Abstract
Exfoliative toxins produced by certain strains of Staphylococcus hyicus mediate exudative epidermitis in pigs. In this study the genes coding for four different exfoliative toxin from S. hyicus (ExhA, ExhB, ExhC, and ExhD) were cloned and sequenced. The coding sequence of the four toxin genes ranged from 816 to 834 bp. The amino acid sequences of these four toxins were homologous to the earlier described exfoliative toxins SHETB from S. hyicus and ETA, ETB, and ETD from Staphylococcus aureus. The homology between the S. hyicus toxins was at the same level as the homology to the exfoliative toxins from S. aureus. The toxins showed similarity to serine proteases, including preservation of the catalytic tract in ExhA, ExhB, and ExhC. However, in ExhD, Asp in the putative catalytic tract was replaced with Glu. The recombinant toxins could be expressed in Escherichia coli, and three of the four toxins were recognized by monoclonal antibodies raised against native exfoliative toxins.
Certain strains of Staphylococcus hyicus can cause the skin disease exudative epidermitis in pigs. Exudative epidermitis is characterized by separation of the cells in the epidermis in the upper stratum spinosum, exfoliation of the skin, erythema, and serous exudation (4, 32). Spontaneous exudative epidermitis in pigs is a generalized infection of the skin caused by strains of S. hyicus, which are able to produce a disease-causing factor designated exfoliative toxin (28). The exfoliative toxins responsible for the characteristic lesion of exudative epidermitis have been identified and purified from strains in Japan and Denmark. These toxins have been characterized as exoproteins of approximately 27 kDa or 30 kDa (6, 24). The toxins isolated in Japan were designated SHETA and SHETB (25), and the toxins isolated in Denmark were designated ExhA, ExhB, and ExhC. One additional toxin antigenically distinct from these was provisionally designated ExhD (5). The toxins differ in their antigenic properties (5, 26); however, no comparison of the toxins isolated in Japan and in Denmark has been published.
The complex of skin lesions called the staphylococcal scalded skin syndrome in humans (7, 10, 16) has several aspects in common with exudative epidermitis in pigs. Staphylococcal scalded skin syndrome is caused by infection with Staphylococcus aureus strains that produce exfoliative toxin ETA, ETB, or both (14). The exfoliative toxins from S. aureus, ETA and ETB, have been cloned and their nucleotide sequences have been determined (12, 17, 20, 22). Investigations have shown that the gene encoding ETA is located on the chromosome of S. aureus, while the gene encoding ETB is located on a 42-kb plasmid (reviwed by Arbuthnott [7]). The molecular masses of mature ETA and ETB as deduced from the amino acid sequences were 26,951 Da and 27,318 Da, respectively (17). The differences in the composition of the amino acid sequences reflected the previously observed antigenic differences of the two toxins (8, 13, 34). A third exfoliative toxin from S. aureus, designated ETC, was purified and characterized by Sato et al. (23), and recently, a fourth exfoliative toxin, designated ETD, was published by Yamaguchi et al. (35).
In S. hyicus, the synthesis of SHETB has been shown to be plasmid associated (25, 26), and recently the gene encoding SHETB was cloned from a plasmid DNA fraction of S. hyicus strain P-23 (31).
In the present study, the genes encoding the exfoliative toxins produced by selected virulent strains of S. hyicus were cloned in E. coli, sequenced, and expressed. The DNA sequences of the genes for the exfoliative toxins and the deduced amino acid sequences were compared to the corresponding published sequence data on the exfoliative toxins from S. aureus and from S. hyicus.
MATERIALS AND METHODS
Bacterial strains and media.
The S. hyicus strains used for cloning were selected based on their proved virulence and ability to produce ExhA (NTCT 10350) (9, 27), ExhB (1289D-88) (33), and ExhC (842A-88) (33) or virulent and negative for all three of these toxins (A2869C) (4). Additionally, a collection of Danish field isolates was included.
S. aureus strain 958 (eta+) was used for preparation of a DNA probe. Staphylococcus strains were grown in tryptic soy broth supplemented with 10% yeast extract. E. coli strain XL-Blue (Stratagene, La Jolla, Calif.) and TOP10 (Invitrogen, Carlsbad, Calif.), used for propagation of plasmids, was grown in LB medium with or without ampicillin (50 μg/ml).
DNA manipulations.
Purification of Staphylococcus DNA was done as described earlier (2). Purification of plasmids was done by standard phenol-chloroform extraction. Restriction enzyme digestion and agarose gel electrophoresis were done by standard procedures (18).
Southern blot.
Genomic DNA was digested with restriction enzymes, electrophoresed in a 0.8% agarose gel, transferred to nitrocellulose, and hybridized with the labeled probe ON at 55 to 65°C in a hybridization buffer containing 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The filters were washed in 2× SSC-0.1% sodium dodecyl sulfate (SDS) at 55 to 65°C twice for 15 min each, followed by color development with the digoxigenin labeling kit (Boehringer, Mannheim, Germany) following the supplier's recommendations. Probe: A PCR amplicon was purified on spin columns (Qiagen, Hilden, Germany) and digoxigenin labeled with the abovementioned kit.
Cloning.
Genomic libraries were constructed by cloning of S. hyicus DNA partially digested with the restriction enzyme Tsp509I into the EcoRI site of the plasmid vectors pGEX5x-1, pGEX5x-2, and pGEX5x-3 (Pharmacia, Uppsala, Sweden) and electroporated into E. coli XL-Blue at 1.7 kV.
Specific fragments were cloned in the plasmid vector pGEX5x-1, pGEX5x-2, or pGEXx-3 or in pUC18 (Pharmacia, Uppsala, Sweden). Recombinant cells were spread on LB plates containing 50 μg of ampicillin per ml and incubated at 37°C for 6 h and then at room temperature overnight.
The libraries were screened with digoxigenin-labeled DNA probes as described for Southern blots. Alternatively, libraries were screened with monoclonal antibodies. Each overnight plate was overlaid with a sterile 88-mm nitrocellulose filter, and the upside down colonies were transferred to a fresh LB plate containing ampicillin and 1 mM isopropylthiogalactopyranoside (IPTG). The colonies as well as the original plates were incubated at 37°C for 3 h. The filters were washed in phosphate-buffered saline-0.5% SDS for 10 min, followed by three 10-min washes in Tris-buffered saline and incubation with monoclonal antibody MAbEXH5.1 (identifies ExhB) (6) or MabEXH7.7 (identifies ExhA, ExhB, and ExhC) (5) in Tris-buffered saline (12.5 mM NaCl, 50 mM Tris-HCl, pH 9.5, 0.5% Tween 20) at 4°C overnight. Toxin-producing colonies were visualized by incubation with peroxidase-conjugated rabbit anti-mouse immunoglobulin (Dako, Glostrup, Denmark) diluted 1:2000 and staining with tetramethylbenzidine and hydrogen peroxide.
From pGEX, the toxin genes were subcloned in the expression vector pBAD/His. Purification of recombinant antigen was done with a nickel-chelating resin as recommended by the manufacturer (Invitrogen, Carlsbad, Calif.). SDS-PAGE, silver staining and immunoblot with sera and monoclonal antibodies were done as previously described (5).
PCR.
The DNA was PCR amplified for 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min by standard PCR conditions (10 mM Tris-HCl, 50 mM KCl, 2.5 mM MgCl2, 100 pM each of the four deoxynucleotides, 65 ng of each primer, and 0.5 U of Taq polymerase) unless otherwise specified, with the primers listed in Table 1.
TABLE 1.
Primers used for PCR amplification and/or sequencing
| Primer | Sequence (5′ → 3′)a |
|---|---|
| Vector | |
| pGEX5 | GGGCTGGCAAGCCACGTTTGGTG |
| pGEX3 | CCGGGAGCTGCATGTGTCAGAGG |
| m13-F(pUC) | GTTGTAAAACGACGGCCAG |
| m13-R(pUC) | AGGAAACAGCTATGACCA |
| S. aureus | |
| aureus1 | AAGTACTATGGTGTCAATGC |
| aureus2 | TCTCTATCAAGATGAGACAC |
| ExhA | |
| V1-5 | AGTAGAATTGCCTTTTGGTG |
| V1-3 | ATGACCAACAAGGATTTCTG |
| V1-1-1Ar | ATAAACAAGGAGCCTACAGC |
| V1-1-1Bf | TATGGTCAGTGATTATGCAC |
| ExhB | |
| B1 | AATTGCCTTGCATGTGATACTG |
| ExhC | |
| V6ABF | ATAGATGCACCATTTGGAAC |
| V6ABR | GAATTACCTAAATCAGCAGC |
| V6C | AAAGGCGTATATCGATAATC |
| V6D | CTTCATCATTATAACGATTCC |
| ExhD | |
| NN1 | CCWTATCAAKCWGTWGGC |
| NN2 | ATNSSWGAKCCTGAATTWCC |
| MM1 | CAAAAAACCGTTGCTACCG |
| MM2 | TTTTGAACATGCTCGTTAGG |
| V9AR | TCGTTTTCTGCTTGTTTAGC |
K = G or T; N = A, C, G, or T; S = C or G; W = A or T.
Sequencing.
Cloned fragments were PCR amplified as described above. The amplification products were purified with QIAqiuck spin columns (Qiagen, Hilden, Germany) and sequenced on ABI 373A/377 automatic sequencers with the Prism BigDye terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.) and the primers listed in Table 1.
Sequence analysis.
Phylogenetic and molecular evolutionary analyses were conducted with Clustal (30), GeneDoc (K. B. Nicholas and H. B. Nicholas, Jr., 1997, www.psc.edu/biomed/gendoc), and MEGA version 2.1 (15). Predictions of putative protoxin cleavage sites were performed with SignalP (19). Molecular weights were calculated with the Compute pI/Mw tool at www.expasy.ch.
Nucleotide accession numbers.
The toxin sequences were given the following GenBank accession numbers: ExhA, AF515453; ExhB, AF515454; ExhC, AF515455; and ExhD, AF515456.
RESULTS
In earlier studies, three different types of exfoliative toxin were identified among Danish S. hyicus strains, ExhA, ExhB, and ExhC. These have been characterized, and specific sera and monoclonal antibodies have been produced (5). At least one additional toxin could be expected, as a number of virulent isolates failed to react with any of these antibodies. Here, the cloning of ExhA, ExhB, ExhC, and ExhD is described.
ExhB.
From the ETA-positive S. aureus strain 958, a 615-bp fragment was amplified with the primers aureus1 and aureus2 (Table 1). This fragment was digoxigenin labeled and used as the probe in a Southern hybridization with S. hyicus strain 1289D-88 at low stringency (hybridization and washing at 55°C). The probe identified an approximately 1-kb EcoRI fragment. To achieve a clone expressing antigen that could be identified by the monoclonal antibody, genomic EcoRI fragments were excised from an agarose gel and cloned in a pool of plasmid vectors covering all three reading frames (pGEX5x-1, pGEX5x-2, and pGEX5x-3). An ExhB-producing clone was identified by MabEXH5.1, and the insert was sequenced. This clone contained a major part of the ExhB gene, and the remaining part was found on an overlapping 1.5-kb HindIII fragment with the cloned EcoRI fragment as the probe. The HindIII fragment was cloned in pUC18.The complete gene was sequenced with pGEX- and pUC-specific primers and primer B1 (Table 1).
ExhA.
The ExhA gene was identified by screening a genomic library of partially TspI-digested DNA from S. hyicus strain NCTC10350 with monoclonal antibody MabEXH7.7. The gene was found on two different clones containing 4 and 6 kb, respectively, of S. hyicus DNA. The sequence of this gene was obtained with primers V1-5, V1-3, V1-1-1Ar, and V1-1-1Bf and the vector primers (Table 1).
ExhC.
The ExhC gene was isolated from a genomic library of strain 842A-88 as described for ExhA by screening with monoclonal antibody MabEXH7.7. The sequence of the ExhC gene was determined with the vector primers and V6 primers listed in Table 1.
ExhD.
As no antibody or probe was available to screen genomic libraries for ExhD, a different approach was applied to clone this gene. Alignment of the deduced amino acid sequences of ExhA, ExhB, and ExhC revealed two conserved regions (positions 84 and 237 in Fig. 2) that were also conserved in the S. aureus ETB sequence. Thus, a similar sequence might also be present in a putative ExhD sequence. Based on the conserved amino acid regions PYQA/SVG (PYNSVG in S. aureus ETB) (12) and GNSGSP/GI (GNSGSGS in S. aureus ETB), two degenerate PCR primers, NN1 and NN2 (Table 1), were constructed. PCR with genomic DNA from strain A2869C at low stringency (annealing temperature of 50°C) yielded an amplicon that was sequenced for the design of specific primers MM1 and MM2. These two primers were used in a PCR to produce a DNA probe that hybridized in a Southern blot to a 3-kb TaqI restriction fragment. The exhD gene was isolated from a library of strain A2869C TaqI fragments cloned in pUC18. Primers MM1, MM2, and V9AR and vector-specific primers were used for sequencing of the exhD gene.
FIG. 2.
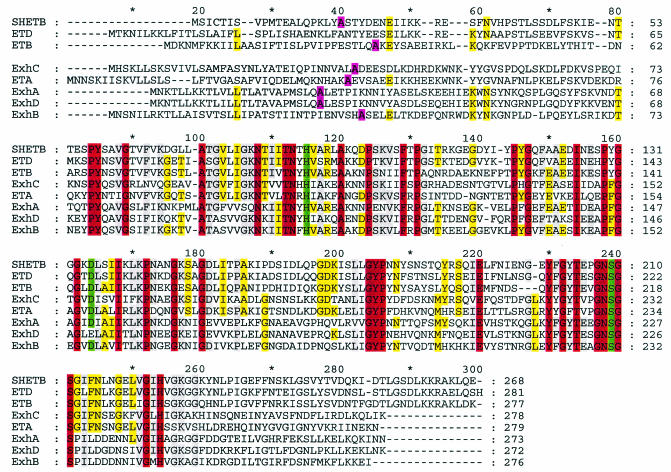
Alignment of the amino acid sequences of ExhA, ExhB, ExhC, ExhD, and SHETB from S. hyicus and ETA, ETB, and ETD from S. aureus. Identical amino acids are shown in red, while grey and yellow indicate six to seven and five identical amino acids, respectively. The putative signal peptide cleavage site around positions 40 is shown in pink. In ETA, His-72, Asp-120, and Ser-195 (ETA numbering) are considered to be involved in the active site of the toxins. These three amino acids are shown in green; His-115 (H), Asp 164 (D), and Ser-239(S). It is noteworthy that Asp-164 in ExhD is replaced with Glu (E).
Recombinant toxins.
For a simple preparation of recombinant toxin, all four genes were subcloned in the expression vector pBad/His. Following a simple nickel-chelating chromatography step, the yield from the four recombinant clones was between 1 and 10 mg of 50 to 80% pure toxin per liter of culture.
The identity of the recombinant toxins ExhA, ExhB, and ExhC was confirmed by immunoblot analysis with monoclonal antibodies MabEXH5.1 and MabEXH7.7 as well as polyclonal rabbit sera raised against the three toxins isolated from S. hyicus (not shown).
Sequence analysis.
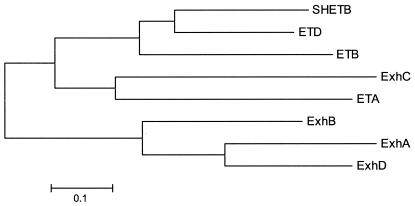
The coding sequences of the four toxins are very similar in length, ranging from 816 to 834 bases. The sequences of the four toxins were compared with the sequence of the earlier published S. hyicus exfoliative toxin SHETB and the genes of the three S. aureus toxin ETA, ETB, and ETD. At the DNA level, there was homology of 45 to 70% with ExhA and ExhD showing the highest degree of identity. A phylogenetic analysis of the proteins is shown in Fig. 1. All of the displayed toxins are related to each other with an identity of 49 to 54%. ExhC had higher similarity to ETA than to the other S. hyicus exfoliative toxins. Also, SHETB from S. hyicus and ETD from S. aureus were more similar to each other than to the other exfoliative toxins from S. hyicus and S. aureus. It is noteworthy that there is no species-specific clustering of the amino acid sequences of the toxins.
FIG. 1.
Relationships between eight exfoliative toxins from S. hyicus and S. aureus. The phylogenetic tree (neighbor joining method) is based on the amino acid sequences. ExhA, ExhB, ExhC, ExhD, and SHETB are from S. hyicus, while ETA, ETB, and ETD are from S. aureus. No species-specific clustering of the toxin genes was observed.
The molecular sizes of the deduced peptides from ExhA, ExhB, ExhC, and ExhD were approximately 30 kDa for the protoxins and approximately 27 kDa for the mature toxins, which is in good agreement with the previous values for ExhA, ExhB, and ExhC determined by SDS-PAGE (5).
A direct comparison of the amino acid sequences of the toxins ExhA, ExhB, ExhC, and ExhD, SHETB, ETA, ETB, and ETD is shown in the alignment displayed in Fig. 2. Two additional published exfoliative toxin genes, ETC and SHETA from S. aureus and S. hyicus, respectively, were not included, as these have been shown to be distinctly different from SHETB and the S. aureus exfoliative toxins (35). There is significant homology among the eight toxins in most of the central part of the molecule, in particular in the proximity of the three amino acids that in ETA were shown to constitute the active site (His-72, Asp-120, and Ser-195, ETA numbering) (17). In Fig. 2 these three amino acids are shown in green as His-115 (H), Asp-164 (D), and Ser-239 (S). The three amino acids of the active site are present in all of the toxins with the exception that in ExhD aspartic acid is replaced with glutamic acid. As an amino acid substitution in the putative active site was unexpected, a part of the exhD gene was PCR amplified and sequenced from 25 ExhD-positive S. hyicus field strains. All of the exhD genes analyzed were found to contain Glu rather than Asp at the position in question.
DISCUSSION
In this study we cloned and sequenced four different exfoliative toxins from S. hyicus. In an earlier investigation on the distribution of three of these toxins, ExhA, ExhB, and ExhC, the existence of yet another exfoliative toxin from S. hyicus, provisionally designated ExhD, was suggested (5). This suggestion was based on the finding that certain strains that were negative for the three abovementioned toxins were still able to induce exudative epidermitis by experimental infection of piglets (5, 33). The cloning and sequencing of a gene from S. hyicus strain A2869 that is similar to ExhA, ExhB, ExhC, and SHETB clearly substantiate the existence of ExhD.
Additionally, in an other investigation (L. O. Andresen and P. Ahrens, Proc. 17th Int. Pig Vet. Soc. Congress, Ames, Iowa, 2002, p 43), S. hyicus strains from 32 pig herds with exudative epidermitis and negative for exhA, exhB, and exhC were examined by a PCR specific for the exhD gene. Here exhD-positive S. hyicus could be found in 24 out of the 32 herds, which could indicate that ExhD is a disease-causing factor. However, it remains to be shown experimentally that the protein expressed from this gene can actually act as an exfoliative toxin in vivo.
All of the exfoliative toxins except ExhD have conserved amino acids corresponding to the catalytic triad of serine proteases, indicating a common mode of action for all the toxins irrespective of the species of origin. The finding of glutamine instead of asparagine in the presumptive catalytic site in ExhD was surprising. However the generality of this finding was confirmed by sequence analysis of exhD genes from 25 Danish field isolates, all of which contained Glu at this specific position. This amino acid substitution might alter the activity of the toxin; however, it should be noted that the Asp to Glu substitution includes the minimal change in biochemical properties of the toxin. Piglets experimentally infected with ExhD-expressing strains of S. hyicus have shown clinical signs of exudative epidermitis (33). This could indicate that ExhD has a specificity that is similar to that of ExhA, ExhB, ExhC, or SHETB.
The four exfoliative toxins described here together with SHETB and the S. aureus toxins ETA, ETB, and ETD form a group of similar molecules. A previous comparison of the amino acid sequences of ETA, ETB, ETC, ETD, SHETA, and SHETB showed that ETC and SHETA are quite different from the other exfoliative toxins, including the sequence of the putative catalytic site (35). Analysis including the amino acid sequences for ExhA, ExhB, ExhC, and ExhD confirmed this (data not shown). Therefore, ETC and SHETA were not included in Fig. 1 and 2.
Like the other exfoliative toxins, ExhA, ExhB, ExhC, and ExhD show a certain similarity to the staphylococcal V8 protease, which is a serine protease. The similarity includes preservation of the catalytic tract of the active site of the serine proteases (Fig. 2) (21).
Recently, the target for epidermolysins ETA, ETB, and ETD, desmoglein-1, a cell-cell adhesion molecule, was identified and characterized (3, 11). These studies showed that the three toxins act as serine proteases with highly focused specificity to cleave human and mouse desmoglein. Given the similarity between these toxins and ExhA, ExhB, ExhC, ExhD, and SHETB, it could be speculated that a similar specificity will be found for these toxins.
The phylogenetic analysis the toxins (Fig. 1) showing that the exfoliative toxins from S. hyicus and S. aureus are almost as similar between the species as within each species could lead to speculations that horizontal gene transfer may occur within staphylococci. An earlier study indicated a host-specific evolution for Staphylococcus intermedius (1). Further analysis of strains of various Staphylococcus species and from different geographic regions may elucidate the evolution of exfoliative toxin genes within this genus.
Recently, the cloning of an exfoliative toxin gene from S. intermedius (SIET) was published (29). This toxin, like SHETA from S. hyicus and ETC from S. aureus, is quite different from the serine protease-like group of exfoliative toxins. Thus, there appears to exist a group of exfoliative toxins that are serine proteases and that are found in two different species. At the same time, other molecules from the same species have been described as exfoliative toxins.
In conclusion, the exfoliative toxins cloned and sequenced in the present study and SHETB from S. hyicus as well as the exfoliative toxins ETA, ETB, and ETD from S. aureus constitute a group of related exfoliative toxins, which all have traits in common with other serine proteases at the amino acid level.
Acknowledgments
The expert technical assistance of Kirsten Vestergaard is gratefully acknowledged.
REFERENCES
- 1.Aarestrup, F. M. 2001. Comparative ribotyping of Staphylococcus intermedius isolated from members of the Canoidea gives possible evidence for host-specificity and coevolution of bacteria and hosts. Int. J. Syst. E vol. Microbiol. 51:1343-13477. [DOI] [PubMed] [Google Scholar]
- 2.Aarestrup, F. M., H. C. Wegener, and V. T. Rosdahl. 1995. Evaluation of phenotypic and genotypic methods for epidemiological typing of Streptococcus aureus isolates from bovine mastitis in Denmark. Vet. Microbiol. 45:139-150. [DOI] [PubMed] [Google Scholar]
- 3.Amagai, M., N. Matsuyoshi, Z. H. Wang, C. Andl, and J. R. Stanley. 2000. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat. Med. 6:1275-1277. [DOI] [PubMed] [Google Scholar]
- 4.Amtsberg, G. 1979. Nachweis von Exfoliation auslösenden Substanzen in Kulturen von Staphylococcus hyicus des Schweines und Staphylococcus epidermidis Biotyp 2 des Rindes. Zentralbl. Vetmed. Reihe B 26:257-272. [PubMed] [Google Scholar]
- 5.Andresen, L. O. 1998. Differentiation and distribution of three types of exfoliative toxin produced by Staphylococcus hyicus from pigs with exudative epidermititis. FEMS Immunol. Med. Microbiol. 20:301-310. [DOI] [PubMed] [Google Scholar]
- 6.Andresen, L. O., V. Bille-Hansen, and H. C. Wegener. 1997. Staphylococcus hyicus exfoliative toxin: purification and demonstration of antigenic diversity among toxins from virulent strains. Microb. Pathog. 22:113-122. [DOI] [PubMed] [Google Scholar]
- 7.Arbuthnott, J. P. 1983. Epidermolytic toxins, p. 599-617. In C. S. F. Easmon and C. Adlam (ed.), Staphylococci and staphylococcal infections. Academic Press, London, United Kingdom.
- 8.Arbuthnott, J. P., and B. Billcliffe. 1976. Qualitative and quantitative methods for detecting staphylococcal epidermolytic toxin. J. Med. Microbiol. 9:191-201. [DOI] [PubMed] [Google Scholar]
- 9.Devriese, L. A., V. Hájek, P. Oeding, S. A. Meyer, and K. H. Schleifer. 1978. Staphylococcus hyicus (Sompolinsky 1953) comb. nov. and Staphylococcus hyicus subsp. chromogenes subsp. nov. Int. J. Syst. Bacteriol. 28:282-290. [Google Scholar]
- 10.Elias, P. M., P. Fritsch, and E. H. Epstein. 1977. Staphylococcal scalded skin syndrome. Clinical features, pathogenesis, and recent microbiological and biochemical developments. Arch. Dermatol. 113:207-219. [DOI] [PubMed] [Google Scholar]
- 11.Hanakawa, Y., N. M. Schechter, C. Lin, L. Garza, H. Li, T. Yamaguchi, Y. Fudaba, K. Nishifuji, M. Sugai, M. Amagai, and J. R. Stanley. 2002. Molecular mechanisms of blister formation in bullous impetigo and staphylococcal scalded skin syndrome. J. Clin. Investig. 110:53-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson, M. P., and J. J. Iandolo. 1986. Sequence of the exfoliative toxin B gene of Staphylococcus aureus. J. Bacteriol. 167:726-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, A. D., L. Spero, J. S. Cades, and B. T. De Cicco. 1979. Purification and characterization of different types of exfoliative toxin from Staphylococcus aureus. Infect. Immun. 24:679-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo, I., S. Sakurai, Y. Sarai, and S. Futaki. 1975. Two types of exfoliative toxin and their distribution in staphylococcal strains isolated from patients with scalded skin syndrome. J. Clin. Microbiol. 1:397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 16.Ladhani, S., C. L. Joannou, D. P. Lochrie, R. W. Evans, and S. M. Poston. 1999. Clinical, microbial, and biochemical aspects of the exfoliative toxin causing staphylococcal scalded-skin syndrome. Clin. Microbiol. Rev. 12:224-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, C. Y., J. J. Schmidt, A. D. Johnson-Winegar, L. Spero, and J. J. Iandolo. 1987. Sequence determination and comparison of the exfoliative toxin A and toxin B gene from Staphylococcus aureus. J. Bacteriol. 169:3904-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning, a laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 19.Nielsen, H., J. Engelbrecht, S. Brunak, and G. Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 20.O'Toole, P. W., and T. J. Foster. 1987. Nucleotide sequence of the epidermolytic toxin A gene of Staphylococcus aureus. J. Bacteriol. 169:3910-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rawlings, N. D., and A. J. Barrett. 1994. Families of serine peptidases. Methods Enzymol. 244:19-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai, S., H. Suzuki, and I. Kondo. 1988. DNA sequence of the eta gene coding for staphylococcal exfoliative toxin serotype A. J. Gen. Microbiol. 134:711-717. [DOI] [PubMed] [Google Scholar]
- 23.Sato, H., Y. Matsumori, T. Tanabe, H. Saito, A. Shimizu, and J. Kawano. 1994. A new type of staphylococcal toxin from Staphylococcus aureus strain isolated from a horse with plegmon. Infect. Immun. 62:3780-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato, H., T. Tanabe, M. Kuramoto, K. Tanaka, T. Hashimoto, and H. Saito. 1991. Isolation of exfoliative toxin from Staphylococcus hyicus subsp. hyicus and its exfoliative activity in the piglet. Vet. Microbiol. 27:263-275. [DOI] [PubMed] [Google Scholar]
- 25.Sato, H., T. Watanabe, K. Higuchi, K. Teruya, A. Ohtake, Y. Murata, H. Saito, C. Aizawa, H. Danbara, and N. Maehara. 2000. Chromosomal and extrachromosomal synthesis of exfoliative toxin from Staphylococcus hyicus. J. Bacteriol. 182:4069-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato, H., T. Watanabe, Y. Murata, A. Ohtake, M. Nakamura, C. Aizawa, H. Saito, and N. Maehara. 1999. New Exfoliative toxin produced by a plasmid-carrying strain of Staphylococcus hyicus. Infect. Immun. 67:4014-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sompolinsky, D. 1950. Impetigo contagiosa suis. Maanedsskrift Dyrlaeger 61:401-453. [Google Scholar]
- 28.Tanabe, T., H. Sato, H. Sato, K. Watanabe, M. Hirano, K. Hirose, S. Kurokawa, K. Nakano, H. Saito, and M. Maehara. 1996. Correlation between occurrence of exudative epidermitis and exfoliative toxin-producing ability of Staphylococcus hyicus. Vet. Microbiol. 48:9-17. [DOI] [PubMed] [Google Scholar]
- 29.Terauchi, R., H. Sato, Y. Endo, C. Aizawa, and N. Maehara. 2003. Cloning of the gene coding for Staphylococcus intermedius exfoliative toxin and its expression in Escherichia coli. Vet. Microbiol. 94:31-38. [DOI] [PubMed] [Google Scholar]
- 30.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe, T., H. Sato, Y. Hatakeyama, T. Matsuzawa, M. Kawai, C. Aizawa, H. Danbara, and N. Maehara. 2000. Cloning of the gene coding for Staphylococcus hyicus exfoliative toxin B and its expression in Escherichia coli. J. Bacteriol. 182:4101-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wegener H. C., and E. W. Skov-Jensen. 1999. Exudative epidermititis, p. 469-474. In B. E. Straw et al. (ed.), Diseases of swine, 8th ed. Iowa State University Press, Ames, Iowa.
- 33.Wegener, H. C., L. O. Andresen, and V. Bille-Hansen. 1993. Staphylococcus hyicus virulence in relation to exudative epidermitis in pigs. Can J. Vet. Res. 57:119-125. [PMC free article] [PubMed] [Google Scholar]
- 34.Wiley, B. B., and M. Rogolsky. 1977. Molecular and serological differentiation of staphylococcal exfoliative toxin synthesized under chromosomal and plasmid control. Infect. Immun. 18:487-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi, T., K. Nishifuji, M. Sasaki, Y. Fudaba, M. Aepfelbacher, T. Takata, M. Ohara, H. Komatsuzawa, M. Amagai, and M. Sugai. 2002. Identification of the Staphylococcus aureus etd pathogenicity island which encodes a novel exfoliative toxin, ETD, and EDIN-B. Infect. Immun. 70:5835-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]