Abstract
Farnesol is an isoprenoid found in many aromatic plants and is also produced in humans, where it acts on numerous nuclear receptors and has received considerable attention due to its apparent anticancer properties. Although farnesol has been studied for over 30 years, its metabolism has not been well characterized. Recently, farnesol was shown to be metabolized by cytochromes P450 in rabbit; however, neither farnesol hydroxylation nor glucuronidation in humans have been reported to date. In the present paper, we show for the first time that farnesol is metabolized to farnesyl glucuronide, hydroxyfarnesol and hydroxyfarnesyl glucuronide by human tissue microsomes, and we identify the specific human UGTs (uridine diphosphoglucuronosyltransferases) involved. Farnesol metabolism was examined by a sensitive LC (liquid chromatography)–MS/MS method. Results indicate that farnesol is a good substrate for glucuronidation in human liver, kidney and intestine microsomes (values in nmol/min per mg). Initial analysis using expressed human UGTs indicated that UGTs 1A1 and 2B7 were primarily responsible for glucuronidation in vitro, with significantly lower activity for all the other UGTs tested (UGTs 1A3, 1A4, 1A6, 1A9 and 2B4). Kinetic analysis and inhibition experiments indicate that, in liver microsomes, UGT1A1 is primarily responsible for farnesol glucuronidation; however, in intestine microsomes, UGT2B7 is probably the major isoform involved, with a very-low-micromolar Km. We also show the first direct evidence that farnesol can be metabolized to hydroxyfarnesol by human liver microsomes and that hydroxyfarnesol is metabolized further to hydroxyfarnesyl glucuronide. Thus glucuronidation may modulate the physiological and/or pharmacological properties of this potent signalling molecule.
Keywords: farnesol, farnesyl glucuronide, glucuronidation, hydroxyfarnesol, nuclear receptor, UGT1A1, UGT2B7
Abbreviations: CYP, cytochrome P450; FP, farnesyl phosphate; FPP, farnesyl pyrophosphate; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; LC, liquid chromatography; MRM, multiple reaction monitoring; PPAR, peroxisome-proliferator-activated receptor; UDPGA, β-D-uridine diphosphoglucuronic acid; UGT, uridine diphosphoglucuronosyltransferase
INTRODUCTION
Farnesol (trans,trans-3,7,11-trimethyl-2,6,10-dodecatrien-1-ol) is a sesquiterpene/isoprenoid originally discovered and isolated from plants, although it is now produced synthetically. FPP (farnesyl pyrophosphate, doubly phosphorylated farnesol) is a key synthetic intermediate in the mevalonate pathway (Scheme 1) [1]. It is the last common intermediate for the synthesis of cholesterol, coenzyme Q and dolichol, and it is also used as the substrate for protein isoprenylation [2].
Scheme 1. The mevalonate synthesis pathway showing the role of farnesol.
Farnesol originates from three sources: (i) synthesis of FPP via the mevalonate pathway, followed by action by FPP phosphatase, (ii) degradation of prenylated proteins (although this has not been proved experimentally), or (iii) external sources, such as dietary or pharmaceutical. The broken arrow indicates the feedback role that farnesol has in controlling HMG-CoA reductase levels. Farnesol can be metabolized to farnesyl phosphate and then to FPP, to hydroxyfarnesol, farnesal or to the farnesyl glucuronide. F, farnesol; FP, farnesyl phosphate; P-450, unknown CYP; ADH, alcohol dehydrogenase.
In recent years, interest in farnesol has focused on its anticancer properties, and farnesol has been shown to be an effective chemopreventive agent [3]. It has been tested on cancer cell lines [4–6], and, in addition to tests in vitro, dietary supplementation has been shown to be effective against pancreatic cancer growth [7]. It is postulated that these actions are mediated via a variety of signalling pathways, including links with PKC (protein kinase C)-dependent signal transduction in farnesol-induced apoptotic cell death [8]; farnesol acts on nuclear receptors PPAR (peroxisome-proliferator-activated receptor) γ and PPARα [9]; farnesol is also a substrate for the bile acid receptor (farnesoid X receptor) [10], and can activate CAR (constitutive androstane receptor) [11].
Apart from its potential use as an anticancer agent, farnesol has also generated interest because of its key role in the mevalonate/cholesterol synthesis pathway. Statins inhibit HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase, the enzyme that synthesizes mevalonate (Scheme 1). Farnesol, in the form of FPP, is a product of this pathway, downstream of HMG-CoA, therefore endogenous FPP (and consequently farnesol) levels are also reduced by statins. In fact, some of the effects of statins can be reversed if farnesol is given [12], which indicates that farnesol is converted into FPP in vivo. In addition to this, farnesol is believed to be a non-sterol regulator for HMG-CoA reductase levels [13], although its effects might be indirect [14], and this means that farnesol could play an important role in regulating cholesterol levels.
Given these important physiological roles, a thorough understanding of farnesol metabolism is essential; however, to date, it has not been well characterized. Apart from the conversion of farnesol into FPP, the only known metabolic pathway in mammals is via CYP (cytochrome P450). Studies have indicated that, in rabbit, farnesol is both a substrate and an inhibitor for CYP [15]. In humans, it has been shown that farnesol can inhibit the metabolism of retinoic acid by CYP [16]. Neither the product(s) from this reaction nor the individual CYP isoforms that metabolize farnesol have been identified, nor have any other direct metabolic pathways.
To date no conjugative (Phase 2) metabolism of farnesol has been identified in any system. In the present paper, we report the kinetics of farnesol glucuronidation in human liver microsomes and identify the human UGTs (uridine diphosphoglucuronosyl- transferases) that are responsible for its metabolism in vitro. In addition, we show that farnesol can be hydroxylated and that this hydroxyfarnesol metabolite can also be glucuronidated.
EXPERIMENTAL
Chemicals and reagents
2-Aminobiphenyl, DMSO, hyodeoxycholic acid, 4-methyl umbelliferone, morphine, morphine 3-β-D-glucuronide, 1-naphthol, octylgallate, propofol, UDPGA (β-D-uridine diphosphoglucuronic acid) and farnesol were purchased from Sigma-Aldrich (Gillingham, Dorset, U.K.). [Glucuronyl-14C(U)]-UDPGA was purchased from PerkinElmer (Beaconsfield, Bucks., U.K.). Methanoic (formic) acid and acetonitrile (HPLC grade) and all other chemicals (highest grade available) were purchased from Merck Eurolab (Poole, Dorset, U.K.).
Sample preparation
Microsomes were prepared from frozen human tissue, obtained as anonymized surgical or donor surplus, by standard methods [17]. Detailed information on donors was not available. Ethical approval for use of human tissue was obtained from the Tayside Committee on Medical Research Ethics. Cell lines expressing human UGTs have been created previously in this laboratory [18–21], except UGT1A4 [kindly donated by Dr Robert Tukey (La Jolla, CA, U.S.A.)]. Frozen cell pellets (originally from two 15-cm-diameter tissue-culture plates) were thawed and resuspended in 200 μl of PBS at pH 7.4. The 200 μl suspension was sonicated for five bursts of 5 s (MSE Soniprep 150, Sanyo Gallenkamp) on ice, with 1 min cooling on ice between bursts. Microsomes were diluted in PBS to 5–10 mg/ml and were sonicated as above. Protein concentrations were determined post-sonication by the method of Lowry et al. [22].
Farnesol glucuronidation assays
Farnesol (5 mM, from 100 mM stock solution in DMSO) was added to the assay mixture containing 350 μg of sonicated microsomes (or 200 μg of sonicated cell lysate) in 100 mM Tris/maleate buffer (pH 7.5) containing 5 mM MgCl2. The reaction was initiated by addition of UDPGA (10 mM, from 100 mM stock in PBS) to give a final reaction volume of 100 μl. Samples (assayed in triplicate) were incubated for 1 h at 37 °C as a standard condition; a time course over a 3 h incubation with human liver microsomes indicated that the reaction was linear for up to 1 h (results not shown). The enzyme reactions were terminated by the addition of 100 μl of cold (−20 °C) acetonitrile to the incubation mixtures. Samples were frozen at −20 °C for 20 min, thawed and centrifuged at 14000 g for 5 min. The supernatant was removed and stored at −20 °C until analysis for the presence of farnesyl glucuronide; farnesyl glucuronide was stable at −20 °C (results not shown).
Sample analysis by LC (liquid chromatography)–MS/MS
Samples were diluted 10-fold in 50% (v/v) acetonitrile/water before analysis by LC–MS/MS using a HP1100 LC system (Agilent Technologies, Stockport, U.K.), connected to a Micromass LC Quattro (Micromass, Manchester, U.K.) with a 10 μl injection per run. The LC separation used a mobile phase of 0.1% methanoic acid (buffer A), and acetonitrile containing 0.1% methanoic acid (buffer B). LC separation and elution were achieved using a 1 min isocratic portion at 80% buffer A followed by a short step to 50% buffer A and a linear gradient of 50–95% buffer B over 2 min. This was followed by a 4 min wash at 95% buffer B and a 3 min reequilibration step at 80% buffer A. Chromatography was performed on a Waters Spherisorb (ODS2) 2 μm, 2.1 mm×150 mm column at a flow rate of 0.3 ml/min with a 2 cm Hypersil (ODS2) guard column.
Mass spectral analysis was performed with direct infusion into the electrospray source, with column diversion during the first 3 min to protect the source from excessive salt. The glucuronide peak from the LC column was analysed using an MRM (multiple reaction monitoring) method in negative-ion mode. Optimized MRM conditions used the transition from 397.3→112.8 m/z at a cone voltage of 30 eV and capillary voltage of 3.0 eV. Collision energy was 20 eV and the collision gas was at 3 mbar (300 Pa). The nebulizing gas was set at 110 l/h, and the desolvation gas at 700 l/h.
To quantify the glucuronide produced, two identical farnesyl glucuronide standards, one radiolabelled and one non-radio-labelled, were generated by parallel incubation of farnesol with human liver microsomes as described above (performed in triplicate). The amount of farnesyl glucuronide produced was calculated by analysis of the [14C]farnesyl glucuronide using a HPLC radiochemical detection assay (see below). As the non-radiolabelled samples were generated in parallel, they contained the same amount of farnesyl glucuronide as the calculated value for the radiolabelled samples. The non-radioactive standard was then used in conjunction with all analyses to quantify the amount of farnesyl glucuronide present.
[14C]UDPGA assay
The method used was based on that described by Ethell et al. [23]. Incubated samples were prepared as before, except the UDPGA stock solution contained 0.2 μCi of [14C]UDPGA. Sample (150 μl) was injected onto a 150 mm×4.6 mm Techsphere ODS2 column (HPLC Technology, Welwyn Garden City, U.K.) connected to a Gilson binary injection pump with mixer. Elution from the column was achieved with a gradient from 95% buffer A (10 mM ammonium acetate, pH 6.5) to 95% buffer B (acetonitrile) over 15 min. Detection of the [14C]UDPGA was achieved using a Reeve 9710 radioactivity monitor (Reeve Analytical) fitted with a 200 μl heterogeneous cerium-activated lithium gas flow cell. Elution of UDPGA was at 2.4 min, and the farnesyl glucuronide was eluted at 8.5 min.
Determination of kinetic parameters
Kinetic parameters for farnesyl glucuronide formation were determined in duplicate using the standard assay above, with variations in the concentration of farnesol (final concentrations were 0, 5, 10, 20, 50, 75, 100, 200, 350, 500, 750, 1000, 5000 and 10000 μM). For human intestine microsomes and cell lines expressing UGT2B7, additional kinetic data were generated as the Km was below 5 μM. Assays were performed as above and with an additional set of low farnesol concentrations (0, 0.1, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 5 and 10 μM). Owing to substrate inhibition, standard Michaelis–Menten curves could not be used to calculate all the kinetic parameters. In these cases, Km and Vmax were determined using a modified Michaelis–Menten equation by non-linear least-squares regression (Kaleidagraph, Synergy Software, Reading, PA, U.S.A.) [24]:
 |
where Ki is the self-inhibition constant. When the Ki was >10 mM, the standard Michaelis–Menten equation was used to calculate Km and Vmax.
To confirm the nature of the kinetics in the human tissue microsomes, where multiple enzymes may be operating, Eadie–Hofstee plots were generated and the multiple Km values were calculated.
Inhibition of farnesol glucuronidation by UGT probe substrates
To investigate further the role of certain UGT isoforms in the glucuronidation of farnesol, a competitive inhibition assay was performed with known human UGT probe substrates [25]. Octylgallate was used for UGT1A1, 2-aminobiphenol for UGTs 1A3 and 1A4, 1-naphthol for UGT1A6, propofol for UGT1A9, and morphine for UGT2B4 and 2B7. The farnesol concentration was fixed at 50 μM, and the other compounds used were at concentrations of 0, 20 μM, 200 μM and 2 mM. Assays were performed for 60 min with human liver microsomes using the same conditions as for the kinetic experiments described above.
To examine the effects of NADPH, farnesol glucuronidation levels were measured as described above, with or without 5 mM UDPGA, and with or without 10 mM NADPH.
Detection of the novel products formed during farnesol incubation with NADPH and UDPGA
To determine whether human liver microsomes could produce any products of oxidative metabolism from farnesol, the standard assay reaction was carried out with NADPH alone or with NADPH and UDPGA together in the same reaction mixture, and the resulting incubation mixtures were analysed using LC–MS. The MS was set to scan in negative-ion mode over a mass range of 200–600 m/z and a scan speed of 2 s/spectrum. The spectrum was generated with a cone voltage of 40 eV and a capillary voltage of 3.0 eV. The desolvation and source block temperatures were 250 °C and 80 °C respectively. The nebulizing gas was applied at a flow rate of 100 l/h, the desolvation gas at 500 l/h. The LC trace was then scanned for any likely metabolites. A daughter ion scan was then performed on these peaks at a mass of 237.3 for monohydroxylated farnesol products and at 413.4 for glucuronide(s) of hydroxyfarnesol. The scan was performed in negative-ion mode over a mass range from 100 to the mass of the main ion, at a speed of 2 s/spectrum. The spectrum was generated with a cone voltage of 40 eV, a capillary voltage of 3.0 eV and with varying collision energy (optimized to give the biggest peak). The collision gas was at 3 mbar, and the desolvation and source block temperatures were 250 °C and 80 °C respectively. Control reaction mixtures were also analysed in the same way.
RESULTS
LC–MS assay
A very effective LC–MS assay was developed to detect farnesyl glucuronide produced from UDP-expressing cell lines or human tissue microsomes (Figure 1). The limit of detection of this method was calculated as 30 fmol, which translates to a minimum detectable UGT reaction rate of 4 fmol/min per mg (for a standard 1 h assay, with 250 μg of protein). This is 2000 times more sensitive than the best radioactive LC assay used routinely to detect glucuronides [23].
Figure 1. LC–MS/MS trace of farnesol glucuronidation in human liver microsomes using MRM detection of farnesyl glucuronide.
For details, see the Experimental section.
Confirmation of farnesyl glucuronide
The structure of farnesol and the predicted structure of the glucuronide are shown in Figure 2. Theoretically, farnesol can form only one glucuronide, through the farnesol alcohol moiety. The mass spectrum of the farnesyl glucuronide is shown in Figure 3(a). The fragmentation peaks are consistent with the breakdown of a glucuronide with characteristic peaks at m/z 113, 157 and 175, which is common for glucuronide fragments in negative-ion mode electrospray [26,27]. No farnesol aglycone ion peak was observed in the fragmentation (which is expected in negative-ion mode), but one of the peaks can be attributed to a farnesyl glucuronide fragment (m/z 279). No peak was observed when control experiments were performed without UDPGA, microsomes or farnesol. Farnesyl glucuronide was stable at 4 °C (for 24 h) and at −20 °C (for 1 month).
Figure 2. Structures of farnesol and the farnesyl glucuronide product.
Figure 3. Mass spectra of farnesyl β-D-glucuronide and hydroxyfarnesyl β-D-glucuronide.
(A) Mass spectrum of farnesyl β-D-glucuronide was run from 100 to 400 m/z in negative-ion mode; the spectrum was derived from a daughter ion scan at the farnesyl glucuronide mass (m/z 397), from an incubation of farnesol with human liver microsomes and UDPGA. The inset shows the proposed fragmentation of farnesyl β-D-glucuronide with main fragment masses. Masses at 175, 157 and 113 m/z are common fragments of glucuronide. No peak was observed for a farnesol aglycone fragment (221 m/z). (B) Mass spectrum of hydroxyfarnesyl β-D-glucuronide. Main peaks observed at 193, 175 and 113 m/z are indicative of the glucuronide, and the peak at 237 is the hydroxyfarnesol aglycone. As in the case of the farnesyl β-D-glucuronide fragmentation, no peak was observed for the farnesol moiety.
Glucuronidation of farnesol by human microsomes and expressed human UGTs
Table 1 shows the formation of farnesyl glucuronide by different human UGT isoforms and human tissue microsomes. It is clear from these data that UGT2B7 and UGT2B4 have the highest rate, followed by UGTs 1A1 and 1A9. With the exception of UGT2B4, these isoforms are the major xenobiotic-metabolizing UGTs [28].
Table 1. Table of kinetic data parameters of farnesol glucuronidation.
| Km (μM) | Vmax (pmol/min per mg) | Ki (μM) | Curve error (%) | |
|---|---|---|---|---|
| 1A1 | 197.0 | 140 | 2 | |
| 1A3 | 333.0 | 7.9 | 2 | |
| 1A4 | 10.6 | 2.2 | 2 | |
| 1A6 | 55.8 | 2.5 | 3 | |
| 1A9 | 9.0 | 23.4 | 10 | |
| 2B4 | 27.5 | 179 | 4627 | 4 |
| 2B7 | 6.4 | 1226 | 183 | 2 |
| HLM | 49.5 | 4195 | 1 | |
| HKM | 8.6 | 1687 | 2593 | 3 |
| HIM | 4.0 | 422 | 168 | 5 |
Kinetics of farnesol glucuronidation in human microsomes and expressed human UGT isoforms
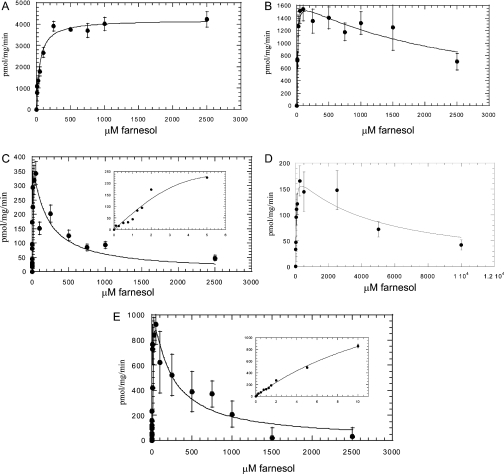
Examples of the rate against substrate concentration curves for the glucuronidation of farnesol in each different system are shown in Figures 4(A)–4(E). The kinetic parameters Km and Vmax for the three human tissue types and UGT isoforms tested were calculated from these data and are presented in Table 1.
Figure 4. Kinetic analysis of farnesol glucuronidation by microsomes or cell lines expressing human UGTs.
(A) Human liver microsomes, (B) human kidney microsomes, (C) human intestine microsomes, (D) human UGT2B4, (E) human UGT2B7. For (C) and (E), data from the low concentration values (0–10 μM) are shown in the insets. The data points are based on the mean of a duplicated experiment.
Substrate inhibition
Human kidney and intestine microsomes and recombinant UGTs 2B4 and 2B7 all exhibited substrate inhibition with farnesol. These two tissues and UGTs were analysed by a modified Michaelis–Menten equation as described in the Experimental section. The calculated inhibition constants are shown in Table 1. The Ki indicates that even submillimolar amounts of farnesol are capable of significantly inhibiting UGT2B7. Intestine microsomes exhibited an identical substrate inhibition profile with that of UGT2B7.
Contribution of each isoform to overall glucuronidation
The Km for farnesol glucuronidation measured with human liver microsomes was significantly different from that of any one individual recombinant UGT isoform, indicating that there is a variety of isoforms in liver with the potential to glucuronidate farnesol. However, the Km in liver is higher than the Kms for UGTs 1A9, 2B4 and 2B7, suggesting a significant contribution from UGT1A1, which is the only significant isoform with a higher Km. In the case of kidney microsomes, the Km for farnesol glucuronidation (very close to the Km of recombinant UGT1A9) indicates that UGT1A1, which has a significantly higher Km, is unlikely to be involved; there is some slight substrate inhibition, which would suggest a contribution from UGT2B7 or UGT2B4. It has been shown previously that there is no UGT1A1 and high levels of UGT1A9 in human kidney [29,30]. In the case of intestine, the kinetic profile supports a view that UGT2B7 is the major contributor to the glucuronidation of farnesol. The extent of substrate inhibition is important in reaching this conclusion: since UGT2B7 is the only UGT isoform that exhibits significant substrate inhibition, any inhibition in tissue microsomes is likely to indicate a significant role for UGT2B7. This therefore confirms the kinetic data, as intestine exhibits high levels of UGT2B7 (substrate inhibition), kidney exhibits medium levels (slight inhibition) and the liver used in the present study shows no major contribution of UGT2B7, i.e. no substrate inhibition. This is also consistent with previous observations of high UGT2B7 mRNA levels in human intestine [31]. Previous indications that there are high levels of UGT1A1 in intestine are based on the substrate oestradiol [32], which has been shown recently to be a substrate for UGTs 1A8 and 1A10 and 2B7 (BD Bioscience, San Jose, CA, U.S.A.; http://www.bdbiosciences.com/discovery_labware/gentest/), which are also present in intestine [33]. Therefore the data would suggest that, in this case, UGT2B7 is the dominant isoform in intestine and not UGT1A1. Knowledge of the tissue distribution of UGT2B4 is limited, therefore a contribution to glucuronidation in any of these tissues cannot be ruled out.
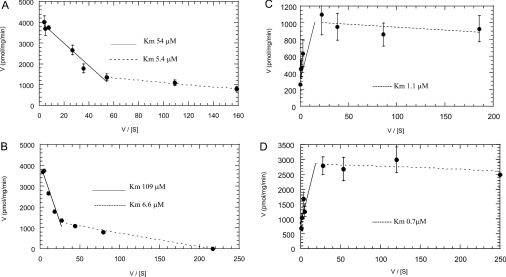
Eadie–Hofstee plots of data from the kinetic experiments (Figures 5A–5D) also demonstrate that multiple isoforms contribute to farnesol glucuronidation in liver and kidney microsomes. In these samples, at least two distinct activities can be observed, suggesting that at least two UGT isoforms glucuronidate farnesol. In the case of intestine microsomes, the substrate inhibition indicates that a UGT with a low-micromolar Km for farnesol is the major isoform, and the plot for intestine microsomes is identical with that of UGT2B7.
Figure 5. Eadie–Hofstee plots of farnesol glucuronidation.
Farnesol glucuronidation was carried out by (A) human liver microsomes, (B) human kidney microsomes, (C) human intestine microsomes or (D) human UGT 2B7. The Km values for the different lines are indicated.
Inhibition of farnesol metabolism in human liver microsomes
The Km for farnesol in human liver microsomes is 49.5 μM, and a concentration of 50 μM was chosen for this experiment. Each of the probe substrates used is generally accepted to identify a specific UGT isoform, although they do exhibit some cross-reactivity for other isoforms. Only octylgallate was found to inhibit farnesol glucuronidation significantly in liver microsomes (85% inhibition at octylgallate concentration of 2000 μM). Octylgallate is predominantly metabolized by human UGT1A1, with some activity for human UGT1A9. Propofol is a selective UGT1A9 substrate, and this showed no inhibition of farnesol glucuronidation. Therefore the inhibition by octylgallate is consistent with our finding that UGT1A1 is the dominant isoform for farnesol glucuronidation in liver.
Oxidative metabolism of farnesol
It is known that farnesol can be metabolized to hydroxyfarnesol in rabbit [15], a reaction almost certainly catalysed by a CYP. In the present study, we wished to determine whether farnesol can be metabolized to hydroxyfarnesol in human tissue and to examine the effect of this reaction on glucuronidation.
Incubation in the presence of NADPH reduced the level of glucuronidation by almost 50%, suggesting that another pathway of farnesol metabolism is operating under these conditions. To confirm that the reduced farnesol glucuronidation in the presence of NADPH was due to the formation of a farnesol CYP metabolite, LC–MS analysis of the products generated following incubation of farnesol with NADPH was carried out. This indicated the formation of a metabolite of the same mass as hydroxylated farnesol, although we could not identify the location of the hydroxy group.
Since farnesol is a good substrate for glucuronidation, it was envisaged that this hydroxylated farnesol might also be a substrate for UGTs. LC–MS analysis of the products from the reaction containing both NADPH and UDPGA incubated with liver microsomes did indeed reveal a metabolite of mass consistent with a hydroxyfarnesyl glucuronide (Figure 3b). This metabolite had similar fragmentation characteristics to farnesyl glucuronide (Figure 3a), with the additional mass corresponding to hydroxyfarnesol. Since this metabolite was found when both UDPGA and NADPH are present, the glucuronidation of hydroxyfarnesol must be able to compete successfully with farnesol glucuronidation itself. Owing to the lack of availability of hydroxylated farnesol, it was not possible to examine the production of the hydroxyfarnesyl glucuronide in more detail.
DISCUSSION
In the present study, we have developed an extremely sensitive assay for farnesol glucuronidation. Detection of the farnesyl glucuronide is 2000-fold more sensitive than that achievable with the next most sensitive technique, use of radiolabelled UDPGA [23]. Although this level of sensitivity is not necessary for the detection of farnesol glucuronidation in liver microsomes, it allowed detection of the lower levels found with some of the minor UGT isoforms and more accurate determination of kinetic data at lower substrate concentrations.
It is important to note that due to significant substrate inhibition with some isoforms, rate measurements do not provide an accurate comparison of the enzyme specificities, therefore complete kinetic analysis must be made. The Km values for recombinant UGT2B7 and for human intestine microsomes are some of the lowest reported for UGT substrates, especially UGT2B7 (usually between 50 and 500 μM) [34], with only retinoic acid (1.3 μM) [35] and androsterone (0.7 μM) [36] being lower. Without a reliable method for determining UGT protein expression levels in each UGT-expressing cell line, it is impossible to compare the Vmax values for farnesol glucuronidation accurately between these cell lines. However, comparison with standard substrates would indicate that farnesol is a better substrate for UGT2B7 than morphine, codeine or AZT (azidothymidine), which are often used as probe substrates for this UGT [37], and better for UGT2B4 than androsterone and morphine [37,38]. The UGT1A family isoforms all exhibited much lower (10–100-fold) Vmax values for farnesol than for their prototypical substrates. UGT1A1 (the UGT that metabolizes bilirubin) had the highest overall rate of farnesol glucuronidation for the 1A family, but this was still 10-fold lower than for octylgallate, one of the best UGT1A1 substrates [39].
The low Km and high Vmax values obtained for farnesol suggest that farnesol clearance in vivo might be rapid. This has important implications with regard to its effect on nuclear receptors, where rapid detoxification is a potential mechanism for modulation of the effects of farnesol via these pathways. We have demonstrated in the present study that glucuronidation is potentially an important regulator of the levels of farnesol.
The in vitro studies of the present paper have demonstrated three routes of glucuronidation of farnesol. One pathway would be through the liver, presumably largely via UGT1A1. Some metabolism could occur in the kidney via different isoforms, but probably UGT2B7 is the dominant isoform. There is also a significant potential metabolic route through the intestine, where UGT2B7 would most likely be the main isoform. Recent studies on the effects of farnesol on lung carcinoma suggested using farnesol via a direct inhalation method [40]. Lung tissue has low levels of UGTs, and may only express UGT1A7 and UGT2B11 [41], which were not available for testing in the present study, although the majority of orally inhaled drugs enter the gastrointestinal tract, where metabolism by UGT2B7 could become significant.
Co-administered drugs are unlikely to have a significant influence on farnesol glucuronidation, because farnesol is predicted to be glucuronidated in multiple organs with high activity. Few compounds are metabolized exclusively by UGT1A1 and UGT2B7; however, one exception is buprenorphine, which is mainly metabolized by these two isoforms [42,43]. The Km values for farnesol glucuronidation by specific UGT isoforms are low, however, especially for UGT2B7, and it is therefore possible that farnesol would competitively inhibit metabolism of drugs that are extensively glucuronidated, especially by UGT2B7, including common drugs, such as morphine, other opiates and some oestrogens [42]. From the kinetic and inhibition data presented, hepatic metabolism of farnesol is likely to involve predominantly UGT1A1, the enzyme responsible for bilirubin glucuronidation. This has potential toxicological implications, particularly since a common UGT1A1 promoter polymorphism is responsible for Gilbert's syndrome, a mild hyperbilirubinaemia present in approx. 15% of Caucasians. Gilbert's individuals have impaired UGT1A1 metabolism, which may affect farnesol glucuronidation, and therefore regulation of its function. Unlike UGT1A1, there are currently no known polymorphisms of UGT2B7 that have any significant effect on substrate specificity.
Since no data are available on the in vivo metabolism of farnesol, it was important to confirm that glucuronidation could occur in competition with other metabolic pathways. It has only been reported recently that farnesol can be metabolized by CYP [15]. The evidence presented here indicates for the first time that glucuronidation and hydroxylation of farnesol can occur simultaneously in human liver microsomes.
Farnesol can also be converted into farnesyl phosphate and FPP. It is not clear whether this is a recovery pathway for the disruption of the mevalonate pathway or a compensation for the FPP phosphatase action [44]. Further investigation would be required to see how this enzyme competes with glucuronidation and hydroxylation pathways. However, it should be noted that administration of farnesol does not result in a large increase in mevalonate pathway catabolites. This would suggest that conversion of farnesol into FPP is not a fast process in vivo, which should be compared with the predicted high clearance rate for farnesol glucuronidation.
Farnesol has both structural and functional similarities to retinoic acid. Retinoic acid, like farnesol, is abundant in the diet, and also acts on certain nuclear receptors. Structurally, they both contain a nucleophilic oxygen at the end of a long methylated alkene chain, retinoic acid having a hexene ring at the terminal end of the chain. It has been shown that farnesol can interfere with retinoic acid hydroxylation [45], suggesting that they may both be hydroxylated by the same CYP (CYP26). Retinoic acid and its hydroxy metabolites have been shown to be glucuronidated, like farnesol, primarily by UGT2B7 [46]; our data support these authors' hypothesis that UGT2B7 regulates the activity of these cellular signalling molecules.
One additional point of note is the extensive substrate inhibition observed with farnesol. This substrate inhibition would appear to be specific to the 2B family, and human UGT2B7 in particular. This suggests that farnesol could be a potential starting point for the design of specific UGT2B family inhibitors.
In the present study, we have demonstrated that farnesol is a substrate for human UGTs and CYP in vitro, and is metabolized to farnesyl glucuronide and hydroxyfarnesol respectively; glucuronidation can compete with CYP metabolism, and, in addition, the hydroxyfarnesol metabolite from this reaction is also glucuronidated. From kinetic and inhibition data, the glucuronidation reaction is catalysed mainly by UGT1A1 in liver and UGT2B7 in intestine. In the case of UGT2B7, farnesol has a low-micromolar Km, indicating that it is an excellent UGT2B7 substrate. Glucuronidation may therefore represent a novel pathway for modulating the action of this potent signalling molecule.
Acknowledgments
We thank Robert Tukey (La Jolla, CA, U.S.A.) for the gift of the UGT1A4 cell lines. This work was supported by a grant from the Wellcome Trust to B. B. and M. W. H. C.
References
- 1.Grunler J., Ericsson J., Dallner G. Branch-point reactions in the biosynthesis of cholesterol, dolichol, ubiquinone and prenylated proteins. Biochim. Biophys. Acta. 1994;1212:259–277. doi: 10.1016/0005-2760(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 2.Parmryd I., Dallner G. Organization of isoprenoid biosynthesis. Biochem. Soc. Trans. 1996;24:677–682. doi: 10.1042/bst0240677. [DOI] [PubMed] [Google Scholar]
- 3.Wargovich M. J., Jimenez A., McKee K., Steele V. E., Velasco M., Woods J., Price R., Gray K., Kelloff G. J. Efficacy of potential chemopreventive agents on rat colon aberrant crypt formation and progression. Carcinogenesis. 2000;21:1149–1155. [PubMed] [Google Scholar]
- 4.Vigushin D. M., Poon G. K., Boddy A., English J., Halbert G. W., Pagonis C., Jarman M., Coombes R. C. Phase I and pharmacokinetic study of D-limonene in patients with advanced cancer. Cancer Chemother. Pharmacol. 1998;42:111–117. doi: 10.1007/s002800050793. [DOI] [PubMed] [Google Scholar]
- 5.Poon G. K., Vigushin D., Griggs L. J., Rowlands M. G., Coombes R. C., Jarman M. Identification and characterization of limonene metabolites in patients with advanced cancer by liquid chromatography mass spectrometry. Drug Metab. Dispos. 1996;24:565–571. [PubMed] [Google Scholar]
- 6.Hudes G. R., Szarka C. E., Adams A., Ranganathan S., McCauley R. A., Weiner L. M., Langer C. J., Litwin S., Yeslow G., Halberr T., et al. Phase I pharmacokinetic trial of perillyl alcohol (NSC 641066) in patients with refractory solid malignancies. Clin. Cancer Res. 2000;6:3071–3080. [PubMed] [Google Scholar]
- 7.Burke Y. D., Stark M. J., Roach S. L., Sen S. E., Crowell P. L. Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids. 1997;32:151–156. doi: 10.1007/s11745-997-0019-y. [DOI] [PubMed] [Google Scholar]
- 8.Voziyan P. A., Haug J. S., Melnykovych G. Mechanism of farnesol cytotoxicity: further evidence for the role of PKC-dependent signal-transduction in farnesol-induced apoptotic cell-death. Biochem. Biophys. Res. Commun. 1995;212:479–486. doi: 10.1006/bbrc.1995.1995. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi N., Kawada T., Goto T., Yamamoto T., Taimatsu A., Matsui N., Kimura K., Saito M., Hosokawa M., Miyashita K., Fushiki T. Dual action of isoprenols from herbal medicines on both PPARγ and PPARα in 3T3-L1 adipocytes and HepG2 hepatocytes. FEBS Lett. 2002;514:315–322. doi: 10.1016/s0014-5793(02)02390-6. [DOI] [PubMed] [Google Scholar]
- 10.Fayard E., Schoonjans K., Auwerx J. Xol INXS: role of the liver X and the farnesol X receptors. Curr. Opin. Lipidol. 2001;12:113–120. doi: 10.1097/00041433-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kocarek T. A., Mercer-Haines N. A. Squalestatin 1-inducible expression of rat CYP2B: evidence that an endogenous isoprenoid is an activator of the constitutive androstane receptor. Mol. Pharmacol. 2002;62:1177–1186. doi: 10.1124/mol.62.5.1177. [DOI] [PubMed] [Google Scholar]
- 12.Nishio E., Tomiyama K., Nakata H., Watanabe Y. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitor impairs cell differentiation in cultured adipogenic cells (3T3-L1) Eur. J. Pharmacol. 1996;301:203–206. doi: 10.1016/0014-2999(96)00063-5. [DOI] [PubMed] [Google Scholar]
- 13.Meigs T. E., Simoni R. D. Farnesol as a regulator of HMG-CoA reductase degradation: characterization and role of farnesyl pyrophosphatase. Arch. Biochem. Biophys. 1997;345:1–9. doi: 10.1006/abbi.1997.0200. [DOI] [PubMed] [Google Scholar]
- 14.Keller R. K., Zhao Z. H., Chambers C., Ness G. C. Farnesol is not the nonsterol regulator mediating degradation of HMG-CoA reductase in rat liver. Arch. Biochem. Biophys. 1996;328:324–330. doi: 10.1006/abbi.1996.0180. [DOI] [PubMed] [Google Scholar]
- 15.Raner G. M., Muir A. Q., Lowry C. W., Davis B. A. Farnesol as an inhibitor and substrate for rabbit liver microsomal P450 enzymes. Biochem. Biophys. Res. Commun. 2002;293:1–6. doi: 10.1016/S0006-291X(02)00178-X. [DOI] [PubMed] [Google Scholar]
- 16.Kim S. Y., Kim C., Han I. S., Lee S. C., Kim S. H., Lee K. S., Choi Y., Byun Y. Inhibition effect of new farnesol derivatives on all-trans-retinoic acid metabolism. Metab. Clin. Exp. 2001;50:1356–1360. doi: 10.1053/meta.2001.27190. [DOI] [PubMed] [Google Scholar]
- 17.Coughtrie M. W. H., Blair J. N. R., Hume R., Burchell A. Improved preparation of hepatic microsomes for in vitro diagnosis of inherited disorders of the glucose-6-phosphatase system. Clin. Chem. 1991;37:739–742. [PubMed] [Google Scholar]
- 18.Fournel-Gigleux S., Jackson M. R., Wooster R., Burchell B. Expression of a human liver cDNA encoding a UDP-glucuronosyltransferase catalyzing the glucuronidation of hyodeoxycholic acid in cell culture. FEBS Lett. 1989;243:119–122. doi: 10.1016/0014-5793(89)80111-5. [DOI] [PubMed] [Google Scholar]
- 19.Jackson M. R., McCarthy L. R., Harding D., Wilson S., Coughtrie M. W. H., Burchell B. Cloning of a human liver microsomal UDP-glucuronosyltransferase cDNA. Biochem. J. 1987;242:581–588. doi: 10.1042/bj2420581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wooster R., Sutherland L., Ebner T., Clarke D., Silva O. D. E., Burchell B. Cloning and stable expression of a new member of the human liver phenol bilirubin UDP-glucuronosyltransferase cDNA family. Biochem. J. 1991;278:465–469. doi: 10.1042/bj2780465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fournel-Gigleux S., Sutherland L., Sabolovic N., Burchell B., Siest G. Stable expression of 2 human UDP-glucuronosyltransferase cDNAs in V79 cell cultures. Mol. Pharmacol. 1991;39:177–183. [PubMed] [Google Scholar]
- 22.Lowry O., Rosebrough N., Farr L., Randall R. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Ethell B. T., Anderson G. D., Beaumont K., Rance D. J., Burchell B. A universal radiochemical high-performance liquid chromatographic assay for the determination of UDP-glucuronosyltransferase activity. Anal. Biochem. 1998;255:142–147. doi: 10.1006/abio.1997.2443. [DOI] [PubMed] [Google Scholar]
- 24.Houston J. B., Kenworthy K. E. In vitro–in vivo scaling of CYP kinetic data not consistent with the classical Michaelis–Menten model. Drug Metab. Dispos. 2000;28:246–254. [PubMed] [Google Scholar]
- 25.Gaiser B. K., Lockley D. J., Staines A. G., Baarnhielm C., Burchell B. Almokalant glucuronidation in human liver and kidney microsomes: evidence for the involvement of UGT1A9 and 2B7. Xenobiotica. 2003;33:1073–1083. doi: 10.1080/00498250310001609129. [DOI] [PubMed] [Google Scholar]
- 26.Yan Z. Y., Caldwell G. W., Jones W. J., Masucci J. A. Cone voltage induced in-source dissociation of glucuronides in electrospray and implications in biological analyses. Rapid Commun. Mass Spectrom. 2003;17:1433–1442. doi: 10.1002/rcm.1071. [DOI] [PubMed] [Google Scholar]
- 27.Casala M., Staines A., Bánhegyi G., Mandl J., Coughtrie M. W. H., Burchell B. Evidence for multiple glucuronide transporters in rat liver microsomes. Biochem. Pharmacol. 2004 doi: 10.1016/j.bcp.2004.05.055. in the press. [DOI] [PubMed] [Google Scholar]
- 28.Burchell B., Brierley C. H., Rance D. Specificity of human UDP-glucuronosyltransferases and xenobiotic glucuronidation. Life Sci. 1995;57:1819–1831. doi: 10.1016/0024-3205(95)02073-r. [DOI] [PubMed] [Google Scholar]
- 29.Soars M. G., Riley R. J., Findlay K. A. B., Coffey M. J., Burchell B. Evidence for significant differences in microsomal drug glucuronidation by canine and human liver and kidney. Drug Metab. Dispos. 2001;29:121–126. [PubMed] [Google Scholar]
- 30.McGurk K. A., Brierley C. H., Burchell B. Drug glucuronidation by human renal UDP-glucuronosyltransferases. Biochem. Pharmacol. 1998;55:1005–1012. doi: 10.1016/s0006-2952(97)00534-0. [DOI] [PubMed] [Google Scholar]
- 31.Strassburg C. P., Kneip S., Topp J., Obermayer-Straub P., Barut A., Tukey R. H., Manns M. P. Polymorphic gene regulation and interindividual variation of UDP-glucuronosyltransferase activity in human small intestine. J. Biol. Chem. 2000;275:36164–36171. doi: 10.1074/jbc.M002180200. [DOI] [PubMed] [Google Scholar]
- 32.Fisher M. B., Van den Branden M., Findlay K., Burchell B., Thummel K. E., Hall S. D., Wrighton S. A. Tissue distribution and interindividual variation in human UDP-glucuronosyltransferase activity: relationship between UGT1A1 promoter genotype and variability in a liver bank. Pharmacogenetics. 2000;10:727–739. doi: 10.1097/00008571-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Tukey R. H., Strassburg C. P. Genetic multiplicity of the human UDP-glucuronosyltransferases and regulation in the gastrointestinal tract. Mol. Pharmacol. 2001;59:405–414. doi: 10.1124/mol.59.3.405. [DOI] [PubMed] [Google Scholar]
- 34.Cheng Z. Q., Rios G. R., King C. D., Coffman B. L., Green M. D., Mojarrabi B., Mackenzie P. I., Tephly T. R. Glucuronidation of catechol estrogens by expressed human UDP-glucuronosyltransferases (UGTs) 1A1, 1A3, and 2B7. Toxicol. Sci. 1998;45:52–57. doi: 10.1006/toxs.1998.2494. [DOI] [PubMed] [Google Scholar]
- 35.Samokyszyn V. M., Gall W. E., Zawada G., Freyaldenhoven M. A., Chen G. P., Mackenzie P. I., Tephly T. R., Radominska-Pandya A. 4-Hydroxyretinoic acid, a novel substrate for human liver microsomal UDP-glucuronosyltransferase(s) and recombinant UGT2B7. J. Biol. Chem. 2000;275:6908–6914. doi: 10.1074/jbc.275.10.6908. [DOI] [PubMed] [Google Scholar]
- 36.Turgeon D., Carrier J. S., Levesque E., Hum D. W., Belanger A. Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology. 2001;142:778–787. doi: 10.1210/endo.142.2.7958. [DOI] [PubMed] [Google Scholar]
- 37.Court M. H., Krishnaswamy S., Hao Q., Duan S. X., Patten C. J., Von Moltke L. L., Greenblatt D. J. Evaluation of 3′-azido-3′-deoxythymidine, morphine, and codeine as probe substrates for UDP-glucuronosyltransferase 2B7 (UGT2B7) in human liver microsomes: specificity and influence of the UGT2B7*2 polymorphism. Drug Metab. Dispos. 2003;31:1125–1133. doi: 10.1124/dmd.31.9.1125. [DOI] [PubMed] [Google Scholar]
- 38.Strassburg C. P., Nguyen N., Manns M. P., Tukey R. H. UDP-glucuronosyltransferase activity in human liver and colon. Gastroenterology. 1999;116:149–160. doi: 10.1016/s0016-5085(99)70239-8. [DOI] [PubMed] [Google Scholar]
- 39.Senafi S. B., Clarke D. J., Burchell B. Investigation of the substrate-specificity of a cloned expressed human bilirubin UDP-glucuronosyltransferase: UDP-sugar specificity and involvement in steroid and xenobiotic glucuronidation. Biochem. J. 1994;303:233–240. doi: 10.1042/bj3030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z., Chen H. T., Roa W., Finlay W. H. Farnesol for aerosol inhalation: nebulization and activity against human lung cancer cells. J. Pharm. Pharm. Sci. 2003;6:95–100. [PubMed] [Google Scholar]
- 41.Weaver R. J. Assessment of drug–drug interactions: concepts and approaches. Xenobiotica. 2001;31:499–538. doi: 10.1080/00498250110060950. [DOI] [PubMed] [Google Scholar]
- 42.Coffman B. L., King C. D., Rios G. R., Tephly T. R. The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y(268) and UGT2B7H(268) Drug Metab. Dispos. 1998;26:73–77. [PubMed] [Google Scholar]
- 43.Soars M. G., Fettes M., O'Sullivan A. C., Riley R. J., Ethell B. T., Burchell B. Cloning and characterization of the first drug-metabolising canine UDP-glucuronosyltransferase of the 2B subfamily. Biochem. Pharmacol. 2003;65:1251–1259. doi: 10.1016/s0006-2952(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 44.Bentinger M., Grunler J., Peterson E., Swiezewska E., Dallner G. Phosphorylation of farnesol in rat liver microsomes: properties of farnesol kinase and farnesyl phosphate kinase. Arch. Biochem. Biophys. 1998;353:191–198. doi: 10.1006/abbi.1998.0611. [DOI] [PubMed] [Google Scholar]
- 45.Klein C. E., Gupta E., Reid J. M., Atherton P. J., Sloan J. A., Pitot H. C., Ratain M. J., Kastrissios H. Population pharmacokinetic model for irinotecan and two of its metabolites, SN-38 and SN-38 glucuronide. Clin. Pharmacol. Ther. 2002;72:638–647. doi: 10.1067/mcp.2002.129502. [DOI] [PubMed] [Google Scholar]
- 46.Iyer R. A., Malhotra B., Khan S., Mitroka J., Bonacorsi S., Waller S. C., Rinehart J. K., Kripalani K. Comparative biotransformation of radiolabeled [C-14]omapatrilat and stable-labeled [C-13(2)]omapatrilat after oral administration to rats, dogs, and humans. Drug Metab. Dispos. 2003;31:67–75. doi: 10.1124/dmd.31.1.67. [DOI] [PubMed] [Google Scholar]