Abstract
Marco Romano, MD, and Antonio Cuomo, MD, discuss the current state of the field regarding H pylori infection, with a view toward future strategies of eradication.
Introduction
Helicobacter pylori is a ubiquitous gram-negative bacterium infecting half the world's population[1,2] and causing chronic active gastritis in virtually all infected individuals.[3] The majority of individuals who acquire chronic H pylori gastritis will exhibit mild gastritis, more prominent in the antrum compared with the corpus. A minority of infected patients develop marked chronic inflammation in the antrum, with mild inflammation in the oxyntic mucosa (antral-predominant gastritis), and are prone to develop duodenal ulcer.[4] Infrequently, some individuals show a corpus-predominant pattern that overlaps with autoimmune gastritis. Recently, epidemiologic and laboratory studies in animals as well as interventional studies in humans strongly suggested that H pylori may play a pathogenic role in the development of adenocarcinoma of the distal stomach.[5-7] In particular, individuals with corpus-predominant gastritis seem more susceptible to development of adenocarcinoma of the distal stomach.[6]
The mechanisms whereby H pylori may cause gastroduodenal disease and contribute to gastric carcinogenesis are still hypothetical.[5-8] However, the production of specific virulence factors by the bacterium, the inflammatory response of the host, and the association with environmental factors may all play a contributory role.[5,6,8]
Therapy of H pylori Infection
The management of H pylori gastritis involves a 3-step approach: diagnose, treat, and confirm cure. The availability of accurate and noninvasive tests, such as the urea breath test or stool antigen test, has rendered confirmation of cure practical. The location of H pylori within the stomach (eg, the mucus lining the surface epithelium or the surface of mucous cells) provides a challenge for antimicrobial therapy. In addition, the gastric lumen is a hostile environment for antimicrobial therapy because the drugs must penetrate thick mucus and may need to be active at pH values below neutral. Successful therapy requires a combination of drugs that prevent the emergence of resistance and reach the bacteria within its various niches. Therapy must ensure that a small population of bacteria does not remain viable. Eradication is defined as the presence of negative tests for H pylori 4 weeks or longer after the end of antimicrobial therapy. Clearance or suppression of H pylori may occur during therapy, and failure to detect H pylori on tests done within 4 weeks of the end of therapy may give false-negative results. The latter is because clearance or suppression is swiftly followed by recurrence of the original infection.
Treatment regimens for H pylori infection have been evolving since the early 1990s, when monotherapy was first recommended. Antimicrobial therapy for this infection is a complex issue, and the results from new combination treatments are often unpredictable. Errors that should be avoided include quick adoption of regimens tested only in small populations and substitution of 1 well-studied, effective medication for another in the same class. Also, it is important to validate the success rate of a treatment regimen in each country, and perhaps even in the specific region of each country, where its use is intended.
Drugs Used to Eradicate H pylori Infection
Proton-Pump Inhibitors (PPIs)
PPI-based triple therapies have shown efficacy in various clinical trials from different geographic areas.[9] PPIs have direct antimicrobial effects in vitro on H pylori. However, the direct antimicrobial effect of PPIs does not seem to play a major role in the eradication of the infection. In fact, in terms of better eradication rates, consistent advantage has not been demonstrated for any particular PPI.[10]
The mechanism(s) mediating the synergy of PPIs combined with antimicrobials to increase the clinical efficacy of antimicrobial therapy against H pylori has (have) not been fully elucidated. Antisecretory drugs, such as PPIs, can interfere with the indirect delivery of antibiotics (as has already been suggested for metronidazole and clarithromycin),[11] may decrease gastric juice volume, and may reduce the washout of antibiotics, hence increasing luminal antibiotic concentration.[12-14] In addition, the increased absorption and tissue penetration of antimicrobial agents that occur with elevated gastric mucosal levels caused by omeprazole may contribute to the observed synergy.[12-14] Furthermore, acid suppression may reduce the chemical degradation or increase the drug (antimicrobial) stability at a higher gastric pH.[12]
Bismuth
Bismuth was one of the first agents used against H pylori infection. There is evidence that bismuth is directly bactericidal, although its minimum inhibitory concentration (MIC) is relatively high for H pylori. Like other heavy metals such as zinc and nickel, bismuth compounds interfere with the activity of urease enzyme, the high activity of which is a characteristic feature of H pylori. Also, bismuth compounds exert their topical antimicrobial activity, acting directly on bacterial cell walls to disrupt their integrity by accumulating in the periplasmic space and along membranes.
Metronidazole
H pylori is generally highly sensitive to metronidazole, which is actively secreted into gastric juice and saliva, with activity independent of pH. Metronidazole is a prodrug that must undergo activation by bacterial nitroreductases. There are a number of H pylori enzymes with the potential to reduce metronidazole, and it is possible that increased drug dosage and resulting very high concentrations in the stomach allow sufficient drug to become activated to kill the organism. Reduced nitroimidazoles (eg, metronidazole) cause loss of the helical structure of bacterial DNA, strand breakage, and thus, impairment of bacterial function.
Clarithromycin
Clarithromycin, a 14-membered ring macrolide antibiotic, is a derivative of erythromycin, with a similar spectrum of activity and clinical application. However, clarithromycin is more acid-resistant, has more consistent absorption, and has a longer elimination half-time compared with erythromycin. Results of studies showing approximately 90% H pylori eradication with triple-therapy regimens using clarithromycin have led to widespread use of this antibiotic. However, the increasing prevalence of clarithromycin-resistant H pylori strains must be kept in mind before using this antimicrobial agent. In this setting, unlike the situation with metronidazole, there is no evidence that increasing the dosage of drug will overcome the problem of bacterial resistance.
Amoxicillin
Amoxicillin is a close chemical and pharmacologic relative of ampicillin. This agent is stable in acid and inhibits the synthesis of the bacterial cell wall, acting both topically and systemically after absorption into the bloodstream and subsequent delivery into the gastric lumen. H pylori demonstrates good sensitivity to this antibiotic in vitro, but gastric antisecretory cotherapy is required for any significant efficacy.
Tetracyclines
The tetracyclines are close derivatives of the polycyclic naphtacenecarboxamides. The site of action of tetracyclines is the bacterial ribosome, which results in the interruption of protein biosynthesis — but at least 2 processes appear to be required for these antibiotics to gain access to the ribosomes of gram-negative bacteria. The first of these is their passive diffusion through hydrophilic pores in the outer cell membrane. The second process involves an energy-dependent active transport system that pumps all tetracyclines through the inner cytoplasmic membrane. Once the tetracyclines gain access to the bacterial cell, they inhibit protein synthesis and bind specifically to the 30-S ribosomal subunit. The latter thereby prevents aminoacyl tRNA access to the acceptor site on the mRNA-ribosome complex, and thus precludes the addition of amino acids to the growing peptide chain. Tetracycline has demonstrated in vitro efficacy against H pylori and is active at low pH.
Whom Should We Treat?
According to the guidelines put forth in the Maastricht 2-2000 Consensus Report,[15] eradication is strongly recommended in all patients with peptic ulcer, including those with complications; in patients with low-grade mucosa-associated lymphoid tissue (MALT) lymphoma; in individuals with atrophic gastritis; and after gastric cancer resection. Eradication of H pylori infection is also strongly recommended for patients who are first-degree relatives of individuals with gastric cancer.
Whether patients with functional dyspepsia, patients on chronic nonsteroidal anti-inflammatory drug (NSAID) therapy, or individuals with gastroesophageal reflux disease (GERD) should have their H pylori eradicated remains a matter of debate. There is no definitive evidence that eradication of H pylori infection has an impact on dyspeptic symptoms.[16-18] However, it is well known that H pylori-infected individuals with nonulcer dyspepsia and corpus-predominant gastritis are more susceptible to the development of gastric adenocarcinoma compared with individuals with peptic ulcer disease who have antral-predominant gastritis.[7] Therefore, eradication treatment should be advised in patients with nonulcer dyspepsia, particularly if they show a corpus-predominant gastritis at histology.
An argument against eradication of H pylori infection in patients scheduled for chronic NSAID therapy derives from the concept that the organism protects the gastric mucosa from the damaging effect of the drugs due to the increased cyclooxygenase activity and prostaglandin production.[19] However, eradication of H pylori prior to use of NSAIDs reduces the incidence of peptic ulcer.[20] Additionally, NSAID-related peptic ulcer disease can be safely and efficiently prevented by instituting PPI therapy. Therefore, H pylori eradication should be advisable in patients on chronic NSAID therapy.
It has been shown that curing H pylori infection may provoke reflux esophagitis.[21] Moreover, it has been suggested that H pylori infection may enhance the ability of PPIs to reduce intragastric acidity, and therefore, that patients with H pylori-positive esophagitis heal faster with PPIs than uninfected individuals.[21] Furthermore, rebound acid hypersecretion has been observed in H pylori-negative patients after stopping PPI therapy.[22] Therefore, there is concern that treatment of H pylori in patients with GERD may exacerbate the disease, reduce the ability of PPIs to treat symptoms effectively, and promote rebound acid hypersecretion once the drug is discontinued. However, H pylori eradication does not increase relapse rates in GERD patients, and treating H pylori infection does not dramatically impair the efficacy of PPI therapy.[23] Additionally, patients with H pylori infection are at risk of developing gastric mucosal atrophy, and a cohort study[24] suggested that long-term PPI therapy for GERD may accelerate this process.
Therefore, the decision as to whether H pylori eradication therapy should be offered to infected GERD patients rests on the individual beliefs of clinicians about the risk of developing corpus atrophy and intestinal metaplasia during prolonged acid suppression. In this author's opinion, H pylori-infected GERD patients should be advised to eradicate their H pylori infection.
How Should We Treat?
Despite use of the currently most effective treatment regimens, approximately 10% to 20% of patients will fail to achieve eradication of their infection, and thus will remain H pylori positive.[25] Because retreatment is always difficult, choosing the best available first-line treatment regimen still represents the best rescue strategy. However, in designing a treatment strategy, we should not focus on the results of primary therapy alone. An adequate strategy for treating this infection should employ 2 therapies that, if applied consecutively, come as close to a 100% cure rate as possible.
The choice of second-line treatment depends on which treatment approach was used initially, because retreatment with the same regimen is not recommended. If a clarithromycin-based regimen was used initially, a metronidazole-based regimen should be used as follow-up (in combination with a PPI, tetracycline, and bismuth), and vice versa. Prolonging the treatment period to 14 days is probably necessary. Because bacterial resistance to metronidazole or clarithromycin results primarily from previous treatment failures, first-choice treatment should never combine clarithromycin and metronidazole in the same regimen. In fact, even though this combination is highly effective, patients who are not cured will have at least single, and usually double, resistance,[26] and no viable empirical treatment remains afterwards. If an empirical (ie, nonsusceptibility testing-based) treatment is chosen, the performance of a test culture after first eradication failure is not necessary; in clinical practice, assessment of the sensitivity of H pylori to antibiotics is suggested only after failure of second-line treatment.
First-line therapy should be a PPI-based triple therapy employing a PPI (standard dose twice daily) combined with clarithromycin (500 mg twice daily) and amoxicillin (1 g twice daily), for a minimum of 7 days. Second-line therapy should be quadruple therapy with a PPI (standard dose twice daily), bismuth salt (subsalicylate or subcitrate 120 mg 4 times daily), metronidazole (500 mg thrice daily) and tetracycline (500 mg 4 times daily) for 14 days. Further failures should be managed by specialists. The Table summarizes the suggested therapeutic regimens for eradication of H pylori infection.
Table.
Suggested Therapeutic Regimens for Eradication of H pylori Infection
|
Fist-line Therapy (7–10 days) PPI standard dose twice daily + Clarithromycin 500 mg twice daily + Amoxicillin 1 g twice daily |
|
Second-line Therapy (10–14 days) PPI standard dose twice daily + Metronidazole 500 mg thrice daily or amoxicillin 1 g twice daily + Tetracycline 500 mg 4 times daily + Bismuth subcitrate 120 mg 4 times daily |
|
Rescue Therapies PPI standard dose twice daily + Rifabutin 300 mg once daily + Amoxicillin 1g twice daily for 7 days |
| or |
| PPI standard dose twice daily + Amoxicillin 1g twice daily. + Levofloxacin 500 mg once daily for 7 days |
| or |
| PPI standard dose twice daily. + Amoxicillin 1g twice daily + for 5 days |
| followed by |
| PPI standard dose twice daily + Clarithromycin 500 mg twice daily + Tinidazole 500 mg twice daily for 5 days |
What rescue regimen should be used after initial treatment failure? Recently, rifabutin-based rescue therapies (twice-daily standard-dose PPI plus amoxicillin 1 g twice daily or levofloxacin 500 mg once daily plus rifabutin 300 mg daily, for 7 days) have been shown to represent an encouraging strategy for eradication failures because they are effective against H pylori strains resistant to clarithromycin or metronidazole.[27,28] However, rifabutin is very costly, and concerns still remain about the widespread use of this drug because of the possibility for accelerating development of drug resistance. Results of a recent study[29] suggest that a 10-day rescue therapy regimen based on the use of rabeprazole (20 mg twice daily) plus amoxicillin (1 g twice daily) plus levofloxacin (500 mg once daily) is more effective than standard quadruple regimen as a second-line option for H pylori eradication. Additionally, a 7-day quadruple-therapy regimen containing amoxicillin and tetracycline has recently been proven more effective than standard quadruple therapy with metronidazole and tetracycline to rescue failed triple therapy, by overcoming the antimicrobial resistance of H pylori.[30]
Antimicrobial Resistance
Resistance of H pylori to the limited range of antibiotics that have efficacy in its treatment can severely affect attempts to eradicate the bacteria. Bacterial resistance to antimicrobials can be either primary (ie, present before therapy) or secondary (ie, develop as the result of failed therapy). Resistance to tetracycline or amoxicillin is extremely rare.[31,32] The issue of resistance primarily concerns the nitroimidazoles (metronidazole or tinidazole) and macrolides (clarithromycin).[33-35] Prevalence of H pylori resistance to metronidazole is approximately 25%.[36,37] In vitro resistance to metronidazole is not always predictive of results in vivo. Increasing the dosage of metronidazole administered (eg, from 1.0 to 1.5 g/day) generally improves the results of therapy when treating metronidazole-resistant H pylori strains.
Resistance to clarithromycin is becoming more prevalent in some European countries, where the prevalence may be as high as 17%.[36,37] The clinical effect of clarithromycin resistance is essentially complete loss of any clarithromycin anti-H pylori effect; outcome of therapy can generally be predicted on the basis of what could be expected if only the other antimicrobials in the regimen are used.[35]
The increasing prevalence of antimicrobial resistance jeopardizes the success of therapeutic regimens aimed at the eradication of the infection.[38] Currently, most physicians treat H pylori infection without relying on antimicrobial susceptibility testing to select the best regimen. Choosing the treatment regimen on the basis of pretreatment antimicrobial susceptibility testing may significantly decrease the number of treatment failures compared with that obtained with standard triple therapy. In fact, a prospective study conducted at our institution involving 150 H pylori-infected patients showed that an eradication regimen based on the results of pretreatment antimicrobial-susceptibility testing was associated with a higher eradication rate and a significantly lower rate of treatment failure in this population (H pylori-infected dyspeptic subjects; Figure).[39] Comparable results have been obtained by Toracchio and colleagues.[40] Moreover, a cost-benefit analysis taking into account the cost estimates for a number of variables including office visits; performance of endoscopy plus biopsy, rapid urease test, histology, H pylori test culture and antimicrobial susceptibility testing, 13C- urea breath test; and cost-per-tablet of omeprazole, amoxicillin, tetracycline, clarithromycin, metronidazole, and bismuth subcitrate showed that eradication based on the results of antimicrobial susceptibility testing saved up to US $5 per patient. When this was normalized with respect to the effectiveness of the different treatments, H pylori eradication obtained through pretreatment susceptibility testing-based therapy resulted in a cost-effectiveness ratio of approximately US $15 per patient lower when compared with that achieved with standard triple therapy.[39]
Figure.
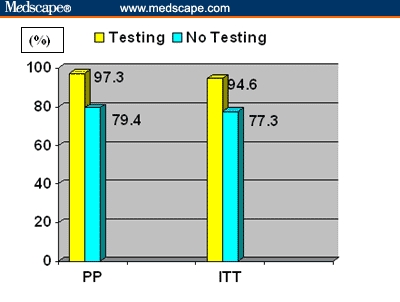
H pylori infection eradication rate in 75 patients treated on the basis of results of susceptibility testing (Testing) and in 75 patients treated with standard triple therapy (No Testing)
Key: PP: Per Protocol (75 patients per group); ITT: Intention-to-Treat (73 patients per group)
We postulate that because antimicrobial-resistant strains are becoming increasingly prevalent, therapeutic regimens based on susceptibility testing should always be used as first-line therapy, especially in areas with a high prevalence of resistance to clarithromycin and metronidazole.[41] This approach to treating H pylori infection is more effective and cost-saving and thus may help prevent the emergence of secondary resistance.
Future Directions
Therapeutic regimens directed against H pylori infection will continue to evolve. What is required is a well-tolerated monotherapy specific for H pylori, which therefore does not induce resistance in other organisms. This possibility may be realized now that the H pylori genome has been sequenced. However, with no new development of anti-H pylori antimicrobial agents expected anytime soon, research efforts will continue to focus on improved eradication rates through adjustments in dosing and combinations of available medications. In this regard, Zullo and colleagues[42] have recently reported that 5-day treatment with rabeprazole 20 mg twice daily plus amoxicillin 1 g twice daily followed by 5-day triple-drug treatment with rabeprazole 20 mg twice daily plus tinidazole 500 mg twice daily plus clarithromycin 500 mg twice daily was significantly more effective than the standard 7-day regimen in a randomized, controlled study involving approximately 1000 H pylori-infected patients. The eradication rate associated with this sequential regimen was 92% compared with 77% obtained with standard triple therapy.
It has also been reported that probiotics may exert a favorable effect on H pylori gastritis and significantly reduce the incidence of side effects related to antimicrobial therapy.[43] Moreover, the efficacy of a standard PPI-based triple-therapy regimen was significantly increased by adding bovine lactoferrin in an open, randomized, single-center study,[44] whereas human recombinant lactoferrin was ineffective in the treatment of human H pylori infection.[45]
In our opinion, given the limited number of effective drugs available, clinicians should try to optimize the use of these drugs by treating H pylori infection as they would any infection, thus establishing the in vitro sensitivity of the clinical isolates to antimicrobials before initiating treatment. The major obstacle to this approach is the invasive nature of the endoscopy exam used to obtain gastric mucosal samples for culture and testing and, therefore, efforts should be made to obtain samples of gastric mucosa in a less invasive manner. Development of H pylori strains with no infectivity may become a reality, and further knowledge of genomics may help in identifying the virulent strains. Targeting these virulent strains and developing aggressive eradication strategies aimed specifically at population subgroups may represent the path forward.
Contributor Information
Marco Romano, Associate Professor, Dipartimento di Internistica Clinica e Sperimentale-Cattedra di Gastroenterologia, Seconda Università di Napoli, Napoli, Italy.
Antonio Cuomo, Staff Physician, Dipartimento di Internistica Clinica e Sperimentale-Cattedra di Gastroenterologia, Seconda Università di Napoli, Napoli, Italy.
References
- 1.Megraud F, Brassens-Rabbe MP, Denis F. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol. 1989;27:1870–1873. doi: 10.1128/jcm.27.8.1870-1873.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go MF. Natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16(Suppl. 1):3–15. doi: 10.1046/j.1365-2036.2002.0160s1003.x. [DOI] [PubMed] [Google Scholar]
- 3.Blaser MJ. Hypothesis: the changing relationships of Helicobacter pylori and humans: implications for health and disease. J Infect Dis. 1999;179:1523–1530. doi: 10.1086/314785. [DOI] [PubMed] [Google Scholar]
- 4.Rauws EAJ, Tytgat GNY. Cure of duodenal ulcer disease associated with eradication of Helicobacter pylori. Lancet. 1990;2:12233–12235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- 5.Zarrilli R, Ricci V, Romano M. Molecular response of gastric epithelial cells to Helicobacter pylori-induced cell damage. Cell Microbiol. 1999;1:93–99. doi: 10.1046/j.1462-5822.1999.00018.x. [DOI] [PubMed] [Google Scholar]
- 6.Ricci V, Zarrilli R, Romano M. Voyage of Helicobacter pylori in human stomach: Odyssey of a bacterium. Digest Liver Dis. 2002;34:2–8. doi: 10.1016/s1590-8658(02)80051-2. [DOI] [PubMed] [Google Scholar]
- 7.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. Abstract. [DOI] [PubMed] [Google Scholar]
- 8.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nature Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 9.Vergara M, Vallve M, Gisbert JP, Calvet X. Meta-analysis: comparative efficacy of different proton-pump inhibitors in triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2003;18:647–654. doi: 10.1046/j.1365-2036.2003.01746.x. Abstract. [DOI] [PubMed] [Google Scholar]
- 10.Iwahi T, Satoh H, Hakao M. Lansoprazole: a novel benzimidazole proton pump inhibitor and its related compounds have selective activity against Helicobacter pylori. Antimicrob Agents Chemother. 1991;35:490–496. doi: 10.1128/aac.35.3.490. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goddard AF, Jessa MJ, Barrett DA, et al. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology. 1996;111:358–367. doi: 10.1053/gast.1996.v111.pm8690200. Abstract. [DOI] [PubMed] [Google Scholar]
- 12.Gustarson LE, Kaiser JF, Edmonds AL. Effect of omeprazole on concentrations of clarithromycin in plasma and gastric tissue at steady state. Antimicrob Agents Chemother. 1995;39:2078–2083. doi: 10.1128/aac.39.9.2078. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calafatti AS, Santos A, Da Silva CMF, et al. Transfer of metronidazole to gastric juice: impact of Helicobacter pylori infection and omeprazole. Scand J Gastroenterol. 2000;35:699–704. doi: 10.1080/003655200750023354. Abstract. [DOI] [PubMed] [Google Scholar]
- 14.Pedrazzoli J, Jr, Calafatti SA, Ortiz RA, et al. Transfer of clarithromycin to gastric juice is enhanced by omeprazole in Helicobacter pylori-infected individuals. Scand J Gastroenterol. 2001;36:1248–1253. doi: 10.1080/003655201317097074. Abstract. [DOI] [PubMed] [Google Scholar]
- 15.Malfertheiner P, Megraud F, O Morain C. Current concepts in the management of Helicobacter pylori infection. The Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2000;16:167–180. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 16.Blum AL, Talley NJ, O'Morain C, et al. Lack of effect of treating Helicobacter pylori in patients with non-ulcer dyspepsia. N Engl J Med. 1998;339:1871–1875. doi: 10.1056/NEJM199812243392602. Abstract. [DOI] [PubMed] [Google Scholar]
- 17.McColl K, Murray L, El-Omar E, et al. Symptomatic benefit from eradicating Helicobacter pylori infection in patients with non-ulcer dyspepsia. N Engl J Med. 1998;339:1869–1874. doi: 10.1056/NEJM199812243392601. Abstract. [DOI] [PubMed] [Google Scholar]
- 18.Malfertheiner P, Mossner J, Fishbach W, et al. Helicobacter pylori eradication is beneficial in the treatment of functional dyspepsia. Aliment Pharmacol Ther. 2003;18:615–626. doi: 10.1046/j.1365-2036.2003.01695.x. Abstract. [DOI] [PubMed] [Google Scholar]
- 19.Feldman M, Cryer B, Mallat D, Go MF. Role of Helicobacter pylori infection in gastroduodenal injury and gastric prostaglandin synthesis during long term/low dose aspirin therapy: a prospective placebo-controlled, double-blind randomized trial. Am J Gastroenterol. 2001;96:1751–1757. doi: 10.1111/j.1572-0241.2001.03928.x. Abstract. [DOI] [PubMed] [Google Scholar]
- 20.Chan FK, Sung JJ, Chung SC, et al. Randomised trial of eradication of Helicobacter pylori before non-steroidal anti-inflammatory drug therapy to prevent peptic ulcers. Lancet. 1997;350:975–979. doi: 10.1016/s0140-6736(97)04523-6. Abstract. [DOI] [PubMed] [Google Scholar]
- 21.Labenz J, Blum AL, Bayerdorffer E, Meining A, Stolte M, Borsch G. Curing Helicobacter pylori infection in patients in with duodenal ulcer may provoke reflux esophagitis. Gastroenterology. 1997;112:1442–1447. doi: 10.1016/s0016-5085(97)70024-6. Abstract. [DOI] [PubMed] [Google Scholar]
- 22.Gillen D, Wirz AA, Ardille JE, McColl KEL. Rebound hypersecretion after omeprazole and its relation to on-treatment acid suppression and Helicobacter pylori status. Gastroenterology. 1999;116:239–247. doi: 10.1016/s0016-5085(99)70118-6. Abstract. [DOI] [PubMed] [Google Scholar]
- 23.Moayyedi P, Bardhan C, Young L, Dixon MF, Brown L, Axon ATR. Helicobacter pylori eradication does not exacerbate reflux symptoms in gastroesophageal reflux disease. Gastroenterology. 2001;121:1120–1126. doi: 10.1053/gast.2001.29332. Abstract. [DOI] [PubMed] [Google Scholar]
- 24.Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux oesophagitis treated with omeprazole or fundoplication. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199604183341603. Abstract. [DOI] [PubMed] [Google Scholar]
- 25.Gisbert JP, Pajares JM, Racz I. The year in Helicobacter pylori therapy 2001. Curr Opin Gastroenterol. 2001;17(Suppl 1):S47–S54. [Google Scholar]
- 26.Peitz U, Sulliga M, Wolle K, et al. High rate of post-therapeutic resistance after failure of macrolide-nitroimidazole triple therapy to cure Helicobacter pylori infection: impact of two second-line therapies in a randomised study. Aliment Pharmacol Ther. 2002;16:315–324. doi: 10.1046/j.1365-2036.2002.01173.x. Abstract. [DOI] [PubMed] [Google Scholar]
- 27.Perri F, Festa V, Clemente R, Quitadamo M, Andriulli A. Rifabutin-based “rescue therapy” for Helicobacter pylori infected patients after failure of standard regimens. Aliment Pharmacol Ther. 2000;14:311–316. doi: 10.1046/j.1365-2036.2000.00719.x. Abstract. [DOI] [PubMed] [Google Scholar]
- 28.Wong WM, Gu Q, Lam SK, et al. Randomized controlled study of rabeprazole, levofloxacin and rifabutin triple therapy vs quadruple therapy as second-line treatment for Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:553–560. doi: 10.1046/j.1365-2036.2003.01459.x. Abstract. [DOI] [PubMed] [Google Scholar]
- 29.Nista EC, Candelli M, Cremonini F, et al. Levofloxacin-based triple therapy vs quadruple therapy in second-line Helicobacter pylori treatment: a randomized trial. Aliment Pharmacol Ther. 2003;18:627–633. doi: 10.1046/j.1365-2036.2003.01676.x. Abstract. [DOI] [PubMed] [Google Scholar]
- 30.Chi C-H, Lin C-Y, Sheu B-S, Yang H-B, Huang A-H, Wu J-J. Quadruple therapy containing amoxicillin and tetracycline is an effective regimen to rescue failed triple therapy by overcoming the antimicrobial resistance of Helicobacter pylori. Aliment Pharmacol Ther. 2003;18:347–353. doi: 10.1046/j.1365-2036.2003.01653.x. Abstract. [DOI] [PubMed] [Google Scholar]
- 31.Gerrits MM, Schuijffel D, van Zwet AA, Kuipers EJ, Vandenbroucke-Grauls CM, Kusters JG. Alterations in penicillin-binding protein 1A confer resistance to beta-lactam antibiotics in Helicobacter pylori. Antimicrob Agents Chemother. 2002;46:2229–2233. doi: 10.1128/AAC.46.7.2229-2233.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon DH, Kim JJ, Lee M, et al. Isolation and characterization of tetracycline-resistant clinical isolates of Helicobacter pylori. Antimicrob Agents Chemother. 2000;44:3203–3205. doi: 10.1128/aac.44.11.3203-3205.2000. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Megraud F. Epidemiology and mechanism of antibiotic resistance in Helicobacter pylori. Gastroenterology. 1998;115:1272–1278. doi: 10.1016/s0016-5085(98)70101-5. Abstract. [DOI] [PubMed] [Google Scholar]
- 34.Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology. 1998;115:1272–1277. doi: 10.1016/s0016-5085(98)70100-3. Abstract. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Hunt RH. The importance of clarithromycin dose in the management of Helicobacter pylori infection: a meta-analysis of triple therapies with a proton pump inhibitor, clarithromycicn and amoxicillin or metronidazole. Aliment Pharmacol Ther. 1999;13:719–729. doi: 10.1046/j.1365-2036.1999.00530.x. Abstract. [DOI] [PubMed] [Google Scholar]
- 36.Iovene MR, Romano M, Pilloni AO, et al. Prevalence of antimicrobial resistance in eighty clinical isolates of Helicobacter pylori. Chemotherapy. 1999;45:8–14. doi: 10.1159/000007159. Abstract. [DOI] [PubMed] [Google Scholar]
- 37.Debets-Ossenkopp YJ, Herscheid AJ, Pot RG, Kuipers EJ, Kusters JG, Vanderbroucke-Grauls CM. Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxicillin, tetracycline and trovafloxacin in The Netherlands. J Antimicrob Chemother. 1999;43:415–511. doi: 10.1093/jac/43.4.511. Abstract. [DOI] [PubMed] [Google Scholar]
- 38.Deltenre MA. Economics of Helicobacter pylori eradication therapy. Eur J Gastroenterol Hepatol. 1997;9(Suppl 1):S27–S29. [PubMed] [Google Scholar]
- 39.Romano M, Marmo R, Cuomo A, et al. Pretreatment antimicrobial susceptibility testing is cost-saving in the eradication of Helicobacter pylori. Clin Gastroenterol Hepatol. 2003;1:273–278. [PubMed] [Google Scholar]
- 40.Toracchio S, Cellini L, Di Campli E, et al. Role of antimicrobial susceptibility testing on efficacy of triple therapy in Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:1639–1643. doi: 10.1046/j.1365-2036.2000.00870.x. Abstract. [DOI] [PubMed] [Google Scholar]
- 41.Breuer T, Graham DY. Costs of diagnosis and treatment of Helicobacter pylori infection: when does choosing the treatment regimen based on susceptibility testing become cost-effective? Am J Gastroenterol. 1999;94:725–729. doi: 10.1111/j.1572-0241.1999.00943.x. [DOI] [PubMed] [Google Scholar]
- 42.Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther. 2003;17:719–726. doi: 10.1046/j.1365-2036.2003.01461.x. Abstract. [DOI] [PubMed] [Google Scholar]
- 43.Canducci F, Cremonini F, Armuzzi A, et al. Probiotics and Helicobacter pylori eradication. Digest Liver Dis. 2000;34(suppl 2):S81–S83. doi: 10.1016/s1590-8658(02)80172-4. [DOI] [PubMed] [Google Scholar]
- 44.Di Mario F, Aragona G, Dal B N, et al. Use of bovine lactoferrin for Helicobacter pylori eradication. Dig Liver Dis. 2003;35:706–711. doi: 10.1016/s1590-8658(03)00409-2. Abstract. [DOI] [PubMed] [Google Scholar]
- 45.Guttner Y, Windsor HM, Viiala CH, Marshall BJ. Human recombinant lactoferrin is ineffective in the treatment of human Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:125–130. doi: 10.1046/j.1365-2036.2003.01395.x. Abstract. [DOI] [PubMed] [Google Scholar]


