Abstract
Rat liver glutathione S-transferase (GST) 3-3 is composed of two identical subunits, each containing three cysteine residues, Cys-86, Cys-114 and Cys-173. We have shown previously that Cys-86 is not involved in the enzymic activity of GST 3-3 [Hsieh, Huang, Chen, Lai & Tam (1991) Biochem, J. 278, 293-297]. At 50 degrees C, iodoacetamide can inactivate the enzyme by modifying Cys-86 and Cys-114. Cys-114 can be protected against iodoacetamide inhibition by S-(dinitrophenyl)glutathione. Site-directed mutagenesis was used to construct mutants in which serine replaced one (C114S and C173S) or all three (CallS) cysteine residues. These mutants were over-expressed in Spodoptera frugiperda cells in a baculovirus system and were found to be fully active. Replacing Cys-86 or Cys-114 with alanine (C86A and C114A) does not diminish the activity of the protein. The results suggest that cysteines are not involved in the enzymic mechanism, and Cys-114 is possibly located at the active site of GST 3-3.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad H., Wilson D. E., Fritz R. R., Singh S. V., Medh R. D., Nagle G. T., Awasthi Y. C., Kurosky A. Primary and secondary structural analyses of glutathione S-transferase pi from human placenta. Arch Biochem Biophys. 1990 May 1;278(2):398–408. doi: 10.1016/0003-9861(90)90277-6. [DOI] [PubMed] [Google Scholar]
- Awasthi Y. C., Bhatnagar A., Singh S. V. Evidence for the involvement of histidine at the active site of glutathione S-transferase psi from human liver. Biochem Biophys Res Commun. 1987 Mar 30;143(3):965–970. doi: 10.1016/0006-291x(87)90345-7. [DOI] [PubMed] [Google Scholar]
- Bhargava M. M., Listowsky I., Arias I. M. Ligandin. Bilirubin binding and glutathione-S-transferase activity are independent processes. J Biol Chem. 1978 Jun 25;253(12):4112–4115. [PubMed] [Google Scholar]
- Carne T., Tipping E., Ketterer B. The binding and catalytic activities of forms of ligandin after modification of its thiol groups. Biochem J. 1979 Feb 1;177(2):433–439. doi: 10.1042/bj1770433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. H., Wang L. Y., Tam M. F. The single cysteine residue on an alpha family chick liver glutathione S-transferase CL 3-3 is not functionally important. Biochem Biophys Res Commun. 1991 Oct 15;180(1):323–328. doi: 10.1016/s0006-291x(05)81295-1. [DOI] [PubMed] [Google Scholar]
- Christ-Hazelhof E., Nugteren D. H. Purification and characterisation of prostaglandin endoperoxide D-isomerase, a cytoplasmic, glutathione-requiring enzyme. Biochim Biophys Acta. 1979 Jan 29;572(1):43–51. doi: 10.1016/0005-2760(79)90198-x. [DOI] [PubMed] [Google Scholar]
- Cowan S. W., Bergfors T., Jones T. A., Tibbelin G., Olin B., Board P. G., Mannervik B. Crystallization of GST2, a human class alpha glutathione transferase. J Mol Biol. 1989 Jul 20;208(2):369–370. doi: 10.1016/0022-2836(89)90398-7. [DOI] [PubMed] [Google Scholar]
- Daniel V., Sharon R., Tichauer Y., Sarid S. Mouse glutathione S-transferase Ya subunit: gene structure and sequence. DNA. 1987 Aug;6(4):317–324. doi: 10.1089/dna.1987.6.317. [DOI] [PubMed] [Google Scholar]
- Del Boccio G., Pennelli A., Whitehead E. P., Lo Bello M., Petruzzelli R., Federici G., Ricci G. Interaction of glutathione transferase from horse erythrocytes with 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole. J Biol Chem. 1991 Jul 25;266(21):13777–13782. [PubMed] [Google Scholar]
- Desideri A., Caccuri A. M., Polizio F., Bastoni R., Federici G. Electron paramagnetic resonance identification of a highly reactive thiol group in the proximity of the catalytic site of human placenta glutathione transferase. J Biol Chem. 1991 Feb 5;266(4):2063–2066. [PubMed] [Google Scholar]
- Dirr H. W., Mann K., Huber R., Ladenstein R., Reinemer P. Class pi glutathione S-transferase from pig lung. Purification, biochemical characterization, primary structure and crystallization. Eur J Biochem. 1991 Mar 28;196(3):693–698. doi: 10.1111/j.1432-1033.1991.tb15867.x. [DOI] [PubMed] [Google Scholar]
- Gribskov M., Burgess R. R. Sigma factors from E. coli, B. subtilis, phage SP01, and phage T4 are homologous proteins. Nucleic Acids Res. 1986 Aug 26;14(16):6745–6763. doi: 10.1093/nar/14.16.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hoesch R. M., Boyer T. D. Localization of a portion of the active site of two rat liver glutathione S-transferases using a photoaffinity label. J Biol Chem. 1989 Oct 25;264(30):17712–17717. [PubMed] [Google Scholar]
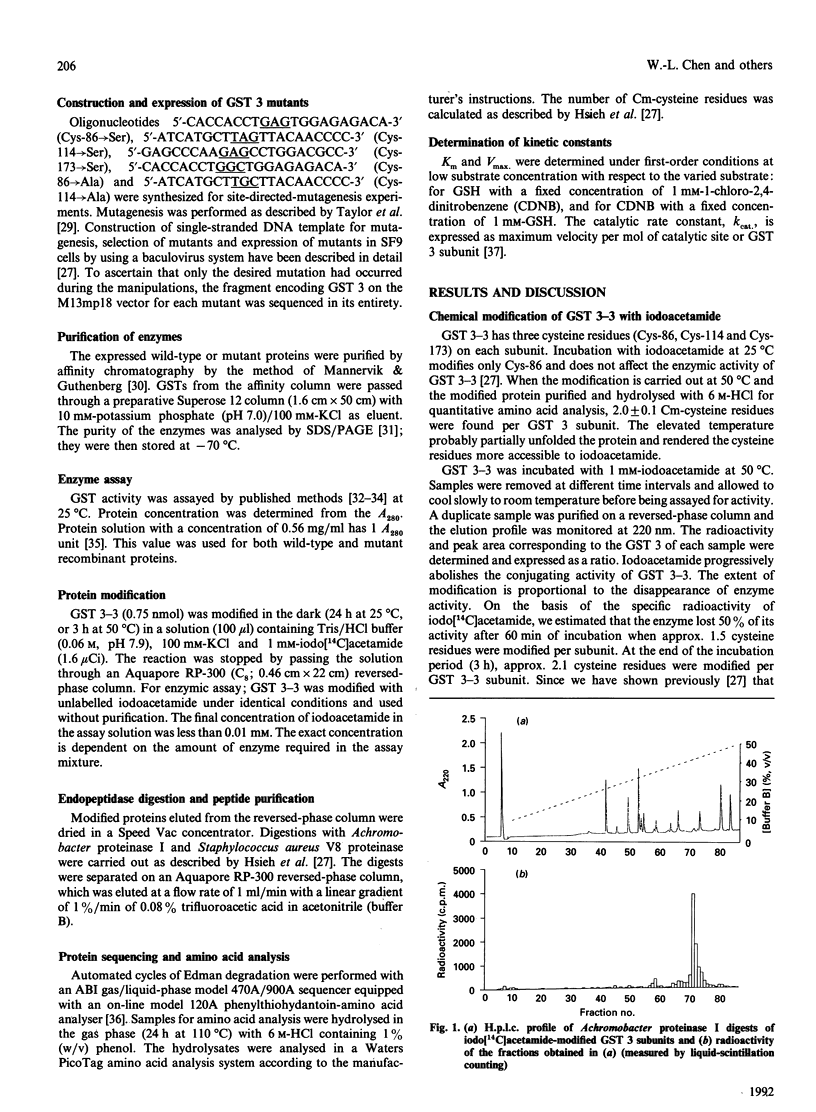
- Hsieh J. C., Huang S. C., Chen W. L., Lai Y. C., Tam M. F. Cysteine-86 is not needed for the enzymic activity of glutathione S-transferase 3-3. Biochem J. 1991 Aug 15;278(Pt 1):293–297. doi: 10.1042/bj2780293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J. C., Liu L. F., Chen W. L., Tam M. F. Expression of Yb1 glutathione S-transferase using a baculovirus expression system. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1147–1154. doi: 10.1016/0006-291x(89)90793-6. [DOI] [PubMed] [Google Scholar]
- Ishigaki S., Abramovitz M., Listowsky I. Glutathione-S-transferases are major cytosolic thyroid hormone binding proteins. Arch Biochem Biophys. 1989 Sep;273(2):265–272. doi: 10.1016/0003-9861(89)90483-9. [DOI] [PubMed] [Google Scholar]
- Jakobson I., Warholm M., Mannervik B. Multiple inhibition of glutathione S-transferase A from rat liver by glutathione derivatives: kinetic analysis supporting a steady-state random sequential mechanism. Biochem J. 1979 Mar 1;177(3):861–868. doi: 10.1042/bj1770861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. R. Tinkering with enzymes: what are we learning? Science. 1987 Jun 5;236(4806):1252–1258. doi: 10.1126/science.3296192. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai H. C., Grove G., Tu C. P. Cloning and sequence analysis of a cDNA for a rat liver glutathione S-transferase Yb subunit. Nucleic Acids Res. 1986 Aug 11;14(15):6101–6114. doi: 10.1093/nar/14.15.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H. C., Li N., Weiss M. J., Reddy C. C., Tu C. P. The nucleotide sequence of a rat liver glutathione S-transferase subunit cDNA clone. J Biol Chem. 1984 May 10;259(9):5536–5542. [PubMed] [Google Scholar]
- Lai H. C., Tu C. P. Rat glutathione S-transferases supergene family. Characterization of an anionic Yb subunit cDNA clone. J Biol Chem. 1986 Oct 15;261(29):13793–13799. [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lindwall G., Boyer T. D. Excretion of glutathione conjugates by primary cultured rat hepatocytes. J Biol Chem. 1987 Apr 15;262(11):5151–5158. [PubMed] [Google Scholar]
- Lo Bello M., Petruzzelli R., De Stefano E., Tenedini C., Barra D., Federici G. Identification of a highly reactive sulphydryl group in human placental glutathione transferase by a site-directed fluorescent reagent. FEBS Lett. 1990 Apr 24;263(2):389–391. doi: 10.1016/0014-5793(90)81421-j. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Coles B., Pemble S. E., Gilmore K. S., Fraser G. M., Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochem J. 1991 Mar 1;274(Pt 2):409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. W., Lo Bello M., Federici G. Crystallization of glutathione S-transferase from human placenta. J Mol Biol. 1990 May 20;213(2):221–222. doi: 10.1016/s0022-2836(05)80183-4. [DOI] [PubMed] [Google Scholar]
- Ploemen J. H., van Ommen B., van Bladeren P. J. Irreversible inhibition of human glutathione S-transferase isoenzymes by tetrachloro-1,4-benzoquinone and its glutathione conjugate. Biochem Pharmacol. 1991 Jun 1;41(11):1665–1669. doi: 10.1016/0006-2952(91)90167-4. [DOI] [PubMed] [Google Scholar]
- Principato G. B., Danielson U. H., Mannervik B. Relaxed thiol substrate specificity of glutathione transferase effected by a non-substrate glutathione derivative. FEBS Lett. 1988 Apr 11;231(1):155–158. doi: 10.1016/0014-5793(88)80722-1. [DOI] [PubMed] [Google Scholar]
- Reinemer P., Dirr H. W., Ladenstein R., Schäffer J., Gallay O., Huber R. The three-dimensional structure of class pi glutathione S-transferase in complex with glutathione sulfonate at 2.3 A resolution. EMBO J. 1991 Aug;10(8):1997–2005. doi: 10.1002/j.1460-2075.1991.tb07729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci G., Del Boccio G., Pennelli A., Aceto A., Whitehead E. P., Federici G. Nonequivalence of the two subunits of horse erythrocyte glutathione transferase in their reaction with sulfhydryl reagents. J Biol Chem. 1989 Apr 5;264(10):5462–5467. [PubMed] [Google Scholar]
- Schäffer J., Gallay O., Ladenstein R. Glutathione transferase from bovine placenta. Preparation, biochemical characterization, crystallization, and preliminary crystallographic analysis of a neutral class PI enzyme. J Biol Chem. 1988 Nov 25;263(33):17405–17411. [PubMed] [Google Scholar]
- Sesay M. A., Ammon H. L., Armstrong R. N. Crystallization and a preliminary X-ray diffraction study of isozyme 3-3 of glutathione S-transferase from rat liver. J Mol Biol. 1987 Sep 20;197(2):377–378. doi: 10.1016/0022-2836(87)90133-1. [DOI] [PubMed] [Google Scholar]
- Shen H. X., Tamai K., Satoh K., Hatayama I., Tsuchida S., Sato K. Modulation of class Pi glutathione transferase activity by sulfhydryl group modification. Arch Biochem Biophys. 1991 Apr;286(1):178–182. doi: 10.1016/0003-9861(91)90025-e. [DOI] [PubMed] [Google Scholar]
- Suguoka Y., Kano T., Okuda A., Sakai M., Kitagawa T., Muramatsu M. Cloning and the nucleotide sequence of rat glutathione S-transferase P cDNA. Nucleic Acids Res. 1985 Sep 11;13(17):6049–6057. doi: 10.1093/nar/13.17.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K., Satoh K., Tsuchida S., Hatayama I., Maki T., Sato K. Specific inactivation of glutathione S-transferases in class Pi by SH-modifiers. Biochem Biophys Res Commun. 1990 Feb 28;167(1):331–338. doi: 10.1016/0006-291x(90)91769-o. [DOI] [PubMed] [Google Scholar]
- Tamai K., Shen H. X., Tsuchida S., Hatayama I., Satoh K., Yasui A., Oikawa A., Sato K. Role of cysteine residues in the activity of rat glutathione transferase P (7-7): elucidation by oligonucleotide site-directed mutagenesis. Biochem Biophys Res Commun. 1991 Sep 16;179(2):790–797. doi: 10.1016/0006-291x(91)91886-h. [DOI] [PubMed] [Google Scholar]
- Tan K. H., Meyer D. J., Coles B., Ketterer B. Thymine hydroperoxide, a substrate for rat Se-dependent glutathione peroxidase and glutathione transferase isoenzymes. FEBS Lett. 1986 Oct 27;207(2):231–233. doi: 10.1016/0014-5793(86)81494-6. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telakowski-Hopkins C. A., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Construction of a cDNA clone complementary to a Yc mRNA and prediction of the complete amino acid sequence of a Yc subunit. J Biol Chem. 1985 May 10;260(9):5820–5825. [PubMed] [Google Scholar]
- Townsend A. J., Goldsmith M. E., Pickett C. B., Cowan K. H. Isolation, characterization, and expression in Escherichia coli of two murine Mu class glutathione S-transferase cDNAs homologous to the rat subunits 3 (Yb1) and 4 (Yb2). J Biol Chem. 1989 Dec 25;264(36):21582–21590. [PubMed] [Google Scholar]
- Tu C. P., Qian B. Human liver glutathione S-transferases: complete primary sequence of an Ha subunit cDNA. Biochem Biophys Res Commun. 1986 Nov 26;141(1):229–237. doi: 10.1016/s0006-291x(86)80358-8. [DOI] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B. Molecular and catalytic properties of glutathione transferase mu from human liver: an enzyme efficiently conjugating epoxides. Biochemistry. 1983 Jul 19;22(15):3610–3617. doi: 10.1021/bi00284a011. [DOI] [PubMed] [Google Scholar]
- Widersten M., Holmström E., Mannervik B. Cysteine residues are not essential for the catalytic activity of human class Mu glutathione transferase M1a-1a. FEBS Lett. 1991 Nov 18;293(1-2):156–159. doi: 10.1016/0014-5793(91)81175-8. [DOI] [PubMed] [Google Scholar]
- Zhang P. H., Graminski G. F., Armstrong R. N. Are the histidine residues of glutathione S-transferase important in catalysis? An assessment by 13C NMR spectroscopy and site-specific mutagenesis. J Biol Chem. 1991 Oct 15;266(29):19475–19479. [PubMed] [Google Scholar]
- van Ommen B., Ploemen J. H., Bogaards J. J., Monks T. J., Gau S. S., van Bladeren P. J. Irreversible inhibition of rat glutathione S-transferase 1-1 by quinones and their glutathione conjugates. Structure-activity relationship and mechanism. Biochem J. 1991 Jun 15;276(Pt 3):661–666. doi: 10.1042/bj2760661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ommen B., den Besten C., Rutten A. L., Ploemen J. H., Vos R. M., Müller F., van Bladeren P. J. Active site-directed irreversible inhibition of glutathione S-transferases by the glutathione conjugate of tetrachloro-1,4-benzoquinone. J Biol Chem. 1988 Sep 15;263(26):12939–12942. [PubMed] [Google Scholar]


