Abstract
Background
Artificial and bioartificial liver support systems may 'bridge' patients with acute or acute‐on‐chronic liver failure to liver transplantation or recovery.
Objectives
To evaluate beneficial and harmful effects of artificial and bioartificial support systems for acute and acute‐on‐chronic liver failure.
Search methods
Trials were identified through the Cochrane Hepato‐Biliary Group Controlled Trials Register (September 2002), The Cochrane Library (Issue 3, 2002), MEDLINE (1966 to September 2002), EMBASE (1985 to September 2002), and The Chinese Biomedical Database (September 2002), manual searches of bibliographies and journals, authors of trials, and pharmaceutical companies.
Selection criteria
Randomised clinical trials on artificial or bioartificial support systems for acute or acute on‐chronic liver failure were included irrespective of blinding, publication status, or language. Non‐randomised studies were included in explorative analyses.
Data collection and analysis
Data were extracted independently by three authors. Results were presented as relative risks (RR) with 95% confidence intervals (CI). Sources of heterogeneity were explored through sensitivity analyses and meta‐regression. The primary outcome was mortality.
Main results
Twelve trials on artificial or bioartificial support systems versus standard medical therapy (483 patients) and two trials comparing different artificial support systems (105 patients) were included. Most trials had unclear methodological quality. Compared to standard medical therapy, support systems had no significant effect on mortality (RR 0.86; 95% CI 0.65 to 1.12) or bridging to liver transplantation (RR 0.87; 95% CI 0.73 to 1.05), but a significant beneficial effect on hepatic encephalopathy (RR 0.67; 95% CI 0.52 to 0.86). Meta‐regression indicated that the effect of support systems depended on the type of liver failure (P = 0.03). In subgroup analyses, artificial support systems appeared to reduce mortality by 33% in acute‐on‐chronic liver failure (RR 0.67; 95% CI 0.51 to 0.90), but not in acute liver failure (RR 0.95; 95% CI 0.71 to 1.29). Two trials comparing artificial support systems showed significant mortality reductions with intermittent versus continuous haemofiltration (RR 0.58; 95% CI 0.36 to 0.94) and no significant difference between five versus ten hours of charcoal haemoperfusion (RR 1.03; 95% CI 0.65 to 1.62). The incidence of adverse events was inconsistently reported.
Authors' conclusions
This Review indicates that artificial support systems may reduce mortality in acute‐on‐chronic liver failure. Artificial and bioartificial support systems did not appear to affect mortality in acute liver failure. However, considering the strength of the evidence additional randomised clinical trials are needed before any support system can be recommended for routine use.
Keywords: Humans; Liver, Artificial; Chronic Disease; Hemofiltration; Hepatic Encephalopathy; Hepatic Encephalopathy/therapy; Liver Failure, Acute; Liver Failure, Acute/therapy; Randomized Controlled Trials as Topic
Plain language summary
The benefit of artificial and bioartificial liver support systems looks promising
Liver failure may develop without pre‐existing liver disease (acute liver failure) or with pre‐existing liver disease (acute‐on‐chronic liver failure). Both disorders are serious and may lead to death. Artificial and bioartificial support systems may 'bridge' patients to liver transplantation or spontaneous recovery. Evidence from twelve small randomised trials were combined. Most trials had unclear methodologic quality. Overall, support systems did not appear to affect mortality or bridging to transplantation, but had a beneficial effect on hepatic encephalopathy. The risk of adverse events could not be established. Further evidence is needed before support systems can be recommended for routine use.
Background
Liver failure is characterised by the development of hepatic encephalopathy, jaundice, and coagulopathy (Rahman 1999). The disorder may rapidly progress to multiorgan failure (Rahman 1999; Riordan 1999). Liver failure can occur without underlying liver disease precipitated by, e.g., viral hepatitis, drugs, toxins, or cardiac related hepatic ischaemia (Lee 1993). Acute liver failure is defined as hyperacute if hepatic encephalopathy develops within ten days of the onset of jaundice and fulminant when the range is ten to 30 days (Tandon 1999). Few patients develop subacute liver failure occurring after 30 days of illness (Tandon 1999). Liver failure can also occur in chronic liver disease precipitated by metabolic stress, e.g., bleeding or infections (acute‐on‐chronic liver failure) or because of slowly progressing end‐stage liver disease (Sorkine 2001; Allen 2001).
Liver transplantation cures approximately 90 per cent of patients from a terminal or pre‐terminal state of liver failure (Russell 1999; Allen 2001). However, the procedure is expensive and there is a serious shortfall of donors (Bonn 1996; Neuberger 1998). This partly explains why mortality rates associated with acute liver failure still approach 80 per cent (Lee 1993). Artificial and bioartificial support systems may serve as a 'bridge' to liver transplantation or facilitate recovery from acute or acute‐on‐chronic liver failure (Riordan 1999; Allen 2001).
The role of artificial liver support encompass removal of toxins (e.g., aromatic amino acids and ammonia), synthesis of products (e.g., coagulation factors and albumin), and reversal of the inflammatory process in the liver (Rahman 1999). The early support systems used haemodialysis, haemofiltration, and haemoperfusion. These systems were strictly artificial and directed against removal of water‐soluble and protein‐bound toxins (Rahman 1999; Riordan 1999; Allen 2001). Haemodialysis requires a semipermeable dialysis membrane through which fluid and small solutes pass via diffusion (Davenport 1991; Rahman 1999). Haemoperfusion removes toxins and low to middle molecular weight molecules by using different adsorbents, e.g., charcoal (O'Grady 1988). The more recently developed artificial systems use haemodiabsorption, which combines haemodialysis with adsorption using charcoal or albumin. These systems include the BioLogic‐DT (Ash 1992; Ash 1994; Hughes 1994a; Ash 1994; Wilkinson 1998b; Ellis 1999; Ash 2000) and the Molecular Adsorbent Recirculating System also known as MARS (Stange 1995; Stange 1999a; Mitzner 2000a; Stange 2000b; Mitzner 2001; Mitzner 2001b; Mitzner 2001c).
Advanced technology has now made it possible to include biological components in support systems. These bioartificial support systems utilise living hepatocytes housed within a bioreactor through which the patient's blood or plasma is pumped in an extracorporeal circuit (Ellis 1996a; Hughes 1996; Miwa 1996; Sussman 1997; Hughes 1998b; Eguchi 2000). The hepatocytes may be xenogenic or human. Two such systems include the bioartificial liver device (BAL) containing pig hepatocytes (Hughes 1998b; Stockmann 2000) and the extracorporeal liver assist device (ELAD) that uses cells from a well‐differentiated hepatoblastoma (Ellis 1996a).
Objectives
To assess the beneficial and harmful effects of artificial and bioartificial liver support systems for acute and acute‐on‐chronic liver failure.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials were included irrespective of blinding, publication status, or language. Quasi‐randomised trials, prospective observational studies, and observational studies with a historical control group were excluded from the primary analyses, but were evaluated in exploratory sensitivity analyses.
Types of participants
Patients with acute or acute‐on‐chronic liver failure irrespective of aetiology or stage of hepatic encephalopathy were included. Liver failure was defined as the development of hepatic encephalopathy, jaundice, and coagulopathy. Acute and acute‐on‐chronic liver diseases were defined as symptoms developing without or with pre‐existing liver disease, respectively. Trials reporting that the included patients had acute or acute‐on‐chronic liver failure were included regardless of how this diagnosis was made.
Types of interventions
Randomised comparisons of artificial and bioartificial liver support systems versus standard medical therapy or different support systems were included irrespective of treatment duration. Co‐interventions (including treatment of complications) were allowed if administered equally to all intervention arms.
Types of outcome measures
All outcomes were assessed at maximum follow‐up. The primary outcome measure was all‐cause mortality. The secondary outcome measures were bridging to liver transplantation (number of patients not improved to allow transplantation), number of patients without improvement of hepatic encephalopathy (i.e., steady state or worsening), adverse events, and health economics (e.g., number of days in hospital). Serious adverse events were defined as any untoward medical occurrence that resulted in death, was life‐threatening, resulted in persistent or significant disability, or any important medical event which may have jeopardised the patient or required intervention to prevent it (ICH‐GCP 1997). All other adverse events were considered non‐serious.
Search methods for identification of studies
The Cochrane Hepato‐Biliary Group Controlled Trials Register (September 2002), the Cochrane Controlled Trials Register on The Cochrane Library Issue 3, 2002, MEDLINE (1966 to September 2002), EMBASE (1985 to September 2002), and The Chinese Medical Database (September 2002) were searched using the following MeSH terms: artificial liver or liver failure or (rand* or controlled [all fields]) (See Appendix 1). The reference lists of relevant articles were scanned. Correspondence with authors of identified trials and pharmaceutical companies involved in the development or production of support systems.
Data collection and analysis
Three authors (JL, LLK, and BAN) independently selected the trials to be included and performed the data extraction. Disagreements were resolved through discussion or through the fourth author (CG). Papers not in Chinese, Danish, English, French, German, or Spanish were translated with the help of The Cochrane Hepato‐Biliary Group. Data on all patients, irrespective of compliance or follow‐up, were sought to allow intention‐to‐treat analyses. Authors were contacted if the necessary data were not available in the published reports. Data on included trials are listed under 'Characteristics of included studies' and ongoing trials are listed under 'Ongoing studies'. Excluded studies are listed under 'Characteristics of excluded studies' with the reason for exclusion. Quasi‐randomised trials and non‐randomised studies included in the explorative sensitivity analyses are listed in Table 1.
1. Non‐randomised studies.
| Study ID | Methods | Participants | Interventions | Outcomes | Notes |
| Bion 1993 | ‐ Design: Prospective observational study with parallel control group. | ‐ Sample size experimental group: 6 patients. ‐ Sample size control group: 4 patients. ‐ Number of patients with missing data: none reported. ‐ Inclusion criteria: fulminant liver failure with grade III or IV encephalopathy. ‐ Exclusion criteria: not reported. ‐ Aetiology: paracetamol (50%), viral hepatitis (40%), and alcohol (10%). ‐ Mean age: 36 years. ‐ Proportion of males: 60%. | ‐ Experimental group: continuous arteriovenous haemofiltration. ‐ Control group: standard medical therapy. | ‐ Outcomes: mortality and bridging to liver transplantation. ‐ Length of follow up: until death or recovery. | ‐ Country: United Kingdom. ‐ Publication status: full paper article. |
| Davenport 1989 | ‐ Design: Quasi‐randomised trial. | ‐ Sample size experimental group: 4 patients. ‐ Sample size control group: 3 patients. ‐ Number of patients with missing data: none reported. ‐ Inclusion criteria: fulminant liver hepatorenal failure and grade IV encephalopathy. ‐ Exclusion criteria: not reported. ‐ Aetiology: paracetamol (72%), viral hepatitis (14%), and unidentified (14%). ‐ Age: range 18 to 49 years. ‐ Proportion of males: 57%. | ‐ Experimental group: continuous arteriovenous haemofiltration. ‐ Control group: daily machine haemofiltration. | ‐ Outcomes: mortality, bridging to liver transplantation and adverse events. ‐ Length of follow up: until death or improvement of mean arterial blood pressure. | ‐ Country: United Kingdom. ‐ Publication status: full paper article. |
| Denis 1978 | ‐ Design: Case series with historical controls. | ‐ Sample size experimental group: 41 patients. ‐ Sample size control group: 117 patients. ‐ Number of patients with missing data: none reported. ‐ Inclusion criteria: fulminant liver failure and hepatic encephalopathy. ‐ Exclusion criteria: not reported. ‐ Aetiology (of patients in treatment group): viral hepatitis B (80%), unknown (17%), and drugs (3%). ‐ Mean age: 39 years. ‐ Proportion of males: 46%. | ‐ Experimental group: haemodialysis. ‐ Control group: standard medical therapy. | ‐ Outcomes: mortality, hepatic encephalopathy, and adverse events. ‐ Length of follow up: until death or recovery of consciousness. | ‐ Country: France. ‐ Publication status: full paper article. |
| Gazzard 1974 | ‐ Design: Case series with historical controls. | ‐ Sample size experimental group: 22 patients. ‐ Sample size control group: 92 patients. ‐ Number of patients with missing data. ‐ Inclusion criteria: fulminant liver failure with grade IV encephalopathy. ‐ Exclusion criteria: not reported. ‐ Aetiology (of the 22 patients in the treatment group): viral hepatitis (45%) and drugs (55%). ‐ Mean age: 30 years. ‐ Males: not reported. | ‐ Experimental group: haemoperfusion with activated charcoal. ‐ Control group: standard medical therapy. | ‐ Outcomes: mortality, hepatic encephalopathy, and adverse events. ‐ Length of follow up: until death or recovery of consciousness. | ‐ Country: United Kingdom. ‐ Publication status: full paper article. |
| Gimson 1982 | ‐ Design: Case series with historical controls. | ‐ Sample size experimental group: 76 patients. ‐ Sample size control group: 53 patients. ‐ Number of patients with missing data: none reported ‐ Inclusion criteria: fulminant liver failure with grade III to IV encephalopathy. ‐ Exclusion criteria: not reported. ‐ Aetiology: drugs (82%) and viral hepatitis (18%). ‐ Mean age: 31 years. ‐ Males: 32%. | ‐ Experimental group: haemoperfusion with activated charcoal. ‐ Control group: standard medical therapy. | ‐ Outcomes: mortality, hepatic encephalopathy, and adverse events. ‐ Length of follow up: until death or recovery of consciousness. | ‐ Country: United Kingdom. ‐ Publication status: full paper article. |
| Jiang 2000 | ‐ Design: Prospective observational study with parallel control group. | ‐ Sample size experimental group: 51 patients. ‐ Sample size control group: 39 patients. ‐ Number of patients with missing data: none reported. ‐ Inclusion criteria: liver failure (with encephalopathy) due to severe hepatitis. ‐ Exclusion criteria: not reported. ‐ Aetiology (of the 51 patients in the treatment group): viral hepatitis (92%) and alcoholic hepatitis (8%). ‐ Mean age: 39 years. ‐ Males: 100%. | ‐ Experimental group: plasmapheresis, haemodialysis, and haemoperfusion. ‐ Control group: standard medical therapy. | ‐ Outcomes: mortality, encephalopathy, and adverse events. ‐ Length of follow up: until death, improvement of consciousness, or discharge. | ‐ Country: China. ‐ Publication status: full paper article. |
| Li 1997 | ‐ Design: Prospective observational study with parallel control group. | ‐ Sample size experimental group: 45 patients. ‐ Sample size control group: 41 patients. ‐ Number of patients with missing data: none reported. ‐ Inclusion criteria: severe viral hepatitis with acute or acute‐on‐chronic liver failure. ‐ Exclusion criteria: not reported. ‐ Aetiology: viral hepatitis (100%). ‐ Age: range 20 to 72 years. ‐ Proportion of males: 82%. | ‐ Experimental group: plasmapheresis, haemodialysis, haemofiltration, plasma adsorption, and haemoperfusion. ‐ Control group: standard medical therapy. | ‐ Outcomes: mortality, hepatic encephalopathy, and adverse events. ‐ Length of follow up: until death, recovery of consciousness, or discharge. | ‐ Country: China. ‐ Publication status: full paper article. |
| Margulis 1989 | ‐ Design: Prospective observational study with parallel control group. | ‐ Sample size experimental group: 59 patients. ‐ Sample size control group: 67 patients. ‐ Number of patients with missing data: none reported. ‐ Inclusion criteria: acute or acute‐on‐chronic liver failure. ‐ Exclusion criteria: not reported. ‐ Aetiology: not reported for all patients. ‐ Age: range 18 to 85 years. ‐ Males: not reported. | ‐ Experimental group: haemoperfusion using a suspension of active pig hepatocytes and charcoal. ‐ Control group: standard medical therapy. | ‐ Outcomes: mortality, hepatic encephalopathy, and adverse events. ‐ Length of follow up: until death or recovery of consciousness. | ‐ Country: Russia. ‐ Publication status: full paper article. |
| Silk 1978 | ‐ Design: Prospective observational study with parallel intervention group. | ‐ Sample size experimental group: 136 patients. ‐ Sample size control group: 53 patients. ‐ Number of patients with missing data: none reported. ‐ Inclusion criteria: fulminant liver failure with grade IV encephalopathy. ‐ Exclusion criteria: not reported. ‐ Aetiology: paracetamol (42%), hepatitis (42%), and 'others' (16%). ‐ Mean age: not reported. ‐ Males: not reported. | ‐ Experimental group: charcoal haemoperfusion. ‐ Control group: standard medical therapy. | ‐ Outcomes assessed: mortality, encephalopathy, and adverse events. ‐ Length of follow up: to recovery. | ‐ Country: United Kingdom. ‐ Publication status: full paper article. |
The randomisation methods, blinding, and follow‐up were extracted as a measure of methodological quality (Schulz 1995; Moher 1998; Kjaergard 2001). The following definitions were used:
Generation of the allocation sequence: adequate (table of random numbers, computer generated random numbers, or similar) or unclear (not reported). Trials with inadequate generation of the allocation sequence (i.e., quasi‐randomised trials) were excluded from the primary analyses.
Allocation concealment: adequate (central randomisation, serially numbered opaque sealed envelopes, or similar), unclear (not reported), or inadequate (open table or similar).
Blinding: whether blinded outcome assessment was used. Considering the nature of the intervention, we did not assess double‐blinding although lack of double blinding may lead to significantly exaggerated estimates of intervention benefits (Schulz 1995; Kjaergard 2001; Juni 2001).
The extracted data included characteristics of trials, patients, interventions, and outcome measures. Trial characteristics were the country of origin, methodological quality, sample size calculations, and study setting. Patient characteristics were inclusion and exclusion criteria, mean age of included patients, proportion of men, aetiology, type of liver failure, precipitating factors of acute‐on‐chronic liver failure, number of patients, and number and reasons for dropouts and withdrawals. The type of support system, number of times and hours the system was used, and administration of co‐interventions were also extracted.
The statistical package (RevMan version 4.0) provided by The Cochrane Collaboration and STATA version 6.0 for Windows were used for the statistical analyses. As we anticipated that the trials were heterogeneous, all analyses were performed using random effects models. Dichotomous data were presented as relative risk (RR) and continuous data as weighted mean difference (WMD), both with 95% confidence intervals (CI). Intertrial heterogeneity was estimated by chi‐square tests. The analyses included all randomised patients ('intention‐to‐treat') irrespective of follow up. If outcome data were missing, we used 'carry forward' of the last observed response (Hollis 1999).
The extent to which the patient, intervention, and trial characteristics could explain heterogeneity was explored through simple random effects meta‐regression. The outcome was mortality (log RR). Weights were assigned according to the estimated variance (standard error [SE] to the log RR). The following covariates were entered: type of liver failure, mean age, proportion of men, year of publication, type of support system, methodological quality, and publication status. If the meta‐regression indicated a significant association between covariates and intervention effects, RR and 95% confidence interval (CI) were calculated in stratified meta‐analysis.
The risk of bias was explored through statistical testing of funnel plot asymmetry (Egger 1997). Explorative meta‐regression analyses including non‐randomised studies were also performed.
In a post‐hoc sensitivity analysis, we recalculated our primary meta‐analysis without one trial published several years before the remaining trials. We also performed a post‐hoc 'worst case scenario analysis' in which patients with missing outcome data were considered as treatment failures.
Results
Description of studies
Search results We identified 528 references through the initial searches, including 32 from The Cochrane Hepato‐Biliary Group Controlled Trials Register, 21 from the Cochrane Controlled Trials Register, 158 from MEDLINE, 255 from EMBASE, 45 from BIOSIS, and 17 from The Chinese Biomedical Database. After reading titles and abstracts, 473 of these references were excluded because they were duplicates, non‐clinical studies, or had an objective different from ours. The remaining articles were retrieved for further assessment. Of these, 32 were excluded because they did not meet our inclusion criteria. Three referred to ongoing trials, which we were unable to include because data were unavailable. Of the remaining 23 articles, one referred to a quasi‐randomised trial, eight referred to non‐randomised studies, and 14 referred to randomised trials. These 14 trials fulfilled our inclusion criteria. Two of the trials were published in abstract form (Mazariegos 1997; Stevens 2001). The remaining 12 trials were published as full paper articles. Twelve trials compared artificial or bioartificial support systems versus standard medical therapy (Redeker 1973; O'Grady 1988; Hughes 1994a; Ellis 1996a; Mazariegos 1997; Kramer 1998; Wilkinson 1998; Ellis 1999; Mitzner 2000a; He 2000a; Stevens 2001; Heemann 2001). Two trials compared different regimens of artificial support systems (O'Grady 1988; Davenport 1993). Randomised trials on artificial and bioartificial support systems versus standard medical therapy The included trials compared the following support systems with standard medical therapy:
One trial assessed whole blood exchange (Redeker 1973). In this trial, patients were transfused with freshly drawn whole blood containing heparin through large cannulae, one in each arm. Each transfusion consisted of 6000 ml blood and required between 1.5‐3 hours.
One trial assessed charcoal haemoperfusion (O'Grady 1988). Haemoperfusion removes toxins and low to middle molecular weight molecules. During a ten hour haemoperfusion, two charcoal columns were used and blood flow was maintained at 150 to 200 ml per minute. Heparin was administered as anticoagulant.
One trial assessed plasma exchange in combination with haemoperfusion (He 2000a).
Five trials assessed the BioLogic‐DT (Hughes 1994a; Mazariegos 1997; Kramer 1998; Wilkinson 1998; Ellis 1999). The BioLogic‐DT uses haemodiabsorption, which combines haemodialysis and adsorption. The system consists of a flat bed cellulose membrane for haemodialysis against a suspension of powdered charcoal and cation exchange resin and requires little or no anticoagulation.
Two trials assessed the molecular adsorbent recirculating system (MARS) (Mitzner 2000a; Heemann 2001). MARS uses haemodiabsorption. The system removes albumin‐bound products via a membrane impregnated on both sides with albumin and by the addition of albumin to the dialysate with subsequent recirculation over sorbents. The system is nearly impermeable to proteins but enables the exchange of water‐soluble and protein bound toxins and requires little or no anticoagulation.
One trial assessed the extracorporeal liver assist device (ELAD) (Ellis 1996a). The system uses perfusion of heparin anticoagulated whole blood through a bioreactor containing about 200 g of cells originating from a well‐differentiated hepatoblastoma.
One trial assessed the HepatAssist bioartificial liver support system (Stevens 2001). The system uses a hollow fibre carriage containing porcine hepatocytes coupled with charcoal in a plasma perfused circuit
In all trials, the control groups received standard medical therapy aimed at complications associated with severe liver failure. Standard medical therapy included electrolyte substitution, fluid substitution, antacid therapy, coagulation therapy, and N‐acetylcysteine.
The total number of patients was 483 of whom 354 (73%) had acute liver failure and 129 (27%) had acute‐on chronic liver failure. All trials reported the proportion of men (median 58%, range 33% to 87%) and the mean age of the included patients (median 38 years, range 26 to 53 years). One trial (Heemann 2001) reported the underlying liver disease and precipitating factors in acute‐on‐chronic liver failure. Ten patients died before receiving the experimental or control intervention and two were withdrawn due to adverse events. One trial (Mazariegos 1997) lacked data on four of five patients randomised to standard medical therapy (see Table of included studies). The primary outcome was 30 days survival in two trials. The planned duration of follow‐up was not clear in the remaining trials. Based on the reported survival data, the median duration of follow‐up was 28 days (range 0 to 33 days). All trials were performed at intensive care units.
Randomised trials comparing different artificial support systems Two trials compared different regimens of artificial liver support (O'Grady 1988; Davenport 1993). The total number of patients was 105. One trial compared intermittent versus continuous haemofiltration with or without dialysis (Davenport 1993). The trial included 22 patients with acute liver failure. The mean age was 33 years and 50% were male. One trial compared five versus ten hours of haemoperfusion (O'Grady 1988). The trial included 75 patients with fulminant liver failure. The mean age was 38 years and 50% were male.
Non‐randomised studies Eight non‐randomised studies compared liver support systems to standard medical therapy and one quasi‐randomised trial compared different support systems. These studies were included for the exploratory sensitivity analyses (Table 1).
Five of the observational studies were prospective with a parallel control group and included 501 patients. These studies compared artificial support systems (Silk 1978; Bion 1993; Li 1997; Jiang 2000) or bioartificial support systems containing pig hepatocytes (Margulis 1989) to standard medical therapy. Two studies included patients with acute liver failure (Silk 1978; Bion 1993) and three studies included patients with acute or acute‐on‐chronic liver failure (Margulis 1989; Li 1997; Jiang 2000).
Three observational studies were case series with historical control groups and included 401 patients (Gazzard 1974; Denis 1978; Gimson 1982). These studies compared haemodialysis (Denis 1978) or haemofiltration with activated charcoal (Gazzard 1974; Gimson 1982) to standard medical therapy for acute liver failure.
One study was quasi‐ randomised and compared continuous haemofiltration to daily haemofiltration (Davenport 1989). This trial included seven patients with acute liver failure and grade IV hepatic encephalopathy.
Risk of bias in included studies
Of the 12 trials comparing liver support systems versus standard medical therapy, five trials had adequate allocation sequence generation (O'Grady 1988; Ellis 1996a; Kramer 1998; Mitzner 2000a; Heemann 2001). The allocation concealment was adequate in nine trials (Ellis 1996a; Kramer 1998; Mitzner 2000a; Heemann 2001; Redeker 1973; Hughes 1994a; Mazariegos 1997; Wilkinson 1998; Ellis 1999; O'Grady 1988). The outcome assessment was blinded in one trial (Heemann 2001).
Of the two trials comparing different support systems, one had adequate generation of the allocation sequence and unclear allocation concealment (O'Grady 1988) and one had unclear allocation sequence generation and allocation concealment (Davenport 1993). Neither had blinded outcome assessment.
Effects of interventions
Randomised trials on artificial and bioartificial support systems versus standard medical therapy Mortality Mortality was reported in all 12 trials. The control group mortality rate was 51% (123/239). Overall, support systems did not appear to reduce mortality significantly compared with standard medical therapy (RR 0.86; 95% CI 0.65 to 1.12; n = 12 trials). The intertrial heterogeneity was significant (Chi‐square 19.1 with 10 df; P = 0.04).
In meta‐regression analysis, there was evidence of a significant association between the effect of support systems and the type of liver failure (P = 0.03). In a stratified meta‐analysis, artificial support systems seemed to reduce mortality by 33% in acute‐on‐chronic liver failure (RR 0.67; 95% CI 0.51 to 0.90). Artificial and bioartificial support systems did not seem to have a significant effect on mortality in acute liver failure (RR 0.91; 95% CI 0.71 to 1.29). The meta‐regression analyses showed little evidence of an association between the effect of support systems on mortality and the following covariates: type of support system (P = 0.10), publication year (P = 0.20), mean age of patients (P = 0.06), or proportion of men (P = 0.58).
In a post‐hoc sensitivity analysis, we recalculated the primary meta‐analysis on mortality without one trial, which was published in 1973. After exclusion of this trial, the effect of support systems on mortality approached statistical significance (RR 0.78; 95% CI 0.61 to 1.00). The intertrial heterogeneity became not statistically significant (P = 0.20). We also performed a post‐ hoc 'worst case scenario' analysis in which patients with missing outcome data were considered as treatment failures. In this analysis, support systems did not seem to have a significant effect on mortality (RR 0.82; 95% CI 0.62 to 1.08). The intertrial heterogeneity was statistically significant (P = 0.02).
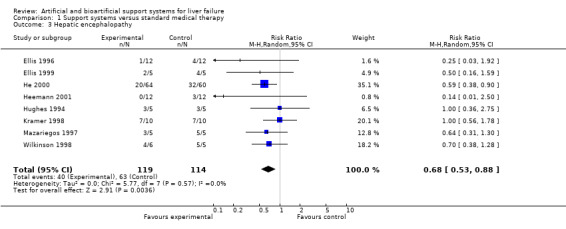
Bridging to transplantation and hepatic encephalopathy We were able to extract data on bridging to liver transplantation from four trials and improvement of hepatic encephalopathy from eight trials. Support systems did not significantly reduce the number of patients without bridging to transplantation (RR 0.87; 95% CI 0.73 to 1.05). Intertrial heterogeneity was not statistically significant (P = 0.54). Support systems reduced the number of patients without improvement of hepatic encephalopathy by 33% (RR 0.67; 95% CI 0.52 to 0.86). Intertrial heterogeneity was not statistically significant (P = 0.57).
Adverse events Support systems were associated with several serious and non‐serious adverse events. The registration of adverse events associated with standard medical therapy was incomplete and we were unable to perform a reliable meta‐analysis of this outcome. The most important adverse event appeared to be bleeding, which was registered as serious in three trials (3/27 patients). Other serious adverse events were systemic infection (5/76 patients; n = 2 trials), disseminated intravascular coagulation (1/10 patients; n = 1 trial), and allergic shock (1/64 patients; n = 1 trial). Non‐serious adverse event included minor bleeding, increase in intracranial pressure, hypotension, and hypersensitivity.
Quality of life and health economics None of the included trials assessed quality of life or health economics.
Randomised trials comparing different artificial support systems Two trials compared different artificial liver support systems. One trial (Davenport 1993) found that intermittent haemofiltration significantly reduced mortality of patients with acute liver failure compared with continuous haemofiltration (RR 0.58; 95% CI 0.36 to 0.94). In the other trial (O'Grady 1988), mortality rates of patients treated with five versus ten hours of charcoal haemoperfusion were not significantly different (RR 1.03; 95% CI 0.65 to 1.62).
Exploratory analysis including non‐randomised studies We performed an explorative analysis in which the eight non‐randomised studies were included. A meta‐regression analysis indicated that the estimated effect of support systems on mortality was significantly different in randomised trials and non‐randomised studies (P = 0.01). In randomised trials, 123/239 patients (51%) allocated to the control group died compared with 130/204 patients (64%) in studies with contemporary controls and 222/262 patients (85%) in studies with historical controls. Accordingly, the method of allocation (randomised or non‐randomised) was associated with control group event rates (P = 0.001). Support systems did not have a significant effect on mortality in randomised trials (RR 0.86; 95% CI 0.65 to 1.12) but appeared to reduce mortality significantly in studies with contemporary (RR 0.72; 95% CI 0.55 to 0.95) or historical controls (RR 0.68; 95% CI 0.58 to 0.80).
Discussion
This review of 12 randomised trials compared the effect of artificial and bioartificial support systems with standard medical therapy for severe liver failure. In the primary meta‐analysis, support systems did not appear to significantly affect mortality. However, there was significant intertrial heterogeneity and meta‐regression analyses indicated that the effect of support systems was associated with the type of liver failure. In a stratified meta‐analysis, artificial support systems reduced mortality by 33% in acute‐on‐chronic liver failure. None of the identified randomised trials assessed the effect of bioartificial support systems for acute‐on‐chronic liver failure. Artificial and bioartificial support systems did not appear to reduce mortality in acute liver failure. However, these subgroup analyses can only be considered as hypothesis generating. Although the evidence seems promising, additional randomised trials are needed before support systems can be recommended for routine use.
The included trials were performed at specialised intensive care units. Transport to these units may be an additional hazard to patients with severe liver disease. This aspect cannot be answered by this review, but should be included in the overall assessment of intervention benefits in future trials in which patients are moved to specialised centres. Another question is whether the intervention is associated with long‐term benefits. Most of the included patients were followed up for about a month. However, the primary purpose of support systems is to bridge patients with severe liver failure to liver transplantation or recovery. Short‐term follow‐up is therefore important.
Mortality in severe liver failure depends on the degree of liver damage and regenerative ability. Support systems may provide a bridge during treatment of bleeding or infections, which are the most common causes of acute‐on‐chronic liver failure. Precipitating factors in acute liver failure include drug toxicity and viral hepatitis, which are difficult to treat. This may explain why our analyses indicated that support systems are effective in acute‐on‐chronic but not in acute liver failure.
We observed a positive effect of support systems on hepatic encephalopathy but not on bridging to liver transplantation. Support systems seemed to be associated with several potentially life‐threatening adverse events. The most frequently reported were bleeding and infections. However, the included patients had severe liver disease, and it may be difficult to estimate whether the treatment or the underlying disease caused the adverse events. Due to incomplete reporting, we were unable to perform a reliable analysis of the occurrence of adverse events. We were also unable to assess the effect on quality of life and health economics. Additional evidence addressing these issues is warranted.
We found that support systems had a significant effect on acute‐on‐chronic, but not acute liver failure. This may reflect the reduced statistical power of the subgroup analyses. However, the findings could also reflect differences in the course and prognosis of the disorder. Acute‐on‐chronic liver failure is the result of stress added to a pre‐existing liver disease (Allen 2001; Sorkine 2001). Liver support systems may provide a bridge during treatment of bleeding or infections, which are the most common causes of acute‐on‐chronic liver failure. Acute liver failure is the result of exogenous stress in a previously healthy liver (Lee 1993). Precipitating factors in acute liver failure include drug toxicity and viral hepatitis, which are difficult to treat.
Charcoal is an effective adsorbent for a range of water‐soluble molecules, but not for ammonia or protein bound compounds. Charcoal haemoperfusion seemed to have no effect on acute liver failure (O'Grady 1988). Several toxins are more effectively removed in more recently developed systems including the BioLogic‐DT (Hughes 1994a; Mazariegos 1997; Kramer 1998; Wilkinson 1998; Ellis 1999) and MARS (Mitzner 2000a; Heemann 2001). This may explain the beneficial effect on hepatic encephalopathy. Our results concur with results published by Ash and colleagues (Mazariegos 1997) who performed a summary of four trials including 37 patients with acute‐on‐chronic liver failure. This summary indicated that the BioLogic‐DT has a significant beneficial effect on physiologic and neurological state, but no effect on clinical outcomes (Mazariegos 1997). On the other hand, hepatic encephalopathy is a soft outcome measure that may be influenced by the convictions of the assessor (Schulz 1995; Kjaergard 2001; Juni 2001). This risk should be considered since only one trial used blinded outcome assessment (Heemann 2001).
This Review has potential weaknesses. Meta‐analyses are by nature observational and may therefore be affected by bias or confounding. We performed a limited number of predefined subgroup analyses. The results of these analyses should be interpreted with caution and prospective validation is needed before causal inferences can be made. Another limitation is that the included trials were relatively small. The risk of a type II error (false negative conclusions) is therefore considerable. Small trials also have an increased risk of type I error (false positive conclusions) due to random error and publication bias (Egger 1997).
When the pre‐set sample size is not reported it is impossible to determine whether the pre‐set sample size was reached. The one trial reporting sample size calculation (Heemann 2001) was prematurely stopped due to an interim analysis which indicated a significant effect of the intervention. This decision is debatable, as the significance level was only three per cent. The conflict in monitoring trial results is to balance the interests of all patients with the disease in question and future patients entering the trial (Pocock 1993). The right time to stop a trial must be when there is certainty that the results are reliable enough to influence clinical practice in the future. The risk of interim analyses is false positive results due to premature termination of a trial at a 'random high'. It is therefore widely recognised that early interim analyses require very small significance levels before a trial is stopped (O'Brien 1979). Later interim analyses require significance levels closer to the normal five per cent (Pocock 1993). The number of interim analyses can be pre‐planned or flexible. The O'Brien and Flemming method is often used for pre‐planned interim analyses (O'Brien 1979). This method suggests that the first of five interim analyses requires a significance level of P = 0.00000001 and the fifth analysis P = 0.009. A more flexible method is the Peto‐Haybrittle that specifies a significance level of P less than 0.001 (Peto 1976).
Several aspects may bias meta‐analyses. First, the chance of getting small trials published is significantly increased if the outcome is positive. This may lead to false positive conclusions, as unpublished trials are more difficult to identify than published trials (Egger 1997). Inadequate methodological quality can also lead to false positive conclusions. On average, trials with unclear randomisation exaggerate intervention effects significantly, as do trials without double blinding (Schulz 1995; Moher 1998; Kjaergard 2001; Juni 2001). Most of the included trials used adequate methods to conceal allocation, which should reduce the risk of selection bias. However, only one trial had blinded assessment of outcomes. This caveat may introduce collateral intervention bias as well as detection bias.
The benefits of non‐randomised studies have been stressed during recent years (Benson 2000). It is argued that observational studies have greater external validity as patients who are willing to enter a randomised trial may differ from patients who are not. However, the question of external validity becomes irrelevant if a study lacks internal validity. The main problem with observational studies is the risk of selection bias. If the intervention groups are not comparable regarding known and unknown prognostic factors, it is impossible to determine whether differences between intervention groups reflect the intervention or baseline prognosis (Sacks 1982; Kunz 1998; Pocock 2000; Kjaergard 2001). We found that control group event rates were higher and intervention effects more positive in non‐randomised studies compared to randomised trials. This indicates a skewed distribution of prognostic factors in the two intervention groups of observational studies supporting previous evidence, which show that non‐randomised studies have a considerable risk of generating false positive conclusions (Kunz 1998; Kunz 2003).
In serious disorders such as liver failure, it can be difficult to assess whether unwanted events are caused by an intervention. This Review indicates that both artificial and bioartificial support systems are associated with several serious and non‐serious adverse events. Although the seriousness of liver failure may lead to adverse events, it is imperative that all adverse events are considered in treatment recommendations. Unfortunately, our data did not allow for an analysis of the risk of adverse events.
Authors' conclusions
Implications for practice.
Patients with acute‐on‐chronic liver failure may benefit from treatment with the more recently developed artificial support systems. The evidence concerning bioartificial support systems and treatment of patients with acute liver failure seemed less conclusive. However, considering the potential limitations of the evidence, liver support system cannot be recommended for routine use.
Implications for research.
Randomised trials on artificial and bioartificial support systems versus standard medical therapy are warranted. The trend of the data justifies larger trials, preferably involving several clinical sites to increase the external validity.
It is of central importance that the therapeutic potential of one support system compared to standard medical therapy becomes sufficiently studied before such systems are introduced as control interventions to more sophisticated support systems. Therfore, hepatologists ought to collaborate in randomised trials allocating patients to one support system plus 'standard medical therapy' versus the same 'standard medical therapy'. Such trials should be adequately designed, including centralised randomisation (using stratification for important prognostic factors) as well as blinded outcome assessment. Such trial should adequately describe aetiology of liver failure. Among outcome measures, all pertinent outcomes and adverse events should be carefully registered. Further, such trials should use an independent data monitoring and safety committee having adequate instructions regarding early arrest of the trial. In the reporting of future trials, authors should follow the recommendations of the CONSORT Statement (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 9 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Drs Rodger Williams (UK), Stephen R Ash (USA), Ludwig Kramer (Austria), Andrew Davenport (UK), Uwe Heemann (Germany), and Jan Stange (Germany) who kindly providing additional data on their trials. We are indebted to Dimitrinka Nikolova for her helpful secretarial assistance and Dr Wendong Chen who validated data extraction of the Chinese trials.
Appendices
Appendix 1. Search Strategies
| Database | Year range | Search strategy |
| MEDLINE | August 1966 to September 2002 | 1 Artificial liver/ 2 Liver failure/ 3 OR 1‐2 4 Random allocation 5 Randomized controlled trials [all fields] 6 3 AND (4 OR 5) |
| EMBASE | 1985 to September 2002 | 1 Artificial liver 2 Liver failure 3 1 AND 2 4 Randomization 5 Randomized controlled trials 6 3 AND (4 OR 5) |
| Chinese BioMedical Databse CD‐ROM | August 1979 to September 2002 | 1 Explode "artificial liver" 2 Liver failure/tw 3 OR 1‐2 4 Clinical trials/tw 5 Randomization/tw 6 3 AND (4 OR 5) |
| The Cochrane Library | Issue 3, 2002 | 1 Liver failure*ME 2 Liver failure 3 (1 OR 2) 4 Artificial liver*ME 5 Artificial liver 6 (4 OR 5) 7 (3 AND 6) 8 Random‐allocation 9 Randomized‐controlled‐trial 10 7 AND (8 OR 9) |
Data and analyses
Comparison 1. Support systems versus standard medical therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 12 | 483 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.65, 1.14] |
| 2 Bridging to transplantation | 4 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.09] |
| 3 Hepatic encephalopathy | 8 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.53, 0.88] |
| 4 Adverse events | 6 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [0.76, 7.47] |
| 4.1 Bleeding | 6 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.34, 8.99] |
| 4.2 Infection | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 67.06] |
| 4.3 Coagulopathy | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [0.52, 30.76] |
1.1. Analysis.

Comparison 1 Support systems versus standard medical therapy, Outcome 1 Mortality.
1.2. Analysis.

Comparison 1 Support systems versus standard medical therapy, Outcome 2 Bridging to transplantation.
1.3. Analysis.
Comparison 1 Support systems versus standard medical therapy, Outcome 3 Hepatic encephalopathy.
1.4. Analysis.

Comparison 1 Support systems versus standard medical therapy, Outcome 4 Adverse events.
Comparison 2. Different support systems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Intermittent versus continuous haemofiltration | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.95] |
| 1.2 Five hours versus ten hours haemoperfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.

Comparison 2 Different support systems, Outcome 1 Mortality.
Comparison 3. Sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality in acute and acute‐on‐chronic liver failure | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Acute (fulminant) | 8 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.71, 1.29] |
| 1.2 Acute‐on‐chronic | 6 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| 2 Mortality in worst‐case scenario analysis | 11 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.60, 1.13] |
3.1. Analysis.

Comparison 3 Sensitivity analyses, Outcome 1 Mortality in acute and acute‐on‐chronic liver failure.
3.2. Analysis.

Comparison 3 Sensitivity analyses, Outcome 2 Mortality in worst‐case scenario analysis.
Comparison 4. Non‐randomised studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Randomised trials | 12 | 483 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.12] |
| 1.2 Prospective observational studies | 5 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.55, 0.95] |
| 1.3 Case series with historical controls | 3 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.58, 0.80] |
4.1. Analysis.

Comparison 4 Non‐randomised studies, Outcome 1 Mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bion 1993.
| Methods | Excluded (see Additional tables: Non‐randomised studies). | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Davenport 1989.
| Methods | Excluded (see Additional tables: Non‐randomised studies). | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Davenport 1993.
| Methods | ‐ Generation of the allocation sequence: not reported. ‐ Allocation concealment: not reported. ‐ Sample size calculation: not reported. ‐ Intention‐to‐treat analyses: Did not explicitly report that intention to treat analyses were used, but accounted for all patients in the analyses. Blinding: not performed. | |
| Participants | ‐ Country: United Kingdom. ‐ Sample size: 22 patients. ‐ Number of patients with missing data: none reported. ‐ Inclusion criteria: i) acute liver failure with acute oliguric renal failure. ‐ Exclusion criteria: none reported ‐ Aetiology: paracetamol (69%), viral hepatitis (13%), undetermined (9%), ischaemic hepatitis (6%), fatty liver of pregnancy (3%). ‐ Mean age: 33 years (range 19 to 72 years). ‐ Proportion of males: 50%. | |
| Interventions | ‐ Experimental group: intermittent haemofiltration (32 treatments). Anticoagulation was not routinely used. ‐ Control group: continuous arteriovenous haemofiltration with or without dialysis. ‐ Treatment and control group received heparin keeping thromboplastin times between 100 to 140 seconds if required. |
|
| Outcomes | ‐ Outcomes: mortality. Adverse events in treatment group. ‐ Length of follow up: end of treatment. | |
| Notes | ‐ Serious adverse events: none reported. ‐ Non‐serious adverse events in experimental group: hypotension, bleeding, and increase in intracranial pressure (Incidence not reported). ‐ Publication status: full paper article. | |
Denis 1978.
| Methods | Excluded (see Additional tables: Non‐randomised studies). | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Ellis 1996.
| Methods | ‐ Generation of the allocation sequence: table of random numbers. ‐ Allocation concealment: sealed envelopes. ‐ Sample size calculation: not reported. ‐ Intention‐to‐treat analyses: yes. All patients were accounted for in the analyses. Blinding: not performed. | |
| Participants | ‐ Country: United Kingdom. ‐ Sample size: 24 patients. ‐ Number of patients with missing data: none reported. Two patients were withdrawn from the experimental group because of adverse events (see notes). ‐ Inclusion criteria: acute liver failure with a potentially recoverable lesion or listed for liver transplantation. ‐ Exclusion criteria: i) pregnancy, ii) other organs failure, iii) recent upper gastrointestinal haemorrhage. ‐ Aetiology: acetaminophen (71%), viral hepatitis (21%), drug reactions (8%). ‐ Mean age: 30 years (range 14 to 65). ‐ Proportion of males: 50%. | |
| Interventions | ‐ Experimental group: ELAD* (performed for a median of 62 hours). Heparin was used as anticoagulant. ‐ Control group: standard medical therapy. |
|
| Outcomes | ‐ Outcomes: mortality, bridging to transplantation, hepatic encephalopathy, and adverse events. ‐ Length of follow up: maximum reported 31 days. | |
| Notes | ‐ Serious adverse events in experimental group: ‐ Bleeding and pyrexia (2 of 12 patients). ‐ Non‐serious adverse events: Decrease of platelet count and anti‐thrombin III level, hypotension, and hypersensitivity (in 5 of 12 patients). ‐ Publication status: full paper article. | |
Ellis 1999.
| Methods | ‐ Generation of the allocation sequence: not reported. ‐ Allocation concealment: sealed envelopes. ‐ Sample size calculation: not reported. ‐ Intention‐to‐treat analyses: Did not explicitly report that intention to treat analyses were used, but accounted for all patients in the analyses. Blinding: not performed. | |
| Participants | ‐ Country: United Kingdom. ‐ Sample size: 10 patients. ‐ Number of patients with missing data: none. ‐ Inclusion criteria: i) acute alcoholic hepatitis with at least grade II encephalopathy. ‐ Exclusion criteria: i) pregnancy, ii) failure of other organs with a mean arterial blood pressure of less than 50 mmHg for one hour, iii) previous cerebrovascular event iv) recent gastrointestinal haemorrhage, v) poorly controlled epilepsy, vi) recent myocardial infarction/ischemia. ‐ Aetiology: alcoholic liver disease (100%). ‐ Mean age: 45 years. ‐ Proportion of males: 70%. | |
| Interventions | ‐ Experimental group: BioLogic‐DT** 6 hours/day for three days (i.e., maximum 18 hours). Anticoagulation was not routinely used. ‐ Control group: standard medical therapy. |
|
| Outcomes | ‐ Outcomes: mortality and adverse events. ‐ Length of follow up: survival to hospital discharge (maximum reported follow up 33 days). | |
| Notes | ‐ Adverse events: no adverse events were registered. ‐ Publication status: full paper article. | |
Gazzard 1974.
| Methods | Excluded (see Additional tables: Non‐randomised studies). | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Gimson 1982.
| Methods | Excluded (see Additional tables: Non‐randomised studies). | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
He 2000.
| Methods | ‐ Generation of the allocation sequence: not reported. ‐ Allocation concealment: not reported. ‐ Sample size calculation: not reported. ‐ Intention‐to‐treat analyses: Did not explicitly report that intention to treat analyses were used, and did not account for all patients in the analyses. Blinding: not performed. | |
| Participants | ‐ Country: China. ‐ Sample size: 124 patients. ‐ Number of patients with missing data: none reported. ‐ Inclusion criteria: severe viral hepatitis with or without pre‐existing liver disease. ‐ Exclusion criteria: none reported ‐ Aetiology: viral hepatitis (100%). ‐ Mean age: 36 years. ‐ Proportion of males: 87%. | |
| Interventions | ‐ Experimental group: plasma exchange/perfusion and haemoperfusion (mean 2.6 treatments). ‐ Control group: standard medical therapy. |
|
| Outcomes | ‐ Outcomes: mortality and hepatic encephalopathy. Adverse events in experimental group. ‐ Length of follow up: death or recovery (maximum 7 days). | |
| Notes | ‐ Serious adverse events in experimental group: allergic shock ( 1 of 64 patients), systemic infection (4 of 64 patients). ‐ Non‐serious adverse events: total incidence (24 of 64 patients) including serious asthma, non‐serious bleeding, plasma hypersensitivity, arrhythmia, and electrolyte imbalances. ‐ Publication status: full paper article. | |
Heemann 2001.
| Methods | ‐ Generation of the allocation sequence: computer generated. ‐ Allocation concealment: serially numbered opaque sealed envelopes. ‐ Sample size calculation: yes (estimated sample size required was 28 patients finishing 30 observation periods). ‐ Intention‐to‐treat analyses: yes. All patients were included in the analyses. Missing data were handled by using carry forward of last observed response. Blinding: outcome assessor blinded. | |
| Participants | ‐ Country: Germany. ‐ Sample size: 24 patients. ‐ Number of patients with missing data: one patient in the control group was excluded from analysis due to not receive treatment. ‐ Inclusion criteria: chronic liver disease with liver failure and cholestatic icterus, ii) age 18 to 65 years. The mean hepatic encephalopathy grade of included patients grade was I. ‐ Exclusion criteria: i) jaundice not caused by chronic liver disease (acute liver failure or obstructive jaundice), ii) active bleeding, iii) additional disease associated with a poor outcome (e.g., cancer, necrotic pancreatitis, severe cardiopulmonary disease, or coma of non‐hepatic‐origin). ‐ Underlying liver disease: alcoholic cirrhosis (75%), viral hepatitis (10%), drugs (10%), and primary biliary cirrhosis (5%). Precipitating events: infection (45%), unknown/alcohol? (45%), drugs (15%), bleeding (10%), viral hepatitis (5%). ‐ Mean age: 53 years. ‐ Proportion of males: 58%. | |
| Interventions | ‐ Experimental group: molecular adsorbent recycled system (MARS) (six‐hour treatments per patient). Patients received little or no anticoagulation. ‐ Control group: standard medical therapy. |
|
| Outcomes | ‐ Outcomes: mortality, hepatic encephalopathy, and adverse events. ‐ Length of follow up: 30 days. | |
| Notes | ‐ Serious adverse events: none. ‐ Non‐serious adverse events: In the treatment group 17 of 12 patients experienced an adverse event (including anaemia, coagulopathy, hypotension, bleeding, and fever) versus 4 of 12 patient in the control group (fluid overload and/or uraemia requiring real support). ‐ Publication status: unpublished data. | |
Hughes 1994.
| Methods | ‐ Generation of the allocation sequence: not described. ‐ Allocation concealment: sealed envelopes. ‐ Sample size calculation: not reported. ‐ Intention‐to‐treat analyses: Did not explicitly report that intention to treat analyses were used, but accounted for all patients in the analyses. Blinding: not performed. | |
| Participants | ‐ Country: United Kingdom. ‐ Sample size: 10 patients. ‐ Number of patients with missing data: none reported. ‐ Inclusion criteria: fulminant hepatic failure with grade IV encephalopathy. ‐ Exclusion criteria: renal failure or listing for emergency liver transplantation. ‐ Aetiology: paracetamol overdose (60%) or hepatitis (40%). ‐ Mean age: 37 years (range 19 to 64 years). ‐ Proportion of males: 70%. | |
| Interventions | ‐ Experimental group: BioLogic‐DT** 6 hours/day for up to 5 days (i.e., maximum 30 hours) or until death or recovery. Patients did not receive anticoagulation. ‐ Control group: standard medical therapy. |
|
| Outcomes | ‐ Outcomes: mortality, hepatic encephalopathy, and adverse events. ‐ Length of follow up: maximum 17 days. | |
| Notes | ‐ Adverse events: no apparent adverse events were registered. Patients in the treatment group had a significant loss of platelets, decrease in plasma fibrinogen, and rise in blood activated clotting time (not seen in controls. Incidence not reported). ‐ Publication status: full paper article. | |
Jiang 2000.
| Methods | Excluded (see Additional tables: Non‐randomised studies). | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Kramer 1998.
| Methods | ‐ Generation of the allocation sequence: computer generated random numbers. ‐ Allocation concealment: serially numbered opaque sealed envelopes. ‐ Sample size calculation: not reported. ‐ Intention‐to‐treat analyses: yes. All patients were accounted for in the analyses. | |
| Participants | ‐ Country: Austria. ‐ Sample size: 20 patients. ‐ Number of patients with missing data: none. ‐ Inclusion criteria: i) cirrhosis, ii) abnormal liver function tests, iii) hepatic encephalopathy grade II to III. ‐ Exclusion criteria: i) renal failure, ii) hypotension, iii) multiorgan failure, iv) temperature above 38.5 degrees Celsius v) multiorgan failure vi) respiratory failure, vii) active bleeding, viii) self‐administration of sedatives within preceding two days, ix) insulin dependent diabetes. ‐ Aetiology: alcoholic cirrhosis (65%), viral hepatitis (25%), autoimmune hepatitis (5%), and unknown (5%). ‐ Mean age: 56 years (range 38 to 71). ‐ Proportion of males: 65%. | |
| Interventions | ‐ Experimental group: BioLogic‐DT** (six‐hour treatments). ‐ Control group: standard medical therapy. |
|
| Outcomes | ‐ Outcomes: mortality, hepatic encephalopathy, and adverse events. ‐ Length of follow up: maximum not reported. | |
| Notes | ‐ Serious adverse events in experimental group: upper gastrointestinal bleeding (1 of 10 patients) and disseminated intravascular coagulation (1 of 10 patients). ‐ Non‐serious adverse events: decrease in platelet count. ‐ Publication status: full paper article. | |
Li 1997.
| Methods | Excluded (see Additional tables: Non‐randomised studies). | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Margulis 1989.
| Methods | Excluded (see Additional tables: Non‐randomised studies). | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Mazariegos 1997.
| Methods | ‐ Generation of the allocation sequence: not reported. ‐ Allocation concealment: sealed envelopes. ‐ Sample size calculation: not reported. ‐ Intention‐to‐treat analyses: Did not explicitly report that intention to treat analyses were used, but accounted for all patients in the analyses. Blinding: not performed. | |
| Participants | ‐ Country: USA. ‐ Sample size: 10 patients (preliminary data on 6 patients). ‐ Number of patients with missing data: four patients in the control group. One patient was withdrawn from the treatment group, but reason not stated ‐ Inclusion criteria: acute liver failure with coma. ‐ Exclusion criteria: not reported. ‐ Aetiology: not reported. ‐ Mean age: 48 years (range 35 to 65). ‐ Proportion of males: 33%. | |
| Interventions | ‐ Experimental group: BioLogic‐DT** (three six‐hour treatments). ‐ Control group: standard medical therapy. |
|
| Outcomes | ‐ Outcomes: mortality, hepatic encephalopathy, bridging to transplantation, and adverse events. ‐ Length of follow up: two days after treatment. | |
| Notes | ‐ Adverse events: one patient died from variceal bleeding. No other adverse events reported ‐ Publication status: abstract. | |
Mitzner 2000.
| Methods | ‐ Generation of the allocation sequence: computer generated random numbers. ‐ Allocation concealment: sealed envelopes. ‐ Sample size calculation: not reported. ‐ Intention‐to‐treat analyses: Did not explicitly report that intention to treat analyses were used, but accounted for all patients in the analyses. Blinding: not performed. | |
| Participants | ‐ Country: Germany. ‐ Sample size: 13 patients. ‐ Number of patients with missing data: none reported. ‐ Inclusion criteria: i) hepatorenal syndrome with rapidly progressive reduction of renal function (type I), ii) acute deterioration of chronic liver disease with ascites, clinical, biochemical and ultrasonographic signs of liver cirrhosis, iii) need of haemodialysis/filtration, iv) age 18‐60 years, and v) informed consent. ‐ Exclusion criteria: i) sepsis unresponsive to treatment, ii) severe acute haemorrhages, iii) malignancies, iv) obstructive/chronic liver failure, v) pregnancy, and vi) severe cardiopulmonary disease. ‐ Aetiology: alcoholic liver disease (54%), hepatitis (30%), primary biliary cirrhosis (8%), or secondary biliary cirrhosis (8%). ‐ Mean age: 47 years. ‐ Proportion of males: 38%. | |
| Interventions | ‐ Experimental group: MARS*** 6‐8 hours/day for up to 10 days (i.e., maximum 80 hours). MARS treatment was only performed if bilirubin increased after single treatments. ‐ Control group: standard medical therapy. ‐ Both the treatment and control group underwent additional haemofiltration and received heparin. |
|
| Outcomes | ‐ Outcomes: mortality and adverse events. ‐ Length of follow up: 30 days. | |
| Notes | ‐ Adverse events: no apparent adverse events were registered. Patients in the treatment group had mild thrombocytopenia. ‐ Publication status: full paper article. | |
O'Grady 1988.
| Methods | ‐ Generation of the allocation sequence: table of random numbers. ‐ Allocation concealment: not reported. ‐ Sample size calculation: not reported. ‐ Intention‐to‐treat analyses: Did not explicitly report that intention to treat analyses were used, but accounted for all patients in the analyses. Blinding: not performed. | |
| Participants | ‐ Country: United Kingdom. ‐ Sample size: 75 patients. ‐ Number of patients with missing data: none reported. ‐ Inclusion criteria: fulminant liver failure with grade III encephalopathy and no pre‐existing liver disease. Fulminant hepatic failure was defined as the development of encephalopathy within eight weeks after the onset of symptoms. ‐ Exclusion criteria: i) refractory hypotension, ii) concomitant treatment with interferon. ‐ Aetiology: acetaminophen (71%), viral hepatitis (25%), halothane/drug reactions (4%). ‐ Mean age: 38 years. ‐ Proportion of males: 50%. | |
| Interventions | ‐ Experimental group: five hours of haemoperfusion (maximum 6 sessions). ‐ Control group: ten hours of haemoperfusion (maximum 4 sessions). |
|
| Outcomes | ‐ Outcomes: mortality. ‐ Length of follow up: maximum 26 days. | |
| Notes | ‐ Adverse events: not reported. ‐ Publication status: full paper article. | |
Redeker 1973.
| Methods | ‐ Generation of the allocation sequence: not described. ‐ Allocation concealment: sealed envelopes. ‐ Sample size calculation: not reported. ‐ Intention‐to‐treat analyses: Did not explicitly report that intention to treat analyses were used, but accounted for all patients in the analyses. Blinding: not performed. | |
| Participants | ‐ Country: USA. ‐ Sample size: 23 patients. ‐ Number of patients with missing data: seven patients died before receiving the experimental treatment. ‐ Inclusion criteria: acute liver failure with grade IV encephalopathy caused by viral hepatitis. ‐ Exclusion criteria: disease course longer than four weeks. ‐ Etiology: viral hepatitis (100%). ‐ Mean age: 26 years. ‐ Proportion of males: 50%. | |
| Interventions | ‐ Experimental group: exchange transfusions with 6000 ml of freshly drawn whole blood. ‐ Control group: standard medical therapy. |
|
| Outcomes | ‐ Outcomes: mortality. ‐ Length of follow up: until death or consciousness. | |
| Notes | ‐ Adverse events: not reported. ‐ Publication status: full paper article. | |
Silk 1978.
| Methods | Excluded (see Additional tables: Non‐randomised studies). | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Stevens 2001.
| Methods | ‐ Generation of the allocation sequence: not reported. ‐ Allocation concealment: not reported. ‐ Sample size calculation: not reported. ‐ Intention‐to‐treat analyses: Not explicitly report. Blinding: not performed. | |
| Participants | ‐ Country: USA and Europe. ‐ Sample size: 147 patients. ‐ Number of patients with missing data: Not reported. ‐ Inclusion criteria: fulminant liver failure with or without pre‐existing liver disease. 147 patients had grade III to IV encephalopathy. ‐ Exclusion criteria: Not specified. ‐ Aetiology: acetaminophen 39 (26%), viral hepatitis 32 (22%), and other 76 (52%). ‐ Mean age: no data. ‐ Proportion of males: no data. | |
| Interventions | ‐ Experimental group: HepatAssist bioartificial liver support system (BAL, six‐hour treatments until transplantation, neurologic recovery to stage I encephalopathy for 72 hours, or any serious adverse event that prevented further treatment). ‐ Control group: standard medical therapy. |
|
| Outcomes | ‐ Outcomes: mortality, bridging to transplantation, hepatic encephalopathy, and adverse events. ‐ Length of follow up: 30 days or to death. | |
| Notes | ‐ Serious adverse events: sepsis (15% control vs 7% BAL), renal failure (20% control vs 14%). No original data was available. ‐ None‐serious adverse events: thrombocytopenia (30% each group), and hypotension (16% control vs 17% BAL). No original data was available. ‐ Publication status: abstract. | |
Wilkinson 1998.
| Methods | ‐ Generation of the allocation sequence: not reported. ‐ Allocation concealment: sealed envelopes. ‐ Sample size calculation: not reported. ‐ Intention‐to‐treat analyses: Did not explicitly report that intention to treat analyses were used, but accounted for all patients in the analyses. Blinding: not performed. | |
| Participants | ‐ Country: USA. ‐ Sample size: 11 patients. ‐ Number of patients with missing data: One patient was retransplanted before trial. Data not available for this patient (see also interventions) . ‐ Inclusion criteria: fulminant liver failure with or without pre‐existing liver disease. Eight patients had grade III to IV encephalopathy and three had hepatic coma. ‐ Exclusion criteria: i) renal failure, ii) listing for emergency liver transplantation, iii) active bleeding. ‐ Aetiology: viral hepatitis (36%), alcoholic cirrhosis (27%), chronic autoimmune hepatitis (9%), cryptogenic cirrhosis (9%), haemochromotosis (9%), and heat chock (9%). ‐ Mean age: 49 years (range 27 to 66). ‐ Proportion of males: 62%. | |
| Interventions | ‐ Experimental group: BioLogic‐DT** (six‐hour treatments for up to five days). ‐ Control group: standard medical therapy. One patient in the control group was withdrawn to be treated with the BioLogic‐DT. |
|
| Outcomes | ‐ Outcomes: mortality, bridging to transplantation, hepatic encephalopathy, and adverse events. ‐ Length of follow up: more than five days (not clearly reported). | |
| Notes | ‐ Serious adverse events: none reported. ‐ None‐serious adverse events: decrease of platelet counts (incidence not reported). ‐ Publication status: full paper article. | |
*extracorporeal liver assist device. **sorbent suspension dialysis. **molecular adsorbent recirculating system.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ash 1992 | ‐ Design: case series. |
| Chen 1996 | ‐ Design: case series. |
| Chen 1997 | ‐ Design: case series. |
| Chen 1999 | ‐ Trial on ultraviolet blood irradiation oxygen therapy versus standard medical therapy. |
| Eiseman 1965 | ‐ Design: case series. |
| Fu 1999 | Trial on haemodialysis with Chinese medicines versus conventional medical therapy. ‐ Excluded due to the co‐intervention with Chinese medicines. |
| Fujita 1982 | ‐ Design: case series. |
| Hughes 1998 | ‐ Randomised clinical trial assessing extracorporeal liver assist devices for liver failure on plasma cytokines. We were unable to obtain data on clinical outcomes. |
| Iizuka 1995 | ‐ Design: case series. |
| Jones 1998 | ‐ Randomised clinical trial on membranes for dialysis of acute renal failure. We were unable to extract data on patients with liver failure. |
| Koudahl 1971 | ‐ Design: case series. |
| Kukita 1990 | ‐ Design: case series. |
| Larsen 1994 | ‐ Design: case series. |
| Lepore 1970 | ‐ Design: case series. |
| Li 1999 | ‐ Design: case series. |
| Matsumura 1987 | ‐ Design: case series. |
| Millis 1999a | ‐ Design: case series. |
| Millis 1999b | ‐ Design: case series. |
| Mitzner 1996 | ‐ Design: case series. |
| Morgan 1997 | ‐ Design: case series. |
| Morsiani 2002 | ‐ Design: case series in phase I and phase II clinical trial. |
| Munoz 1989 | ‐ Design: case series. |
| Parbhoo 1971 | ‐ Design: case series. |
| Ranek 1971 | ‐ Design: case series. |
| Schmidt 2000 | ‐ Design: case series. |
| Sicot 1971 | ‐ Design: case series. |
| Stange 1999 | ‐ Design: case series. |
| Stange 2000 | ‐ Design: retrospective cohort study. |
| Sussman 1994 | ‐ Design: case series. |
| Watanabe 1997a | ‐ Design: case series. |
| Watanabe 1997b | ‐ Design: case series. |
| Yoshiba 1996 | ‐ Design: case series. |
| Zhou 2002 | ‐ Design: case series. |
Characteristics of ongoing studies [ordered by study ID]
Hayes 2000.
| Trial name or title | Development of bioartificial liver assist device for fulminant liver failure. |
| Methods | |
| Participants | Inclusion criteria: fulminant liver failure. |
| Interventions | Bioartificial assist device. |
| Outcomes | Unknown |
| Starting date | 01/07/1996 |
| Contact information | Prof PC Hayes Dept of Medicine, The Royal Infirmary of Edinbergh, Lauriston Place, Edinburgh, EH 3 9YW Scotland |
| Notes | From The National Research Register. ‐ Letter sent to author 11/01/2001. |
Jalan 2002.
| Trial name or title | Molecular adsorbents recirculating system for acute‐on‐chronic liver failure. |
| Methods | |
| Participants | Inclusion criteria: acute decompensation of chronic liver disease. |
| Interventions | Molecular adsorbents recirculating system. |
| Outcomes | Unknown |
| Starting date | 2001 |
| Contact information | Prof R Williams. Institute of Hepatology, University College London Hospitals, London. |
| Notes | R Jalan, C Steiner, S Sen, Authors: D Kapoor, A Alisi*, R Williams. Institute of Hepatology, University College London Hospitals, London, and * Cromwell Hospital, London |
VitaGen 2000.
| Trial name or title | Extracorporeal liver assist device for fulminant liver failure. |
| Methods | |
| Participants | Inclusion criteria: Patients with fulminant liver failure |
| Interventions | Extracorporeal assist device. |
| Outcomes | Unknown |
| Starting date | Unknown |
| Contact information | VitaGen Incorporated, U.S.A. |
| Notes | ‐ Letter sent to author 11/01/2001. |
Contributions of authors
JPL identified trials, extracted data, and drafted the protocol and review. LLG identified trials, extracted data, redrafted the protocol and review, and performed the statistical analyses. BAN identified trials and extracted data. CG validated the identification of trials and data extraction. All reviewers contributed to the writing of the protocol and review and all have approved of the final version.
Sources of support
Internal sources
The Copenhagen Trial Unit, Copenhagen University Hospital, Denmark.
External sources
The Danish Medical Council Grant on Getting Research into Practice (GRIP), Denmark.
The Copenhagen Hospital Coporation's Research Council Grant on GRIP, Denmark.
The 1991 Pharmacy Foundation, Denmark.
Declarations of interest
None known. We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (e.g., employment, consultancy, stock ownership, honoraria, expert testimony).
Edited (no change to conclusions)
References
References to studies included in this review
Bion 1993 {published data only}
- Bion JF, Bowden MI, Chow B, Honisberger L, Weatherley BC. Atracurium infusions in patients with fulminant hepatic failure awaiting liver transplantation. Intensive Care Medicine 1993;19:S94‐S98. [DOI] [PubMed] [Google Scholar]
Davenport 1989 {published data only}
- Davenport A, Will EJ, Davison AM, Swindells S, Cohen AT, Miloszewski KJA, et al. Changes in intracranial pressure during haemofiltration in oliguric patients with grade IV hepatic encephalopathy. Nephron 1989;53:142‐6. [DOI] [PubMed] [Google Scholar]
Davenport 1993 {published data only}
- Davenport A, Will EJ, Davison AM. Effect of renal replacement therapy on patients with combined acute renal and fulminant hepatic failure. Kidney International 1993;43(Suppl 41):S245‐S251. [PubMed] [Google Scholar]
Denis 1978 {published data only}
- Denis J, Opolon P, Nusinovici V, Granger A, Darnis F. Treatment of encephalopathy during fulminant hepatic failure by haemodialysis with high permeability membrane. Gut 1978;19:787‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellis 1996 {published data only}
- Ellis AJ, Hughes RD, Wendon JA, Dunne J, Langley PG, Kelly JH, et al. Pilot‐controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology 1996;24(6):1446‐51. [DOI] [PubMed] [Google Scholar]
- Ellis AJ, Wendon J, Hughes R, Langley P, Sussman NL, Kelly JH, et al. A controlled trial of the Hepatix extracorporeal liver assist device (ELAD) in acute liver failure [abstract]. Hepatology 1994;20(Suppl):140A. [DOI] [PubMed] [Google Scholar]
Ellis 1999 {published data only}
- Ellis AJ, Hughes RD, Nicholl D, Langley PG, Wendon JA, O'Grady JG, et al. Temporary extracorporeal liver support for severe acute alcoholic hepatitis using the BioLogic‐DT. The International Journal of Artificial Organs 1999;22(1):27‐34. [PubMed] [Google Scholar]
Gazzard 1974 {published data only}
- Gazzard BG, Portmann B, Weston MJ, Langley PG, Murray‐Lyon IM, Dunlop EH, et al. Charcoal hemoperfusion in the treatment of fulminant hepatic failure. The Lancet 1974;1(7870):1301‐7. [DOI] [PubMed] [Google Scholar]
Gimson 1982 {published data only}
- Gimson AES, Braude S, Mellon PJ, Canalese J, Williams R. Earlier charcoal hemoperfusion in fulminant hepatic failure. The Lancet 1982;2(8300):681‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
He 2000 {published data only}
- He JQ, Chen CY, Deng JT, Qi HX, Zhang XQ, Chen JQ. Clinical study on the treatment of fatal hepatitis with artificial liver support system. Chinese Critical Care Medicine 2000;12(2):105‐8. [Google Scholar]
Heemann 2001 {unpublished data only}
- Heemann U, Treichel U, Loock J, Philipp T, Gerken G, Broelsch C, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology 2002;36:949‐58. [DOI] [PubMed] [Google Scholar]
Hughes 1994 {published data only}
- Hughes RD, Pucknell A, Routley D, Langley PG, Wendon JA, Williams R. Evaluation of the BioLogic‐DT sorbent‐suspension dialyser in patients with fulminant hepatic failure. The International Journal of Artificial Organs 1994;17(12):657‐62. [PubMed] [Google Scholar]
Jiang 2000 {published data only}
- Jiang YS, Chen YM, Yao JL, Li XJ, Zeng CH, Deng Y, et al. Evaluation of the therapeutic effect of artificial liver support system on severe viral hepatitis. Chinese Journal of Internal Medicine 2000;39(2):115‐7. [Google Scholar]
Kramer 1998 {published data only}
- Kramer L, Gendo A, Madl C, Ferrara I, Funk G, Schenk P, et al. Biocompatibility of a cuprophane charcoal‐based detoxification device in cirrhotic patients with hepatic encephalopathy. American Journal of Kidney Diseases 2000;36(6):1193‐200. [DOI] [PubMed] [Google Scholar]
- Kramer L, Gendo A, Madl C, Mullen K, Kaminski‐Russ K, Zauner C, et al. A controlled study of the BioLogic‐DT System in chronic hepatic encephalopathy (abstract). Hepatology 1998;28:401A. [Google Scholar]
- Kramer L, Gendo A, Madl C, Mullen KD, Kaminski‐Russ K, Sunder‐Plassmann G, et al. A controlled study of sorbent suspension dialysis in chronic liver disease and hepatic encephalopathy. The International Journal of Artificial Organs 2001;24(7):434‐42. [PubMed] [Google Scholar]
Li 1997 {published data only}
- Li LJ, Huang JR, Chen JH, Zhu Z, Chen YM, Gan MJ. The study of treatment of hepatitis gravis by artificial liver support system. Chinese Journal of Hepatology 1997;5(4):202‐3. [Google Scholar]
Margulis 1989 {published data only}
- Margulis MS, Erukhlmov EA, Andreiman LA, Viksna LM. Temporary organ substitution by hemoperfusion through suspension of active donor hepatocytes in a total complex of intensive therapy in patients with acute hepatic insufficiency. Resuscitation 1989;18:85‐94. [DOI] [PubMed] [Google Scholar]
Mazariegos 1997 {published data only}
- Ash SR, Knab WR, Blake DE, Carr DJ, Harker KD, Levy H. The BioLogic‐DTPF System in treatment of fulminant hepatic failure: preliminary results of a clinical trial [Abstract]. Hepatology 1998;28(4):496A. [DOI] [PubMed] [Google Scholar]
- Ash SR, Kuczek T, Blake DE, Gingrich CH. Liver dialysis in treatment of hepatic failure and hepatorenal failure: randomized clinical trials and clinical experience. ASAIO Journal 2000;46:223. [Google Scholar]
- Ash SR, Leamon KB, Gingrich CH. Hemodiabsorption (The BioLogic‐DT System) in treatment of hepatic failure with encephalopathy: summary of randomized, prospectively controlled clinical trials [Abstract]. Hepatology 1998;28(4):496A. [Google Scholar]
- Ash SR, Steczko J, Knab WR, Blake DE, Carr DJ, Harker KD, et al. Push‐pull sorbent‐based pheresis and hemodiabsorption in the treatment of hepatic failure: preliminary results of a clinical trial with the BioLogic‐DTPF System. Therapeuitic Apheresis 2000;4(3):218‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ash SR, Wilkinson A, Hughes R, Williams R. BioLogic‐DT System in treatment of acute hepatic coma: results of randomized, controlled trials [Abstract]. ASAIO Journal 1996;42(1):11. [Google Scholar]
- Mazariegos GV, Ash SR, Patzer JF. Preliminary results: randomized clinical trial of the BioLogic‐DT in treatment of acute hepatic failure (AHF) with coma (abstract). Artificial Organs 1997;21(6):529. [Google Scholar]
- Mazariegos GV, Linden F, Kramer D, Patzer J, Fung JJ. Randomized clinical trial of the BioLogic‐DT in treatment of acute hepatic failure (AHF) with advanced encephalopathy. Hepatology 1997;4(2):559A. [Google Scholar]
Mitzner 2000 {published data only}
- Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, et al. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transplantation 2000;6(3):277‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Grady 1988 {published data only}
- O'Grady JG, Gimson AES, O'Brien CJ, Pucknell A, Hughes RD, Williams R. Controlled trials of charcoal haemoperfusion and prognostic factors in fulminant hepatic failure. Gastroenterology 1988;94(5 Pt 1):1186‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Redeker 1973 {published data only}
- Redeker AG, Yamahiro HS. Controlled trial of exchange‐transfusion therapy in fulminant hepatitis. The Lancet 1973;1(7793):3‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Silk 1978 {published data only}
- Silk DBA, Williams R. Experiences in the treatment of fulminant hepatic failure by conservative therapy, charcoal haemoperfusion, and polyacrylonitrile haemodialysis. The International Journal of Artificial Organs 1978;1(1):29‐33. [PubMed] [Google Scholar]
Stevens 2001 {published data only}
- Stevens C, Busuttil R, Han S, Baquerizo A, Fair J, Shrestha R, et al. An interim analysis of a phase II/III prospective randomized multicenter controlled trial of the Hepat Assist Bioartificial Liver Support System for the treatment of fulminant hepatic failure [abstract]. Hepatology 2001;4:299A. [Google Scholar]
Wilkinson 1998 {published data only}
- Wilkinson AH, Ash SR, Nissenson AR. Hemodiabsorption in treatment of hepatic failure. Journal of Transplant Coordination 1998;8(1):43‐50. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ash 1992 {published data only}
- Ash SR, Blake DE, Carr DJ, Carter C, Howard T, Makowka L. Clinical effects of a sorbent suspension dialysis system in treatment of hepatic coma (the BioLogic‐DT). The International Journal of Artificial Organs 1992;15(3):151‐61. [PubMed] [Google Scholar]
Chen 1996 {published data only}
- Chen SC, Hewitt WR, Watanabe FD, Eguchi S, Kahaku E, Middleton Y, et al. Clinical experience with a porcine hepatocyte‐based liver support system. The International Journal of Artificial Organs 1996;19(11):664‐9. [PubMed] [Google Scholar]
Chen 1997 {published data only}
- Chen SC, Mullon C, Kahaku E, Watanabe F, Hewitt W, Eguchi S, et al. Treatment of severe liver failure with a bioartificial liver. Annals of the New York Academy of Sciences 1997;831:350‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 1999 {published data only}
- Chen JL, Che DP, Long YC, Xiong LL, Xie B, Cai HQ. Observation on therapeutic effect of ultraviolet blood irradiation oxygen (UBIO) on chronic severe viral hepatitis [in Chinese]. Modern Diagnosis and Treatment 1999;10(2):85‐7. [Google Scholar]
Eiseman 1965 {published data only}
- Eiseman B, Liem DS, Raffucci F. Heterologous liver perfusion in treatment of hepatic failure. Annals of Surgery 1965;162(3):329‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fu 1999 {published data only}
- Fu XY, Wang GS, Huang YC, Zhu JH, Hong LL. Artificial liver support combined with Chinese medicine in treating hepatic failure [in Chinese]. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 1999;9(5):3‐4. [Google Scholar]
Fujita 1982 {published data only}
- Fujita Y, Ohiwa T, Kubota Y, Shimizu H, Yoshida S, Hosoya R, et al. Clinical trial of plasmapheresis in hepatic failure. Transaction ‐ American Society for Artificial Internal Organs 1982;28:225‐8. [PubMed] [Google Scholar]
Hughes 1998 {published data only}
- Hughes RD, Nicolaou N, Langley PG, Ellis AJ, Wendon JA, Williams R. Plasma cytokine levels and coagulation and complement activation during use of the extracorporeal liver assist device in acute liver failure. Artificial Organs 1998;22(10):854‐8. [DOI] [PubMed] [Google Scholar]
Iizuka 1995 {published data only}
- Iizuka K, Kanesaka S, Akizawa T, Niikura K, Taira T, Kadokawa M, et al. Artificial liver support for the treatment of fulminant viral hepatic failure. Japanese Journal of Apheresis 1995;14(1):43‐5. [Google Scholar]
Jones 1998 {published data only}
- Jones CH, Goutcher E, Newstead CG, Will EJ, Dean SG, Davison AM. Hemodynamics and survival of patients with acute renal failure treated by continuous dialysis with two synthetic membranes. Artificial Organs 1998;22(8):638‐43. [DOI] [PubMed] [Google Scholar]
Koudahl 1971 {published data only}
- Koudahl G, Juul‐Nielsen J, Schmidt N, Winkler K, Tygstrup N. The function of the isolated pig liver cross‐perfused with anhepatic pigs and patients with liver failure. Scandinavian Journal of Gastroenterology 1971;9(Suppl):155‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Kukita 1990 {published data only}
- Kukita K, Kawamura A, Ariyama T, Meguro J, Yonekawa M, Imai K, et al. Rationality of plasma exchange therapy based on periodic liver CT volume calculation in patients with fulminant hepatitis. Progress in Clinical and Biological Research (Apheresis) 1990;337:237‐8. [PubMed] [Google Scholar]
Larsen 1994 {published data only}
- Larsen FS, Hansen BA, Jorgensen LG, Secher NH, Bondesen S, Linkis P, et al. Cerebral blood flow velocity during high volume plasmapheresis in fulminant hepatic failure. The International Journal of Artificial Organs 1994;17(6):353‐61. [PubMed] [Google Scholar]
Lepore 1970 {published data only}
- Lepore MJ, Martel AJ. Plasmapheresis with plasma exchange in hepatic coma. Annals of Internal Medicine 1970;72(2):165‐74. [DOI] [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li LJ, Huang JR, Chen YM, Yang Q, Chen YG, Ma WH, et al. Use of artificial liver support system in the treatment of viral hepatitis gravis [in Chinese]. Chinese Journal of Infectious Diseases 1999;17(4):228‐30. [Google Scholar]
Matsumura 1987 {published data only}
- Matsumura KN, Guevara GR, Huston H, Hamilton WL, Riklmaru M, Yamasaki G, et al. Hybrid bioartificial liver in hepatic failure: preliminary clinical report. Surgery 1987;101:99‐103. [PubMed] [Google Scholar]
Millis 1999a {published data only}
- Millis JM, Maguire P, Cronin DC, Conjeevarum H, Johnson R, Conlin C, et al. ELAD continuous liver support system: report of cell function. Hepatology 1999;30(4):168A. [Google Scholar]
Millis 1999b {published data only}
- Millis JM, Maguire P, Cronin DC, Conjeevarum H, Johnson R, Conlin C, et al. Continuous human liver support as a bridge to transplantation. Hepatology 1999;30(4):168A. [Google Scholar]
Mitzner 1996 {published data only}
- Mitzner SR, Stange J. Treatment of acute and chronic hepatic failure with the MARS system‐ clinical results. ASAIO Journal 1996;42(1):12. [Google Scholar]
Morgan 1997 {published data only}
- Morgan GR, Chen D, Goldenberg A, Tobias H, Diflo T, Teperman L. Plasma exchange (PEx) as a bridge to successful liver transplantation (OLT) in the critically ill patient. Hepatology 1997;26(4 Pt. 2):565A. [Google Scholar]
Morsiani 2002 {published data only}
- Morsiani E, Pazzi P, Puviani AC, Brogli M, Valieri L, Gorini P, et al. Early experiences with a porcine hepatocyte‐based bioartificial liver in acute hepatic failure patients. The International Journal of Artificial Organs 2002;25(3):192‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Munoz 1989 {published data only}
- Munoz SJ, Ballas SK, Moritz MJ, Martinez J, Friedman LS, Jarrell BE, et al. Perioperative management of fulminant and subfulminant hepatic failure with therapeutic plasmapheresis. Transplantation Proceedings 1989;21(3):3535‐6. [PubMed] [Google Scholar]
Parbhoo 1971 {published data only}
- Parbhoo SP, Kennedy J, James IM, Chalstrey LJ, Ajdukiewicz A, Brock PJ, et al. Extracorporeal pig‐liver perfusion in treatment of hepatic coma due to fulminant hepatitis. The Lancet 1971;1(7701):659‐65. [DOI] [PubMed] [Google Scholar]
Ranek 1971 {published data only}
- Ranek L, Hansen RI, Hilden M, Ramsoe K, Schmidt A, Winkler K, et al. Pig liver perfusion in the treatment of acute hepatic failure. Scandinavian Journal of Gastroenterology 1971;9(Suppl):161‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Schmidt 2000 {published data only}
- Schmidt LE, Sorensen VR, Svendsen LB, Larsen FS, Stange J, Hansen BA. Improvement of systemic vascular resistance and arterial pressure in patients with acute on chronic liver failure during treatment with the molecular adsorbent recycling system (MARS). Hepatology 2000;32(4 Pt. 2):401A. [Google Scholar]
Sicot 1971 {published data only}
- Sicot C, Frejaville JP, Roche J, Rueff B, Benhamou JP, Fauvert R. 6 cases of severe hepatitis treated by interhuman crossed circulation. Annales de Medecine Interne (Paris) 1971;122(3):381‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Stange 1999 {published data only}
- Stange J, Mitzner SR, Risler T, Erley CM, Lauchart W, Goehl H, et al. Molecular adsorbent recycling system (MARS): clinical results of a new membrane‐based blood purification system for bioartificial liver support. Artificial Organs 1999;23(4):319‐30. [DOI] [PubMed] [Google Scholar]
Stange 2000 {published data only}
- Stange J, Mitzner SR, Klammt S, Freytag J, Peszynski P, Loock J, et al. Liver support by extracorporeal blood purification: a clinical observation. Liver Transplantation 2000;6(5):603‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sussman 1994 {published data only}
- Sussman NL, Gislason GT, Conlin CA, Kelly JH. The Hepatix extracorporeal liver assist device: initial clinical experience. Artificial Organs 1994;18:390‐6. [DOI] [PubMed] [Google Scholar]
Watanabe 1997a {published data only}
- Watanabe FD, Mullon CJ, Hewitt WR, Arkadopoulos N, Kahaku E, Eguchi S, et al. Clinical experience with a bioartificial liver in the treatment of severe liver failure: a phase I clinical trial. Annals of Surgery 1997;225(5):484‐94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watanabe 1997b {published data only}
- Watanabe FD, Shackleton CR, Cohen SM, Goldman DE, Arnaout WS, Hewitt W, et al. Treatment of acetaminophen‐induced fulminant hepatic failure with a bioartificial liver. Transplantation Proceedings 1997;29:487‐8. [DOI] [PubMed] [Google Scholar]
Yoshiba 1996 {published data only}
- Yoshiba M, Inoue K, Sekiyama K, Koh I. Favorable effect of new artificial liver support on survival of patients with fulminant hepatic failure. Artificial Organs 1996;20(11):1169‐72. [DOI] [PubMed] [Google Scholar]
Zhou 2002 {published data only}
- Zhou X, Wang X, Yang Y, Zhao L, Miao J, Ding J, Fan D. Clinical research of patients with acute or chronic hepatic failure treated with molecular adsorbent recirculating system [in Chinese]. Chinese Journal of Hepatology 2002;10(3):213‐5. [MEDLINE: ] [PubMed] [Google Scholar]
References to ongoing studies
Hayes 2000 {published data only}
- Hayes PC, et al. Developement of bioartificial liver assist device for use in patients with fulminant hepatic failure. The National Research Register 2000, issue 4.
Jalan 2002 {published data only}
- Jalan R, Steiner C, Kapoor d, Alisi A, Williams R. Molecular adsorbents recirculating system: early clinical experience in acute decompensation of chronic liver disease. Personal communication 2000.
VitaGen 2000 {unpublished data only}
- VitaGen Incorporated. FDA approved Phase I/II clinical trial to evaluate the safety of the ELAD artificial liver device in patients with FHF at six sites. VitaGen Incorporated 2000.
Additional references
Allen 2001
- Allen JW, Hassanein T, Bhatia SN. Advances in bioartificial liver devices. Hepatology 2001;34(3):447‐55. [DOI] [PubMed] [Google Scholar]
Ash 1994
- Ash SR. Hemodiabsorption in treatment of acute hepatic failure and chronic cirrhosis with ascites. The International Journal of Artificial Organs 1994;18(5):355‐62. [DOI] [PubMed] [Google Scholar]
Ash 2000
- Ash SR, Steczko J, Knab WR, Blake DE, Carr DJ, Herker KD, Levy H. Push‐pull sorbent‐based pheresis and hemodiabsorption in the treatment of hepatic failure: preliminary results of a clinical trial with the BioLogic‐DTPF System. Therapeutic Apheresis 2000;4(3):218‐28. [DOI] [PubMed] [Google Scholar]
Benson 2000
- Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. The New England Journal of Medicine 2000;342:1878‐86. [DOI] [PubMed] [Google Scholar]
Bonn 1996
- Bonn D. Hybrid devices offer hope for the failing liver. The Lancet 1996;348(9038):1372. [DOI] [PubMed] [Google Scholar]
Davenport 1991
- Davenport A, Will EJ, Davison AM. Continuous vs. intermittent forms of haemofiltration and/or dialysis in the management of acute renal failure in patients with defective cerebral autoregulation at risk of cerebral oedema. Contributions to Nephrology 1991;93:225‐33. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eguchi 2000
- Eguchi S, Kawazoe Y, Sugiyama N, Kawashita Y, Fujioka H, Furui J, et al. Effects of recombinant human hepatocyte growth factor on the proliferation and function of porcine hepatocytes. ASAIO Journal 2000;46(1):56‐9. [DOI] [PubMed] [Google Scholar]
Ellis 1996a
- Ellis AJ, Hughes RD, Wendon JA, Dunne J, Langley PG, Kelly JH, Gislason GT, Sussman NL, et al. Pilot‐controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology 1996;24(6):1446‐51. [DOI] [PubMed] [Google Scholar]
He 2000a
- He JQ, Chen CY, Deng JT, Qi HX, Zang XQ, Chen JQ. Charcoal hemoperfusion in the treatment of fulminant hepatic failure. Chinese Critical Care Medicine 2000;12(2):105‐8. [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 1994a
- Hughes RD, Pucknell A, Routley D, Langley PG, Wendon JA, Williams R. Evaluation of the BioLogic‐DT sorbent‐suspension dialyser in patients with fulminant hepatic failure. The International Journal of Artificial Organs 1994;17(12):657‐62. [PubMed] [Google Scholar]
Hughes 1996
- Hughes RD, Williams R. Use of bioartificial and artificial liver support devices. Seminars in Liver Disease 1996;16(4):435‐44. [DOI] [PubMed] [Google Scholar]
Hughes 1998b
- Hughes RD, Nicolaou N, Langley PG, Ellis AJ, Wendon JA, Williams R. Plasma cytokine levels and coagulation and complement activation during use of the extracorporeal liver assist device in acute liver failure. Artificial Organs 1998;22(10):854‐8. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. Code of Federal Regulations & International Conference on Harmonisation Guidelines. Pennsylvania: Parexel/Barnett, 1997; Vol. 1.
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Kunz 1998
- Kunz R, Oxman A. The unpredictability paradox: review of empirical comparisons of randomised and non‐randomised clinical trials. BMJ 1998;317:1185‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunz 2003
- Kunz R, Vist GE, Oxman AD. Randomisation to protect against selection bias in healthcare trials. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.MR000012.pub2] [DOI] [PubMed] [Google Scholar]
Lee 1993
- Lee WM. Acute liver failure. The New England Journal of Medicine 16‐12‐1993;329(25):1862‐72. [DOI] [PubMed] [Google Scholar]
Mitzner 2000a
- Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, et al. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transplantation 2000;6(3):277‐86. [DOI] [PubMed] [Google Scholar]
Mitzner 2001
- Mitzner SR, Stange J, Klammt S, Peszynski P, Schmidt R. Albumin dialysis using the molecular adsorbent recirculating system. Current Opinion in Nephrology and Hypertension 2001;10(6):777‐83. [DOI] [PubMed] [Google Scholar]
Mitzner 2001b
- Mitzner SR, Klammt S, Peszynski P, Hickstein H, Korten G, Stange J, et al. Improvement of multiple organ functions in hepatorenal syndrome during albumin dialysis with the molecular adsorbent recirculating system. Therapeutic Apheresis 2001;5(5):417‐22. [DOI] [PubMed] [Google Scholar]
Mitzner 2001c
- Mitzner SR, Stange J, Klammt S, Peszynski P, Schmidt R, Noldge‐Schomburg G. Extracorporeal detoxification using the molecular adsorbent recirculating system for critically ill patients with liver failure. Journal of American Society of Nephrology 2001;12 Suppl 17:S75‐S82. [PubMed] [Google Scholar]
Miwa 1996
- Miwa Y, Ellis AJ, Hughes RD, Langley PG, Wendon JA, Williams R. Effect of ELAD liver support on plasma HGF and TGF‐beta 1 in acute liver failure. The International Journal of Artificial Organs 1996;19(4):240‐4. [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses. The Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Neuberger 1998
- Neuberger J, Adams D, MacMaster P, Maidment A, Speed M. Assessing priorities for allocation of donor liver grafts: survey of public and clinicians. BMJ 1998;317(7152):172‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 1979
- O'Brien PC, Flemming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549‐56. [PubMed] [Google Scholar]
Peto 1976
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomised controlled trials requiring prolonged observation of each patient. 1. Introduction and design. British Journal of Cancer 1976;34:585‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pocock 1993
- Pocock SJ. Statistical and ethical issues in monitoring clinical trials. Statistics in Medicine 1993;12:1459‐69. [DOI] [PubMed] [Google Scholar]
Pocock 2000
- Pocock SJ, Elbourne DR. Randomized trials or observational tribulations?. The New England Journal of Medicine 2000;342:1907‐9. [DOI] [PubMed] [Google Scholar]
Rahman 1999
- Rahman TM, Hodgson HJ. Review article: liver support systems in acute hepatic failure. Alimentary Pharmacology and Therapeutics 1999;13(10):1255‐72. [DOI] [PubMed] [Google Scholar]
Riordan 1999
- Riordan SM, Williams R. Extracorporeal support and hepatocyte transplantation in acute liver failure and cirrhosis. Journal of Gastroenterology and Hepatology 1999;14:757‐70. [DOI] [PubMed] [Google Scholar]
Russell 1999
- Russell PS. Understanding resource use in liver transplantation. JAMA 1999;281(15):1431‐2. [DOI] [PubMed] [Google Scholar]
Sacks 1982
- Sacks H, Chalmers TC, Smith H. Randomized versus historical controls for clinical trials. American Journal of Medicine 1982;72(2):233‐40. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes R, Altman D. Empirical evidence of bias. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sorkine 2001
- Sorkine P, Ben Abraham R, Szold O, Biderman P, Kidron A, Merchav H, et al. Role of the molecular adsorbent recycling system (MARS) in the treatment of patients with acute exacerbation of chronic liver failure. Critical Care Medicine 2001;29(7):1332‐6. [DOI] [PubMed] [Google Scholar]
Stange 1995
- Stange J, Mitzner S, Strauss M, Fischer U, Lindemann S, Peters E, et al. Primary or established liver cells for a hybrid liver? Comparison of metabolic features. ASAIO Journal 1995;41(3):M310‐M315. [DOI] [PubMed] [Google Scholar]
Stange 1999a
- Stange J, Mitzner SR, Risler T, Erley CM, Lauchart W, Goehl H, et al. Molecular adsorbent recycling system (MARS): clinical results of a new membrane‐based blood purification system for bioartificial liver support. Artificial Organs 1999;23(4):319‐30. [DOI] [PubMed] [Google Scholar]
Stange 2000b
- Stange J, Mitzner SR, Klammt S, Freytag J, Peszynski P, Loock J, et al. Liver support by extracorporeal blood purification: a clinical observation. Liver Transplantation 2000;6(5):603‐13. [DOI] [PubMed] [Google Scholar]
Stockmann 2000
- Stockmann HB, Hiemstra CA, Marquet RL, Ijzermans JN. Extracorporeal perfusion for the treatment of acute liver failure. Annals of Surgery 2000;231:460‐70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sussman 1997
- Sussman NL, Kelly JH. Extracorporeal liver support: cell‐based therapy for the failing liver. American Journal of Kidney Disease 1997;30(5 Suppl 4):S66‐S71. [DOI] [PubMed] [Google Scholar]
Tandon 1999
- Tandon BN, Bernauau J, O'Grady J, Gupta SD, Krisch RE, Liaw YF, et al. Recommendations of the International Association for the Study of the Liver Subcommittee on nomenclature of acute and subacute liver failure. Journal of Gastroenterology and Hepatology 1999;14(5):403‐4. [DOI] [PubMed] [Google Scholar]
Wilkinson 1998b
- Wilkinson AH, Ash SR, Niessenson AR. Hemodiabsorption in treatment of hepatic failure. Journal of Tansplant Coordination 1998;8(1):43‐50. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kjaergard 2003
- Kjaergard LL, Liu J, Als‐Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute‐on‐chronic liver failure. A systematic review. JAMA 2003;289(2):217‐22. [DOI] [PubMed] [Google Scholar]


