 kDa. Computer analysis showed that this amino acid sequence shows high-level similarity (93.1%) between residues 327 and 380 (C-terminal domain) to that of the LuxR helix-turn-helix DNA binding domain (smart00421) (Fig. 3A). In addition, the sequence between residues 192 and 227 shows mild similarity (53.7%) to that of the PAS sensor domain (smart00091) (Fig. 3B). This novel gene (called teiR [testosterone-inducible regulator]) encodes a protein with 28% identity (44% similarity) over an aligned length of 384 amino acid residues with a putative protein of Novosphingobium aromaticivorans (accession number ZP 00092325.1). Other related proteins are putative transcription regulator proteins of Mesorhizobium loti (accession number NP 103400.1), Bradyrhizobium japonicum (accession number NP 770508.1), Ralstonia solanacearum (accession number NP 523029.1), and a DNA-binding protein of Vibrio vulnificus (accession number NP 761512.1). A 60-amino-acid region in the C-terminal domain (where a potential helix-turn-helix DNA binding motif is located along with several highly conserved amino acids such as those found in CsgD [COG2771], GerE [pfam00196], and LuxR [smart00421] regulatory proteins) is typically present in these proteins.
FIG. 3.
(A) Alignment of the helix-turn-helix DNA binding domains of three transcriptional regulators (CsgD [COG2771], LuxR [smart00421], and GerE [pfam00196]) and amino acids 327 to 380 of the TeiR protein (accession number AY363220). (B) Alignment of the PAS sensor domain (smart00091) and amino acids 192 to 227 of the TeiR protein. For residues that were identical in all the aligned proteins, the characters representing the residues appear on a dark-gray background. For residues that were identical in 60% of the aligned proteins, the characters representing the residues appear on a light-gray background.
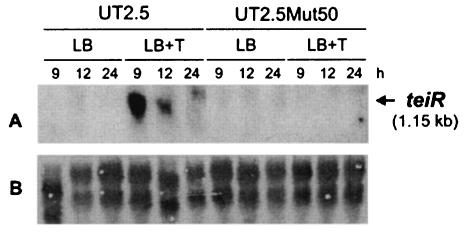
teiR gene expression was investigated in C. testosteroni UT2.5 and UT2.5Mut50 strains growing on LB medium with or without testosterone. Total RNA was extracted at different culture times under each set of experimental conditions, and Northern blot assays were performed. The teiR probe recognized strong signals corresponding to a 1,150-nucleotide transcript when C. testosteroni UT2.5 bacteria were grown in the presence of testosterone, indicating that this is a steroid-inducible gene. In contrast, no teiR expression was found when the UT2.5Mut50 RNA was probed, thus indicating the absence of teiR expression in the UT2.5Mut50 strain (Fig. 4).
FIG. 4.
Expression of teiR in C. testosteroni UT2.5 and UT2.5Mut50 strains in response to the presence of testosterone. Total RNA samples (20 μg per lane) were prepared from bacteria grown on LB medium in the absence (LB) or presence (LB+T) of testosterone during 9, 12, and 24 h of culture growth. (A) The membrane was hybridized with a DNA fragment complementary to the teiR gene. (B) The samples were analyzed by electrophoresis on formaldehyde-agarose gels, transferred to a nylon membrane, and stained with methylene blue.
Expression analysis of other steroid-inducible genes in C. testosteroni UT2.5Mut50.
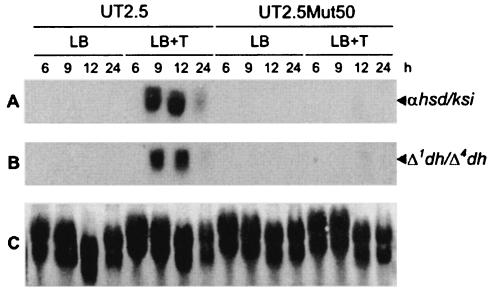
To establish whether the teiR gene is required for the transcription of other steroid-inducible genes, Northern blot assays were performed. Total RNAs from C. testosteroni UT 2.5 and UT2.5Mut50 strains grown on LB medium with or without testosterone were isolated. DNA fragment probes complementary to the sequences of the steroid-inducible genes encoding α-HSD-KSI and Δ1-DH-Δ4-DH recognized the corresponding strong signals in the RNA samples obtained from C. testosteroni UT 2.5 (the control strain) grown in the presence of testosterone. In contrast, no steroid-inducible gene expression was observed in C. testosteroni UT2.5Mut50 strain (Fig. 5). These results demonstrate that in addition to sip48-β-HSD gene transcription, the expression of other testosterone-inducible genes such as the α-HSD-KSI and Δ1-DH-Δ4-DH genes is impaired by teiR gene disruption.
FIG. 5.
Expression of steroid-inducible genes in C. testosteroni UT2.5 and UT2.5Mut50 strains. Total RNA samples (20 μg per lane) were prepared from bacteria grown on LB medium in the presence or absence of testosterone during 6, 9, 12, and 24 h of culture growth. (A and B) The membrane was hybridized with a DNA fragment complementary to α-HSD and Δ5-KSI (αhsd/ksi) (A) and to Δ1-DH and Δ4-DH (Δ1dh/Δ4-dh) (B) steroid-inducible genes. (C) The samples were analyzed by electrophoresis on formaldehyde-agarose gels, transferred to a nylon membrane, and stained with methylene blue.
Altered phenotypes in C. testosteroni UT2.5Mut50.
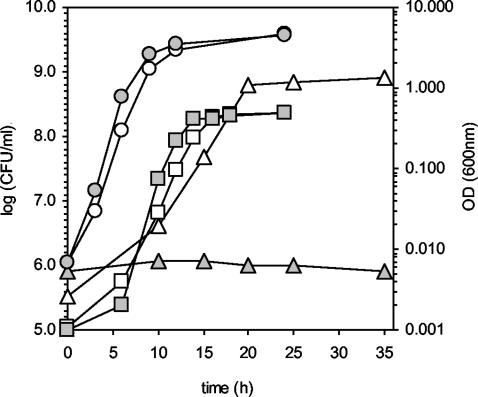
Having obtained evidence that teiR disruption abolishes not only sip48-β-HSD gene testosterone-inducible expression but also the transcription of other steroid-inducible genes, we analyzed the growth of C. testosteroni UT2.5Mut50 in M9 minimal medium supplemented with testosterone as the sole carbon and energy source. The results (shown in Fig. 6) indicate that the mutant strain is completely unable to use testosterone as the sole carbon source. Moreover, measurement of testosterone degradation in C. testosteroni UT2.5Mut50 indicates that this strain cannot transform testosterone into androstenedione (data not shown). Nevertheless, the mutant and wild-type strains showed identical duplication times (70 min) when they were grown in LB medium or M9 minimal medium supplemented with acetate (Fig. 6). These results demonstrate that teiR disruption abolishes testosterone metabolism and (as a consequence) C. testosteroni growth in medium containing this steroid as the sole carbon source.
FIG. 6.
Growth of C. testosteroni UT2.5 (white shading) and UT2.5Mut50 (grey shading) strains in LB medium (circle), M9 medium plus acetate (square), and M9 medium plus testosterone (triangle). Growth of bacteria in LB medium or M9 plus acetate was monitored by measuring OD600. Growth of bacteria in M9 medium plus testosterone was monitored by counting colonies (in CFU per milliliter) that appeared on LB plates (on which appropriately diluted cultures have been spread) after incubation at 30°C.
Insertional transcription inactivation downstream of the teiR coding sequence.
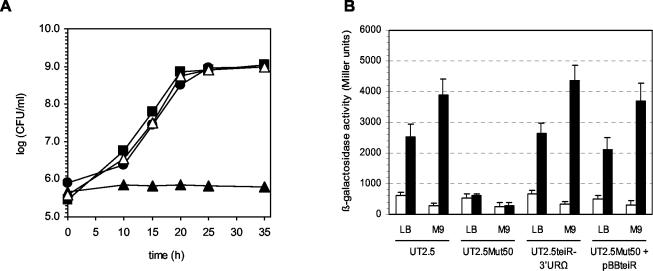
To investigate whether the phenotype observed in the C. testosteroni UT2.5Mut50 strain was due to a polar mutation caused by teiR disruption, we inserted a Sp cassette after the coding sequence of this gene. A new C. testosteroni UT2.5 mutant strain was constructed by insertion of the Sp interposon into the NdeI restriction site located 50 bp downstream of a teiR stop codon. Southern blot analysis of the selected mutant strain (C. testosteroni UT2.5teiR-3′URΩ) allowed us to confirm that the wild-type sequence was completely replaced by the interposon-disrupted construct (data not shown). The UT2.5teiR-3′URΩ mutant strain grew on LB medium or M9 minimal medium plus acetate at the same growth rate (70 min) as C. testosteroni UT2.5. Moreover, the C. testosteroni UT2.5teiR-3′URΩ strain was able to grow on M9 minimal medium supplemented with testosterone as the sole carbon source (Fig. 7A), indicating that no genes required for growth on testosterone are 3′ of teiR and cotranscribed with teiR. In addition, the level of sip48-β-HSD gene promoter activity (measured as the level of β-galactosidase activity in C. testosteroni UT2.5teiR-3′URΩ grown in testosterone-containing medium) was equal to that of the control strain (UT2.5) (Fig. 7B). Thus, no genes required for testosterone-inducible gene expression are located downstream of teiR coding sequence and cotranscribed with it.
FIG. 7.
Growth and sip48-β-HSD gene promoter activity in C. testosteroni UT2.5, UT2.5Mut50, and UT2.5teiR-3′URΩ strains and in the UT2.5Mut50 strain complemented with teiR. (A) Growth of C. testosteroni UT2.5 (black circle), UT2.5teiR-3′URΩ (black square), and UT2.5Mut50 (black triangle) strains and of the UT2.5Mut50 strain complemented with pBBteiR (white triangle). Growth of bacteria in LB medium or M9 plus acetate was monitored by measuring OD600. Growth of bacteria in M9 minimal medium plus testosterone was monitored by counting colonies (in CFU per milliliter) that appeared on LB plates (on which appropriately diluted cultures have been spread) after incubation at 30°C. (B) β-Galactosidase activity levels of sip48-β-HSD gene promoter-lacZ transcriptional fusion in C. testosteroni strains UT2.5, UT2.5Mut50, UT2.5teiR-3′URΩ, and UT2.5Mut50 complemented with pBBteiR (UT2.5Mut50 + pBBteiR) growing in LB medium and in M9 minimal medium supplemented with acetate (M9) in the presence (black bars) and the absence (white bars) of testosterone. β-Galactosidase activities were measured with permeabilized cells as described in Materials and Methods. Each value is the average of the results from three independent experiments (error bars indicate standard deviations).
Genetic complementation of teiR mutant.
To demonstrate that teiR encodes a protein involved in the testosterone-inducible expression of sip48-β-HSD genes and to confirm that this gene is essential for growth on testosterone as the sole carbon source, we performed a complementation assay. The aim of this experiment was to analyze the expression of the reporter lacZ fusion in C. testosteroni UT2.5Mut50 after trans-complementation with teiR and to ascertain whether the complemented strain regains the growing phenotype of the C. testosteroni UT2.5 parental strain. The complementation procedure was performed with pBBteiR plasmid containing the teiR gene. As expected, the testosterone-inducible sip48-β-HSD gene promoter activity was restored in the complemented UT2.5Mut50 strain. The β-galactosidase levels determined when the cells were growing in the presence of testosterone were similar to those of the isogenic UT2.5 strain (Fig. 7B). In addition, the complemented bacterium was able to grow when testosterone was used as the sole carbon source (Fig. 7A). In both experiments, the behavior of a complemented strain carrying pBBR1MSC2 without an insert was indistinguishable from that of C. testosteroni UT2.5Mut50 (data not shown). Northern blot assays performed with the complemented strain also demonstrated steroid-inducible expression of the sip48-β-HSD gene, the α-HSD-KSI gene, and the Δ1-DH-Δ4-DH genes (data not shown). Altogether, these data confirm that teiR is essential for the steroid-inducible transcription of different steroid-inducible genes and for steroid metabolism in C. testosteroni.
DISCUSSION
In this work, we report the identification and characterization of teiR, a novel steroid-inducible gene that is necessary for testosterone degradation in C. testosteroni strains. The C-terminal domain of TeiR has a high level of similarity to the LuxR DNA binding domain (belonging to the family of LuxR-type transcription regulators). It has been established by genetic analyses that LuxR is composed of two functional modules or domains: an amino-terminal domain with an autoinductor binding region and a carboxy-terminal transcription regulatory domain (28). LuxR-like proteins bind autoinducers that have achieved a critical threshold concentration, after which the LuxR-autoinducer complexes generally activate gene transcription. Two interesting features among LuxR-type transcriptional regulators are the high level of homology in the DNA binding domain and the variability in the sensor domain (17). In this regard, the TeiR C-terminal domain shows high-level homology to the helix-turn-helix DNA binding motif present in LuxR and other related transcriptional regulators. In addition to the LuxR helix-turn-helix DNA binding domain (residues 327 to 380), a PAS sensor domain located between amino acids 192 and 227 was observed. This domain distribution resembles that described for different LuxR-type proteins; it is particularly similar to that of TraR, a specific transcriptional regulator involved in quorum sensing in Agrobacterium tumefaciens (whose three-dimensional structure has been recently described) (41).
It has been suggested that quorum sensing is an integral component of gene regulatory networks in gram-negative bacteria (43). Particularly, C. testosteroni teiR-disrupted strains are unable to induce the expression of several steroid-inducible genes, such as those encoding a steroid-inducible protein of 48 kDa, β-HSD, α-HSD, Δ5-KSI, Δ1-DH, and Δ4-DH enzymes. The absence of these proteins probably determines the complete impairment of the C. testosteroni teiR mutant strain with respect to the use of different steroid compounds as a sole carbon and energy source. The results of the complementation assay and the transcriptional interruption downstream of the teiR coding sequence clearly demonstrate the importance of this gene for the observed phenotypes. Taken together, these results suggest that TeiR is a global regulator of the steroid catabolic pathway in C. testosteroni.
One or more regulatory proteins often control the expression of bacterial catabolic pathways for aromatic compounds, and the effectors of these regulatory proteins are usually either the initial substrates or catabolic intermediates of the pathways (39). It has been reported in particular that the regulation of α-HSD gene expression appears to be a derepression mechanism in which the steroidal inducer (testosterone) prevents the binding of two repressor proteins (RepA and RepB) to the α-HSD gene promoter and mRNA, respectively (45). The complete lack of activation of α-HSD gene steroid-inducible transcription when the teiR-disrupted mutant was grown in the presence of testosterone indicates that this steroid is not the true inducer of this pathway, suggesting that the mechanisms regulating α-HSD gene expression might be more complex than previously reported. In agreement with our results, the data obtained by Horinouchi et al. (20) suggest that expression of tesB (encoding a meta-cleavage steroid-inducible enzyme) in C. testosteroni TA441 is induced by an intermediate compound produced in the course of testosterone degradation.
In conclusion, mutation of teiR is sufficient to block the ability of C. testosteroni to use testosterone as a sole carbon source, indicating that TeiR is an integral part of the degradation portion of the steroid catabolic pathway. Furthermore, teiR encoding a LuxR-type transcription factor is required for the expression of several steroid-inducible genes, suggesting that a quorum-sensing mechanism might be involved in its regulation.
Acknowledgments
We are grateful to Victor de Lorenzo for kindly providing pUTminiTn5 (Tcr), to Athan Kuliopolus for providing the pAK1370 plasmid encoding the C-terminal sequences of the 3α-HSD and Δ5--KSI genes, and to Patrick Plessiat for providing the pTEK21 plasmid encoding Δ1-DH and the N end of Δ4-DH. We are grateful to Luis Patrito, Alfredo Flury, and Graciela Panzetta-Dutari for discussions and critical reading of the manuscript.
This work was supported by grants from the Consejo Nacional de Ciencia y Tecnología (CONICET) and the Secretaría de Ciencia y Tecnología de Universidad Nacional de Córdoba (SECyT). J.L.P.-P. was supported by a fellowship from the SECyT.
REFERENCES
- 1.Abalain, J., S. Di Stefano, M. L. Abalain-Colloc, and H. H. Floch. 1995. Cloning, sequencing and expression of Pseudomonas testosteroni gene encoding 3α-hydroxysteroid dehydrogenase. J. Steroid Biochem. Mol. Biol. 55:233-238. [DOI] [PubMed] [Google Scholar]
- 2.Abalain, J. H., S. Di Stéfano, Y. Amet, E. Quemeneur, M. L. Abalain-Colloc, and H. H. Floch. 1993. Cloning, DNA sequencing and expression of (3-17)-β hydroxysteroid dehydrogenase from Pseudomonas testosteroni. J. Steroid Biochem. Mol. Biol. 44:133-139. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad, D., R. Masse, and M. Sylvestre. 1990. Cloning and expression of genes involved in 4-chlorobiphenyl transformation by Pseudomonas testosteroni, homology to polychlorobiphenyl-degrading genes in other bacteria. Gene 86:53-61. [DOI] [PubMed] [Google Scholar]
- 4.Altschul, S. F., W. Gish, E. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 5.Arai, H., S. Akahira, T. Ohishi, and T. Kudo. 1999. Adaptation of Comamonas testosteroni TA441 to utilization of phenol by spontaneous mutation of the gene for a trans acting factor. Mol. Microbiol. 33:1132-1140. [DOI] [PubMed] [Google Scholar]
- 6.Arai, H., T. Yamamoto, T. Ohishi, T. Shimizu, T. Nakata, and T. Kudo. 1999. Genetic organization and characteristics of the 3-(3-hydroxyphenyl)propionic acid degradation pathway of Comamonas testosteroni T441. Microbiology 145:2813-2820. [DOI] [PubMed] [Google Scholar]
- 7.Bortone, S. A., and R. P. Cody. 1999. Morphological masculinization in poeciliid females from a paper mill effluent receiving tributary of the St. Johns River, Florida, USA. Bull. Environ. Contam. Toxicol. 63:150-156. [DOI] [PubMed] [Google Scholar]
- 8.Cabrera, J. E., G. Panzetta-Dutari, J. L. Pruneda, and S. Genti-Raimondi. 1997. A new Comamonas testosteroni steroid-inducible gene: cloning and sequence analysis. J. Steroid Biochem. Mol. Biol. 63:91-98. [DOI] [PubMed] [Google Scholar]
- 9.Cabrera, J. E., J. L. Pruneda Paz, and S. Genti-Raimondi. 2000. Steroid-inducible transcription of the 3β/17β-hydroxysteroid dehydrogenase gene (3β/17β-hsd) in Comamonas testosteroni. J. Steroid Biochem. Mol. Biol. 73:147-152. [DOI] [PubMed] [Google Scholar]
- 10.Cooper, R. L., and R. J. Kavlock. 1997. Endocrine disruptors and reproductive development: a weight-of-evidence overview. J. Endocrinol. 152:159-166. [DOI] [PubMed] [Google Scholar]
- 11.Coulter, A. W., and P. Talalay. 1968. Studies on the microbiological degradation of steroid ring A. J. Biol. Chem. 243:3238-3247. [PubMed] [Google Scholar]
- 12.Choi, K. Y., and W. F. Benisek. 1988. Nucleotide sequence of the gene for the Δ5-3-ketosteroid isomerase of Pseudomonas testosteroni. Gene 69:121-129. [DOI] [PubMed] [Google Scholar]
- 13.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5 and Tn10-derived mini-transposons. Methods Enzymol. 235:386-405. [DOI] [PubMed] [Google Scholar]
- 14.Feinberg, A. P., and B. Vogelstein. 1983. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 132:6-13. [DOI] [PubMed] [Google Scholar]
- 15.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Florin, C., T. Köhler, M. Grandguillot, and P. Plesiat. 1996. Comamonas testosteroni 3-ketosteroid-Δ4 (5α)-dehydrogenase: gene and protein characterization. J. Bacteriol. 178:3322-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuqua, C., M. R. Parsek, and E. P Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 18.Genti-Raimondi, S., M. Tolmasky, L. Patrito, A. Flury, and L. Actis. 1991. Molecular cloning and expression of the β-hydroxysteroid dehydrogenase gene from Pseudomonas testosteroni. Gene 105:43-49. [DOI] [PubMed] [Google Scholar]
- 19.Goyal, A. K., and G. J. Zylstra. 1996. Molecular cloning of novel genes for polycyclic aromatic hydrocarbon degradation from Comamonas testosteroni GZ39. Appl. Environ. Microbiol. 62:30-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horinouchi, M., T. Yamamoto, K. Taguchi, H. Arai, and T. Kudo. 2001. Meta-cleavage enzyme gene tesB is necessary for testosterone degradation in Comamonas testosteroni TA441. Microbiology 147:3367-3375. [DOI] [PubMed] [Google Scholar]
- 21.Horinouchi, M., T. Hayashi, H. Koshino, T. Yamamoto, and T. Kudo. 2003. Gene encoding the hydrolase for the product of the meta-cleavage reaction in testosterone degradation by Comamonas testosteroni. Appl. Environ. Microbiol. 69:2139-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins, R., R. A. Angus, H. McNatt, W. M. Howell, J. A. Kemppainen, M. Kirk, and E. M. Wilson. 2001. Identification of androstenedione in a river containing paper mill effluent. Environ. Toxicol. Chem. 20:1325-1331. [DOI] [PubMed] [Google Scholar]
- 23.Knauff, V. C., and E. W. Nester. 1982. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid 8:45-54. [DOI] [PubMed] [Google Scholar]
- 24.Kovach, M. E., P. H. Elzer, D. Steven Hill, G. T. Robertson, M. A. Farris, R. Martin Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176 [DOI] [PubMed] [Google Scholar]
- 25.Kuliopulos, A., D. Shortle, and P. Talalay. 1987. Isolation and sequencing of the gene encoding Δ5-3-ketosteroid isomerase of Pseudomonas testosteroni: overexpression of the protein. Proc. Natl. Acad. Sci. USA 84:8893-8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcus, P. I., and P. Talalay. 1956. Induction and purification of α- and β-hydroxysteroid dehydrogenases. J. Biol. Chem. 218:661-674. [PubMed] [Google Scholar]
- 27.Miller, J. H. 1972. Experiments in molecular genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 29.Möbus, E., and E. Maser. 1998. Molecular cloning, overexpression and characterization of steroid-inducible 3α-hydroxysteroid dehydrogenase/carbonyl reductase from Comamonas testosteroni. A novel member of the short-chain dehydrogenase/reductase superfamily. J. Biol. Chem. 273:30888-30896. [DOI] [PubMed] [Google Scholar]
- 30.Morgan, C. A., and R. C. Wyndam. 1996. Isolation and characterization of resin acid degrading bacteria found in effluent from a bleached kraft pulp mill. Can. J. Microbiol. 42:423-430. [DOI] [PubMed] [Google Scholar]
- 31.Munkittric, K. R., M. E. McMaster, L. H. McCarthy, M. R. Servos, and G. H. Van der Kraak. 1998. An overview of recent studies on the potential of pulp-mill effluents to alter reproductive parameters in fish. J. Toxicol. Environ. Health Part B Crit. Rev. 1:347-371. [DOI] [PubMed] [Google Scholar]
- 32.Plesiat, P. M., S. Grandguillot, S. Harayama, S. Vragar, and Y. Michel-Briand. 1991. Cloning, sequencing and expression of the Pseudomonas testosteroni encoding 3-oxosteroid Δ1-dehydrogenase. J. Bacteriol. 173:7219-7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 35.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skowasch, D., E. Möbus, and E. Maser. 2002. Identification of a novel Comamonas testosteroni gene encoding a steroid-inducible extradiol dehydrogenase. Biochem. Biophys. Res. Commun. 294:560-566. [DOI] [PubMed] [Google Scholar]
- 37.Taguchi, K., M. Motoyama, and T. Kudo. 2001. PCB/biphenyl degradation gene cluster in Rhodococcus rhodochrous K37, is different from the well-known bph gene clusters in Rhodococcus sp. P6, RHA1, and TA421. Riken Rev. 42:23-26. [Google Scholar]
- 38.Talalay, P., M. Dobson, and D. F. Tapley. 1952. Oxidative degradation of testosterone by adaptative enzymes. Nature 170:620-621. [DOI] [PubMed] [Google Scholar]
- 39.Teramoto, M., S. Harayama, and K. Watanabe. 2001. PhcS Represses gratuitous expression of phenol-metabolizing enzymes in Comamonas testosteroni R5. J. Bacteriol. 183:4227-4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acid Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vannini, A., C. Volpari, C. Gargioli, E. Muraglia, R. Cortese, R. De Francesco, P. Neddermann, and S. Di Marco. 2002. The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J. 21:4393-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe, M., D. Lefebvre, Y. Lefebvre, and L. Po. 1980. Membrane-bound dehydrogenases of Pseudomonas testosteroni. J. Steroid Biochem. 13:821-827. [DOI] [PubMed] [Google Scholar]
- 43.Whiters, H., S. Swift, and P. Williams. 2001. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Curr. Opin. Microbiol. 4:186-193. [DOI] [PubMed] [Google Scholar]
- 44.Xiong, G., and E. Maser. 2001. Regulation of the steroid-inducible 3α-hydroxysteroid dehydrogenase/carbonyl reductase gene in Comamonas testosteroni J. Biol. Chem. 276:9961-9970. [DOI] [PubMed] [Google Scholar]
- 45.Xiong, G., H. J. Martin, A. Blum, C. Schäfers, and E. Maser. 2003. A model on the regulation of 3α-hydroxysteroid dehydrogenase/carbonyl reductase expression in Comamonas testosteroni. Chem. Biol. Interact. 130:723-726. [DOI] [PubMed] [Google Scholar]