Abstract
The substrate specificity of polyhydroxyalkanoate (PHA) synthase 1 (PhaC1Pp, class II) from Pseudomonas putida GPo1 (formerly known as Pseudomonas oleovorans GPo1) was successfully altered by localized semirandom mutagenesis. The enzyme evolution system introduces multiple point mutations, designed on the basis of the conserved regions of the PHA synthase family, by using PCR-based gene fragmentation with degenerate primers and a reassembly PCR. According to the opaqueness of the colony, indicating the accumulation of large amounts of PHA granules in the cells, 13 PHA-accumulating candidates were screened from a mutant library, with Pseudomonas putida GPp104 PHA− as the host. The in vivo substrate specificity of five candidates, L1-6, D7-47, PS-A2, PS-C2, and PS-E1, was evaluated by the heterologous expression in Ralstonia eutropha PHB−4 supplemented with octanoate. Notably, the amount of 3-hydroxybutyrate (short-chain-length [SCL] 3-hydroxyalkanoate [3-HA] unit) was drastically increased in recombinants that expressed evolved mutant enzymes L1-6, PS-A2, PS-C2, and PS-E1 (up to 60, 36, 50, and 49 mol%, respectively), relative to the amount in the wild type (12 mol%). Evolved enzyme PS-E1, in which 14 amino acids had been changed and which was heterologously expressed in R. eutropha PHB−4, not only exhibited broad substrate specificity (49 mol% SCL 3-HA and 51 mol% medium-chain-length [MCL] 3-HA) but also conferred the highest PHA production (45% dry weight) among the candidates. The 3-HA and MCL 3-HA units of the PHA produced by R. eutropha PHB−4/pPS-E1 were randomly copolymerized in a single polymer chain, as analytically confirmed by acetone fractionation and the 13C nuclear magnetic resonance spectrum.
Polyhydroxyalkanoates (PHAs), a species of biological polyester, are synthesized and accumulated in various bacteria when a source of excess carbon is present and one essential growth nutrient is limited (24, 31). Such polymers have the same characteristics as biodegradable thermoplastics and elastomers and have attracted marked attention. They have thus been considered for various agricultural, industrial, and medicinal applications (3, 23). PHAs are normally classified into two groups, according to Steinbüchel and Valentin (32). One group, short-chain-length PHAs (SCL PHAs), comprises 3-hydroxyalkanoate (3-HA) monomers with chain lengths ranging from C3 to C5. The other group, medium-chain-length PHAs (MCL PHAs), consists of 3-HA monomers with chain lengths ranging from C6 to C14. Recently, PHAs with both types of repeating units have been classified as hybrids of SCL PHAs and MCL PHAs (14). SCL PHAs, such as homopolymer polyhydroxybutyrate (PHB), have a higher melting temperature and are stiffer than MCL PHAs, whereas MCL PHAs, such as polyhydroxyoctanoate, exhibit the characteristics of elastomers with poor tensile strength and high extension to breakage (23). Recently, Matsusaki et al. (19) and Sudesh et al. (34) reported that a random copolymer of hybrids of SCL PHAs and MCL PHAs with high 3-hydroxybutyrate (3-HB) content (94 mol%) had mechanical properties, such as tensile strength, Young's modulus, and elongation to breakage, that were very similar to those of low-density polyethylene. This hybrid copolymer is expected to have various commercial applications that are similar to those of low-density polyethylene.
PHA synthase is the critical enzyme, with β-hydroxyacyl-coenzyme A (CoA) as the substrate, in synthesizing PHAs (3, 17, 25). More than 59 PHA synthase genes have been cloned from 45 species of bacteria and broadly categorized into four different classes, based on their in vivo substrate specificities, primary amino acid sequences, and subunit composition (26). Class I (Ralstonia eutropha) and class III (Chromatium vinosum) PHA synthases are preferentially active towards CoA thioesters of various SCL 3-HAs that have three to five carbon atoms, except class III PHA synthases, which comprise two different species of subunits, a PhaC subunit and a PhaE subunit. Class IV (Bacillus megaterium) PHA synthases are similar to class III PHA synthases but with PhaE replaced by PhaR. Class II (Pseudomonas aeruginosa) PHA synthases are preferentially active towards CoA thioesters of various MCL 3-HAs, which are comprised of 6 to 14 carbon atoms.
In contrast, PHA synthases from Aeromonas punctata (1) and Aeromonas caviae (7) (which are highly similar to class I PHA synthase) catalyze the synthesis of a copolyester of 3-HB (C4) and 3-hydroxyhexanoate (C6). The PHA synthases from Thiocapsa pfennigii (class III PHA synthase, based on sequence homology and subunit composition) (16) and Pseudomonas sp. 61-3 (containing PhaC1 and PhaC2, which are very similar to class II PHA synthases) (12, 18) have been identified to exhibit a broad range of substrate specificity and to incorporate SCL (C3-C5) and MCL (C6-C14) 3-hydroxyacyl-CoA.
PHA synthase mutants with wide substrate specificity permit the synthesis of hybrids of SCL PHAs and MCL PHAs, even when novel monomer units are introduced to the PHAs, which are rarely present in natural polyesters. Rational protein evolution based on a known three-dimensional structure has been used for altering enzyme specificity (21, 39). However, no crystal structure of PHA synthase has yet been reported. Class I and class II PHA synthases share a rather low amino acid sequence identity (below 40%) (33), and the residues that determine the substrate specificity could not be deduced by comparing sequences. Random approaches, such as error-prone PCR and DNA shuffling, have recently been applied to the protein engineering of PHA synthases (13, 27, 35, 37).
In this study, a localized semirandom mutagenesis approach targeted to the conserved regions of PHA synthases is presented. Unlike random mutagenesis by error-prone PCR (41) and DNA shuffling (42), the point mutations generated by this method were previously designed on the basis of the sequences of the amino acids in the conserved regions, which were applied to alter the substrate specificity of PHA synthase 1 (class II) derived from Pseudomonas putida GPo1 (11). This study reports four evolved PHA synthase 1 enzymes, which exhibited a drastic increase in their affinity to incorporate 3-HB (C4) into PHA.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Strains and plasmids used in this study are described below. Escherichia coli XL1-Blue (Stratagene) and plasmid pBBR1MCS-2 (15) were used for constructing the mutant library. PHA mutant strains P. putida GPp104 PHA− (11) and R. eutropha PHB−4 were used for screening active evolved PHA synthases and further evaluating the substrate specificity of candidates. The PHA synthase 1 gene of P. putida GPo1 was amplified by PCR with primers POC1F/POC1R (Table 1) and cloned in the vector pET-23a (Novagen) based on previously reported sequences (11).
TABLE 1.
Primers used in the mutant library construction
| Name | Primer sequencea | Relative positions on phaC1pp (nt) |
|---|---|---|
| Gene specific primers | ||
| POC1F | 5′-GGAGCGTCGCATATGAGTAACAAGAAC-3′ | |
| POC1R | 5′-GCTGAAGCTTAACGCTCGTGAACGTA-3′ | |
| EcoR123SDF | 5′-CGGAATTCGAAGGAGATATACATATG-3′ | |
| 23BamHIR | 5′-AGCGGATCCTGGTGCTCGAGTGC-3′ | |
| Degenerate primers | ||
| F1 | 5′-ATCAACAAGTWCTACRTCYTSGACCT-3′ | 686-711 |
| F2 | 5′-AACSYSVTSGGCKDCTGCRTCGGCGG-3′ | 884-909 |
| F3 | 5′-TGGAMCTAYKKSGTCKACAACTACCT-3′ | 1139-1164 |
| F4 | 5′-TGGAACRSCGACRSCSCSMRSMTGCC-3′ | 1202-1227 |
| F5 | 5′-GGSCAYATCSMSRGCRTCVTCAACCC-3′ | 1445-1470 |
| F6 | 5′-TGGTGGCCSSACTGGCASGRMTGGMT-3′ | 1565-1590 |
| R1 | 5′-AKCCAKYCCWGCCAGTSSGGCCACCA-3′ | 1590-1565 |
| R2 | 5′-GGGTTGABGAYGCMSRCGATRTGSCC-3′ | 1470-1445 |
| R3 | 5′-GGCAKSYKSGTGSYGTCGBYGTTCCA-3′ | 1227-1202 |
| R4 | 5′-AGGTAGTTGTYGACSMMRTAGKTCCA-3′ | 1164-1139 |
| R5 | 5′-CCGCCGACGCAGDMGCCSABSRSGTT-3′ | 909-884 |
| R6 | 5′-AGGTCSARGAYGTAGWACTTGTTGAT-3′ | 711-686 |
Restriction enzyme sequences are underlined; the ribosome binding site sequence is italicized.
DNA manipulation.
General DNA manipulation, such as the isolation of genomic DNA, digestion of the restriction enzyme, and agarose gel electrophoresis, was carried out by standard procedures (28). DNA fragments from agarose gels were isolated by using a QIAquick gel extraction kit (QIAGEN). DNA sequence was determined by the modified dideoxy chain termination method as described by Sanger et al. (29) with a 3100 DNA analyzer (Applied Biosystems). The sequencing reaction was performed in accordance with the manual supplied with the dye terminator cycle sequencing kit (Applied Biosystems).
Localized semirandom mutagenesis.
The evolution system consisted of the following four steps. First, multiple sequence alignment and degenerate primer design were performed. Thirteen PHA synthase genes were subjected to multiple sequence alignments as previously described (30). Twelve degenerate primers (Table 1), including forward and reverse primers, were designed based on the six conserved regions found among class I and class II PHA synthase genes (25, 26, 30). Second, PCR-based gene fragmentation was carried out. The PHA synthase gene was fragmented into seven fragments, termed fragments A to G, with corresponding degenerate primer pairs (see Fig. 2A). The PCR mixture contained 1× PCR amplification buffer (QIAGEN), 2.2 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, a 2 μM concentration of each primer, 4% dimethyl sulfoxide (DMSO; Sigma), 1.25 U of Proofstart DNA polymerase (QIAGEN), and plasmid pPOC1-29347 (pET-23a vector containing the phaC1Pp gene) as a template in a 50-μl reaction mixture volume. The thermal cycle program consisted of 95°C for 5 min, 40°C for 30 s, and 72°C for 60 s, and 35 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 40 s; this program was followed by incubation at 72°C for 1 min and a final incubation at 4°C. All steps were performed on a GeneAmp PCR system 9700 (Perkin Elmer). Amplified fragments were separated and purified with electrophoresis to remove the DNA template and primers (QIAquick gel extraction kit; QIAGEN,). Third, a reassembly PCR was performed. Seven gel-purified gene fragments (A to G) were mixed in equal molar ratios to produce the DNA mixture. Approximately 100 ng of the DNA mixture was suspended in 20 μl of the PCR mixture containing 1× PCR amplification buffer (Finnzymes), 2.2 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, 4% DMSO, 0.3 U of DyNAzyme II DNA polymerase (Finnzymes), and 0.3 U of Pfu DNA polymerase (Promega) to perform the primerless reassembly PCR. The PCR program consisted of 95°C for 5 min and then 40 cycles of 94°C for 30 s, 40°C for 30 s, and 72°C for 40 s (increased by 2 s per cycle), followed by 72°C for 2 min and a final incubation at 4°C. All steps were performed on a GeneAmp PCR system 2400 (Perkin Elmer). The final step of the evolution system was the amplification of the chimera gene. The 50-μl PCR mixture contained 1 μl of reassembly PCR product, a 1 μM concentration of each primer (EcoRI23SDF and 23BamHIR), and 4% DMSO; PCR was performed for 20 cycles (94°C for 30 s, 52°C for 30 s, and 72°C for 2 min), and the predicted product was obtained (see Fig. 2C).
FIG. 2.
The strategy of constructing and screening the mutant library. (A) The DNA fragments, their corresponding position within the phaC1Pp gene, and their predicted sizes. A, B, C, D, E, F, and G are the DNA fragments produced by PCR with the corresponding primers EcoRISDF/R6, F1/R5, F2/R4, F3/R3, F4/R2, F5/R1, and F6/23BamHR, respectively. phaC1Pp, the PHA synthase 1 gene of P. putida GPo1. (B) The agarose gel electrophoresis profile of purified DNA fragments generated from phaC1Pp by PCR. Lane 1, A fragment (721 bp); lane 2, B fragment (224 bp); lane 3, C fragment (281 bp); lane 4, D fragment (89 bp); lane 5, E fragment (269 bp); lane 6, F fragment (146 bp); lane 7, G fragment (158 bp); lane M, 100 bp DNA ladder (BioLab). (C) Reamplification of reassembly PCR products with primer pair EcoRISDF/23BamHR. Lane M, λ/HindIII DNA marker. The reaction volume is 20 μl. The arrow indicates the predicted whole phaC1Pp DNA size (∼1.7 kb).
Construction of the mutant library.
After digestion of the PCR product with terminal restriction enzymes (EcoRI and BamHI) and gel purification, the reassembled fragments were ligated with the vector pBBR1MCS-2 treated with the same restriction enzymes and alkaline phosphatase (New England Biolabs). The ligation mixture was transformed into E. coli XL1-Blue with an electroporator (Bio-Rad) and plated on 2xYT plates containing 50 μg of kanamycin per ml. All resulting colonies were collected, and plasmids were extracted with a QIAprep spin miniprep kit (QIAGEN).
Competent cell preparation.
E. coli XL1-Blue and P. putida GPp104 were cultured in a 250-ml baffled flask containing 50 ml of 2xYT medium at 25°C (20°C for R. eutropha PHB−4) until the optical density at 600 nm was around 0.5, and then they were incubated on ice for 1 h. Cells were washed twice with ice-cold 10% glycerol and resuspended in 100 μl of ice-cold 10% glycerol.
Library screening.
The plasmid library was transformed into P. putida GPp104 PHA− by using an electroporation approach (2.5 kV, 200 Ω, 2.5 μF, cuvette gap of 0.2 cm) and plated on mineral salt (MS) medium (24) plates containing 50 μg of kanamycin per ml and 0.5% sodium octanoate as the carbon source. The clones that appeared as opaque colonies were screened after incubation at 30°C for 2 to 4 days. Each screened clone was further purified by streaking onto an MS plate containing octanoate to obtain a well-separated and opaque colony, which was subjected to a PHA accumulation test and gas chromatography (GC) analysis.
GC analysis of PHA in cells.
The PHA was measured qualitatively and quantitatively by GC. Candidates (P. putida GPp104 PHA− cells harboring evolved enzyme plasmid) were stimulated to accumulate PHA by a two-stage culture approach (24). Cells were first grown at 30°C for 14 h in 10 ml of 2xYT broth; cells were then washed with MS medium and transferred to 250-ml baffled flasks containing 30 ml of MS medium with 0.5% sodium octanoate for an additional 40 h of incubation at 30°C. Liquid cultures were centrifuged at 5,000 × g, washed with distilled water, and lyophilized overnight. About 10 mg of lyophilized cells was subjected to methanolysis in the presence of 15% sulfuric acid as previously described (40). The resulting methyl esters of constituent 3-hydroxyalkanoic acids were assayed by GC according to the method of Brandl et al. (5). GC analysis was performed on a Shimadzu GC-17A system equipped with a J&W DB-5 capillary column (length, 30 m; internal diameter, 0.25 mm; film thickness, 1 μm) and a flame ionization detector.
In vivo substrate specificity.
The substrate specificity of the PHA synthase 1 and of the predicted evolved enzymes was analyzed by transferring their respective plasmids into R. eutropha PHB−4, which provides SCL and MCL 3-hydroxyacyl-CoAs, when cultivated in MS medium and sodium octanoate as a carbon source (18). Cells harboring the plasmids were cultivated for 48 h, and PHA content and composition were analyzed by GC analysis. The PHA content was indicative of the relative substrate specificity of the evolved PHA synthases compared with the wild-type PHA synthase 1.
PHA isolation and chemical structure analysis.
PHA was extracted from the lyophilized cells with hot chloroform refluxed in a Soxhlet apparatus, filtered through a Whatman number 2 filter, and purified by precipitation with 10 volumes of ice-cold methanol. 13C-nuclear magnetic resonance (NMR) analysis was performed on PHA samples, which were fractionated with hot acetone as described by Kato et al. (12) and then dissolved in deuterated chloroform (35 mg/ml). The 125-MHz 13C-NMR spectra were recorded on a Bruker Avance 500 NMR spectrometer at a probe temperature of 25°C.
RESULTS
Localized semirandom mutagenesis of PHA synthase 1 from P. putida GPo1.
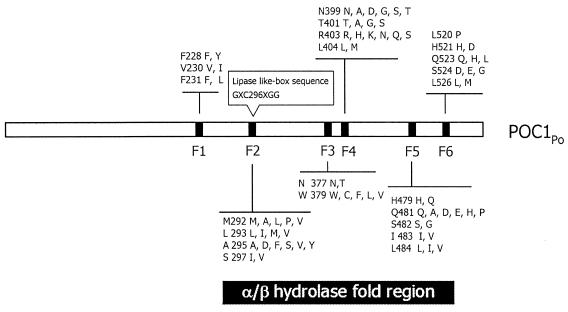
The mutagenesis approach developed herein comprised multiple sequence alignments, degenerate primer design, PCR-based gene fragmentation, reassembly PCR, and mutated gene reamplification, all of which were used in constructing a multiple-point mutation library of PHA synthase 1 derived from P. putida GPo1. The multiple sequence alignment results reveal that six conserved regions, termed F1, F2, F3, F4, F5, and F6, which, except for F1, were localized on the α/β hydrolase fold region of the PHA synthase family (Fig. 1), were used for designing degenerate primers. By using the degenerate primers, sets of designed amino acids were specifically substituted into 23 amino acid sites distributed across six regions (Fig. 1). According to the number of selected amino acid residue positions and the variety of amino acids in each position, an ideal library of the desired mutations would have the following number of members (from the indicated region): 2 × 2 × 2 (F1 region) × 5 × 4 × 6 × 2 (F2 region) × 2 × 5 (F3 region) × 6 × 4 × 6 ×2 (F4 region) × 2 × 6 × 2 × 2 × 3 (F5 region) × 1 × 2 × 3 × 3 × 2 (F6 region), or 28,665,446,400 members. Figure 2B shows the gene fragmentation of PHA synthase and multiple point mutations introduced into the gene by PCR; the sizes of the PCR fragments A, B, C, D, E, F, and G were as theoretically predicted (Fig. 2A). Equal moles of gene fragments were mixed to perform primerless reassembly PCR. Despite the use of various amounts of DNA (50, 100, and 200 ng), the correct size of the reassembled genes was not observed (data not shown) until they were amplified by using terminal primers (Fig. 2C). Additionally, an E. coli-recognizing ribosome binding site (AAGGAG) was introduced in front of the ATG codon by using a 5′ terminal primer to express normally the evolved PHA synthase in P. putida GPp104 and R. eutropha PHB−4. Following digestion with EcoRI and BamHI restriction enzymes, the 1.7-kb PCR product was ligated with plasmid pBBR1MCS-2 and transformed to E. coli XL1-Blue. About 20,000 kanamycin-resistant clones were obtained from 2xYT plates supplemented with 50 μg of kanamycin per ml.
FIG. 1.
The outline of predicted positions and amino acid variants introduced to the phaC1Pp gene. F1, F2, F3, F4, F5, and F6 are the conserved regions among PHA synthases. There are 23 amino acid sites distributed throughout six conserved regions involved in the protein evolution. In the designations, the first letter (left) indicates the residue of the wild-type protein, the number indicates the position of the amino acid residue, and the final letters (right) indicate the possible residues capable of being introduced by degenerate primers.
Screening active evolved PHA synthases from a mutant library.
A plasmid library was prepared by extracting plasmids from the E. coli XL1-Blue library, transforming them to P. putida GPp104, and growing the culture on plates with MS medium supplemented with 0.5% octanoate and 50 μg of kanamycin per ml. P. putida GPp104 exhibits a β-oxidation pathway, which provides intermediates from fatty acids. Related inherited enzymes convert these intermediates into SCL and MCL β-hydroxyacyl-CoA as PHA synthase substrates (6). Additionally, P. putida and R. eutropha recognize the lac promoter upstream of the evolved PHA synthase gene and constitutively express it without induction (9, 15). Therefore, the mutant library was normally expressed, and suitable substrates were provided for PHA biosynthesis. Based on the opaque appearance of the colonies, indicating the accumulation of PHA granules in the cells, 13 candidates were visually screened from about 10,000 transformants.
Table 2 lists the monomer composition and the PHA content that accumulated in recombinant strains of P. putida GPp104. The 13 candidates were then classified into two groups, predicted hybrid-PHA producers and MCL PHA producers, according to the mol% values of 3-HB (C4) in PHA. Predicted hybrid-PHA producers (D7-47, L1-2, L1-6, PS-A2, PS-C2, PS-D1, and PS-E1) revealed higher mol% values of 3-HB (7, 7, 8, 10, 9, 8, and 13 mol%, respectively) than wild-type PHA synthase (6 mol%) (Table 2). MCL PHA producers (PS-B1, PS-C1, PS-D3, PS-E2, PS-G1, and PS-H2) revealed lower mol% values of 3-HB (C4) (4, 5, 4, 4, 6, and 4 mol%, respectively) than wild-type PHA synthase (6 mol%).
TABLE 2.
Composition analysis of PHA accumulated by recombinant strains of P. putida GPp104 PHA− harboring evolved PHA synthase plasmidsa
| Plasmid | % PHA content (wt/wt) | Polymer composition (mol%)b
|
|||
|---|---|---|---|---|---|
| 3-HB | 3-HHx | 3-HO | 3-HD | ||
| pBBR1MCS-2 | 0.3 | ND | ND | ND | ND |
| pBHR-POC1-29347 | 40 ± 7.0 | 6 ± 0.3 | 20 ± 0.3 | 72 ± 0.7 | 1 ± 0.5 |
| pD7-47 | 38 ± 2.2 | 7 ± 0.2 | 19 ± 0.5 | 72 ± 1.0 | 1 ± 0.1 |
| pL1-2 | 34 ± 0.9 | 7 ± 0.4 | 19 ± 0.3 | 73 ± 0.5 | 1 ± 0.2 |
| pL1-6 | 32 ± 1.8 | 8 ± 0.1 | 18 ± 0.3 | 73 ± 0.2 | 1 ± 0.1 |
| pPS-A2 | 23 ± 2.2 | 10 ± 1.8 | 22 ± 3.1 | 66 ± 4.1 | 2 ± 0.5 |
| pPS-C2 | 30 ± 2.6 | 9 ± 1.1 | 19 ± 0.4 | 70 ± 1.5 | 2 ± 0.1 |
| pPS-D1 | 15 ± 3.6 | 8 ± 0.8 | 21 ± 0.8 | 69 ± 1.7 | 2 ± 0.2 |
| pPS-E1 | 32 ± 2.8 | 13 ± 1.9 | 19 ± 0.5 | 66 ± 1.9 | 2 ± 0.5 |
| pPS-B1 | 30 ± 1.7 | 4 ± 0.3 | 23 ± 2.4 | 70 ± 2.0 | 3 ± 0.4 |
| pPS-C1 | 11 ± 3.0 | 5 ± 1.5 | 15 ± 0.3 | 75 ± 2.4 | 5 ± 0.2 |
| pPS-D3 | 18 ± 4.9 | 4 ± 0.8 | 20 ± 1.0 | 71 ± 2.4 | 5 ± 0.7 |
| pPS-E2 | 11 ± 2.3 | 4 ± 1.5 | 23 ± 0.5 | 68 ± 0.9 | 5 ± 0.8 |
| pPS-G1 | 12 ± 0.5 | 6 ± 1.4 | 16 ± 0.1 | 74 ± 0.9 | 4 ± 0.5 |
| pPS-H2 | 14 ± 1.5 | 4 ± 1.2 | 15 ± 0.7 | 78 ± 1.5 | 3 ± 0.5 |
Cells cultivated on 2xYT were washed with MS medium and transferred to 250-ml baffled flasks containing 30 ml of MS medium with 0.5% sodium octanoate for an additional 40-h incubation at 30°C at 125 rpm. The data are presented as means ± standard deviations of three or four independent experiments. ND, not detected.
3-HHx, 3-hydroxyhexanoate; 3-HO, 3-hydoxyoctanoate; 3-HD, 3-hydroxydecanoate.
Analysis of substrate specificity of evolved PHA synthases.
The respective plasmids of predicted hybrid-PHA producers (D7-47, L1-6, PS-A2, PS-C2, and PS-E1) were further transformed into R. eutropha PHB−4, which provides SCL 3-HA-CoA and MCL 3-HA-CoAs as substrates for PHA synthase when grown on fatty acids (18), to determine whether the substrate specificity of the evolved PHA synthases changed. The transformant cells cultivated in 2xYT broth were transferred to MS medium to promote the biosynthesis of PHA from octanoate. GC results indicated that the mol% values of 3-HB in the PHA produced in R. eutropha PHB−4 containing the plasmids pL1-6, pPS-A2, pPS-C2 and pPS-E1 were drastically enhanced up to 60, 36, 40, and 49 mol%, respectively, compared with a level of 12 mol% in the wild type (Table 3). The substrate specificity of the evolved enzymes L1-6, PS-A2, PS-C2, and PS-E1 exhibited increases of about 5, 3, 4.2, and 4.1 times, respectively, in the affinity for the incorporation of 3-HB (C4). Evolved enzyme L1-6 exhibits the most changed affinity for incorporating 3-HB into PHA. However, none of these evolved enzymes exhibited a substrate specificity similar to that of class I PHA synthase. Nonetheless, the GC results strongly support the claim that evolved enzymes L1-6, PS-A2, PS-C2, and PS-E1 inherited a broad range of substrate specificity and could efficiently copolymerize C4, C6, C8, and C10 3-HA units. Additionally, PHA produced by evolved enzyme D7-47 exhibited the same monomer compositions, whether it was produced in P. putida GPp104 or R. eutropha PHB−4 (Tables 2 and 3).
TABLE 3.
Composition analysis of PHA accumulated by recombinant strains R. eutropha PHB−4 harboring evolved PHA synthase plasmidsa
| Plasmid | % PHA content (wt/wt) | Polymer composition (mol%)b
|
||
|---|---|---|---|---|
| 3-HB | 3-HHx | 3-HO | ||
| pBBRIMCS-2 | 0.5 | 100 | ND | ND |
| pBHR-POC1-29347 | 22 | 12 | 27 | 61 |
| pL1-6 | 16 | 60 | 9 | 31 |
| pD7-47 | 22 | 7 | 22 | 71 |
| pPS-A2 | 22 | 36 | 20 | 44 |
| pPS-C2 | 9 | 50 | 14 | 36 |
| pPS-E1 | 45 | 49 | 13 | 38 |
Cells cultivated on 2xYT were washed with MS medium and transferred to a 250-ml baffled flask containing 30 ml of MS medium supplemented with 0.2% (wt/vol) sodium octanoate for culturing for 48 h at 30°C at 125 rpm. An additional 0.2% sodium octanoate was added during culturing. 3-Hydroxydecanoate was not detected in any of the plasmids.
3-HHx, 3-hydroxyhexanoate; 3-HO, 3-hydoxyoctanoate; ND, not detected.
The evolved enzyme PS-E1 exhibited not only broad substrate specificity characteristics (almost 50 mol% 3-HB and 50 mol% MCL 3-HA units of the PHA that accumulated in R. eutropha PHB−4) but also the highest PHA-accumulating ability (45% dry weight) of all predicted candidates. Furthermore, R. eutropha PHB−4/pPS-E1 could produce homogenous PHB (SCL PHA) when 1.5% gluconate was the sole carbon source (10% dry weight). Evolved PHA synthase PS-E1 thus exhibited high enzyme activity and a broad range of substrate specificity.
Sequence analysis of active evolved PHA synthases.
The DNA sequences of the 13 evolved genes were determined. Table 4 presents the amino acid substitutions in the evolved enzymes in which multiple point mutations were generated in a manner consistent with the theoretical design; however, some unanticipated point mutations occurred in nonconserved regions. These mutations in the α/β hydrolase fold region were likely to be harmful to the alteration of substrate specificity and PHA productivity because only the MCL PHA producers possessed them (Table 4). The sequences of D7-47 and L1-6 differed by only one residue at A547V; however, L1-6 could synthesize hybrids of SCL and MCL PHA in R. eutropha PHB−4 (60 mol% 3-HB), but D7-47 did not synthesize hybrids of PHA either in P. putida GPp104 (7 mol% 3-HB) or R. eutropha PHB−4 (7 mol% 3-HB). This finding highlighted the fact that the unanticipated mutant, A547V, was likely to affect substrate specificity. Comparing the sequences of the predicted hybrid PHA and MCL PHA producers demonstrated that region F4 was highly conserved in hybrid PHA producers even though four sites on F4 were designed to evolve (Fig. 1). Five designed point mutations, V230I, S297V, Q482A, L520P, and Q523, and an unanticipated mutation, K207N, did not seem to influence substrate specificity, because these mutations also occurred in the predicted hybrid PHA producers and MCL PHA producers. However, no evidence was found that specific residues differed between the predicted hybrid PHA producers and MCL PHA producers.
TABLE 4.
Mutation analysis of the active PHA-accumulating candidates
| Enzyme | Amino acid substitutions in conserved and nonconserved regions at residue:a
|
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NR
|
F1
|
F2
|
NR
|
F3
|
F4
|
F5
|
NR
|
F6
|
NR
|
||||||||||||||||||||||||
| 2 | 56 | 66 | 207 | 228 | 230 | 231 | 292 | 293 | 295 | 297 | 316 | 363 | 364 | 377 | 379 | 399 | 401 | 403 | 404 | 481 | 482 | 483 | 484 | 490 | 520 | 521 | 523 | 524 | 532 | 534 | 547 | ||
| Wild type Predicted hybrid PHA producer | S | G | G | K | F | V | F | M | L | A | S | K | A | K | N | W | N | T | R | L | Q | S | I | L | P | L | H | Q | S | E | E | A | |
| L1-6 | G | N | I | L | V | V | V | T | L | A | C | I | P | L | G | K | V | ||||||||||||||||
| PS-A2 | N | Y | I | L | A | V | V | V | A | G | I | P | L | D | K | G | |||||||||||||||||
| PS-C2 | N | Y | I | L | A | M | V | V | V | M | A | G | P | L | G | K | |||||||||||||||||
| PS-E1 | N | I | L | T | V | V | M | A | G | V | P | L | G | K | |||||||||||||||||||
| L1-2 | N | I | L | V | V | V | T | A | C | I | P | L | G | K | |||||||||||||||||||
| PS-D1 | N | Y | I | L | L | M | V | V | V | T | A | C | V | V | L | P | D | L | E | K | |||||||||||||
| MCL PHA producer | |||||||||||||||||||||||||||||||||
| D7-47 | G | N | I | L | V | V | V | T | L | A | C | I | P | L | G | K | |||||||||||||||||
| PS-B1 | N | I | L | A | V | V | G | Q | T | F | S | M | A | C | I | P | L | D | K | ||||||||||||||
| PS-C1 | S | N | I | G | A | A | V | V | P | D | L | G | K | ||||||||||||||||||||
| PS-D3 | N | I | V | V | T | M | A | V | V | P | L | E | K | K | |||||||||||||||||||
| PS-E2 | N | I | A | I | V | T | V | M | A | G | V | V | P | L | E | K | |||||||||||||||||
| PS-G1 | N | I | L | V | V | R | N | A | C | V | P | L | D | K | |||||||||||||||||||
| PS-H2 | L | N | Y | L | P | I | S | V | D | A | H | A | G | I | P | L | G | K | |||||||||||||||
F1 to F6 are conserved regions. The sequence of the wild type is given in bold for reference. NR, nonconserved region.
The evolved PHA synthase randomly copolymerized SCL and MCL 3-HA units in a single polymer chain.
A total of 231 mg of polyester produced by R. eutropha PHB−4/pPS-E1 was fractionated for 5 h with hot acetone and analyzed by GC to confirm whether it was a copolymer of SCL and MCL 3-HA units or a blend of SCL and MCL PHAs. The acetone-soluble fraction was nearly 100% (by weight) of the initial polyester, which contained 54 mol% 3-HB, 13 mol% 3-hydroxyhexanoate, and 33 mol% 3-hydroxyoctanoate, whereas the acetone-insoluble fraction contained almost no polyester (<0.2 mg). GC analysis revealed that the compositions of nonacetone-fractionated polyester and acetone-soluble fractionated polyester were identical (data not shown), preliminarily indicating that the polyester yielded by R. eutropha PHB−4/pPS-E1 comprised hybrids, not blends, of SCL and MCL PHA. Figure 3 presents the 125-MHz 13C-NMR spectrum of the acetone-soluble fraction of polyester. The chemical shift assignments for each carbon resonance and an expanded spectrum of carbonyl resonances are the same as those reported in previous investigations (10, 12). The carbonyl carbon resonances (169.1 to 169.5 ppm) were obviously resolved into three peaks, and the findings were entirely consistent with those of Kato (12), who found that different diad sequences of connected 3-HB and MCL 3-HA units caused a chemical shift. The peak at 169.1 ppm corresponded to carbonyl resonance in 3HB*-3HB, that at 169.42 ppm corresponded to resonance in 3HA*-3HA, and that at 169.26 ppm corresponded to resonance in the 3HB*-3HA and 3HA*-3HB sequences of connected SCL and MCL units. NMR analysis further confirmed that the 3-HB (SCL 3-HA unit) and MCL 3-HA units of the copolyester generated by R. eutropha PHB−4/pPS-E1 were randomly copolymerized on a single polymer chain. Acetone fractionation, GC analysis, and 13C-NMR results strongly reveal that evolved enzyme PS-E1 possesses a broad range of substrate specificity in vivo and can efficiently synthesize hybrids of SCL and MCL PHAs in cells.
FIG. 3.
The 125-MHz 13C-NMR spectrum of the acetone-soluble fraction of polyester yielded by R. eutropha PHB−4/pPS-E1 in chloroform. 3HHx, 3-hydroxyhexonoate; 3HO, 3-hydroxyoctanote.
DISCUSSION
PHA synthase 1, derived from P. putida GPo1, is a classic class II PHA synthase, which prefers MCL 3-acyl-CoA as a substrate for synthesizing PHA (11, 38). The three types of PHA synthases exhibit rather low sequence similarity (25, 26, 33), so the residues that govern substrate specificity cannot be deduced from the results of gene alignment. One study stated that PHA synthases belong to the α/β hydrolase superfamily (35), in which the catalytic residues always constitute a highly conserved triad. Close to the catalytic site, loops were inserted to shape the substrate-binding site of the α/β hydrolase domain (20, 22). Consequently, the position of the substrate-recognizing residues in class I and class II PHA synthases should be similar and should localize on the α/β hydrolase fold region. Although the sequences differ greatly between class I and class II PHA synthases (25, 26), multiple alignment results imply that they involve six conserved regions (25, 26, 30), which are present throughout all PHA synthases in which variants of amino acids are naturally evolved. Additionally, the conserved regions are all in the α/β hydrolase fold region, except for F1 (Fig. 1), theoretically, which strongly implies that the substrate specificity of PHA synthases can be altered by introducing naturally evolved amino acids.
Recently, numerous reports have addressed directed evolution, such as error-prone PCR, to increase the activity of PHA synthases or change the substrate specificity to alter the composition of PHA (1, 2, 13, 27, 36, 37). The point mutations occurred at F420S of the PHB synthase from R. eutropha (36), at F518I of the PHA synthase of A. punctata (1), and at S325 and Q481 of the PHA synthase 1 of Pseudomonas sp. 61-3 (37), markedly enhancing the enzyme activity or the PHA accumulation without changing the substrate specificity. Point mutations occurred at C296S or H453Q of the PHA synthase 1 from P. aeruginosa, increasing the affinity for incorporating 3-hydroxyhexanoyl-CoA (C6) and 3-hydroxydodecanoyl-CoA (C12) into PHA, but none of the mutants could use 3-hydroxybutyryl-CoA (C4) (2). These point mutations are all localized on the α/β hydrolase fold region of the PHA synthase family. Accordingly, the α/β hydrolase fold region of the PHA synthase family should involve enzyme activity and substrate recognition based on these references. However, point mutations that occurred at N149S and D171G, which are not localized in the α/β hydrolase fold region, of the PHA synthase from A. caviae produced a higher 3-hydroxyhexanoate (C6) fraction (up to 16 mol% and 18 mol%, respectively) than in the wild type (10 mol%) (13).
Evolved enzymes L1-6, PS-A2, PS-C2, and PS-E1 exhibited a broad range of substrate specificity and contained multiple point mutations (Table 4), which were very diverse. No individual mutations were identified as being associated with a change in substrate specificity. Therefore, the alteration in the substrate specificity of PHA synthase 1 herein seemed to be caused by global changes in protein structure, with the introduction of multiple point mutations, but this change was not determined by a single mutation. These results support those of Rehm et al. (27).
A phenotype method was used to screen the library rapidly and obtain target mutants; the colonies appeared opaque when large amounts of PHA granules accumulated in the cells. This approach is not very sensitive but facilitated the screening procedure for obtaining candidates with high PHA accumulation. Two PHA-negative mutants, P. putida GPp104 and R. eutropha PHB−4, which primarily provide MCL 3-hydroxyacyl-CoA and SCL 3-hyroxyacyl-CoA, respectively, from fatty acids were employed to evaluate the in vivo substrate specificity of each candidate. The transformation efficiency of P. putida GPp104 by electroporation greatly exceeded that of R. eutropha PHB−4, when competent cells were grown in 2xYT medium at 25°C (8). Accordingly, the compromise strategy was preliminarily to screen the library on P. putida GPp104 and further verify promising candidates on R. eutropha PHB−4, by using the PHA accumulation test and GC analysis. However, two promising candidates, L1-2 and PS-D1, could not be transformed into R. eutropha PHB−4. This result is not yet understood.
Sequence analysis of 13 candidates revealed that some unanticipated point mutations were also generated by PCR, even though the proofreading function of thermal DNA polymerase was used. Adding DMSO to the PCR reaction mixture to reduce the fidelity of the thermal DNA polymerase supposedly causes such unanticipated point mutations (4). All candidates had two unanticipated point mutations on K207N and E534K. K207N was deduced from the early step of gene fragmentation by PCR. However, E534K appeared on the PHA synthase 1 gene cloned from P. putida GPo1 chomosomal DNA by PCR; seven PCR clones were checked and yielded the same results. A further sequence comparison with published class II PHA synthases demonstrated that the amino acid of PhaC1Pp at position 534 was K rather than E (data not shown). All instances of mutagenesis of the predicted hybrid PHA producers occurred on the conserved regions as previously designed, except for mutagenesis occurring at the N and C termini; furthermore, the predicted hybrid PHA producers also retained high PHA productivity (>30% dry weight), except for PS-D1 (at 10% dry weight), for which an unanticipated mutation occurred at P490L and was localized in the α/β fold region. Mutagenesis of the MCL PHA producers occurred in the F3 and F4 regions, and unanticipated mutations in the α/β fold region were more prevalent, resulting in low PHA yields (all MCL PHA producers showed <20% dry weight except for PS-B1). This result implies that evolution in the F3 and F4 regions or unanticipated mutagenesis in the α/β hydrolase fold region may reduce enzyme activity.
In conclusion, localized semirandom mutagenesis substantially changed the substrate specificity of PhaC1Pp. The limited number of amino acid residues clearly changes the substrate specificity of PhaC1Pp. Additionally, this method can be applied to class I and class III PHA synthases to develop more information on substrate specificity.
Acknowledgments
The authors thank M. E. Kovach and K. M. Peterson for providing plasmid pBBR1MCS-2, A. Steinbüchel and B. Witholt for R. eutropha PHB−4 and P. putida GPo1, respectively, and G.-R. Her and Y.-R. Chen for help with the GC analysis.
This work is partially supported by grant NSC 91-2313-B-002-353 from the National Science Council, Taipei, Taiwan.
REFERENCES
- 1.Amara, A. A., S. Steinbüchel, and B. H. A. Rehm. 2002. In vivo evolution of the Aeromonas punctata polyhydroxyalkanoate (PHA) synthase: isolation and characterization of modified PHA synthases with enhanced activity. Appl. Microbiol. Biotechnol. 59:477-482. [DOI] [PubMed] [Google Scholar]
- 2.Amara, A. A., and B. H. A. Rehm. 2003. Replacement of the catalytic nucleophile cysteine-296 by serine in class II polyhydroxyalkanoate synthase from Pseudomonas aeruginosa-mediated synthesis of a new polyester: identification of catalytic residues. Biochem. J. 374:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, A. J., and E. A. Dawes. 1990. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 54:450-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borns, M., and H. Hogrefe. 2000. Herculase enhanced DNA polymerase delivers high fidelity and great performance. Strategies Newslett. 13:1-3. [Google Scholar]
- 5.Brandl, H., R. A. Gross, R. W. Lenz, and R. C. Fuller. 1988. Pseudomonas oleovorans as source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Environ. Microbiol. 54:1977-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggink, G., P. de Waard, and G. N. M. Huijberts. 1992. The role of fatty acid biosynthesis and degradation in the supply of substrates for poly(3-hydroxyalkanoate) formation in Pseudomonas putida. FEMS Microbiol. Rev. 103:159-164. [Google Scholar]
- 7.Fukui, T., and Y. Doi. 1997. Cloning and analysis of the poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) biosynthesis genes of Aeromonas caviae. J. Bacteriol. 179:4821-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hengen, P. N. 1996. Preparing ultra-competent Escherichia coli. Trends Biochem. Sci. 21:75-76. [PubMed] [Google Scholar]
- 9.Hoffmann, N., A. Steinbüchel, and B. H. A. Rehm. 2000. The Pseudomonas aeruginosa phaG gene product is involved in the synthesis of polyhydroxyalkanoic acid consisting of medium-chain-length constituents from non-related carbon sources. FEMS Microbiol. Lett. 184:253-259. [DOI] [PubMed] [Google Scholar]
- 10.Huijberts, G. M., T. C. de Rijk, P. de Waard, and G. Eggink. 1994. 13C nuclear magnetic resonance studies of Pseudomonas putida fatty acid metabolic routes involved in poly(3-hydroxyalkanoate) synthesis. J. Bacteriol. 176:1661-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huisman, G. W., E. Wonink, R. Meima, B. Kazemier, P. Terpstra, and B. Witholt. 1991. Metabolism of poly(3-hydroxyalkanoates) (PHAs) by Pseudomonas oleovorans. Identification and sequences of genes and function of the encoded proteins in the synthesis and degradation of PHA. J. Biol. Chem. 266:2191-2198. [PubMed] [Google Scholar]
- 12.Kato, M., H. J. Bao, C.-K. Kang, T. Fukui, and Y. Doi. 1996. Production of a novel copolyester of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids by Pseudomonas sp. 61-3 from sugars. Appl. Microbiol. Biotechnol. 45:363-370. [Google Scholar]
- 13.Kichise, T., S. Taguchi, and Y. Doi. 2002. Enhanced accumulation and changed monomer composition in polyhydroxyalkanoate (PHA) copolyester by in vitro evolution of Aeromonas caviae PHA synthase. Appl. Environ. Microbiol. 68:2411-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, Y. B., and R. W. Lenz. 2001. Polyesters from microorganisms. Adv. Biochem. Eng. Biotechnol. 71:51-79. [DOI] [PubMed] [Google Scholar]
- 15.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 16.Liebergesell, M., F. Mayer, and A. Steinbüchel. 1993. Analysis of polyhydroxyalkanoic acid-biosynthetic genes of anoxygenic phototrophic bacteria reveals synthesis of a polyester exhibiting an unusual composition. Appl. Microbiol. Biotechnol. 40:292-300. [Google Scholar]
- 17.Madison, L. L., and G. W. Huisman. 1999. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsusaki, H., S. Manji, K. Taguchi, M. Kato, T. Fukui, and Y. Doi. 1998. Cloning and molecular analysis of the poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyalkanoate) biosynthesis genes in Pseudomonas sp. strain 61-3. J. Bacteriol. 180:6459-6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsusaki, H., H. Abe, and Y. Doi. 2000. Biosynthesis and properties of poly(3-hydroxybutyrate-co-hydroxyalkanoates) by recombinant strains of Pseudomonas sp. 61-3. Biomacromolecules 1:17-22. [DOI] [PubMed] [Google Scholar]
- 20.Nardini, M., and B. W. Dijkstra. 1999. α/β hydrolase fold enzymes: the family keeps growing. Curr. Opin. Struct. Biol. 9:732-737. [DOI] [PubMed] [Google Scholar]
- 21.Nickerson, D. P., C. F. Harford-Corss, S. R. Fulcher, and L. L. Wong. 1997. The catalytic activity of cytochrome P450cam towards styrene oxidation is increased by site-specific mutagenesis. FEBS Lett. 405:153-156. [DOI] [PubMed] [Google Scholar]
- 22.Ollis, D. L., E. Cheah, M. Cygler, B. Dijkstra, F. Frolow, S. M. Franken, M. Harel, S. J. Remington, I. Silman, J. Schrag, J. L. Sussman, K. H. G. Verschueren, and A. Goldman. 1992. The α/β hydrolase fold. Protein Eng. 5:197-211. [DOI] [PubMed] [Google Scholar]
- 23.Poirier, Y., C. Nawrath, and C. Somerville. 1995. Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Bio/Technology 13:142-150. [DOI] [PubMed] [Google Scholar]
- 24.Ramsay, B. A., K. Lomaliza, C. Chavarie, B. Dubé, P. Bataille, and J. A. Ramsay. 1990. Production of poly-(β-hydroxybutyric-co-β-hydroxyvaleric) acids. Appl. Environ. Microbiol. 56:2093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehm, B. H. A., and A. Steinbüchel. 1999. Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int. J. Biol. Macromol. 25:3-19. [DOI] [PubMed] [Google Scholar]
- 26.Rehm, B. H. A. 2003. Polyester synthases: natural catalysts for plastics. Biochem. J. 376:15-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehm, B. H. A., R. V. Antonio, P. Spiekermann, A. A. Amaro, and A. Steinbüchel. 2002. Molecular characterization of the poly(3-hydroxybutyrate) (PHB) synthase from Ralstonia eutropha: in vitro evolution, site-specific mutagenesis and development of a PHB synthase protein model. Biochim. Biophys. Acta 1594:178-190. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5462-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheu, D.-S., Y.-T. Wang, and C.-Y. Lee. 2000. Rapid detection of polyhydroxyalkanoate-accumulating bacteria isolated from the environment by colony PCR. Microbiology 146:2019-2025. [DOI] [PubMed] [Google Scholar]
- 31.Steinbüchel, A., and H. G. Schlegel. 1991. Physiology and molecular genetics of poly(β-hydroxyalkanoic acid) synthesis in Alcaligenes eutrophus. Mol. Microbiol. 5:535-542. [DOI] [PubMed] [Google Scholar]
- 32.Steinbüchel, A., and H. E. Valentin. 1995. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 128:19-228. [Google Scholar]
- 33.Steinbüchel, A., and S. Hein. 2001. Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv. Biochem. Eng. Biotechnol. 71:81-123. [DOI] [PubMed] [Google Scholar]
- 34.Sudesh, K., H. Abe, and Y. Doi. 2000. Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog. Polym. Sci. 25:1503-1555. [Google Scholar]
- 35.Taguchi, S., A. Maehara, K. Takase, M. Nakahara, H. Nakamura, and Y. Doi. 2000. Analysis of mutational effects of a polyhydroxybutyrate (PHB) polymerase on bacterial PHB accumulation using an in vivo assay system. FEMS Microbiol. Lett. 198:65-71. [DOI] [PubMed] [Google Scholar]
- 36.Taguchi, S., H. Nakamura, T. Hiraishi, I. Yamato, and Y. Doi. 2002. In vitro evolution of a polyhydroxybutyrate synthase by intragenic suppression-type mutagenesis. J. Biochem. 131:801-806. [DOI] [PubMed] [Google Scholar]
- 37.Takase, K., S. Taguchi, and Y. Doi. 2003. Enhanced synthesis of poly(3-hydroxybutyrate) in recombinant Escherichia coli by means of error-prone PCR mutagenesis, saturation mutagenesis, and in vitro recombination of the class II polyhydroxyalkanoate synthase gene. J. Biochem. 133:139-145. [DOI] [PubMed] [Google Scholar]
- 38.van Beilen, J. B., S. Panke, S. Lucchini, A. G. Franchini, M. Röthlisberger, and B. Witholt. 2001. Analysis of Pseudomonas putida alkane-degradation gene clusters and flanking insertion sequences: evolution and regulation of the alk genes. Microbiology 147:1621-1630. [DOI] [PubMed] [Google Scholar]
- 39.van den Heuvel, R. H. H., M. W. Fraaije, M. Ferrer, A. Mattevi, and W. J. H. van Berkel. 2000. Inversion of stereospecificity of vanillyl-alcohol oxidase. Proc. Natl. Acad. Sci. USA 97:955-9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, H.-A., D.-S. Sheu, and C.-Y. Lee. 2003. Rapid differentiation between short-chain-length and medium-chain-length polyhydroxyalkanoate-accumulating bacteria with spectrofluorometry. J. Microbiol. Methods 53:131-135. [DOI] [PubMed] [Google Scholar]
- 41.You, L., and F. H. Arnold. 1996. Directed evolution of subtilisin E in Bacillus subtilis to enhance total activity in aqueous dimethylformamide. Protein Eng. 9:77-83. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, J.-H., G. Dawes, and W. P. C. Stemmer. 1997. Directed evolution of a fucosidase from a galactosidase by DNA shuffling and screening. Proc. Natl. Acad. Sci. USA 94:4504-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]