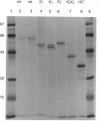
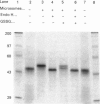
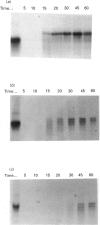
Abstract
Tissue-type plasminogen activator (t-PA) is synthesized in mammalian cells as a mixture of two forms that differ in their extent of N-linked glycosylation. We have investigated the mechanism underlying this variation in glycosylation, using a cell-free system that consists of a rabbit reticulocyte lysate optimized for the formation of disulphide bonds and supplemented with dog pancreas microsomal membranes. Molecules of human t-PA synthesized in vitro are enzymically active and responsive to natural activators and inhibitors, and are glycosylated in a pattern identical with that of the protein produced in vivo. This demonstrates that t-PA synthesized in vitro folds into the same conformation as the protein synthesized in vivo. We show that the extent of glycosylation of individual t-PA molecules is dependent on the state of folding of the polypeptide chain, since the probability of addition of an oligosaccharide side chain at Asn-184 is decreased under conditions that promote the formation of enzymically active molecules. This variation in glycosylation is independent of the rate of protein synthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid N. J., Curling E., Freedman R. B., Jenkins N. Source of heterogeneity in secreted interferon-gamma. A study on products of translation in vitro. Biochem J. 1990 Jun 15;268(3):777–781. doi: 10.1042/bj2680777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid N. J., Freedman R. B. Cotranslational glycosylation of proteins in systems depleted of protein disulphide isomerase. EMBO J. 1990 Nov;9(11):3527–3532. doi: 10.1002/j.1460-2075.1990.tb07561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid N. J., Freedman R. B. The transcription and translation in vitro of individual cereal storage-protein genes from wheat (Triticum aestivum, cv. Chinese Spring). Evidence for translocation of the translation products and disulphide-bond formation. Biochem J. 1988 Sep 15;254(3):805–810. doi: 10.1042/bj2540805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980 Jun 18;43(2):77–89. [PubMed] [Google Scholar]
- Curling E. M., Hayter P. M., Baines A. J., Bull A. T., Gull K., Strange P. G., Jenkins N. Recombinant human interferon-gamma. Differences in glycosylation and proteolytic processing lead to heterogeneity in batch culture. Biochem J. 1990 Dec 1;272(2):333–337. doi: 10.1042/bj2720333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Adler B., Boose J. A., Gerard R. D., Madison E. L., McGookey D., Meidell R. S., Roman L. M., Sambrook J. Variants of human tissue-type plasminogen activator that lack specific structural domains of the heavy chain. EMBO J. 1988 Sep;7(9):2731–2740. doi: 10.1002/j.1460-2075.1988.tb03127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg D. P., Creighton T. E. Gel electrophoresis in studies of protein conformation and folding. Anal Biochem. 1984 Apr;138(1):1–18. doi: 10.1016/0003-2697(84)90761-9. [DOI] [PubMed] [Google Scholar]
- Hayes M. L., Castellino J. F. Carbohydrate of the human plasminogen variants. I. Carbohydrate composition, glycopeptide isolation, and characterization. J Biol Chem. 1979 Sep 25;254(18):8768–8771. [PubMed] [Google Scholar]
- Hille A., Waheed A., von Figura K. The ligand-binding conformation of Mr 46,000 mannose 6-phosphate-specific receptor. Acquisition of binding activity during in vitro synthesis. J Biol Chem. 1989 Aug 15;264(23):13460–13467. [PubMed] [Google Scholar]
- Kaderbhai M. A., Austen B. M. Studies on the formation of intrachain disulphide bonds in newly biosynthesised bovine prolactin. Role of protein-disulphide isomerase. Eur J Biochem. 1985 Nov 15;153(1):167–178. doi: 10.1111/j.1432-1033.1985.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Levin E. G. Latent tissue plasminogen activator produced by human endothelial cells in culture: evidence for an enzyme-inhibitor complex. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6804–6808. doi: 10.1073/pnas.80.22.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison E. L., Goldsmith E. J., Gerard R. D., Gething M. J., Sambrook J. F. Serpin-resistant mutants of human tissue-type plasminogen activator. Nature. 1989 Jun 29;339(6227):721–724. doi: 10.1038/339721a0. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Miletich J. P., Broze G. J., Jr Beta protein C is not glycosylated at asparagine 329. The rate of translation may influence the frequency of usage at asparagine-X-cysteine sites. J Biol Chem. 1990 Jul 5;265(19):11397–11404. [PubMed] [Google Scholar]
- Ny T., Elgh F., Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5355–5359. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Pless D. D., Lennarz W. J. Enzymatic conversion of proteins to glycoproteins. Proc Natl Acad Sci U S A. 1977 Jan;74(1):134–138. doi: 10.1073/pnas.74.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl G., Källström M., Bergsdorf N., Wallén P., Jörnvall H. Tissue plasminogen activator: peptide analyses confirm an indirectly derived amino acid sequence, identify the active site serine residue, establish glycosylation sites, and localize variant differences. Biochemistry. 1984 Jul 31;23(16):3701–3707. doi: 10.1021/bi00311a020. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Hanahan D., Rodgers L., Gething M. J. Expression of human tissue-type plasminogen activator from lytic viral vectors and in established cell lines. Mol Biol Med. 1986 Dec;3(6):459–481. [PubMed] [Google Scholar]
- Sawyer J. T., Doyle D. Assembly of a heterooligomeric asialoglycoprotein receptor complex during cell-free translation. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4854–4858. doi: 10.1073/pnas.87.12.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G., Jacoby R. Conformational changes associated with proteolytic processing of presecretory proteins allow glutathione-catalyzed formation of native disulfide bonds. J Biol Chem. 1982 Oct 25;257(20):12277–12282. [PubMed] [Google Scholar]
- Sonderfeld-Fresko S., Proia R. L. Synthesis and assembly of a catalytically active lysosomal enzyme, beta-hexosaminidase B, in a cell-free system. J Biol Chem. 1988 Sep 15;263(26):13463–13469. [PubMed] [Google Scholar]
- Vidal S., Mottet G., Kolakofsky D., Roux L. Addition of high-mannose sugars must precede disulfide bond formation for proper folding of Sendai virus glycoproteins. J Virol. 1989 Feb;63(2):892–900. doi: 10.1128/jvi.63.2.892-900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]