Abstract
Through a combined metabolomics and transcriptomics approach we analyzed the events that took place during the first 5 d of infesting intact tomato (Lycopersicon esculentum) plants with spider mites (Tetranychus urticae). Although the spider mites had caused little visible damage to the leaves after 1 d, they had already induced direct defense responses. For example, proteinase inhibitor activity had doubled and the transcription of genes involved in jasmonate-, salicylate-, and ethylene-regulated defenses had been activated. On day four, proteinase inhibitor activity and particularly transcript levels of salicylate-regulated genes were still maintained. In addition, genes involved in phospholipid metabolism were up-regulated on day one and those in the secondary metabolism on day four. Although transcriptional up-regulation of the enzymes involved in the biosynthesis of monoterpenes and diterpenes already occurred on day one, a significant increase in the emission of volatile terpenoids was delayed until day four. This increase in volatile production coincided with the increased olfactory preference of predatory mites (Phytoseiulus persimilis) for infested plants. Our results indicate that tomato activates its indirect defenses (volatile production) to complement the direct defense response against spider mites.
Plants employ diverse strategies to resist or evade arthropod herbivores. Some plants accumulate constitutively high levels of compounds that function as biochemical defenses; some minimize herbivore damage through rapid growth or development, dispersion, or choice of habitat (Gatehouse, 2002). When a herbivore starts feeding on a plant it inflicts mechanical damage resulting in a wound response that in general is aspecific (Schaller and Frasson, 2001). However, herbivores can also produce cues that enable plants to respond in a much more targeted fashion. Such cues can be products in herbivore saliva or regurgitant (McCloud and Baldwin, 1997) but also plant products that result from herbivore damage (Pare et al., 1998). In general, the plant's direct defense response upon herbivory is characterized by the activation of signaling cascades, which leads to the formation of specific products and physiological changes that interfere with the performance of the herbivore. Additionally, it has been found that plants can produce herbivore-induced volatile metabolites. It has been proposed that this happens when direct defenses are inadequate (Kahl et al., 2000), since in many cases it appeared that these volatiles had the property of making the plant more attractive to predators or parasitoids of herbivores (Pare and Tumlinson, 1999). The attraction of natural enemies of a pest species is referred to as the indirect defense response (Sabelis et al., 2001). It is believed that the direct and the indirect defense strategies act in concert (Baldwin and Preston, 1999). However, hardly anything is known about the properties of their interaction and their interdependence.
In tomato, the herbivore-induced direct defense response is characterized by an increase in signaling compounds like jasmonate (JA), ethylene (Et), salicylate (SA) and systemin, which results in local and systemic changes, such as the accumulation of wound-inducible proteinase inhibitors (WIPIs), polyphenol oxidase, peroxidase (POD), chitinases, and callose synthase (Walling, 2000). The direct defense responses of tomato plants against stylet-feeders (such as phloem-feeding whiteflies, aphids, and mesophyll-feeding mites and thrips) and leaf-chewing insects (such as lepidopteran larvae) appears to be different. Caterpillars stimulate polyphenol oxidase and lipoxygenase activity, leaf miners only POD activity, and spider mites POD and lipoxygenase activity (Stout et al., 1994). Also, spider mites feeding on tomato induce the accumulation of WIPI-II transcripts, whereas whiteflies do not (Walling, 2000).
Li et al. (2002) showed that the two-spotted spider mite Tetranychus urticae induces a rapid jasmonate-regulated direct defense-response in tomato. This mesophyll-feeding mite has been recorded from over 900 plant species comprising 124 different plant families (Egas et al., 2003). The predatory mite Phytoseiulus persimilis is the natural enemy of T. urticae and is commonly used to control spider mites on tomato (Drukker et al., 1997; Garthwaite, 2000). It has been well established that such predatory species discriminate between prey-infested and uninfested plants on the basis of odors (for reviews, see Dicke et al., 1998; Sabelis et al., 1999).
The volatiles produced by tomato plants are predominantly monoterpenes, but they also produce a variety of sesquiterpenes, aromatics, aldehydes, ketones, alcohols, and esters (Andersson et al., 1980; Lundgren et al., 1985; Buttery et al., 1987; Smith et al., 1996). When infested with spider mites, leaves of the Lycopersicon esculentum Mill cv Moneymaker start to emit more of the phenolic methyl salicylate (MeSA) and the homoterpene 4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT; Dicke et al., 1998). However, different herbivore species can induce different volatiles. For example, upon feeding by larvae of Spodoptera exigua, leaves of the Lycopersicon esculentum Mill cv Castlemart do not produce MeSA or TMTT (Thaler et al., 2002), whereas in the cultivar Moneymaker, larvae of Spodoptera littoralis do induce the emission of MeSA. This emission appeared to fluctuate in a complex manner, partly related to the light-dark cycle (Vercammen et al., 2001). Foraging predatory mites can somehow cope with such fluctuations and there is consensus that some induced volatiles (e.g. linalool, MeSA, and trans-β-ocimene) can attract predatory mites when offered as pure chemical (Dicke et al., 1990).
Here we have investigated tomato's defense responses to spider mites during the first 5 d of infestation because in this period no eggs hatched. This enabled us to simultaneously assess the temporal progress of leaf damage, spider mite performance, transcriptome changes (through microarray and RNA gel-blot analysis), changes in volatile emission, and the attraction of predatory mites.
RESULTS
Performance of Spider Mites
Spider mites caused chlorotic lesions when feeding on tomato leaf tissue. We took the total necrotic area of an infested leaflet as a measure to determine the optimal infestation density. Figure 1A shows the average damage in millimeters squared over a period of 5 d for four different mite densities. Fifty mites necrotized approximately 24% of the leaflet area within 5 d. To retain enough viable plant material during the experiment we therefore chose an infestation-dose of 15 adult female spider mites per leaflet. Moreover, with 50 spider mites per leaflet the damage per mite per day was less than that with 15 mites. Figure 1C shows an example of the damage inflicted by 0, 1, 5, 15, and 50 spider mites per leaflet after a period of 5 d. At a density of 15 spider mites per leaflet, each individual spider mite damaged on average 1.4 ± 0.2 mm2/d. The total damage on day five corresponded to an average leaf area of approximately 14%.
Figure 1.
Spider mite performance and damage to tomato leaves. A, Represents the average damaged leaf area in millimeters squared over a period of 5 d inflicted by 1, 5, 15, and 50 adult female spider mites. The vertical bars represent the ses. B, Represents the number of eggs (black line) and juveniles (dotted line) produced by adult females (2-d-old at day 1 of the experiment). The vertical bars represent the se. C, Spider mites caused chlorotic lesions when feeding on tomato leaf tissue. The photo shows the accumulated damage on day five inflicted by 0, 1, 5, 15, and 50 adult female spider mites.
In all our experiments we infested tomato plants with adult female spider mites. The number of eggs produced per adult female was used as a measure of their performance. Eggs started to hatch after 5 d (Fig. 1B), leading to a population increase. Therefore, we decided to do our experiments within a 5-day time frame. Two-day-old adult females laid on average 7.4 ± 0.5 eggs per day during this period (Fig. 1B).
Microarray Analysis and Northern-Blot Analysis
A dedicated microarray with 428 tomato expressed sequence tags (ESTs) was made to investigate which genes were regulated through spider mite feeding. These ESTs were selected on the basis of their potential relevance to plant-herbivore and plant-pathogen interactions, and signaling in general. Data on the ESTs that were used and the details of the microarray can be found in the Supplemental Data (available at www.plantphysiol.org). The microarray slide also contained 839 PCR-amplified cDNA fragments and 170 ESTs from petunia (Petunia hybrida) flowers and 46 controls for unrelated experiments. To distinguish between early and late responses of tomato upon spider mite infestation, transcript levels were determined after 1 and 4 d, in 3 independent experiments.
On day one we found that the transcript levels of 60 genes (45 from tomato and 15 from petunia) were at least 1.6 times higher in plants infested by spider mites than in control plants. We have chosen a 1.6-fold difference in transcript level as reliable and relevant since this difference in transcript levels is reproducible with northern blots as shown for diacylglycerol kinase (DGK; Fig. 2). Two additional criteria, a P-value (adjusted for multiple testing) smaller than 0.05 and a signal to noise (S:N) ratio larger than 2.0 were also applicable to the data of these 45 tomato and 15 petunia clones. An adjusted P-value smaller than 0.05 corresponds to a significance level (α) of 0.007 and 0.006 for day one and four respectively, indicating the significant difference between transcript levels in control and spider mite-infested plants. Cross-hybridization between tomato cDNA probes and petunia cDNA is expected under the hybridization conditions used for the microarray slides due to their high homology. Specific cross-hybridization between tomato DNA probes and petunia RNA was validated for salicylic acid methyl transferase and vice versa for 1-deoxy-d-xylulose-5-P synthase (DOXP) through RNA gel-blot analysis (data not shown). On day four, 67 genes were more than 1.6 times up-regulated, of which 34 originated from tomato and 33 from petunia. Five genes (two from tomato, three from petunia) were down-regulated on day one and four genes on day four (all four from tomato). Genes that could be assigned to a signaling pathway and were differentially expressed on either day one or day four are listed in Table I and will be discussed accordingly.
Figure 2.
Spider mite-induced gene expression. Three leaves of 3-week-old tomato plants were each infested with 15 spider mites and RNA was isolated after 1, 2, 3, and 4 d. RNA gel-blots (Verdonk et al., 2003) were hybridized with pathogenesis related protein P6 (TC 115911), diacylglycerol kinase (DGK; BE434771), or polyubiquitin (UBQ) as loading control. The ethidium bromide-stained gel (EtBr) is shown to confirm equal loading. Control plants were grown under identical conditions without spider mites.
Table I.
Spider mite-regulated genes
| Plant | TC/AT/GB | Annotation | Category | Avg Ratio Day One | P- Value Day One | Avg Ratio Day Four | P-Value Day Four |
|---|---|---|---|---|---|---|---|
| Le | TC124387 | Wound-induced proteinase inhibitor I | JA | 42.4 | 0.020 | 16.2 | 0.001 |
| Le | TC124100 | Leu aminopeptidase | JA | 28.7 | 0.003 | 8.7 | 0.000 |
| Le | K03291 | Wound-induced proteinase inhibitor II | JA | 19.9 | 0.016 | 13.7 | 0.000 |
| Le | TC123946 | Cathepsin D inhibitor | JA | 13.6 | 0.016 | 9.1 | 0.002 |
| Le | TC116040 | Wound-induced proteinase inhibitor II | JA | 10.3 | 0.006 | 11.1 | 0.000 |
| Le | TC124388 | Wound-induced proteinase inhibitor I | JA | 6.9 | 0.025 | 5.0 | 0.000 |
| Le | TC116240 | Wound-induced proteinase inhibitor I | JA | 6.4 | 0.064 | 2.0 | 0.008 |
| Le | AI488671 | Proteinase inhibitor I | JA | 2.9 | 0.139 | 2.2 | 0.003 |
| Le | TC117620 | Allene oxide cylase | JA | 2.8 | 0.000 | 1.9 | 0.000 |
| Le | TC115727 | PR-1b | JA | 1.7 | 0.079 | 1.9 | 0.005 |
| Le | TC130310 | Patatin | JA | 1.7 | 0.000 | 1.3 | 0.008 |
| Le | TC124098 | Proteinase inhibitor; auxin-induced | JA, auxin | 21.2 | 0.005 | 5.5 | 0.001 |
| Le | TC115911 | PR-P6 | SA | 8.8 | 0.000 | 7.0 | 0.007 |
| Le | TC116619 | Chitinase | SA | 3.3 | 0.069 | 3.0 | 0.005 |
| Le | TC124531 | Acidic endochitinase | SA | 2.5 | 0.016 | 3.1 | 0.030 |
| Le | TC124012 | TSI-1 protein | SA | 1.9 | 0.003 | 2.3 | 0.013 |
| Le | TC116545 | Osmotin-like | SA, ET | 2.0 | 0.011 | 2.3 | 0.104 |
| Le | TC117926 | Subtilisin-like proteinase | SA, JA | 2.3 | 0.017 | 2.0 | 0.017 |
| Le | TC118684 | MLO-like protein | SA, PATHO | 2.6 | 0.045 | 2.8 | 0.014 |
| Le | TC121425 | Callose synthase | SA, PATHO | 2.0 | 0.012 | 1.5 | 0.239 |
| Le | TC116386 | Ethylene-responsive small GTP-binding protein | ET | 2.7 | 0.046 | 1.6 | 0.007 |
| Le | TC116831 | Ethylene response factor 3 | ET | 2.3 | 0.089 | 1.6 | 0.037 |
| Le | TC116989 | Ethylene-inducible protein | ET | 1.7 | 0.003 | −1.4 | 0.000 |
| Le | TC130320 | Geranylgeranyl pyrophosphate synthetase | SEC MET | 13.1 | 0.002 | 7.1 | 0.001 |
| Le | TC125929 | Anthocyanidine rhamnosyl-transferase | SEC MET | 2.3 | 0.170 | 1.8 | 0.024 |
| Le | TC125242 | Diphosphomevalonate decarboxylase | SEC MET | −1.1 | 0.802 | 1.6 | 0.009 |
| Le | TC128225 | 1-Phosphatidylinositol 4-kinase | ST | 3.1 | 0.068 | 2.7 | 0.018 |
| Le | TC120983 | Protein phosphatase type 2C | ST | 3.1 | 0.038 | 3.9 | 0.072 |
| Le | BE434771 | Diacylglycerol kinase | ST | 2.6 | 0.015 | 1.6 | 0.093 |
| Le | TC125973 | 3-Phosphatidylinositol 5-kinase, FAB1 | ST | 2.3 | 0.029 | 1.6 | 0.907 |
| Le | TC129237 | Phosphoinositide-specific phospholipase C | ST | 2.2 | 0.019 | 1.8 | 0.003 |
| Le | TC129136 | Leu rich repeat containing protein kinase | ST | 2.1 | 0.047 | 1.8 | 0.087 |
| Le | TC122585 | Phosphoinositide-specific phospholipase Dβ2 | ST | 1.9 | 0.020 | 1.5 | 0.242 |
| Le | TC125158 | Phosphoinositide-specific phospholipase Dα1 | ST | 1.8 | 0.011 | 1.0 | 0.995 |
| Le | TC120843 | bHLH transcription factor JAF13-like | ST | 1.7 | 0.015 | 1.2 | 0.001 |
| Le | TC117471 | SINA2 protein | ST | 1.3 | 0.038 | −1.6 | 0.003 |
| Le | TC115865 | Catalase | ST | 1.2 | 0.777 | 1.6 | 0.000 |
| Le | TC125541 | LSD1 homolog 2 | ST | −1.7 | 0.000 | 1.1 | 0.985 |
| Le | TC117304 | Triacylglycerol lipase | LIPASE | 2.2 | 0.005 | 1.3 | 0.009 |
| Le | TC115712 | Plastidic aldolase | PLASTID | 1.6 | 0.640 | −1.8 | 0.000 |
| Le | TC123753 | Rubisco small subunit | PLASTID | −1.7 | 0.005 | −1.4 | 0.019 |
| Ph | L21978 | ACC oxidase | ET | 13.1 | 0.037 | 4.0 | 0.932 |
| Ph | AY044235 | AP2 domain protein, ethylene response factor | ET | 2.9 | 0.043 | −1.2 | 0.300 |
| Ph | M90294 | ACC oxidase | ET | 2.1 | 0.038 | 1.2 | 0.776 |
| Ph | AF210049 | Gibberellin induced protein, gip1 | GA | −1.1 | 0.383 | 3.7 | 0.019 |
| Ph | At1075280 | Isoflavone reductase | SEC MET | 21.5 | 0.047 | 4.6 | 0.922 |
| Ph | BT002340 | DOXP synthase | SEC MET | 3.6 | 0.040 | 1.3 | 0.678 |
| Ph | AAL50566 | Malonyl CoA: anthocyanin 5-O-glucoside-6′′′-O-malonyltransferase | SEC MET | 3.3 | 0.020 | 1.9 | 0.949 |
| Ph | CAA79856 | DAPH synthase | SEC MET | 2.3 | 0.011 | 1.5 | 0.675 |
| Ph | AT3G23810.1 | S-adenosyl-l-homo-Cys synthase | SEC MET | 1.9 | 0.011 | −1.4 | 0.017 |
| Ph | AF233639 | Dihydroflavonol 4-reductase, DfrA | SEC MET | 1.4 | 0.071 | 4.8 | 0.046 |
| Ph | Y07721 | Glutathione S-transferase, An9 | SEC MET | 1.4 | 0.978 | 4.7 | 0.012 |
| Ph | AF020545 | bHLH transcription factor, jaf13 | SEC MET | 1.3 | 0.934 | 4.3 | 0.024 |
| Ph | U9478 | MYB-like transcription factor, An11 | SEC MET | 1.3 | 0.800 | 5.1 | 0.007 |
| Ph | AF233638 | Chalcone isomerase, chiA | SEC MET | 1.3 | 0.960 | 5.3 | 0.042 |
| Ph | AF155332 | Flavonoid 3′-hydroxylase, ht1 | SEC MET | 1.2 | 0.778 | 4.1 | 0.004 |
| Ph | AF260919 | bHLH transcription factor, An1 | SEC MET | 1.1 | 0.747 | 5.6 | 0.006 |
| Ph | AF146702 | MYB-like transcription factor, An2 | SEC MET | 1.1 | 0.617 | 5.8 | 0.001 |
| Ph | AF509865 | NAM-like, nh2 | ST | 6.7 | 0.041 | 5.7 | 0.807 |
| Ph | AT1G67900.1 | Expressed protein phototaxis | ST | 6.5 | 0.033 | 2.6 | 0.699 |
| Ph | n.a. | NAM-like, nh23 | ST | 5.5 | 0.044 | 5.6 | 0.647 |
The cDNAs from the dedicated microarray that were either 1.6-fold up- or down-regulated on day one or day four are shown. The ratios indicate the relative transcript abundance in spider mite-infested plants over uninfested plants. The cDNAs were either from tomato (Le) or Petunia (Ph). Where possible the corresponding TC numbers, GenBank numbers (GB), or AT numbers with the highest homology are shown (n.a. = not applicable). The cDNAs were subdivided in various categories according to their function: ET, ethylene-related; GA, gibberellin related; JA, jasmonate related; SA, salicylic acid related; PATHO, pathogen related; SEC MET, secondary metabolism; ST, signal transduction. The P-values (adjusted for multiple testing) denote the significant difference of the average ratios (Avg Ratio) of infested over uninfested plants in three independent experiments.
JA, SA, and Ethylene Responsive Genes Are Induced by Spider Mites
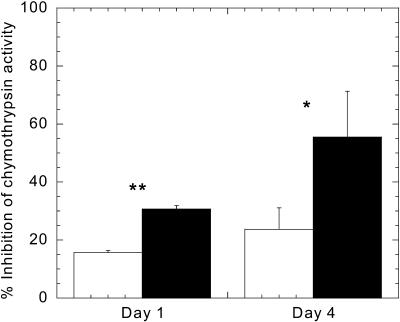
When the transcript levels in uninfested and spider mite-infested leaves were compared, a clear increase was observed for many members of the family of WIPIs (WIPI-I and WIP-II, Table I) during infestation. Two members of these WIPIs have previously been reported to be induced by spider mites (Li et al., 2002). Measurements of proteinase inhibitor activity showed an approximately 2-fold increase in spider mite-infested leaves compared to control leaves, both at day one and day four (Fig. 3), showing that the transcriptional activation of this family of genes also lead to higher protein levels. These WIPIs are known to be induced by JA (Farmer et al., 1992), levels of which are known to increase in tomato upon spider mite infestation (Li et al., 2002). Our microarray array contains several cDNAs related to JA biosynthesis, but our data only showed a sustained increase in transcripts levels of allene oxide cyclase but not of allene oxide synthase or lipoxygenase. A patatin-like protein that is potentially involved in releasing precursors for JA biosynthesis (Dhondt et al., 2002) was also up-regulated. The transcript levels of two other genes that are induced by JA, Leu aminopeptidase and PR-1b, were already higher after 1 d. In general, most JA-dependent genes were more prevalent on day one. Moreover, transcript levels of several genes related to ethylene biosynthesis or signaling were up-regulated by spider mites on day one. Two 1-aminocyclopropane-1-carboxylate (ACC) oxidase genes were induced by spider mites, but only one was still up-regulated on day four. An ethylene-inducible protein was only induced by spider mites on day one while two ethylene inducible GTP-binding proteins and two genes with high homology to ethylene response factors, one from tomato and one from petunia, were induced on both days. An osmotin-like protein was also up-regulated by spider mites as previously shown for spider mite-infested lima beans (Phaseolus lunatus) in which ethylene emission had increased (Arimura et al., 2000, 2002). The pathogenesis-related gene, PR-P6 (Fig. 2), was strongly and rapidly induced by spider mites with sustained, though somewhat variable, transcript levels. Transcript levels of other genes also known to be activated through the SA-pathway, such as various acidic chitinases and the pathogenesis-related TSI-1 (tomato stress induced-1), were high in infested leaves at both time-points.
Figure 3.
Proteinase inhibitor activity is induced by spider mites. Three leaves of 3-week-old tomato plants were each infested with 15 spider mites in duplicate experiments. After 1 and 4 d, infested leaves were collected and pooled per plant. Leaves were also collected and pooled from uninfested control plants. Proteinase inhibitor activity (percent inhibition of 10 μg chymotrypsin activity/500 μg protein) in extracts of infested (black bars) and uninfested leaves (white bars) was determined following the protocol of Stout et al. (1994). The vertical bars indicate the means and sds of each treatment group. An asterisk (*) denotes a significant difference at P < 0.05, double asterisks (**) at P < 0.01 (Student's t test).
Several players in lipid signaling such as phosphatidylinositol-4-kinase, phospholipase C, and DGK were also up-regulated, albeit to different extents, on day one. The combined action of these three enzymes can lead to the formation of phosphatidic acid. Phosphatidic acid levels have been shown to increase in tomato upon pathogen attack (Munnik, 2001). Two phospholipase D genes, PLDα1 and PLDβ2, which can produce phosphatidic acid from phosphatidylcholine and are putatively involved in JA-signaling (Wang et al., 2000), were up-regulated on day one. Other miscellaneous genes related to signal transduction events such as a protein phosphatase 2C, a Leu rich protein kinase, catalase, and a petunia phototaxis-related protein were also up-regulated by spider mites. Two genes encoding zinc-finger proteins were regulated inversely, with lesion stimulated disease-1 (LSD1) down-regulated on day one and SINA-2 on day four. The zinc-finger protein encoded by the Arabidopsis LSD1 gene that acts as a negative regulator of the hypersensitive response has been previously shown to be down-regulated by methyl jasmonate and SA (Schenk et al., 2000). Interestingly, transcript levels of an MLO-2 homolog, a negative regulator of cell death (Stein and Somerville, 2002), were 2- and 3-fold higher after 1 and 4 d, respectively. Two NAM-like proteins from petunia, nh2 and nh23, were also higher on day one, indicating that tomato contains homologous transcripts. The NAM proteins belong to the family of NAC proteins, of which several are induced by elicitor or pathogen treatment (Collinge and Boller, 2001). The transcript levels of the small subunit of Rubisco were lower in spider mite-infested leaves, similar to the situation in tobacco (Nicotiana tabacum) subjected to Manduca sexta herbivory (Halitschke et al., 2003).
Secondary (Volatile) Metabolism Is Modulated by Spider Mites
Genes encoding enzymes that produce precursors for terpenoid biosynthesis, such as geranylgeranyl pyrophosphate (GGPP) synthase, DOXP-synthase, and diphosphomevalonate decarboxylase were significantly up-regulated by spider mites, indicating that both the mevalonate and DOXP-routes were activated (Table I). Transcript levels of genes related to anthocyanin production such as chalcone isomerase A, dihydroflavonol 4-reductase, and flavonoid 3′-hydroxylase were only up-regulated after 4 d. Correspondingly, transcript levels of tomato genes with high homology to several of the petunia cDNAs that are involved in regulating the transcription of genes involved in the biosynthesis of anthocyanins (an1, an2, an11, and jaf13; Quattrocchio et al., 1993; Spelt et al., 2000; Spelt et al., 2002), were up-regulated by spider mites after 4 d. In petunia, AN1 is known to directly activate the expression of the dfrA gene and myb27, a MYB-domain protein of unknown function that was also 4-fold up-regulated by spider mites on day four, albeit at a lower significance level (P = 0.084; Supplemental Data). In tomato, overexpression of the an2 homolog Ant1 also activates anthocyanin pathways (Mathews et al., 2003). In general more genes involved in secondary metabolism were up-regulated on day four.
Volatile Metabolome Analysis
From the above it was clear that spider mites induce the transcription of direct defense-related genes. In order to determine the onset of the indirect defense response, the temporal emission of volatile organic compounds by infested and uninfested intact tomato plants was analyzed by means of gas chromatography-mass spectrometry. We identified 3 phenolics (p-cymene, MeSA, and putatively di-tert-butylphenol), 9 monoterpenes (2-carene, α-terpinene, β-phellandrene, limonene, α-terpinene, γ-terpinolene, β-myrcene, trans-β-ocimene, and linalool), 15 sesquiterpenes (δ-elemene, α-cubebene, α-copaene, β-elemene, β-caryophyllene, α-gurjunene, α-humulene, γ-muurolene, α-muurolene, β-farnesene, cis-nerolidol, trans-nerolidol, and cis,cis-farnesol; two could not be identified), and 2 homoterpenes (4,8-dimethyl-1,3,7-nonatriene [DMNT] and TMTT; Table II). During 5 d one control (uninfested) tomato plant produced around 440 μg of volatiles. On average this blend consisted of 77% TMTT, 8% MeSA, and 4% β-phellandrene. In contrast, one spider mite-infested plant produced around 900 μg of volatiles, of which TMTT (77%), MeSA (16%), and β-phellandrene (2%) were the main components as well. This means that TMTT emission was doubled while MeSA emission was increased 4-fold. In contrast, β-phellandrene emission was unaffected.
Table II.
Volatiles emitted by tomato plants
| Compound
|
Calculated IK
|
Total Emission by Control Plants
|
Total Emission by Spider Mite-Infested Plants
|
P-Value (Infested Plants Versus Control Plants)
|
|---|---|---|---|---|
| μg/plant/5 d | μg/plant/5 d | |||
| β-myrcene | 988.1 | 0.008 ± 0.008 | 0.02 ± 0.02 | 0.558 |
| 2-carene | 1000.0 | 0.1 ± 0.1 | 0.5 ± 0.3 | 0.247 |
| α-terpinene | 1017.5 | 0.0003 ± 0.0003 | 0.03 ± 0.03 | 1.000 |
| p-cymene | 1022.3 | 1.1 ± 0.8 | 0.2 ± 0.1 | 0.144 |
| β-phellandreneA | 1025.0 | 19.5 ± 5.8 | 19.6 ± 5.6 | 0.757 |
| limonene | 1029.7 | 5.0 ± 3.0 | 3.4 ± 1.8 | 0.374 |
| trans-β-ocimene | 1046.9 | 0.1 ± 0.08 | 1.2 ± 0.3 | 0.001* |
| γ-terpinolene | 1089.3 | 0.6 ± 0.3 | 0.4 ± 0.2 | 0.721 |
| linalool | 1097.6 | 0.1 ± 0.03 | 0.5 ± 0.1 | 0.001* |
| DMNT | 1114.1 | 0.6 ± 0.3 | 1.0 ± 0.4 | 0.221 |
| MeSA | 1198.8 | 37.6 ± 11.1 | 144.2 ± 56.8 | 0.001* |
| δ-elemeneA | 1338.6 | 0.9 ± 0.3 | 1.3 ± 0.4 | 0.341 |
| α-cubebene | 1353.6 | 0.01 ± 0.006 | 0.04 ± 0.02 | 0.577 |
| α-copaene | 1380.0 | 2.7 ± 1.4 | 3.2 ± 1.0 | 0.597 |
| β-elemeneA | 1393.6 | 1.4 ± 0.6 | 1.7 ± 0.5 | 0.634 |
| longifoleneA | 1415.7 | 0.8 ± 0.2 | 1.2 ± 0.3 | 0.644 |
| β-caryophyllene | 1429.6 | 2.6 ± 0.6 | 3.8 ± 0.8 | 0.224 |
| α-gurjuneneA | 1434.2 | 3.3 ± 1.6 | 4.5 ± 2.1 | 0.610 |
| β-farnesene | 1450.8 | 0.2 ± 0.1 | 0.2 ± 0.05 | 0.255 |
| α-humulene | 1462.8 | 2.1 ± 1.1 | 2.1 ± 1.4 | 0.731 |
| γ-muuroleneA | 1477.5 | 1.0 ± 0.3 | 1.0 ± 0.2 | 0.733 |
| α-muuroleneA | 1499.3 | 3.0 ± 1.2 | 2.8 ± 1.6 | 0.362 |
| tert-butylphenolA | 1500.9 | 6.1 ± 2.6 | 8.0 ± 2.4 | 0.851 |
| cis-nerolidol | 1527.7 | 0.3 ± 0.07 | 0.4 ± 0.1 | 0.277 |
| Unk. sesquiterpene AA | 1538.3 | 3.5 ± 1.7 | 6.2 ± 2.4 | 0.102 |
| Unk. sesquiterpene BA | 1551.4 | 2.5 ± 1.1 | 3.9 ± 1.6 | 0.598 |
| trans-nerolidol | 1560.0 | 0.4 ± 0.05 | 0.9 ± 0.2 | 0.004* |
| TMTT | 1572.9 | 342.9 ± 170.1 | 690.0 ± 218.5 | 0.050* |
| cis,cis-farnesolA | 1720.7 | 1.4 ± 0.7 | 1.9 ± 0.6 | 0.360 |
Compounds in the table are ordered according to their calculated Kovats index (IK). The compounds marked with A were not available as pure standards and therefore the amounts for these compounds have been estimated on the basis of the nearest compound on the RT-scale of the GC-spectrum, provided this compound was of the same chemical class and a pure standard was available. The values indicate the means and ses of each treatment group. The P-values have been calculated per compound with one-way ANOVA (df = 1) on the total amount produced per plant in 5 d (n = 9). The compounds that are marked with an asterisk (*) tested significantly different (F ≥ 7.2/P ≤ 0.05) for infested plants versus control plants.
To test whether there was a consistent difference between infested and uninfested plants over a period of 5 d, the data of nine independent replicate experiments was analyzed by repeated measures ANOVA. Five volatiles were significantly different (P < 0.05 for all tests): MeSA (Fig. 4A), trans-β-ocimene (Fig. 4B), trans-nerolidol (Fig. 4C), linalool (Fig. 4D), and TMTT. To test whether some volatiles exhibited a significant difference on only a subset of days we also tested each day separately. Apart from MeSA, TMTT, trans-nerolidol, trans-β-ocimene, and linalool, there were only three other volatiles that showed a significant difference on a particular day. Four times more cis-nerolidol (P = 0.03; Fig. 4F), and twice as much limonene (P = 0.02; data not shown) were produced by the control plants on the first day. β-farnesene was produced in 4-fold greater quantities by control plants on the second day (P = 0.02; Fig. 4E). The significant differences between infested and uninfested plants for trans-nerolidol and linalool were apparent from day three onwards and for MeSA, TMTT, and trans-β-ocimene on day four and five. The total amount of monoterpenes and the total amount of sesquiterpenes produced by infested plants were not significantly different from the amounts produced by uninfested plants (results not shown).
Figure 4.
Volatile compounds emitted by 3-week-old tomato plants infested by spider mites (black lines) and by uninfested control plants (dotted lines). In each replicate experiment we sampled the volatiles of 3 control plants and 3 treatment plants at 24 h intervals during 5 d. The graphs show the average relative production during 5 d for: (A) methyl salicylate; (B) trans-β-ocimene; (C) trans-nerolidol; (D) linalool; (E) β-farnesene; and (F) cis-nerolidol, calculated from 9 independent replicate experiments. The vertical bars represent the ses.
In a separate analysis, we also tested whether the cumulative amounts of each volatile emitted by infested and uninfested plants were significantly different. For this we calculated the total amounts produced over 5 d per replicate and tested them through a one-way ANOVA. Only MeSA, TMTT, trans-β-ocimene, trans-nerolidol, and linalool were significantly different (Table II). We did not find volatiles whose total production was significantly down-regulated in the infested plants compared to the uninfested plants.
Olfactory Choice Assays
To determine whether the enhanced emission of linalool and trans-nerolidol on day three and four, and the increased emission of TMTT, MeSA, and trans-β-ocimene on day four represented the induction of indirect plant defenses, we performed olfactory choice-assays on predatory mites. Predatory mites were submitted to the odors of infested and uninfested plants and their behavioral response was recorded. On day three predatory mites did not prefer infested over clean plants (Fig. 5). Moreover, a substantial number of predatory mites did not make a choice at all, indicating the absence of a stimulus. A replicated test for goodness-of-fit showed that day three replicates as well as the day four replicates were not heterogeneous (P = 0.27/degrees of freedom [df] = 3 and P = 0.44/df = 3, respectively) thus allowing for pooling. The pooled data of the day three replicates were not significantly different from the zero-hypothesis (H0 = a 1:1 ratio) with P = 0.26 (df = 1). The pooled data of the day four results did test significantly different from the H0 with P = 0.001 (df = 1). This demonstrates that predatory mites were able to single out infested plants on day four.
Figure 5.
Olfactory response of P. persimilis to tomato plants infested with T. urticae and uninfested control plants. The figure shows the fraction of predatory mites that chose for the odors of infested plants (black bars), for uninfested plants (gray bars), or that did not make a choice within 5 min (white bars). Two replicate choice tests (n = 24 and n = 34) on day 3 and 2 replicates (n = 60 and n = 50) on day 4 are presented. An asterisk (*) denotes significant deviation from H0 (predatory mites chose infested plants and uninfested plants in a ratio of 1:1) according to a replicated test for goodness-of-fit at α = 0.01.
DISCUSSION
Our results indicate that when tomato plants are infested with spider mites their direct defense responses are activated within 1 d, but that their indirect defense-response takes 3 d to be mounted. The microarray results showed an early increase in transcript levels of various genes related to defense proteins and metabolites. In general, the early defense response was characterized by simultaneous activation of JA, ethylene, and SA signal transduction pathways, comparable to the response activated in Arabidopsis and tobacco by leaf-chewing insects (Reymond et al., 2000; Halitschke et al., 2003).
Spider Mite-Regulated Gene Expression
In contrast to mechanical wounding (Strassner et al., 2002), spider mites induced PR-gene expression in tomato. Perhaps this was the result of continuous wounding by spider mites compared with a single mechanical wound, but we favor the possibility that the increase in PR-transcript levels is a specific response to spider mites. A similar response was induced in detached lima bean leaves by spider mites where both PR-gene expression and SA levels were up-regulated (Arimura et al., 2000, 2002). Our microarray did not contain ESTs directly related to SA biosynthesis but we did measure a clear increase in MeSA emission. It was shown earlier that MeSA is made from SA (Shualev et al., 1997).
Both the microarray data (Table I) and the proteinase inhibitor assay (Fig. 3) indicated a rapid increase in inhibitory compounds upon infestation. Proteinase inhibitors as well as Leu aminopeptidase and callose synthase are all known to be up-regulated in tomato by wounding (Strassner et al., 2002). In addition, Li et al. (2002) showed that the induction of proteinase inhibitors by spider mites in tomato is jasmonate dependent. Our data also show that genes related to ethylene biosynthesis and ethylene responsiveness were up-regulated in mite-infested intact tomato just as ethylene production was induced in infested lima bean leaves (Arimura et al., 2002). It seems likely that both hormones promote the expression of wound-inducible genes (Odonnell et al., 1996) and systemic signaling (Verberne et al., 2003).
Our microarray contained many ESTs involved in lipid signaling and we saw that several of them were up-regulated in infested leaves. The transcripts of three enzymes, PI-4-kinase, phospholipase C, and DGK, whose combined action leads to the formation of phosphatidic acid, were all significantly up-regulated. Phosphatidic acid is being recognized as an important second messenger in plants and increases in this lipid have been observed during wounding, oxidative stress, and pathogen attack (Laxalt and Munnik, 2002). Phosphatidic acid is also produced by phospholipase D, and significantly, the transcripts of two family members also increased on mite infestation. It would seem therefore that phosphatidic acid is an important second messenger in the plants response to herbivory by spider mites.
We also discovered that various genes that regulate flavonoid and anthocyanin synthesis were up-regulated in tomato by spider mites. These genes encode several types of transcription factors, of which some are up-regulated by gibberellic acid (GA; Weiss et al., 1993). In this regard it is interesting to note that the levels of the GA-regulated gip1 (Ben-Nissan and Weiss, 1996) were also higher in infested leaves after 4 d.
Spider Mites Induce Genes Involved in Terpenoid Biosynthesis
Our microarray data showed that diphosphomevalonate decarboxylase and DOXP-synthase were induced by spider mites, indicating a higher flux through pathways leading to terpenoid synthesis. 3-hydroxy-3-methyl glutaryl coenzyme A reductase and mevalonate kinase were also up-regulated though less significantly (see Supplemental Data). What is new is our finding that GGPP-synthase was induced by spider mites. Apparently more GGPP has to be produced, since TMTT, one of the major volatiles produced by tomato infested with spider mites, is derived from the GGPP-derivate geranyllinalool (Donath and Boland, 1994). Recently, Faldt et al. (2003) have shown that trans-β-ocimene synthase can be induced by JA and wounding. It will require further analysis to determine whether the above enzymes are specifically induced by spider mites or whether they are part of a common packet response to JA, SA, ethylene, or wounding. Few studies have explored the relationship between direct and indirect defenses, although there is some evidence that JA and SA are involved in orchestrating both responses. In excised bean leaves the emission of herbivore-induced volatiles (predominantly those from the isoprenoid pathway) as well as the accumulation of basic PR-genes was stimulated by exogenous JA (Krumm et al., 1995; Ozawa et al., 2000). However, although JA may be necessary, it is not always sufficient to induce emission of volatiles, since mechanical wounding induced JA-accumulation but did not promote the emission of isoprenoids in cucumber (Cucumis sativus; Bouwmeester et al., 1999) or lima bean (Dicke et al., 1998). Moreover, detached lima bean leaves became attractive to predatory mites after exogenous application of JA but not after mechanical wounding. The JA-treated leaves produced a volatile blend very similar to that induced by spider mites, with the exception of TMTT and MeSA (Dicke et al., 1999). Absence of the latter could simply be due to the relative absence of its precursor SA, since MeSA and SA increased parallel in tobacco upon tobacco mosaic virus infection (Seskar et al., 1998). Spider mites however, do induce MeSA in tomato (Fig. 4A), and our microarray data showed elevated transcript levels of SA-dependent genes over a 4-d period (Table I). It therefore seems justified to assume that SA, in addition to JA, is involved in both direct and indirect defenses.
Timing of Indirect Defenses
The attraction of predatory mites to infested tomato plants occurred when the emission of MeSA, TMTT, linalool, trans-β-ocimene, and trans-nerolidol significantly increased on day four. On day three, linalool and trans-nerolidol levels were significantly higher, but the predatory mites did not yet show a preference for them. Maeda and Takabayashi (2001) showed that the absolute amounts of MeSA and TMTT were positively correlated with the attraction of predatory mites to Phaseolus vulgaris infested with T. urticae. In our experiments with tomato, the production of herbivore-induced volatiles increased with time, even though proteinase inhibitor-activity was high from day one onwards (Fig. 3). Our results therefore show that the indirect defense-response is timed later than both the induction of proteinase inhibitor activity and the transcriptional activation of direct defense-related genes.
Li et al. (2002) found that spider mites reared on bean produced approximately 1 to 2 eggs per day on the cultivar Castlemart. However, our spider mites had been on tomato for over 50 generations prior to the experiments and produced approximately 7 to 8 eggs per day on Castlemart. This suggests that a period of conditioning on tomato is needed to stabilize reproductive performance. In addition, it was shown by Gotoh et al. (1993) that spider mite populations could adapt to tomato after several generations of selection. This adaptation was characterized by increased resistance against methylketones from tomato glandular hairs (Chatzivasileiadis and Sabelis, 1998) and a high reproductive success and low mortality of the adapted strain compared with maladapted strains (Magowski et al., 2003). Therefore, comparing the plant defense response to adapted and maladapted herbivores could teach us whether adapted herbivores induce defense responses that they can simply resist, whether they induce a different response that they might not have to resist, or a combination of the two.
Since the indirect defense response is delayed relative to the direct defense response, the question is whether the delay occurs in terms of function or in terms of causal mechanisms. In terms of function the delay might represent a strategy of the predators who spend energy in searching for prey and who will profit more when prey densities are high. Vice versa, it might represent a strategy of the herbivore that attempts to delay the arrival of predators. Alternatively, it might represent a strategy of the plant that only needs to attract predators when the direct defense mechanisms are insufficient. Kahl et al. (2000) proposed that plants reduce their direct defenses and start to produce volatiles after the direct defenses have failed to control an adapted herbivore. These findings suggest that the plant could regulate the balance between direct and indirect defenses. From a causal point of view, however, the delay could just be an unavoidable consequence of the physiological processes needed to mount the two defense responses. This can be addressed by determining which signaling processes mediate the tuning of the defense strategies. From previous studies (Li et al., 2002; Thaler et al., 2002), it has become apparent that JA is involved in both direct and indirect responses. Whether SA and/or ethylene signaling pathways are also involved can be resolved using mutants in these pathways.
MATERIAL AND METHODS
Plant Material and Arthropod Rearing
Tomato (Lycopersicon esculentum Mill cv Castlemart) seedlings were grown in a greenhouse with day/night temperatures of 23° to 18°C and a 16/8 h light/dark regime. Three days prior to each experiment all plants were transferred to a climate room at 23°C to 18°C, a 16/8 h light regime with 300 μE m−2 s−1, and 60% relative humidity (RH).
The two-spotted spider mite Tetranychus urticae Koch was originally obtained in 1993 from tomato plants in a greenhouse (Houten, The Netherlands; Gotoh et al., 1993) and was since maintained on the cultivar Moneymaker (the base-colony). In 2001, one year prior to our experiments, we replaced Moneymaker plants with the cultivar Castlemart. For the experiments involving RNA gel-blots, microarray analysis, volatile-sampling, and olfactory choice-assays, adult females were randomly collected from this culture.
The colony of the predatory mite Phytoseiulus persimilis was originally maintained in the laboratory on detached lima bean leaves infested with spider mites, for more than 3 years. Prior to all experiments they were transferred to intact tomato plants (cultivar Castlemart) infested with spider mites and maintained for approximately 1 month in a climate room at 23°C, a 16/8 h light regime with 100 μE m−2 s−1, and 70% RH. We choose to work with predatory mites reared on Castlemart tomato plants since predatory mites can learn to associate plant odors with their prey (Drukker et al., 2000). Spider mite-infested tomato plants were supplied to the culture once per week. Adult female predatory mites were collected from this culture for the olfactory-choice assays.
Spider Mite Fecundity and Leaf-Damage Assays
Prior to the fecundity-assay, several adult female spider mites were allowed to lay eggs on detached uninfested tomato leaves (on wet cotton wool) to produce an egg-wave. After 24 h the adults were removed and the eggs were maintained. After 16 d (2 d after the last moulting stage) the females were collected. We infested 14 plants with 7 of these 2-d-old females on 1 leaflet per plant. The plants were inspected daily by means of a stereomicroscope and all adults, eggs, and juveniles on both sides of the infested leaflet were counted during 7 d. The experiment was performed in a climate room at 23°C, a 16/8 h light regime with 100 μE m−2 s−1, and 60% RH.
To assess the level of damage inflicted by different densities of spider mites, we infested 3 leaves per plant with either none, 1, 5, 15, or 50 adult females. Ten plants were infested for each density level. To assess the total leaf area that had been damaged, we harvested the infested leaves of 2 plants (6 leaves) at each density-level during 5 d. After collection the leaves were scanned digitally. Each scan included a 4 cm2 reference. The scans were processed in Photoshop 7.0 (Adobe Systems, Mountain View, CA). First the background was selected and deleted. Second, the colored pixels were transformed to black-and-white using the threshold tool in such a way that all damaged areas were set to white and the remaining undamaged leaf-area was set to black. The histogram-tool was then used to count the white pixels (chlorotic lesions) and the black pixels (intact leaf) of each separate leaflet, as well as that of the reference.
Plant Treatments
Adult female spider mites were gently placed on the adaxial surface of the fully expanded terminal leaflets using a soft-bristle paintbrush. Per leaflet 15 mites were introduced, 3 leaflets on each plant in total. Plants contained 4 fully expanded leaves, which were chosen for infestation, and 2 emerging leaves. We never observed spider mites dispersing to adjacent leaflets.
Microarray Analysis
For preparation of the slide 428 ESTs clones were selected from the Institute of Genomic Research based on their relevance in plant-herbivore and plant-pathogen interactions and plant-signaling in general. These cDNAs were PCR amplified with T3 (5′-AAT TAA CCC TCA CTA AAG GG-3′) and T7 (5′-GTA ATA CGA CTC ACT ATA GGG C-3′) primers. The PCR products were purified with MultiScreen plates (Millipore Intertech, Bedford, MA) according to the manufacturer's instructions. The concentrations and lengths of the PCR products were checked with agarose gels. The PCR-products were printed on CMT-CAPS slides (Corning, Corning, NY) with a 12-pin arraying robot (Molecular Dynamics, Sunnyvale, CA). Each spot contained 0.25 ng cDNA. The array also contained spots with PCR products from plasmids with no inserts, bacteriophage lambda DNA in various concentrations, pBluescript DNA in several concentrations, poly-A80 oligonucleotide DNA in different concentrations, water, spotting-buffer, the MSLO cDNA from mouse, CNGC1 cDNA from Arabidopsis, and dilution series of randomly selected PCR pooled products from both petunia and tomato (46 in total). The array also contained 839 randomly selected cDNAs and 170 ESTs from petunia flowers that were also PCR amplified with T3 and T7 primers. All cDNAs were spotted four times per slide. Slides were left to dry over night at 55% RH prior to UV crosslinking (150 mJ). One slide was hybridized with SpotCheck Cy3 labeled nonameres (Genetix, New Milton, Hampshire, UK) to examine the spotting quality.
Leaves were cut at the base and 3 leaves from 3 plants were pooled in 14-mL tubes, directly frozen in liquid nitrogen, and stored at −80°C. Total RNA was isolated with a phenol-LiCl based method and RNA gel-blot analysis was done as described in Verdonk et al. (2003). Poly(A) mRNA was isolated using PolyAtract (Promega, Madison, WI) according to the manufacturer's protocol. cDNA was synthesized from 1 μg mRNA as follows: oligo(dT) 25-mer (final concentration of 1.2 μm) was added to the mRNA and heated to 70°C for 10 min and placed on ice. Reverse transcription was performed for 2.5 h at 42°C using 200 units Superscript II RNAse H− reverse transcriptase (Invitrogen, Carlsbad, CA); 10 mm DTT; 0.1 mm of dATP, dGTP, and dTTP; 0.05 mm of dCTP; and 0.05 mm Cy3-dCTP or Cy5-dCTP in Superscript II buffer. RNA was degraded afterward with 0.25 mm NaOH at 37°C for 15 min. Labeled cDNA fragments were purified using Qiaquick PCR purification columns (Qiagen, Valencia, CA) and incorporation of the fluoriphores as well as cDNA synthesis were measured on a spectrophotometer (Nanodrop Technologies, Rockland, DE). Subsequently, the cDNAs were pooled. The Cy5/Cy3 labeled cDNA was denaturated and loaded onto the slides and covered with a plastic slip. After prehybridization for 2 h at 55°C in 2× SSC, 0.2% SDS, and the slides were hybridized in an automated slide processor (Amersham Bioscience) in the hybridization buffer supplied using the following protocol: the probe was injected and incubated for 16 h at 42°C. Subsequently, the chamber was heated to 50°C and the slides were washed with 1× SSC, 0.2% SDS for 10 min, followed by 0.1× SSC, 0.2% SDS, once for 10 min and once for 4 min. Slides were flushed with 0.1× SSC followed by isopropanol before they were air dried for 200 s. The arrays were scanned for fluorescence emission with a PerkinElmer scanner at a resolution of 10 microns. Signal and background intensity were extracted using ArrayVision 6.0 software (Imaging Research, St. Catharines, Canada).
The data in this article represent 6 hybridizations of 6 independent replicates. Three of these replicates were done on purified RNA of tomato plants infested with spider mites 24 h (day 1) after introduction of 15 adult female spider mites on 3 leaves per plant. The 3 other replicates were done after 96 h (day 4). Leaves from 3 plants (9 infested leaves) were harvested and pooled per replicate.
The spot signal intensities were corrected by subtracting the average background calculated from the second percentile of the petunia spot intensities. For each clone we subsequently calculated the signal-to-noise ratio (S:N) by dividing the Cy3 or the Cy5 signal of a particular clone by the sd of its local background as assessed by ArrayVision software. Data for the two fluorophores were subsequently normalized using a Lowess-normalization procedure performed in R using the Sma-package from CRAN (freely available at: http://cran.r-project.org/). The average ratios (infested to uninfested) and the ses of these ratios were calculated from the normalized, background-subtracted intensity values for each clone. For calculation of the significance of up- or down-regulation we used a nested-design ANOVA on the separate clones, treating the read-outs of the four within-slide replicates per clone as subsamples. Prior to the analysis an irrelevant small value (by default set to 0.001) was added to each value to neutralize zeroes, after which all values were 10log transformed. The ANOVA was structured using three factors: slide, spotlabel-pair nested in slide and treatment, and was performed in Statistica 6.0 as a univariate analysis on all clones. The obtained P-values of the factor treatment for all clones were adjusted for multiple testing using Benjamini and Hochberg's (1995) step-up procedure for controlling the false discovery rate. The false discovery rate procedure was performed in R using the multtest library from Bioconductor (freely available at http://www.bioconductor.org/). After the analysis, clones were selected on the basis of three criteria: (1) the significance of the adjusted P-values (α = 0.05); (2) the average S:N ratio of the spots had to be higher than two for the treatment when up-regulated and for the control when down-regulated; and (3) the minimal treatment to control ratio was set to >1.6 or <−1.6 on the basis of our RNA gel-blots controls. The day one results and the day four results were analyzed separately. All cDNAs in Table I were sequenced.
Sampling of Volatiles
Eighteen-day-old Castlemart tomato plants were transferred to a climate room where they remained in 40-L desiccators for 3 d. The desiccators were ventilated with carbon-filtered pressure-air at 400 mL/min. Per replicate experiment, 3 plants were used for infesting with 15 adult female spider mites on 3 leaves of 3 plants (45 mites per plant) and 3 clean plants were used as control. The head space (the air surrounding a plant) was sampled during 24 h for 5 consecutive days by trapping it on 300 mg Tenax TA in 5-mm wide glass tubes. The desiccators were closed with a glass lid (no grease was used), which had a glass air-inlet and an air-outlet. The air-outlet opening was led directly into a Teflon tube that split in two; one branch was connected to a Tenax-filled sampling tube (connected to a vacuum pump that operated at 200 mL/min), the other released the remainder of the airflow. Before the junction of these two tubes there was a small hatch through which an internal standard could be added to the airstream downstream of the plants because in this set-up part of the headspace was trapped on Tenax, but part was also lost. To correct for this loss we pipetted 1.8 μg benzylacetate dissolved in 20 μL pentane:diethylether (4:1, v/v) into the air stream on a filter paper directly after connecting a new Tenax tube. All connections in the set-up were sealed with Teflon tape. After sampling the tubes were eluted with 2 mL pentane:diethylether (4:1) into amber vials.
Analysis of Volatiles
The headspace collections were analyzed on a Trace GC-MS-Plus (ThermoFinnigan, Austin, TX). The injector port temperature was set at 250°C and the source temperature at 200°C. The HP DB-5 column (30 m long; 0.25 mm i.d.; 0.25 μm stationary phase) was held at 40°C and heated at 8°C/min to 230°C, where it was maintained for 4 min. Helium was used as carrier gas (1 mL min−1). The MS was set at 5 scans per second for a mass-range from 50 to 350 atomic mass units in electron impact mode (–70 eV).
A mixture of pure GC-grade standards of all identified terpenes (Fluka, Milwaukee, WI) was run as external standard, except for trans-β-ocimene (R.C. Treatt, Suffolk, England), β-phellandrene, β-elemene, and α-muurolene, for which the RTs were identified from both citronella grass oil and spearmint oil (Fluka). DMNT, TMTT, and β-farnesene were kindly provided by Prof. Dr. Wilhelm Boland (Jena, Germany). Calculation of absolute amounts (from pure standards) or peak area (from impure standards) was processed with Xcalibur processing software on the basis of the retention time (RT) and mass spectrum, using the benzylacetate as the internal standard. We cross-checked all calculated Kovats retention index (IK) values with the IK-reference guide (Adams, 2001) to confirm identifications. To calculate the IK we used the RTs obtained from an alkane-mixture (Sigma, St. Louis), analyzed under the same GC-MS conditions as were used for our samples. We then used the formula: IK(x) = 100 × ([logRT{x}−logRT{alkane on the left}]/[logRT{alkane on the right}−logRT{alkane on the left}] × [number of carbon atoms of alkane on left]) to calculate the IK for each compound. Two sesquiterpenes remained unidentified and tert-butylphenol was only putatively identified by means of the NIST library.
All experiments were repeated 9 times. We analyzed both the absolute emission (in μg/5 d or peak area/5 d) and the relative emission. Relative emission was calculated by setting the highest amount of a volatile, measured during 5 d in each experiment, to 1 and subsequently expressing the other values of that volatile as fraction of the highest. This was done separately for each replicate. Prior to the statistical analysis, the relative data was arcsin-square-root transformed to achieve normality. The temporal emission was analyzed through a repeated measures ANOVA on the relative data using Statistica 6.0 for Windows. All volatiles were analyzed separately. Total production of each volatile compound in 5 d was tested with a one-way ANOVA.
Olfactory Choice Analysis
Olfactory-choice assays for predatory mites were conducted using the Y-tube olfactometer, as previously described by Bruin et al. (1992) with some minor modifications. The Y-tube olfactometer was connected directly to the outlet of the volatile sampling set-up. Results were analyzed using a replicated G-test under the H0 that there is no difference in attractiveness to either of the two odor sources.
Policy Statement
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Supplementary Material
Acknowledgments
The authors thank Francel Verstappen and Harro Bouwmeester (Plant Research International, Wageningen) for contributing to the GC-MS analyses, Martijn Egas (University of Amsterdam) for discussions regarding statistical analyses, and the MicroArray Division of the University of Amsterdam for assisting in preparing and analyzing the microarrays. Prof. Dr. Wilhelm Boland (Jena, Germany) is kindly acknowledged for providing us with DMNT, TMTT, and β-farnesene. Frank Koudijs (Interscience, Breda, The Netherlands) is acknowledged for his advice with Trace-MS analyses. Francesca Quattrocchio (Free University, Amsterdam) is acknowledged for providing us with 170 petunia ESTs. We are grateful to Dr. Alan Musgrave for his valuable comments on the manuscript.
This work was supported by NWO, The Netherlands Organization for Scientific Research (ALW 812.04.004 to M.K.).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.038315.
References
- Adams RP (2001) Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publishing, Carol Stream, IL
- Andersson BA, Holman RT, Lundgren L, Stenhagen G (1980) Capillary gas chromatograms of leaf volatiles. A possible aid to breeders for pest and disease resistance. J Agric Food Chem 28: 985–989 [Google Scholar]
- Arimura G, Tashiro K, Kuhara S, Nishioka T, Ozawa R, Takabayashi J (2000) Gene responses in bean leaves induced by herbivory and by herbivore-induced volatiles. Biochem Biophys Res Commun 277: 305–310 [DOI] [PubMed] [Google Scholar]
- Arimura G, Ozawa R, Nishioka T, Boland W, Koch T, Kuhnemann F, Takabayashi J (2002) Herbivore-induced volatiles induce the emission of ethylene in neighboring lima bean plants. Plant J 29: 87–98 [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Preston CA (1999) The eco-physiological complexity of plant responses to insect herbivores. Planta 208: 137–145 [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57: 289–300 [Google Scholar]
- Bruin J, Dicke M, Sabelis MW (1992) Plants are better protected against spider-mites after exposure to volatiles from infested conspecifics. Experientia 48: 525–529 [Google Scholar]
- Ben-Nissan G, Weiss D (1996) The petunia homologue of tomato gast1: transcript accumulation coincides with gibberellin-induced corolla cell elongation. Plant Mol Biol 32: 1067–1074 [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Verstappen FWA, Posthumus MA, Dicke M (1999) Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean. The first dedicated step in acyclic C11-homoterpene biosynthesis. Plant Physiol 121: 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery RG, Ling LC, Light DM (1987) Tomato leaf volatile aroma components. J Agric Food Chem 35: 1039–1042 [Google Scholar]
- Chatzivasileiadis EA, Sabelis MW (1998) Variability in susceptibility among cucumber and tomato strains of Tetranychus urticae Koch to 2-tridecanone from tomato trichomes: effects of host plant shift. Exp Appl Acarol 22: 455–466 [Google Scholar]
- Collinge M, Boller T (2001) Differential induction of two potato genes, Stprx2 and StNAC, in response to infection by Phytophthora infestans and to wounding. Plant Mol Biol 46: 521–529 [DOI] [PubMed] [Google Scholar]
- Dhondt S, Gouzerh G, Muller A, Legrand M, Heitz T (2002) Spatio-temporal expression of patatin-like lipid acyl hydrolases and accumulation of jasmonates in elicitor-treated tobacco leaves are not affected by endogenous levels of salicylic acid. Plant J 32: 749–762 [DOI] [PubMed] [Google Scholar]
- Dicke M, Vanbeek TA, Posthumus MA, Bendom N, Vanbokhoven H, Degroot AE (1990) Isolation and identification of volatile kairomone that affects Acarine predator-prey interactions: involvement of host plant in its production. J Chem Ecol 16: 381–396 [DOI] [PubMed] [Google Scholar]
- Dicke M, Takabayashi J, Posthumus MA, Schutte C, Krips OE (1998) Plant-phytoseiid interactions mediated by herbivore-induced plant volatiles: variation in production of cues and in responses of predatory mites. Exp Appl Acarol 22: 311–333 [Google Scholar]
- Dicke M, Gols R, Ludeking D, Posthumus MA (1999) Jasmonic acid and herbivory differentially induce carnivore- attracting plant volatiles in lima bean plants. J Chem Ecol 25: 1907–1922 [Google Scholar]
- Donath J, Boland W (1994) Biosynthesis of acyclic homoterpenes in higher-plants parallels steroid-hormone metabolism. J Plant Physiol 143: 473–478 [Google Scholar]
- Drukker B, Janssen A, Ravensberg W, Sabelis MW (1997) Improved control capacity of the mite predator Phytoseiulus persimilis (Acari: Phytoseiidae) on tomato. Exp Appl Acarol 21: 507–518 [Google Scholar]
- Drukker B, Bruin J, Jacobs G, Kroon A, Sabelis MW (2000) How predatory mites learn to cope with variability in volatile plant signals in the environment of their herbivorous prey. Exp Appl Acarol 24: 881–895 [DOI] [PubMed] [Google Scholar]
- Egas M, Norde DJ, Sabelis MW (2003) Adaptive learning in arthropods: spider mites learn to distinguish food quality. Exp Appl Acarol 30: 233–247 [DOI] [PubMed] [Google Scholar]
- Faldt J, Arimura G, Gershenzon J, Takabayashi J, Bohlmann J (2003) Functional identification of AtTPS03 as (E)-beta-ocimene synthase: a monoterpene synthase catalyzing jasmonate- and wound-induced volatile formation in Arabidopsis thaliana. Planta 216: 745–751 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Johnson RR, Ryan CA (1992) Regulation of expression of proteinase-inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol 98: 995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite D (2000) Changes in biological control usage in Great Britain between 1968 and 1995 with particular reference to biological control on tomato crops. Biocontrol Sci Techn 10: 451–457 [Google Scholar]
- Gatehouse JA (2002) Plant resistance towards insect herbivores: a dynamic interaction. New Phytol 156: 145–169 [DOI] [PubMed] [Google Scholar]
- Gotoh T, Bruin J, Sabelis MW, Menken SBJ (1993) Host race formation in Tetranychus-urticae - Genetic differentiation, host-plant preference, and mate choice in a tomato and a cucumber strain. Entomol Exp Appl 68: 171–178 [Google Scholar]
- Halitschke R, Gase K, Hui D, Schmidt DD, Baldwin IT (2003) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates. Plant Physiol 131: 1894–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gabler R, Kuhnemann F, Preston CA, Baldwin IT (2000) Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta 210: 336–342 [DOI] [PubMed] [Google Scholar]
- Krumm T, Bandemer K, Boland W (1995) Induction of volatile biosynthesis in the Lima bean (Phaseolus lunatus) by leucine- and isoleucine conjugates of 1-oxo- and 1-hydroxyindan-4-carboxylic acid: evidence for amino acid conjugates of jasmonic acid as intermediates in the octadecanoid signalling pathway. FEBS Lett 377: 523–529 [DOI] [PubMed] [Google Scholar]
- Laxalt AM, Munnik T (2002) Phospholipid signaling in plant defence. Curr Opin Plant Biol 5: 332–338 [DOI] [PubMed] [Google Scholar]
- Li CY, Williams MM, Loh YT, Lee GI, Howe GA (2002) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol 130: 494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren L, Norelius G, Stenhagen G (1985) Leaf volatiles from some wild tomato species. Nord J Bot 5: 315–320 [Google Scholar]
- Maeda T, Takabayashi J (2001) Production of herbivore-induced plant volatiles and their attractiveness to Phytoseiulus persimilis (Acari: Phytoseiidae) with changes of Tetranychus urticae (acari: Tetranychidae) density on a plant. Appl Entomol Zool (Jpn) 36: 47–52 [Google Scholar]
- Magowski W, Egas M, Bruin J, Sabelis MW (2003) Intraspecific variation in induction of feeding preference and performance in a herbivorous mite. Exp Appl Acarol 29: 13–25 [DOI] [PubMed] [Google Scholar]
- Mathews H, Clendennen SK, Caldwell CG, Liu XL, Connors K, Matheis N, Schuster DK, Menasco DJ, Wagoner W, Lightner J, et al. (2003) Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. Plant Cell 15: 1689–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloud ES, Baldwin IT (1997) Herbivory and caterpillar regurgitants amplify the wound- induced increases in jasmonic acid but not nicotine in Nicotiana sylvestris. Planta 203: 430–435 [Google Scholar]
- Munnik T (2001) Phosphatidic acid: an emerging plant lipid second messenger. Trends Plant Sci 6: 227–233 [DOI] [PubMed] [Google Scholar]
- Odonnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ (1996) Ethylene as a signal mediating the wound response of tomato plants. Science 274: 1914–1917 [DOI] [PubMed] [Google Scholar]
- Ozawa R, Arimura G, Takabayashi J, Shimoda T, Nishioka T (2000) Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol 41: 391–398 [DOI] [PubMed] [Google Scholar]
- Pare PW, Alborn HT, Tumlinson JH (1998) Concerted biosynthesis of an insect elicitor of plant volatiles. Proc Natl Acad Sci USA 95: 13971–13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare PW, Tumlinson JH (1999) Plant volatiles as defense against insect herbivores. Plant Physiol 121: 325–331 [PMC free article] [PubMed] [Google Scholar]
- Quattrocchio F, Wing JF, Leppen H, Mol J, Koes RE (1993) Regulatory genes controlling anthocyanin pigmentation are functionally conserved among plant species and have distinct sets of target genes. Plant Cell 5: 1497–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelis MW, Janssen A, Pallini A, Venzon M, Bruin J, Drukker B, Scutareanu P (1999) Behavioral responses of predatory and herbivorous arthropods to induced plant volatiles: from evolutionary ecology to agricultural implications. In AA Agrawal, S Tuzun, E Bent, eds, Induced Plant Defenses Against Pathogens and Herbivores. American Phytopathological Society Press, St. Paul, pp 269–296
- Sabelis MW, Janssen A, Kant MR (2001) Ecology: the enemy of my enemy is my ally. Science 291: 2104–2105 [DOI] [PubMed] [Google Scholar]
- Schaller A, Frasson D (2001) Induction of wound response gene expression in tomato leaves by ionophores. Planta 212: 431–435 [DOI] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97: 11655–11660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seskar M, Shulaev V, Raskin I (1998) Endogenous methyl salicylate in pathogen inoculated tobacco plants. Plant Physiol 116: 387–392 [PMC free article] [Google Scholar]
- Shualev V, Silverman P, Raskin I (1997) Airborne signaling by methyl salicylate in plant pathogen resistance. Nature 385: 718–721 [Google Scholar]
- Smith RM, Marshall JA, Davey MR, Lowe KC, Power JB (1996) Comparison of volatiles and waxes in leaves of genetically engineered tomatoes. Phytochemistry 43: 753–758 [Google Scholar]
- Spelt C, Quattrocchio F, Mol JN, Koes R (2000) Anthocyanin1 of petunia encodes a basic helix-loop-helix protein that directly activates transcription of structural anthocyanin genes. Plant Cell 12: 1619–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelt C, Quattrocchio F, Mol J, Koes R (2002) ANTHOCYANIN1 of petunia controls pigment synthesis, vacuolar pH, and seed coat development by genetically distinct mechanisms. Plant Cell 14: 2121–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Somerville SC (2002) MLO, a novel modulator of plant defenses and cell death, binds calmodulin. Trends Plant Sci 7: 379–380 [DOI] [PubMed] [Google Scholar]
- Stout MJ, Workman J, Duffey SS (1994) Differential induction of tomato foliar proteins by arthropod herbivores. J Chem Ecol 20: 2575–2594 [DOI] [PubMed] [Google Scholar]
- Strassner J, Schaller F, Frick UB, Howe GA, Weiler EW, Amrhein N, Macheroux P, Schaller A (2002) Characterization and cDNA-microarray expression analysis of 12- oxophytodienoate reductases reveals differential roles for octadecanoid biosynthesis in the local versus the systemic wound response. Plant J 32: 585–601 [DOI] [PubMed] [Google Scholar]
- Thaler JS, Farag MA, Pare PW, Dicke M (2002) Jasmonate-deficient plants have reduced direct and indirect defences against herbivores. Ecol Lett 5: 764–774 [Google Scholar]
- Verberne MC, Hoekstra J, Bol JF, Linthorst HJM (2003) Signaling of systemic acquired resistance in tobacco depends on ethylene perception. Plant J 35: 27–32 [DOI] [PubMed] [Google Scholar]
- Vercammen J, Pham-Tuan H, Sandra P (2001) Automated dynamic sampling system for the on-line monitoring of biogenic emissions from living organisms. J Chromatogr A 930: 39–51 [DOI] [PubMed] [Google Scholar]
- Verdonk JC, de Vos CHR, Verhoeven HA, Haring MA, van Tunen AJ, Schuurink RC (2003) Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 62: 997–1008 [DOI] [PubMed] [Google Scholar]
- Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19: 195–216 [DOI] [PubMed] [Google Scholar]
- Wang CX, Zien CA, Afitlhile M, Welti R, Hildebrand DF, Wang XM (2000) Involvement of phospholipase D in wound-induced accumulation of jasmonic acid in Arabidopsis. Plant Cell 12: 2237–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D, van der Luit AH, Kroon JT, Mol JN, Kooter JM (1993) The petunia homologue of the Antirrhinum majus candi and Zea mays A2 flavonoid genes; homology to flavanone 3-hydroxylase and ethylene-forming enzyme. Plant Mol Biol 22: 893–897 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.