Abstract
Adequate maternal nutrient supply is critical for normal fetal organogenesis. We previously demonstrated that a global 50% nutrient restriction during the first half of gestation causes compensatory growth of both the left and right ventricles of the fetal heart by day 78 of gestation. Thus, it was hypothesized that maternal nutrient restriction significantly altered gene expression in the fetal cardiac left ventricle (LV). Pregnant ewes were randomly grouped into control (100% national research council (NRC) requirements) or nutrient-restricted groups (50% NRC requirements) from day 28 to day 78 of gestation, at which time fetal LV were collected. Fetal LV mRNA was used to construct a suppression subtraction cDNA library from which 11 cDNA clones were found by differential dot blot hybridization and virtual Northern analysis to be up-regulated by maternal nutrient restriction: caveolin, stathmin, G-1 cyclin, α-actin, titin, cardiac ankyrin repeat protein (CARP), cardiac-specific RNA-helicase activated by MEF2C (CHAMP), endothelial and smooth muscle derived neuropilin (ESDN), prostatic binding protein, NADH dehydrogenase subunit 2, and an unknown protein. Six of these clones (cardiac α-actin, cyclin G1, stathmin, NADH dehydrogenase subunit 2, titin and prostatic binding protein) have been linked to cardiac hypertrophy in other species including humans. Of the remaining clones, caveolin, CARP and CHAMP have been shown to inhibit remodelling of hypertrophic tissue. Compensatory growth of fetal LV in response to maternal undernutrition is concluded to be associated with increased transcription of genes related to cardiac hypertrophy, compensatory growth or remodelling. Counter-regulatory gene transcription may be increased, in part, as a response to moderating the degree of cardiac remodelling. The short- and long-term consequences of these changes in fetal heart gene expression and induction of specific homeostatic mechanisms in response to maternal undernutrition remain to be determined.
Compelling epidemiological and animal studies have demonstrated that nutrient supply during early gestation is critical for the development of fetal organogenesis (Reynolds & Redmer, 1995). Undernutrition during the first half of gestation impairs fetal and placental growth and alters the trajectory of development in mammalian species (Barker, 1995; Godfrey & Robinson, 1998; Godfrey & Barker, 2000). In humans (Barker, 1994; Godfrey & Barker, 2000) and rodents (Langley & Jackson, 1994), offspring from undernourished mothers have a predisposition to obesity, diabetes and cardiovascular disease in adult life. Low weight or thinness at birth in human neonates is associated with increased risk of cardiovascular and metabolic disorders in later life (Barker et al. 1993; Martyn et al. 1996; Stein et al. 1996). The ‘fetal origins’ hypothesis proposes that suboptimal conditions experienced by the fetus (e.g. nutritional deprivation or excess hormonal exposure) result in an altered trajectory of development that can permanently change structure, physiology and metabolism, thereby predisposing individuals to cardiovascular, metabolic and endocrine disease in adult life (Barker, 1995). The process whereby the fetus compensates for a maternal insult (undernutrition, stress, etc.) at a sensitive or critical period of fetal development with consequential long-term effects has been termed fetal programming (Lucas, 1991). In evolutionary terms, this phenomenon is likely to reflect the benefits of developmental flexibility by the fetus, allowing for short-term survival. Such adaptations that are beneficial for short-term fetal survival may be detrimental to health in later life (Stein et al. 1996; Barker, 1998). The concept of fetal programming arose from epidemiological studies carried out by Barker and his coworkers who studied birth records of babies born in the United Kingdom between 1910 and 1930 and related their weight and physical characteristics at birth to their subsequent health status in later life (Barker et al. 1989, 1993). Early work focused on the cardiovascular system, where they found increased risk of cardiovascular disease was associated with low birth weight. Experimental studies using animals have documented the effects of restricting the placental nutrient supply during gestation on fetal organ systems and cardiovascular responsiveness (Thureen et al. 1992; Galan et al. 1998; Symonds et al. 2003; Vonnahme et al. 2003). Studies of the cardiovascular system have tended to focus on the peripheral vasculature (Ozaki et al. 2000; Nishina et al. 2003; Brawley et al. 2004) but there is very little information available on the effects of maternal undernutrition on the heart itself. In the present study we conducted dietary restriction to 50% NRC requirement (50% total digestible nutrient (TDN)) in pregnant ewes from early (day 28) to mid (day 78) gestation, the time of organ system development, differentiation and rapid placental growth. We have previously demonstrated that this level of maternal undernutrition caused compensatory growth of the left ventricular heart by day 78 of gestation when compared to controls (Vonnahme et al. 2003). To further understand the impact of maternal undernutrition on the developing fetal heart, the present study focuses on the results of a global screen of gene transcription in the ovine fetal cardiac left ventricle (LV) by utilizing subtractive cDNA library and differential screening approaches.
Methods
Animal care and tissue collection
All animal procedures were approved by the University of Wyoming Animal Care and Use Committee. Thirteen multiparous ewes of mixed breeding were housed in individual pens in a confinement building with controlled temperature (13–16°C) and light (12 h light/day). Ewes were synchronized for oestrus with two injections of Lutalyse (UpJohn, Kalamazoo, MI, USA) 10 days apart and bred at 12 h intervals from the beginning of oestrus by one of two intact rams. On day 20 of gestation, ewes were weighed so that individual diets could be calculated based on metabolic body weight (weight0.75). The diet consisted of a pelleted beet pulp (79.7% TDN, 93.5% dry matter (DM) and 10.0% crude protein). Final diets were calculated on a DM basis for TDN required for maintenance for an early pregnant ewe (metabolic weight × 3.07% NRC requirements). A mineral—vitamin mixture consisting of 51.43% sodium triphosphate, 47.62% potassium chloride, 0.39% zinc oxide, 0.06% cobalt acetate and 0.50% ADE vitamin pre-mix (17 636 800 IU vitamin A, 1 763 680 IU vitamin D3 and 898 400 IU vitamin E per kg; amount of vitamin pre-mix was formulated to meet the vitamin A requirements) was included with the beet pulp pellets to meet requirements. On day 21 of gestation, all ewes were placed in individual pens and fed maintenance diets. On day 28, ewes were weighed and then randomly assigned to the control-fed (n = 7; fed 100% NRC requirements which included 100% mineral—vitamin pre-mix) and the nutrient-restricted (n = 6; fed 50% NRC requirements which included 50% mineral—vitamin pre-mix) groups. At 7-day intervals, ewes were weighed and rations were adjusted for weight gain or loss. On day 45 of gestation, the numbers of fetuses carried by each ewe was determined by ultrasonography (Ausonics Microimager 1000 sector scanning instrument; Ausonics Pty Ltd, Sydney, Australia). On day 78 of gestation, gravid uterine tissue and fetuses were removed. Data were collected for each fetus and included total weight, crown-rump length, abdominal circumference, sex and weights of liver, pancreas, lung, kidney, adrenal, LV, right ventricle (RV) and septum of the heart. The LV heart sample was snap frozen in liquid nitrogen.
Control fed ewes produced four sets of twins and three singles. One set of control fed twins was deleted from the analysis because fetal LV and RV weights were > 3.3 standard deviations from the mean. Also, one fetus from a different set of control fed twins was included for determination of heart weights (Table 1), but was deleted for RNA processing because of a contaminated LV sample. Thus, five twins and three singles provided LV RNA from control fed ewes for the subtractive cDNA library (n = 8). Two sets of twins and four singles provided fetal LV RNA from nutrient-restricted ewes for the subtractive cDNA library (n = 8).
Table 1.
Fetal heart weights (g) from conceptuses on day 78 of gestation from control-fed and nutrient-restricted ewes (mean ± s.e.m.)
| Fetal heart weight (g) | Organ wt/fetus wt (%) | |||||
|---|---|---|---|---|---|---|
| Item | Control n = 9 | Restricted n = 8 | P | Control n = 9 | Restricted n = 8 | *P |
| Fetus | 319.1 ± 10.78 | 221.2 ± 11.96 | 0.0002 | — | — | — |
| LV | 0.94 ± 0.07 | 0.94 ± 0.07 | NS | 0.29 ± 0.02 | 0.42 ± 0.02 | 0.0041 |
| RV | 0.66 ± 0.04 | 0.61 ± 0.05 | NS | 0.21 ± 0.02 | 0.27 ± 0.02 | 0.0245 |
| Septum | 0.43 ± 0.04 | 0.39 ± 0.04 | NS | 0.14 ± 0.01 | 0.17 ± 0.01 | 0.0773 |
P-value control versus restricted. Data were adopted, in part, from Vonnahme et al. (2003).
Total RNA extraction
Total RNA was extracted from 100 mg of LV homogenized in 1 ml Tri Reagent (Molecular Research Inc., Cincinnati, OH, USA). Homogenates were incubated at 25°C for 10 min, mixed with 0.2 ml chloroform, and incubated at 25°C for an additional 15 min. The homogenate was then centrifuged at 12 000 g, for 15 min, at 4°C, to remove the cellular debris. The RNA in the supernatant was precipitated by adding isopropanol and incubated for 10 min at 25°C. RNA was pelleted by centrifugation at 12 000 g, for 15 min, at 4°C. Pellets were washed with cold 75% ethanol, dried and resuspended in sterile diethylpyrocarbonate-treated water.
Construction of a suppression subtraction cDNA library
Total LV RNA was pooled by treatment group. Pooled fetal LV in each treatment group included four from twins and four from singles. Pooled nutrient-restricted RNA served as tester and control-fed RNA served as a driver for construction of the subtractive cDNA library. First strand cDNA was prepared using the SMART PCR cDNA synthesis kit (Clontech, Palo Alto, CA, USA), including SMART II A Oligonucleotide (5′-AAGCAGTGGTATCAACGCAGAGTACGCGGG-3′) and 3′-SMART CDS Primer II A (5′-AAGCAGTGGTATCAACGCAGAGTACT(30)N−1N-3′; N = A, C, G or T; N−1= A, G or C). Reverse transcription was done using PowerScript reverse transcriptase and temperatures of 70°C for 2 min followed by 42°C for 1 h. PCR for cDNA amplification was performed using 95°C 15 s, 60°C 30 s and 68°C 6 min. The number of PCR cycles (18 cycles) was optimized to ensure that cDNA product was in the exponential phase of amplification. Optimized cDNA was collected for generation of the library following 18 cycles because this represented one cycle prior to the plateau in amplification. PCR-amplified cDNAs were purified using CHROMA SPIN-1000 columns (Clontech, Palo Alto, CA, USA) and were then digested with Rsa I at 37°C for 3 h. Restriction of cDNA was confirmed by electrophoretic resolution on 1.5% agarose gels. Blunt-end-digested cDNA was purified by extraction with phenol—chloroform—isoamyl alcohol followed by ethanol precipitation. Tester and driver cDNAs were ligated with two adaptors in separate reactions. An excess of driver cDNA was added to each adaptor-ligated tester cDNA, heat denatured at 98°C for 1.5 min and allowed to anneal at 68°C for 8 h. Enriched cDNAs were then subjected to a second hybridization at 68°C overnight with fresh denatured driver cDNA to further enrich for differentially expressed genes. Subtracted genes were amplified by two subsequent PCR reactions. Primary PCR was performed at 94°C 30 s, 66°C 30 s, and 72°C 1.5 min. Secondary PCR was performed with 10× diluted primary PCR products at 94°C 30 s, 68°C 30 s and 72°C 1.5 min
Screening of differentially expressed cDNA
Amplified cDNA products from the cDNA subtraction were ligated into pCR II vector (Invitrogen, Carlsbad, CA, USA) at 14°C for 16 h. One Shot competent cells were transformed with the ligation mixture and incubated at 37°C for 24 h on LB agar plates containing X-gal and isopropyl-1-thio-β-d-galactopyranoside (IPTG). Colonies were randomly picked, grown in 96-well plates containing 100 μl of LB-amp medium for 2 h at 37°C with shaking. Subtracted cDNA in each bacterial culture was amplified by PCR with corresponding primers generated from the adapters used in hybridization. Five microlitres of PCR product was denatured by adding 0.6 N NaOH. Then, 1 μl of denatured PCR product was transferred to four Nytran membranes (Schleicher and Schuell, Keene, NH, USA). The blots were neutralized in 0.5 m Tris-HCl (pH 7.5) and washed in water. Blots were baked at 80°C for 2 h. Blots were hybridized with control subtracted and nutrient-restricted subtracted 32P-labelled cDNA at 42°C for 18 h, and after stringent washing were placed on film (Bioworld, Atlanta, GA, USA).
Virtual Northern blot analysis
To confirm the dot blot screening, cDNA was generated as mentioned above and 5 μg of cDNA was loaded onto 1.5% agarose gels in Tris-acetate EDTA (TAE) buffer. The cDNA was transferred to 0.2 μm Nytran membranes by capillary method in 10 × SSC at 25°C for 24 h. Blots were baked at 80°C for 2 h, prehybridized for 3 h in 50% formamide, 5 × SSC, 0.05 m sodium phosphate, 5 × Denhardt's solution, 0.1% SDS, 0.1 mg ml−1 salmon sperm DNA, and then hybridized with radiolabelled cDNA probes at 42°C for 18 h. Membranes were washed 2 × for 15 min in 2 × SSC−0.1% SDS, 2 × for 15 min in 1 × SSC−0.1% SDS at 42°C, and then placed on X-ray film.
DNA sequencing and analysis
Differentially expressed clones were confirmed by virtual Northern blot, amplified and then sequenced using an automatic sequencer (3100 Genetic Analyser, Applied Biosystems Inc., Foster City, CA, USA).
Results
Fetal weight and ventricular weight
As previously reported (Vonnahme et al. 2003), at the time of tissue collection the degree of nutrient restriction had elicited a 7.4% decrease in maternal weight while control fed ewes exhibited a 7.5% increase in body weight. In the same report we have shown that, while absolute left and right ventricle weight at day 78 gestation were not affected by undernutrition, relative to body weight, both left and right ventricular weight were greater in the nutrient-restricted fetuses (Table 1; P < 0.05; from Vonnahme et al. 2003). Fetal LV in the current study were collected from these same animals.
Subtractive library and differential screening
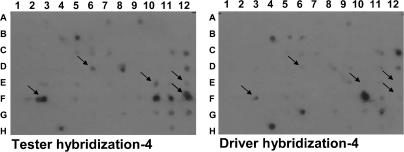
Generation of a subtractive cDNA library enriched for fetal LV cDNA from nutrient-restricted ewes on day 78 of gestation and differential hybridization using subtractive cDNA probes revealed 41 differentially expressed genes (Fig. 1). Virtual Northern blot analysis confirmed that 11 of the 41 clones were differentially expressed in fetal LV from nutrient-restricted when compared to control-fed ewes (Table 2).
Figure 1.
Differential screening of fetal LV nutrient-restricted subtraction cDNA library
This nutrient-restricted (tester) cDNA library was generated by ‘subtracting’ common cDNAs found in fetal LV from control-fed ewes. The same volume of PCR-amplified cDNA library from fetal LV derived from nutrient-restricted ewes was dot blotted onto nylon membranes. 32P-labelled nutrient-restricted (tester) or control-fed (driver) cDNAs were then hybridized with the nutrient-restricted (tester) cDNA library. Differentially expressed cDNAs are identified with the arrows.
Table 2. Differentially expressed genes in ovine fetal heart from conceptuses on day 78 of gestation from control-fed and nutrientrestricted ewes.
Discussion
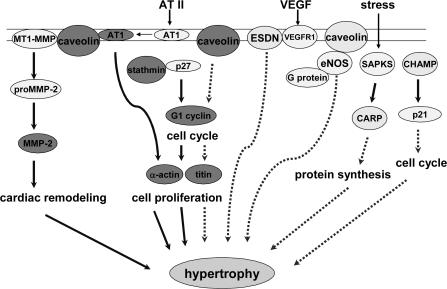
We have previously reported that a 50% global nutrient restriction between days 28 and 78 of ovine gestation leads to an increase in the organ to body weight ratio of day 78 fetal hearts (Vonnahme et al. 2003). In the present study we report possible molecular systems involved in compensatory growth of the fetal heart (Fig. 2). There have been several attempts to discover the genes responsible for cardiac hypertrophy in adults. Presented here is the first report of genes that are up-regulated during increased growth in fetal heart in response to maternal undernutrition. A subtractive cDNA library in which LV cDNAs from nutrient-restricted fetuses were enriched was constructed. Screening of this library using subtracted cDNA probes followed by virtual Northern analysis identified 11 enriched clones: cardiac α-actin, cardiac ankyrin repeating protein (CARP), caveolin-1, cyclin G1, NADH dehydrogenase subunit 2, cardiac-specific helicase activated by MEF2 (CHAMP), stathmin, titin, prostatic binding protein, endothelial and smooth muscle derived neuropilin (ESDN) and a clone (3F2) that has 86% identity with Homo sapiens chromosome 18, clone RP11-268I9.
Figure 2. A model describing up-regulation of fetal LV mRNAs in response to hypertrophy induced by maternal undernutrition.
Binding of MT1-MMP activates proMMP-2, which is then cleaved to form activated MMP-2. Caveolin is also required for the AT2-induced activation of AT1 to initiate signal transduction. Stathmin inhibits CDKI p27, which allows the cell cycle to proceed. These molecules are involved in inducing hypertrophy and mediate cell proliferation and cardiac remodelling. Cardiac α-actin and titin are up-regulated in response to these processes. Caveolin is also known to block G1 cyclin and binds to eNOS to inhibit its activity. CARP is activated by SAPKs in response to stress, which prevents protein synthesis. RNA helicase, also known as CHAMP, inhibits the cell cycle by activation of CDKI p21. These molecules are up-regulated and have inhibitory effects during hypertrophy. It is proposed that hypertrophy of fetal LV in response to maternal undernutrition is a homeostatic response between stimulatory and inhibitory signal transduction pathways. The abbreviations used are: MT1-MMP, membrane type 1 matrix metalloproteinase; AT1, angiotensin II type 1 receptor; ESDN, endothelial and smooth muscle derived neuropilin; eNOS, endothelial NO synthase; SAPKs, stress activated protein kinases; CHAMP, cardiac-specific helicase activated by MEF2. Continuous arrows denote activation and dotted arrows denote inhibition of signalling pathways.
Nutrient restriction during the first half of gestation induced differential transcription of genes in fetal LV that are heavier per unit fetal body weight compared to controls. Cardiac hypertrophy is known to be a result of up-regulation of protein synthesis and over-transcription of fetal genes that serve as markers of cardiac hypertrophy such as skeletal α-actin, β-myosin heavy chain, and atrial natriuretic factor (Adachi et al. 1995). Of the 11 differentially expressed clones confirmed by virtual Northern analysis in the present study, eight genes (cardiac α-actin, cyclin G1, stathmin, NADH dehydrogenase subunit 2, titin, CARP, ESDN and prostatic binding protein) have previously been identified in hypertrophied adult heart tissue (Baumeister et al. 1997; Adachi et al. 1998; Satoh et al. 1999; Aihara et al. 2000; Nozato et al. 2000; Taimor et al. 2001; Pelletier, 2002; Egnaczyk et al. 2003). Notably, of the remaining clones, caveolin and cardiac-specific helicase activated by MEF2 (CHAMP) have been reported to inhibit hypertrophic growth in adults. Finally, one gene product identified in fetal LV had 86% sequence identity to Homo sapiens chromosome 18, clone RP11-268I9, and will be studied in future experiments designed to define the coding region, inferred amino acid sequence, and its function in the fetal heart.
Participation of cell cycle regulation molecules in the development of cardiac hypertrophy has been studied extensively (Li et al. 1998; Tamamori et al. 1998; Nozato et al. 2000; Busk & Hinrichsen, 2003). In adult animals, LV hypertrophy is a compensatory growth of cardiomyocytes as a result of increased working pressure of the heart. During fetal development, cardiomyocytes grow actively by cell division and by increases in cell size (hypertrophy). However, the growth of cardiomyocytes by cell division is lost in the peripartum period (Li et al. 1998). Cyclin-dependent kinases (CDKs) are required with cyclins for mitosis to occur. Because cardiomyocytes undergo irreversible termination of mitosis after birth, increased levels of these cyclins may lead to increased synthesis of RNA and protein that can cause hypertrophy in the heart. Cyclin G1 expression is increased in cardiac myocytes from neonatal rats exposed to angiotensin II (Ang II) (Nozato et al. 2000). When these cardiac myocytes were treated with CDK inhibitors (CDKI), growth was effectively inhibited. It was suggested from these results that certain cell cycle regulators are associated with hypertrophic growth of the heart. In addition, the G1 cyclin/cyclin dependent kinase pathway induces phosphorylation of pRb. Nozato et al. (2000) have proposed that G1 cyclin/CDK and/or phosphor-pRb initiate protein synthesis to cause hypertrophy rather than DNA synthesis through entry into the cell cycle. In addition, the ability of Ang II to induce cardiac hypertrophy through tyrosine kinase-mediated signal transduction pathways such as JAK/STAT (Kodama et al. 1998), mitogen activated protein kinase (MAPK) (Takahashi et al. 1997), PI 3-K (Rabkin et al. 1997), and protein kinase C (PKC) pathways is well documented. However, it is not clear whether cyclins are involved in hypertrophic growth in LV during fetal development because the heart is still undergoing mitotic growth. Nonetheless, it is likely that maternal nutrient restriction resulted in increased cyclin G1 transcription that, in turn, contributed to the increase in fetal LV weight observed.
Fetal LV stathmin, titin, CARP, α-actin and ESDN were also up-regulated in response to maternal undernutrition. The up-regulation of stathmin may lead to cardiac remodelling through up-regulation of both cardiac titin and α-actin (Fig. 2). Stathmin binds to the C-terminal domain of p27 and phosphorylates serine 10, which results in deactivation of this CDKI. Inhibition of p27 leads to cell cycle progression (Boehm et al. 2002). Endothelin-1-induced hypertrophic injury causes over-expression of proteins involved in cytoskeletal reorganization including stathmin (Egnaczyk et al. 2003). Up-regulation of stathmin in the present experiments is likely to play a role in cell remodelling by inactivation of CDKI during the fetal hypertrophic response. Mutations in the muscle protein titin have been linked to dilated cardiomyopathy, a condition in which the heart chambers are enlarged, in humans and in other animal models (Huq et al. 2002). This giant sarcomere protein functions as a molecular spring, the properties of which define the passive mechanical properties of cardiomyocytes, as well as providing essential support for other muscle proteins (Granzier et al. 2002). Interestingly, a recent report has linked CARP and its broader family of muscle ankyrin repeat proteins to titin-based stress/strain signals (Miller et al. 2003). For example, CARP is up-regulated by cardiac muscle stretch and colocalizes with titin N2A epitopes in adult rat heart muscle tissues. These investigators further suggested that the muscle ankyrin repeat protein family provides a link between muscle stretch signal pathways and muscle gene expression. It appears feasible therefore that CARP expression would be increased in the presence of up-regulated titin. There are 23 titin exons unique to fetal and neonatal human myocardium, most of which are highly conserved across species and down-regulated in the adult (Lahmers et al. 2004). Since the PEVK repeats within titin have been suggested to function as protein interaction sites (Gutierrez-Cruz et al. 2001), the increased number of PEVK repeats in fetal titin may increase its role in mediating protein interactions (Lahmers et al. 2004). Which fetal titin isoforms were detected in the present study remains to be determined. It is likely, however, that the up-regulated transcription of titin and α-actin in the ovine fetal LV following maternal undernutrition is, at the very least, an indicator of increased protein synthesis and increased growth. The up-regulation of CARP may indicate that some of the increased growth may involve increased cardiac muscle stretch. The impact of these changes on fetal and postnatal myocardial stiffness and contractile function would provide a direction for future investigation.
In this experiment, fetal LV caveolin and CHAMP have been identified as up-regulated in response to maternal nutrient restriction. Caveolin functions as a general kinase inhibitor (Li et al. 1995; Garcia-Cardena et al. 1997; Ghosh et al. 1998), arresting the cell cycle at G0/1 phase through a p53/p21-dependent mechanism, negatively regulating the cell cycle (Galbiati et al. 2001) and repressing cyclin D1 (Hulit et al. 2000; Peterson et al. 2003). Caveolin 3, and caveolin 1/3 knockout mice induced the activation of G-proteins or Ras signalling and induced dilated LV cardiomyopathy and RV hypertrophy (Park et al. 2002; Woodman et al. 2002). Furthermore, it has been suggested that higher production of NO because of the loss of caveolin can be a contributing factor for these cardiopulmonary defects. Interestingly, Molnar and coworkers (Molnar et al. 2002; Molnar et al. 2003) and others (Ozaki et al. 2000; Brawley et al. 2004; Nishina et al. 2003) have shown alterations in vascular smooth muscle NO signalling in fetal, neonatal and adult sheep exposed to suboptimal pregnancy environments including maternal undernutrition. Increased NO-induced vasodilatory capacity may also contribute to the observation that fetal blood pressure near term is lower in fetuses exposed to early pregnancy nutrient restriction followed by re-alimentation (Hawkins et al. 2000). The role that caveolin regulation plays in altered ovine fetal vascular smooth muscle responsiveness is intriguing and warrants further examination. Since ESDN is thought to play a role in the regulation of vascular cell growth (Kobuke et al. 2001), and CARP has also been implicated in arteriogenesis (Boengler et al. 2003), the up-regulation of caveolin, together with that of ESDN and CARP, indicates increased cardiac vascular smooth muscle growth and/or the presence of increased vascularity. We have confirmed this possibility by noting an increase in mRNA expression of vascular endothelial growth factor (VEGF) and its receptor Flk in the heart tissue from the undernourished group (authors' unpublished observations).
Liu et al. (2001) first described a MEF2C-dependent cardiac specific protein that was expressed in the heart throughout prenatal and postnatal development in the mouse. This protein contained seven conserved motifs characteristic of helicases involved in RNA processing, DNA replication, and transcription and was named CHAMP. CHAMP was later found to inhibit hypertrophic growth and the induction of fetal genes in both prenatal and adult cardiomyocytes in culture (Liu & Olson, 2002). The anti-hypertrophic activity of CHAMP was shown to require a conserved ATPase motif that characterizes the RNA helicase family and the up-regulation of the cell cycle CDKI p21CIP1. CHAMP is probably up-regulated due to increased cyclin G1 transcription, and may act to moderate the impact of maternal undernutrition on myocyte growth and remodelling.
Many of the factors discussed above are influenced by Ang II. Kingdom et al. (1993) reported that in a group of intrauterine growth restriction (IUGR) human fetuses, Ang II concentrations in umbilical venous blood were elevated when compared to uncomplicated term pregnancies, suggesting that the fetal renin—angiotensin system is activated in IUGR fetuses. The role of Ang II receptors in hypertensive adult offspring of rats exposed to prenatal undernutrition is well documented (Sherman & Langley-Evans, 1998; Langley-Evans et al. 1999). There are two known receptors for Ang II in sheep, type 1 (AT1) and type 2 (AT2) with AT1 being constituently expressed while AT2 is transiently expressed primarily during development (Burrell et al. 2001). Global maternal undernutrition (50%) during the last 30 days of pregnancy increased fetal arterial blood pressure in the sheep fetus during late gestation (Edwards & McMillen, 2001). The arterial blood pressure response to Ang II was also higher in the undernutrition group, implying increased AT1 receptor expression, most likely in the feto-placental vascular circuit (Yoshimura et al. 1990). Elevated Ang II concentrations induce a selective increase in LV mass in fetal sheep (Segar et al. 2001). Ang II acts on cardiac remodelling through the AT1 receptor and activates growth pathways in adult animals. AT1 regulates accumulation of extracellular matrix that induces the development of cardiac hypertrophy (Brilla et al. 1995; Weber et al. 1995), perhaps as a result of accumulation of cardiac fibroblasts causing the build up of collagen (Brilla et al. 1995). As already mentioned, Ang II has been implicated in cardiac hypertrophy via cyclin G1 activation in rats (Nozato et al. 2000). Ang II also promotes interaction of AT1 with caveolin (Ushio-Fukai et al. 2001). Ang II also increases caveolin-1 mRNA in rat vascular smooth muscle cells (Ishizaka et al. 1998). These studies were interpreted to mean that caveolin is required for the mechanism of action of Ang II and activation of associated signal transduction pathways. The impact of maternal nutrient restriction on fetal Ang II levels and direct consequences on fetal LV have yet to be ascertained in the present model.
To our knowledge, this is the first animal model that reproduces observations in the human fetus of increased left and right ventricular hypertrophy under conditions of maternal stress (Samson et al. 2000). Other experiments using the fetal lamb have typically resulted in LV hypertrophy without enlargement of the RV (Burrington, 1978; Fishman et al. 1978). Thus, this model of early fetal nutrient deprivation in the sheep may also be relevant for studying the aetiology and consequences of human fetal ventricular hypertrophy. We have found that genes associated with inducing and regulating cellular growth and remodelling in the LV were up-regulated. Changes in expression of these genes can be affected by several factors such as genotype, sex, body size (De Simone et al. 2001) and environmental factors such as nutritional status (Aguilera et al. 2002). The LV undergoes pathological structural changes that go beyond the compensatory needs with increases in extracellular matrix and fibroblast invasion as a consequence (Arnett, 2000). However, limited information is available that describes the genetic basis for LV hypertrophy in the fetus at this early stage of development. In this study, undernutrition caused increased fetal cardiac growth at mid gestation. It is not clear yet whether the increase in growth of heart is compensatory growth, hypertrophy or hyperplasia. As noted above, at mid gestation the fetal heart is still capable of significant increase in cell number.
In the current study, confirmation that clones were up-regulated in nutrient-restricted fetal LV for individual animals was not possible due to limited availability of tissue. Also some of the up-regulated genes in nutrient-restricted fetal LV (i.e. CARP, ESDN and Caveolin), which seems to inhibit hypertrophic growth, suggest that down-regulated genes due to undernutrition also may provide helpful information. Identification of genes that are down-regulated in fetal LV because of maternal undernutrition is the focus of future experiments. Whether the changes discussed above are a cardio-protective response in the face of limited nutrient supply, a response to increased systemic vascular resistance and myocyte stretch, or a response to an altered endocrine milieu also remains the focus of future investigation. More specifically, the encoded proteins need to be studied to determine if they function as a cause or a consequence of altered LV growth.
Acknowledgments
The research was supported, in part, by a grant to T.R.H., S.P.F. and P.J.N. through the University of Wyoming NIH BRIN 1P20RR16474-01, and by an NICHD grant HD21350 to P.J.N. The authors thank Dr K. A. Vonnahme for management of experimental animals and tissue collection and Dr D. J. Perry, Dr B. R. Francis and Dr T. E. Spencer for helpful discussions.
References
- Adachi S, Ito H, Ohta Y, Tanaka M, Ishiyama S, Nagata M, et al. Distribution of mRNAs for natriuretic peptides in RV hypertrophy after pulmonary arterial banding. Am J Physiol. 1995;268:H162–H169. doi: 10.1152/ajpheart.1995.268.1.H162. [DOI] [PubMed] [Google Scholar]
- Adachi S, Ito H, Tamamori M, Tanaka M, Marumo F, Hiroe M. Skeletal and smooth muscle alpha-actin mRNA in endomyocardial biopsy samples of dilated cardiomyopathy patients. Life Sci. 1998;63:1779–1791. doi: 10.1016/s0024-3205(98)00452-4. [DOI] [PubMed] [Google Scholar]
- Aguilera A, Bajo MA, Rebollo F, Diez JJ, Diaz C, Paiva A, et al. Leptin as a marker of nutrition and cardiovascular risk in peritoneal dialysis patients. Adv Perit Dial. 2002;18:212–217. [PubMed] [Google Scholar]
- Aihara Y, Kurabayashi M, Saito Y, Ohyama Y, Tanaka T, Takeda S, et al. Cardiac ankyrin repeat protein is a novel marker of cardiac hypertrophy: role of M-CAT element within the promoter. Hypertension. 2000;36:48–53. doi: 10.1161/01.hyp.36.1.48. [DOI] [PubMed] [Google Scholar]
- Arnett DK. Genetic contributions to left ventricular hypertrophy. Curr Hypertens Rep. 2000;2:50–55. doi: 10.1007/s11906-000-0058-3. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Mothers Babies and Disease in Later Life. London: BMJ Publishing group; 1994. [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Mothers, Babies and Health in Later Life. Edinburgh: Harcourt Brace Ltd; 1998. [Google Scholar]
- Barker DJ, Martyn CN, Osmond C, Hales CN, Fall CH. Growth in utero and serum cholesterol concentrations in adult life. BMJ. 1993;307:1524–1527. doi: 10.1136/bmj.307.6918.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Baumeister A, Arber S, Caroni P. Accumulation of muscle ankyrin repeat protein transcript reveals local activation of primary myotube endcompartments during muscle morphogenesis. J Cell Biol. 1997;139:1231–1242. doi: 10.1083/jcb.139.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M, Yoshimoto T, Crook MF, Nallamshetty S, True A, Nabel GJ, et al. A growth factor-dependent nuclear kinase phosphorylates p27 (Kip1) and regulates cell cycle progression. EMBO J. 2002;21:3390–3401. doi: 10.1093/emboj/cdf343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler K, Pipp F, Fernandez B, Ziegelhoeffer T, Schaper W, Deindl E. Arteriogenesis is associated with an induction of the cardiac ankyrin repeat protein (carp) Cardiovasc Res. 2003;59:573–581. doi: 10.1016/s0008-6363(03)00511-x. [DOI] [PubMed] [Google Scholar]
- Brawley L, Torrens C, Anthony FW, Itoh S, Wheeler T, Jackson AA, et al. Glycine rectifies vascular dysfunction induced by dietary protein imbalance during pregnancy. J Physiol. 2004;554:497–504. doi: 10.1113/jphysiol.2003.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilla CG, Zhou G, Rupp H, Maisch B, Weber KT. Role of angiotensin II and prostaglandin E2 in regulating cardiac fibroblast collagen turnover. Am J Cardiol. 1995;76:8D–13D. doi: 10.1016/s0002-9149(99)80485-8. [DOI] [PubMed] [Google Scholar]
- Burrell JH, Hegarty BD, McMullen JR, Lumbers ER. Effects of gestation on ovine fetal and maternal angiotensin receptor subtypes in the heart and major blood vessels. Exp Physiol. 2001;86:71–82. doi: 10.1113/eph8602075. [DOI] [PubMed] [Google Scholar]
- Burrington JD. Response to experimental coarctation of the aorta and pulmonic stenosis in the fetal lamb. J Thorac Cardiovasc Surg. 1978;75:819–826. [PubMed] [Google Scholar]
- Busk PK, Hinrichsen R. Cyclin D in left ventricle hypertrophy. Cell Cycle. 2003;2:91–95. [PubMed] [Google Scholar]
- De Simone G, Pasanisi F, Contaldo F. Link of nonhemodynamic factors to hemodynamic determinants of left ventricular hypertrophy. Hypertension. 2001;38:13–18. doi: 10.1161/01.hyp.38.1.13. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, McMillen IC. Maternal undernutrition increases arterial blood pressure in the sheep fetus during late gestation. J Physiol. 2001;533:561–570. doi: 10.1111/j.1469-7793.2001.0561a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egnaczyk GF, Pomonis JD, Schmidt JA, Rogers SD, Peters C, Ghilardi JR, et al. Proteomic analysis of the reactive phenotype of astrocytes following endothelin-1 exposure. Proteomics. 2003;3:689–698. doi: 10.1002/pmic.200300407. [DOI] [PubMed] [Google Scholar]
- Fishman NH, Hof RB, Rudolph AM, Heymann MA. Models of congenital heart disease in fetal lambs. Circulation. 1978;58:354–364. doi: 10.1161/01.cir.58.2.354. [DOI] [PubMed] [Google Scholar]
- Galan HL, Hussey MJ, Chung M, Chyu JK, Hobbins JC, Battaglia FC. Doppler velocimetry of growth-restricted fetuses in an ovine model of placental insufficiency. Am J Obstet Gynecol. 1998;178:451–456. doi: 10.1016/s0002-9378(98)70419-3. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Liu J, Capozza F, Frank PG, Zhu L, et al. Caveolin-1 expression negatively regulates cell cycle progression by inducing G0/G1 arrest via a p53/p21 (WAF1/Cip1)-dependent mechanism. Mol Biol Cell. 2001;12:2229–2244. doi: 10.1091/mbc.12.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Gachhui R, Crooks C, Wu C, Lisanti MP, Stuehr DJ. Interaction between caveolin-1 and the reductase domain of endothelial nitric-oxide synthase. Consequences for catalysis. J Biol Chem. 1998;273:22267–22271. doi: 10.1074/jbc.273.35.22267. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71:1344S–1352S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- Godfrey K, Robinson S. Maternal nutrition, placental growth and fetal programming. Proc Nutr Soc. 1998;57:105–111. doi: 10.1079/pns19980016. [DOI] [PubMed] [Google Scholar]
- Granzier H, Labeit D, Wu Y, Labeit S. Titin as a modular spring: emerging mechanisms for elasticity control by titin in cardiac physiology and pathophysiology. J Muscle Res Cell Motil. 2002;23:457–471. doi: 10.1023/a:1023458406346. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Cruz G, Van Heerden AH, Wang K. Modular motif, structural folds and affinity profiles of the PEVK segment of human fetal skeletal muscle titin. J Biol Chem. 2001;276:7442–7449. doi: 10.1074/jbc.M008851200. [DOI] [PubMed] [Google Scholar]
- Hawkins P, Steyn C, Ozaki T, Saito T, Noakes DE, Hanson MA. Effect of maternal undernutrition in early gestation on ovine fetal blood pressure and cardiovascular reflexes. Am J Physiol Regul Integr Comp Physiol. 2000;279:R340–R348. doi: 10.1152/ajpregu.2000.279.1.R340. [DOI] [PubMed] [Google Scholar]
- Hulit J, Bash T, Fu M, Galbiati F, Albanese C, Sage DR, et al. The cyclin D1 gene is transcriptionally repressed by caveolin-1. J Biol Chem. 2000;275:21203–21209. doi: 10.1074/jbc.M000321200. [DOI] [PubMed] [Google Scholar]
- Huq F, Heist EK, Hajjar RJ. Titin — springing back to youth? Sci Aging Knowledge Environ. 2002:pe20. doi: 10.1126/sageke.2002.49.pe20. [DOI] [PubMed] [Google Scholar]
- Ishizaka N, Griendling KK, Lassegue B, Alexander RW. Angiotensin II type 1 receptor: relationship with caveolae and caveolin after initial agonist stimulation. Hypertension. 1998;32:459–466. doi: 10.1161/01.hyp.32.3.459. [DOI] [PubMed] [Google Scholar]
- Kingdom JC, McQueen J, Connell JM, Whittle MJ. Fetal angiotensin II levels and vascular (type I) angiotensin receptors in pregnancies complicated by intrauterine growth retardation. Br J Obstet Gynaecol. 1993;100:476–482. doi: 10.1111/j.1471-0528.1993.tb15276.x. [DOI] [PubMed] [Google Scholar]
- Kobuke K, Furukawa Y, Sugai M, Tanigaki K, Ohashi N, Matsumori A, et al. ESDN, a novel neuropilin-like membrane protein cloned from vascular cells with the longest secretory signal sequence among eukaryotes, is up-regulated after vascular injury. J Biol Chem. 2001;276:34105–34114. doi: 10.1074/jbc.M105293200. [DOI] [PubMed] [Google Scholar]
- Kodama H, Fukuda K, Pan J, Makino S, Sano M, Takahashi T, et al. Biphasic activation of the JAK/STAT pathway by angiotensin II in rat cardiomyocytes. Circ Res. 1998;82:244–250. doi: 10.1161/01.res.82.2.244. [DOI] [PubMed] [Google Scholar]
- Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res. 2004;94:505–513. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 1994;86:217–222. doi: 10.1042/cs0860217. discussion 121. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Sherman RC, Welham SJ, Nwagwu MO, Gardner DS, Jackson AA. Intrauterine programming of hypertension: the role of the renin-angiotensin system. Biochem Soc Trans. 1999;27:88–93. doi: 10.1042/bst0270088. [DOI] [PubMed] [Google Scholar]
- Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, et al. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- Li JM, Poolman RA, Brooks G. Role of G1 phase cyclins and cyclin-dependent kinases during cardiomyocyte hypertrophic growth in rats. Am J Physiol. 1998;275:H814–H822. doi: 10.1152/ajpheart.1998.275.3.H814. [DOI] [PubMed] [Google Scholar]
- Liu ZP, Nakagawa O, Nakagawa M, Yanagisawa H, Passier R, Richardson JA, et al. CHAMP, a novel cardiac-specific helicase regulated by MEF2C. Dev Biol. 2001;234:497–509. doi: 10.1006/dbio.2001.0277. [DOI] [PubMed] [Google Scholar]
- Liu ZP, Olson EN. Suppression of proliferation and cardiomyocyte hypertrophy by CHAMP, a cardiac-specific RNA helicase. Proc Natl Acad Sci U S A. 2002;99:2043–2048. doi: 10.1073/pnas.261708699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A. Programming by Early Nutrition in Man. Chichester, UK: John Wiley and Sons; 1991. [Google Scholar]
- Martyn CN, Barker DJ, Osmond C. Mothers' pelvic size, fetal growth, and death from stroke and coronary heart disease in men in the UK. Lancet. 1996;348:1264–1268. doi: 10.1016/s0140-6736(96)04257-2. [DOI] [PubMed] [Google Scholar]
- Miller MK, Bang ML, Witt CC, Labeit D, Trombitas C, Watanabe K, et al. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol. 2003;333:951–964. doi: 10.1016/j.jmb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Molnar J, Howe DC, Nijland MJ, Nathanielsz PW. Prenatal dexamethasone leads to both endothelial dysfunction and vasodilatory compensation in sheep. J Physiol. 2003;547:61–66. doi: 10.1113/jphysiol.2002.032565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar J, Nijland MJ, Howe DC, Nathanielsz PW. Evidence for microvascular dysfunction after prenatal dexamethasone at 0.7, 0.75, and 0.8 gestation in sheep. Am J Physiol Regul Integr Comp Physiol. 2002;283:R561–R567. doi: 10.1152/ajpregu.00031.2002. [DOI] [PubMed] [Google Scholar]
- Nishina H, Green LR, McGarrigle HH, Noakes DE, Poston L, Hanson MA. Effect of nutritional restriction in early pregnancy on isolated femoral artery function in mid-gestation fetal sheep. J Physiol. 2003;553:637–647. doi: 10.1113/jphysiol.2003.045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozato T, Ito H, Tamamori M, Adachi S, Abe S, Marumo F, et al. G1 cyclins are involved in the mechanism of cardiac myocyte hypertrophy induced by angiotensin II. Jpn Circ J. 2000;64:595–601. doi: 10.1253/jcj.64.595. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Hawkins P, Nishina H, Steyn C, Poston L, Hanson MA. Effects of undernutrition in early pregnancy on systemic small artery function in late-gestation fetal sheep. Am J Obstet Gynecol. 2000;183:1301–1307. doi: 10.1067/mob.2000.107463. [DOI] [PubMed] [Google Scholar]
- Park DS, Woodman SE, Schubert W, Cohen AW, Frank PG, Chandra M, et al. Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. Am J Pathol. 2002;160:2207–2217. doi: 10.1016/S0002-9440(10)61168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G. Effects of estradiol on prostate epithelial cells in the castrated rat. J Histochem Cytochem. 2002;50:1517–1524. doi: 10.1177/002215540205001112. [DOI] [PubMed] [Google Scholar]
- Peterson TE, Guicciardi ME, Gulati R, Kleppe LS, Mueske CS, Mookadam M, et al. Caveolin-1 can regulate vascular smooth muscle cell fate by switching platelet-derived growth factor signaling from a proliferative to an apoptotic pathway. Arterioscler Thromb Vasc Biol. 2003;23:1521–1527. doi: 10.1161/01.ATV.0000081743.35125.05. [DOI] [PubMed] [Google Scholar]
- Rabkin SW, Goutsouliak V, Kong JY. Angiotensin II induces activation of phosphatidylinositol 3-kinase in cardiomyocytes. J Hypertens. 1997;15:891–899. doi: 10.1097/00004872-199715080-00014. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci. 1995;73:1839–1851. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- Samson F, Bonnet N, Heimburger M, Rucker-Martin C, Levitsky DO, Mazmanian GM, et al. Left ventricular alterations in a model of fetal left ventricular overload. Pediatr Res. 2000;48:43–49. doi: 10.1203/00006450-200007000-00010. [DOI] [PubMed] [Google Scholar]
- Satoh M, Takahashi M, Sakamoto T, Hiroe M, Marumo F, Kimura A. Structural analysis of the titin gene in hypertrophic cardiomyopathy: identification of a novel disease gene. Biochem Biophys Res Commun. 1999;262:411–417. doi: 10.1006/bbrc.1999.1221. [DOI] [PubMed] [Google Scholar]
- Segar JL, Dalshaug GB, Bedell KA, Smith OM, Scholz TD. Angiotensin II in cardiac pressure-overload hypertrophy in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2001;281:R2037–R2047. doi: 10.1152/ajpregu.2001.281.6.R2037. [DOI] [PubMed] [Google Scholar]
- Sherman RC, Langley-Evans SC. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin Sci (Lond) 1998;94:373–381. doi: 10.1042/cs0940373. [DOI] [PubMed] [Google Scholar]
- Stein CE, Fall CH, Kumaran K, Osmond C, Cox V, Barker DJ. Fetal growth and coronary heart disease in south India. Lancet. 1996;348:1269–1273. doi: 10.1016/s0140-6736(96)04547-3. [DOI] [PubMed] [Google Scholar]
- Symonds ME, Gopalakrishnan G, Bispham J, Pearce S, Dandrea J, Mostyn A, et al. Maternal nutrient restriction during placental growth, programming of fetal adiposity and juvenile blood pressure control. Arch Physiol Biochem. 2003;111:45–52. doi: 10.1076/apab.111.1.45.15141. [DOI] [PubMed] [Google Scholar]
- Taimor G, Schluter K, Piper HM. Hypertrophy-associated gene induction after beta-adrenergic stimulation in adult cardiomyocytes. J Mol Cell Cardiol. 2001;33:503–511. doi: 10.1006/jmcc.2000.1324. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kawahara Y, Okuda M, Ueno H, Takeshita A, Yokoyama M. Angiotensin II stimulates mitogen-activated protein kinases and protein synthesis by a Ras-independent pathway in vascular smooth muscle cells. J Biol Chem. 1997;272:16018–16022. doi: 10.1074/jbc.272.25.16018. [DOI] [PubMed] [Google Scholar]
- Tamamori M, Ito H, Hiroe M, Terada Y, Marumo F, Ikeda MA. Essential roles for G1 cyclin-dependent kinase activity in development of cardiomyocyte hypertrophy. Am J Physiol. 1998;275:H2036–H2040. doi: 10.1152/ajpheart.1998.275.6.H2036. [DOI] [PubMed] [Google Scholar]
- Thureen PJ, Trembler KA, Meschia G, Makowski EL, Wilkening RB. Placental glucose transport in heat-induced fetal growth retardation. Am J Physiol. 1992;263:R578–R585. doi: 10.1152/ajpregu.1992.263.3.R578. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Hilenski L, Santanam N, Becker PL, Ma Y, Griendling KK, et al. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem. 2001;276:48269–48275. doi: 10.1074/jbc.M105901200. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Hess BW, Hansen TR, McCormick RJ, Rule DC, Moss GE, et al. Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol Reprod. 2003;69:133–140. doi: 10.1095/biolreprod.102.012120. [DOI] [PubMed] [Google Scholar]
- Weber KT, Sun Y, Campbell SE. Structural remodelling of the heart by fibrous tissue: role of circulating hormones and locally produced peptides. Eur Heart J. 1995;16(Suppl. N):12–18. doi: 10.1093/eurheartj/16.suppl_n.12. [DOI] [PubMed] [Google Scholar]
- Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, et al. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Magness RR, Rosenfeld CR. Angiotensin II and alpha-agonist. I. Responses of ovine fetoplacental vasculature. Am J Physiol. 1990;259:H464–H472. doi: 10.1152/ajpheart.1990.259.2.H464. [DOI] [PubMed] [Google Scholar]