Abstract
We have shown that a constitutively active Gα13 (Gα13Q226L) induces differentiation in P19 embryonic carcinoma cells to an endodermal phenotype. In this report, we demonstrate that Ku, a heterodimer of p80 (Ku80) and p70 (Ku70), is upregulated in P19 cells overexpressing Gα13Q226L. Ku is the regulatory subunit of the DNA-dependent protein kinase and is primarily involved in DNA repair and recombination. Ku80 also is a somatostatin receptor. We show that while overexpression of Ku80 drastically reduced P19 cell proliferation, it was not sufficient to induce endodermal differentiation. However, coexpression of Gα13Q226L and an anti-sense Ku80 abrogated the retarded growth rate and endodermal differentiation observed in cells expressing only Gα13Q226L. Over-expression of Gα13Q226L or Ku80 downregulated RNA polymerase I-mediated transcriptional activity and overexpression of antisense Ku80 restored the activity to control level. These results suggest that Ku80 is required for Gα13-mediated endodermal differentiation in P19 cells.
The Ku protein is the heterodimeric regulatory component of the serine/threonine kinase, DNA-dependent protein kinase (DNA-PK) [1]. Ku consists of 80 (Ku80) and 70 kDa (Ku70) subunits [2]. Ku80 is also a somatostatin receptor protein that can regulate the activity of protein phosphatase 2A (PP2A) [3]. The fact that somatostatin is an inhibitor of cell proliferation and that PP2A is involved in cell cycle regulation [4] suggests for Ku80 a putative role in regulating cell growth. Sustained cell growth requires the production of new ribosomes [5]. The rate-limiting step in this process is the synthesis of ribosomal RNA (rRNA). Ku-mediated repression of ribosomal gene transcription in mouse has been reported [6]. Furthermore, a member of the Ku protein family, non-histone protein 1 (NHP1) has been shown to be upregulated in differentiation of mouse myoblasts and human promyelocytes [7].
A central role for heterotrimeric G-protein-mediated signaling in cell proliferation, differentiation, and apoptosis has been established [8]. Various G-proteins have been shown to regulate features of cellular differentiation, such as adipogenesis [9,10]. The P19 embryonic carcinoma cells provide a useful model for murine pre-implantation development [11,12]. Three germ layers, endoderm, mesoderm, and ectoderm, can be derived from P19 cells through the use of appropriate culture conditions and an inducer(s). Retinoic acid stimulates P19 cells to differentiate into primitive endoderm, as defined by the loss of the embryonic antigen SSEA-1 and positive staining with the TROMA antibody, specific for the cytokeratin endo A protein that is the hallmark of the endodermal phenotype [13,14].
In this report, we show that Ku80 is required for Gα13Q226L-induced P19 cell differentiation. Ku80 modulates the RNA polymerase I-mediated transcriptional activity in the P19 cells. However, Ku80 is not sufficient to induce endodermal differentiation in these cells.
Materials and methods
Cell culture
The P19 embryonic carcinoma cells were purchased from the American Type Culture Collection (Manassas, VA). Both the stable transfectants and the wild-type clones were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) in a humidified atmosphere of 6% CO2.
Antibodies
A monoclonal anti-HA tag antibody (12CA5) was purchased from Babco (Berkeley, CA). A goat polyclonal antibody raised against human actin and a rabbit polyclonal antibody to Gα13 were obtained from Santa Cruz (California). Anti-Ku antibody (autoimmune serum), OM [2,15] was a gift from Dr. Akira Suwa (Medical College of Georgia, Augusta, GA).
Plasmids and stable transfection
pCHA-Ku80 plasmid [16] was a gift from Dr. M. Hirakata (Medical College of Georgia, Augusta, GA). An antisense oligo was synthesized against mouse Ku80 cDNA spanning from nucleotide 181 to 219 (GATGAGATTGCGTTAGCTCTCTATGGCACAGATGGCACTG). A blast alignment to this region showed homology only with the Ku protein across species. The two strands (sense and antisense) of the single-stranded oligonucleotides were synthesized by Operon (Alameda, CA). The two strands were then annealed to create the double-stranded antisense fragment of DNA. The fragment was ligated to the XhoI and BglII sites of the pT-Hygr plasmid vector. Plasmids harboring constitutively active mutant form [17] Gα13 (pCDNA3-Gα13Q226L) were provided by Dr. Gary L. Johnson (Department of Pharmacology, University of North Carolina, Chapel Hill, NC).
Cell proliferation assay
For proliferation assays, P19 cells were washed three times in DMEM/FCS, and 1 ×105 cells were cultured in 3 ml of P19 growth medium. Triplicate wells for each time point were established at time zero. Each day for 4 days, the total number of viable cells per well was determined by counting after the addition of trypan blue. Results were plotted with the use of the Delta Graph program (SSPS, Chicago, IL).
Immunoblotting
Total cell lysates were subjected to electrophoresis in SDS on 10% polyacrylamide gels. The resolved proteins were transferred electrophoretically to nitrocellulose blots. The blots were stained with primary antibodies (anti-Ku or anti-Gα13 or anti-HA tag), and the immune complexes were made visible by the electro-chemiluminescence kit (NEN Life Science Products) in tandem with a second antibody coupled with horseradish peroxidase.
RNA polymerase I enzyme activity assay
Engaged RNA polymerase I, the fraction of the total pool of the enzyme, actively transcribes chromatin. In P19 cells, the activity of the engaged RNA polymerase I was measured [18,19]. The nuclei isolated from P19 cells were incubated in the presence of 1 mM ATP, CTP, GTP, 1 μM UTP, and 50 μg/ml α-amanitin. 3[H]UTP (1 μCi) was added to these reactions which were incubated at 37 °C for 15 min. The reactions were stopped by the addition of an equal volume of 0.1% SDS and spotted onto DE-81 filters (Whatman). The filters were washed five times in 5% sodium phosphate to remove unincorporated nucleotides. Filters were dried and [3H]UTP incorporation was determined by liquid scintillation counting. The DNA content in the nuclear extract was quantitated and used to calculate the engaged enzyme activity.
Indirect immunofluorescence staining of SSEA-1 and TROMA
The staining of the embryonic antigen SSEA-1 with mAb MC-480 and the endoderm-specific marker antigen cytokeratin endo A by the monoclonal antibody TROMA was performed with antibodies purchased from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IO). The P19 cells were cultured, stained, and subjected to analysis by epifluorescence microscopy as described previously [14].
Results
Overexpression of constitutively active Ga13 upregulates Ku (p80/p70) protein in P19 cells
Mouse embryonic carcinoma P19 cells were stably transfected with the expression vectors harboring either the pCDNA3 or Gα13Q226L. Overexpressed Gα13Q226L was identified by immunoblots of extracts of P19 cells (Fig. 1). Two of several stable clones were analyzed for this purpose. The basal levels of Gα13 in control, vector-transfected cells were undetectable due to relatively low abundance compared to the overexpressed Gα13Q226L, although P19 cells express endogenous Gα13. To confirm equal loading, immunoblotting was performed using an antibody to actin (Fig. 1, lower panel). A well-characterized Ku autoimmune antiserum (OM serum) that recognizes the Ku heterodimer (Ku80/Ku70) was used to detect the Ku proteins in the P19 cells transfected with either the pCDNA3 or pCDNA3-Gα13Q226L (Fig. 2). In the cells overexpressing Gα13Q226L, the level of both the Ku polypeptides (Ku80/Ku70) was upregulated significantly in the two clones tested compared to the cells harboring the empty vector (Fig. 2A). Another monoclonal antibody that recognizes Ku70 was used to confirm this observation. In the two clones tested, immunoblotting demonstrated a marked increase in the Ku70 level (Fig. 2B). Antibody that recognizes rodent Ku80 only is not available to conduct a similar experiment for Ku80.
Fig. 1.
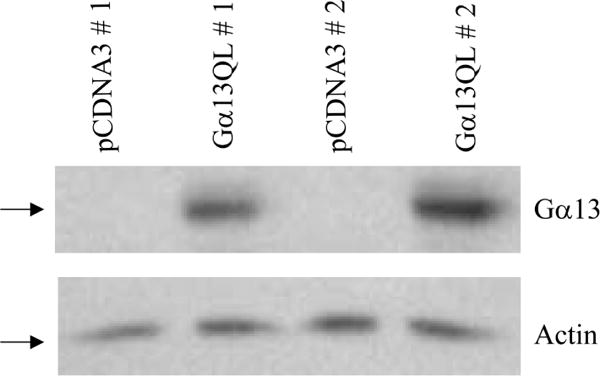
P19 cells transfected with Gα13Q226L plasmid ectopically express the constitutively active Gα13Q226L protein. Total protein was purified from various stable P19 clones. One hundred micrograms of protein/ lane was separated by SDS–polyacrylamide gel electrophoresis on 10% gels, transferred to nitrocellulose membranes, and probed with primary antibodies to Gα13. To confirm equal loading, an antibody against actin was used to detect actin in the various extracts (bottom panel).
Fig. 2.
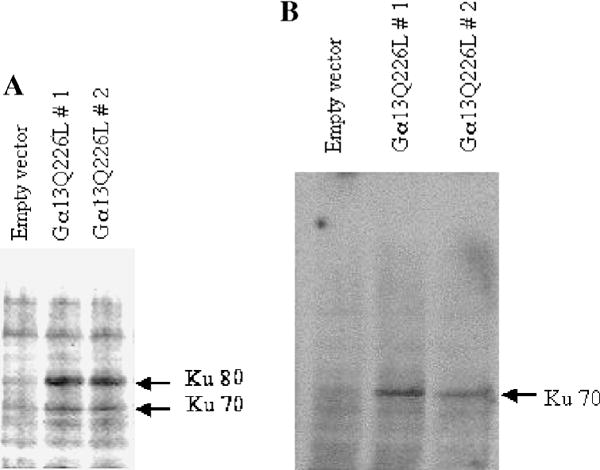
Constitutively active Gα13 upregulates Ku (Ku80/Ku70) protein level in P19 cells. An immunoblot analysis was performed taking 100 μg of total protein in each lane of a 7.5% SDS–polyacryl-amide gel. Anti-Ku antiserum (OM) that recognizes the human and murine Ku heterodimer (A) and a monoclonal antibody (N3H10) that recognizes Ku70 (B) were used. Two different clones of Gα13Q226L expressing cells are shown in comparison to the empty vector control. The Ku polypeptides are marked with arrows.
Ga13Q226L-induced Ku80 upregulation is abrogated by coexpression of an antisense Ku80 nucleotide
In order to delineate the role of Ku in Gα13Q226L-induced differentiation, an antisense 32-mer oligonucleotide was designed from the murine Ku80 cDNA sequence. The double-stranded oligonucleotide fragment was inserted into an expression vector (pTHygr) having the hygromycin-resistance (AS-Ku80). An expression construct pCHA-Ku80 was also used to ectopically overexpress Ku80. Immunoblot analysis of extracts prepared from stable clones harboring pCHA-Ku80 confirmed the expression of the exogenous Ku80 as detected by the use of an anti-HA tag monoclonal antibody (Fig. 3A). The two stable clones overexpressing Gα13Q226L expressed higher levels of Ku80 compared to the control harboring the empty vector. Subsequently, P19 cells were stably transfected with either the empty vector or AS-Ku80 or pCHA-Ku80. Clones of P19 cells stably cotransfected with Gα13Q226L and AS-Ku80 were obtained after doubly selecting them with a combination of G418 and hygromycin. In order to examine the expression of the desired polypeptides from the plasmids in the cells, an immunoblot analysis was performed on the various cell lysates. Cells overex-pressing Gα13Q226L showed a higher level of Ku polypeptides compared to the empty vector control (Fig. 3B). In the cells coexpressing Gα13Q226L and AS-Ku80, both the Ku80 and Ku70 were not detectable. The results suggested a functional antisense interference indicating as well that downregulation of Ku80 level could also result in a decrease in the dimerizing partner, Ku70, probably a matter of stabilizing interrelationship.
Fig. 3.
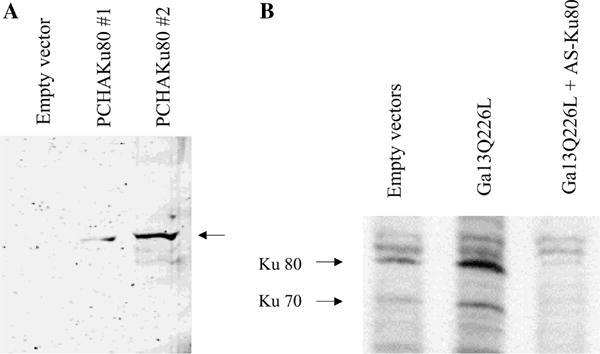
Overexpression of Ku80 in P19 cells is achieved by transfection with pCHA-Ku80 and downregulation of Ku80 is induced by transfection of an antisense Ku80, AS-Ku80. (A) P19 cells were stably transfected with PCHA-Ku80 and pCHA. Immunoblot analysis of the cell extracts was performed using an antibody (anti-HA tag) against the hemaglutinnin tag polypeptide expressed from the C-terminal end of the pCHA-Ku80 vector. The position of HA-Ku80 is marked with an arrow. (B) P19 cells were transfected either with PCDNA3 plus pT-Hygr (lane, empty vectors), or PCDNA3 Gα13Q226L plus pT-Hygr (lane, Gα13Q226L), or PCDNA3 Gα13Q226L plus pT-HygrAS-Ku80 (lane, Gα13Q226L + AS-Ku80). Immunoblot analysis of cell extracts was performed using the anti-Ku antiserum (OM). Arrows indicate the positions of Ku80 and Ku70.
Overexpression of Ku80 retards proliferation rate in P19 cells
A growth-rate analysis showed a reduced proliferation rate in cells expressing Gα13Q226L and Ku80 (Fig. 4). Interestingly, cells overexpressing antisense Ku80 proliferated as fast as cells harboring the empty vector. Furthermore, coexpression of AS-Ku80 with Gα13Q226L resulted in a recovery in the slowed growth rate caused by Gα13Q226L alone. These results demonstrate that retardation of cell growth by Gα13Q226L in P19 cells is mediated through Ku80.
Fig. 4.
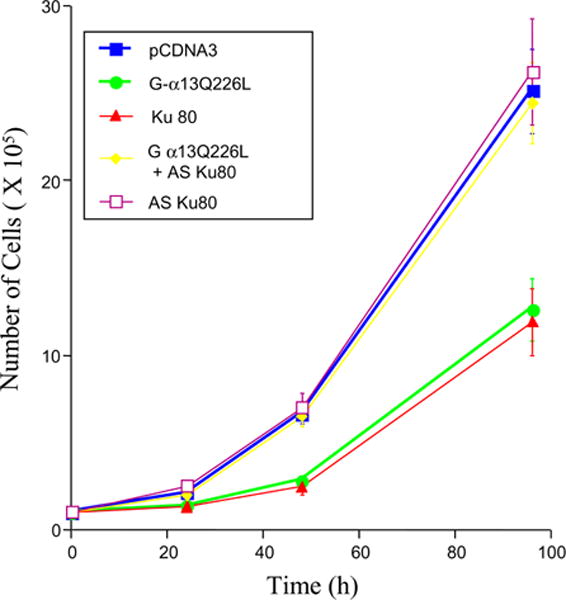
Antisense Ku80 relieves P19 cells from growth retardation induced by the constitutively active Gα13Q226L. Cells were stably transfected separately with PCDNA3, Gα13Q226L, pCHAKu80, Gα13Q226L plus pT-HygrAS-Ku80, and pT-HygrAS-Ku80. Proliferation curve of P19 clones overexpressing different proteins was obtained. Analyses of multiple clones were performed, and a representative clone for each cell line is presented. Triplicate culture wells for each time point were established at time zero. Error bars represent the standard deviation about the mean values.
Effect of overexpression and downregulation of Ku80 on endodermal differentiation of P19 cells
To test if elevated expression of Ku80 is sufficient to induce differentiation of P19 cells, clones stably expressing the Ku80 were used to immunostain for specific differentiation markers. Immunostaining with endodermal (TROMA-1) as well as ectodermal markers (SSEA-1) revealed that these cells were not differentiated into the endodermal phenotype (Fig. 5). Cells overexpressing Gα13Q226L stained positive for endodermal differentiation. In these cells SSEA-1 staining was also sparsely noticed, indicating that these cells partially retained the ectodermal marker expression. However, cells coexpressing Gα13Q226L and AS-Ku80 remained undifferentiated showing no expression of TROMA-1. These cells stained positive for the ectodermal marker, SSEA-1.
Fig. 5.
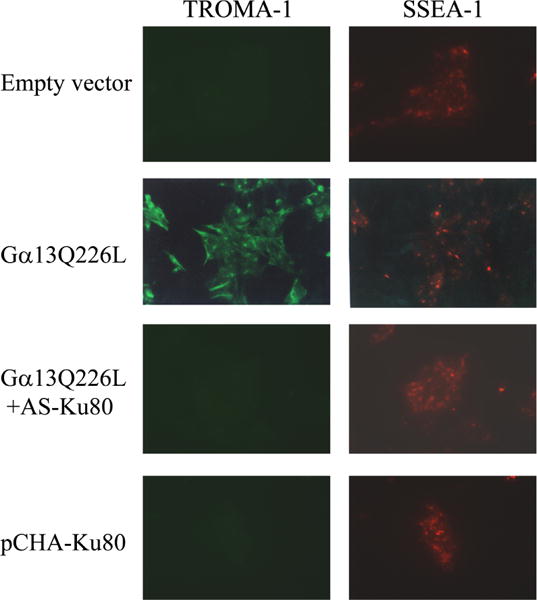
Coexpression of constitutively active Gα13Q226L and antisense Ku80 (AS-Ku80) inhibits P19 cell differentiation and constitutively active Q226L stimulates differentiation to an endodermal phenotype. Stable clones of cells expressing either the empty vectors (PCDNA3 and pT-Hygr) or Gα13Q226L (PCDNA3Gα13Q226L + pT-Hygr) or Gα13Q226L and AS-Ku80 (PCDNA3Gα13Q226L + pTHygrAS-Ku80) were subjected to indirect immunofluorescence after staining with antibodies specific for an embryonal marker (SSEA-1) or endodermal marker (TROMA-1).
P19 cells overexpressing Ku80 show attenuated RNA polymerase I activity
In eukaryotes, the genes that code for ribosomal RNA are transcribed by RNA polymerase I. In order to examine if in the differentiating P19 cells, Ku80 modulates the enzyme activity, engaged RNA polymerase I activity in the cells was measured. Three complementary assays were performed for each experiment. The procedure measured the RNA polymerase I activity that is bound to chromatin actively transcribing DNA. Nuclei were isolated from cells. The enzyme activity was determined by incubating the nuclei with nucleotides in the presence and absence of α-amanitin and heparin. The presence of α-amanitin in the assay inhibits RNA synthesis by both RNA polymerase II and III, but not synthesis by RNA polymerase I. The inclusion of heparin in the assay and exclusion of exogenous template inhibit reinitiation, allowing measurement of the enzyme that is bound to chromatin and engaged in transcription. The enzyme activity was quantitated and normalized for the quantity of DNA in the assay (Table 1). The data show that in P19 cells overexpressing Gα13Q226L, the enzyme activity is approximately 2-fold less than in cells harboring the empty vector. Cells overexpressing Ku80 (PCHA-Ku80) showed a drastic reduction (~4-fold) in the enzyme activity whereas cells harboring the antisense Ku80 (AS-Ku80) demonstrated a slightly enhanced rate (1.5-fold) of enzyme activity compared to that observed in the cells harboring the empty vector. Coexpression of Gα13Q226L and antisense Ku80 resulted in no change in the enzyme activity compared to that in cells harboring the empty vectors. These results indicate that Ku80 is involved in modulating the RNA polymerase I activity in P19 cells that could affect cell growth.
Table 1.
Engaged RNA polymerase I activity in P19 cells transfected with various plasmids
| Transfected cells | [3H]UTP incorporated (dpm) | DNA (mg) | Specific activity (dpm/mg) |
|---|---|---|---|
| PCDNA3 | 11061 | 8.5 | 1301 |
| Gα13Q226L | 5485 | 8.2 | 669 |
| AS-Ku80 | 15680 | 8.5 | 1845 |
| Gα13Q226L + AS-Ku80 | 11266 | 8.3 | 1357 |
| PCHA-Ku80 | 2730 | 7.8 | 350 |
Stable clones of P19 cells harboring various plasmids were used for the experiment. Cells were harvested and nuclei were isolated. Engaged RNA polymerase activity was assayed as decribed in Materials and methods. The results are presented for a typical experiment. These experiments were reproduced three times with similar results.
Discussion
G12 subfamily of proteins that include Gα12 and Gα13 is involved in cell growth and tumorigenesis [20–22]. The involvement of Gα12 and Gα13 in P19 cell differentiation has been documented earlier [13,14,23]. We have previously shown that the signaling cascade downstream of Gα13 in P19 cells involves the MEKK4 pathway [24] culminating in the activation of JNK1. However, other potential targets have not been explored. In this study, we have focused on a protein, Ku, that is a transcription factor as well as a somatostatin receptor [1,3]. Report on the RNA Polymerase I transcription suppressive effects of Ku presents a compelling clue that this protein in association or alone could, in fact, affect cell growth [25] and therefore may play a role in cell differentiation. During F9 cell differentiation induced by retinoic acid, rapidly growing teratocarcinoma cells are converted into various cell types. These cells display a low rate of proliferation and a downregulation of rDNA expression [26]. Cell growth in non-lymphoid cells stimulates ribosomal gene transcription [27]. This notion is corroborated by additional reports on the reduction in rRNA synthesis during myoblast and hematopoietic cell differentiation [28,29]. Ku protein has been reported to inhibit rDNA transcription [27]. A retarded cell growth via repression of RNA polymerase I-mediated transcription has been reported [26,30]. On the contrary, inhibition of Ku heterodimer DNA end binding activity has been shown during granulocytic differentiation of human promyelocytic cell lines although the Ku protein level remained unchanged [31]. In our present study, a significant increase in the Ku (p80 and p70) was observed in cells overexpressing Gα13Q226L. The antisense Ku80 mRNA expression obliterated the enhancement of Ku80 expression in Gα13Q226L-expressing cells, thereby demonstrating a functionally potent antisense effect. The level of Ku70 also decreased in these cells expressing antisense Ku80, demonstrating that the loss of one of the Ku subunits results in a loss of the other subunit. This interdependence of the two Ku subunits for their interaction and stabilization has been documented [32].
The cells coexpressing Gα13Q226L and AS-80 not only proliferated almost at a control level, but also showed no indication of undergoing differentiation. These results are in conformity with the demonstration that Ku70−/− mouse fibroblasts undergo spontaneous neoplastic transformation [33]. In order to test the ability of Ku80 to induce P19 cell differentiation, we expressed Ku80 ectopically in the cells. The results demonstrated that although Ku80 was able to retard cell growth to a low rate as observed in cells overexpressing Gα13Q226L, unlike the latter, it was not sufficient to drive P19 cells to differentiate into an endodermal phenotype. Thus, we show that Ku, especially Ku80, is required but not sufficient for induction of differentiation in P19 cells signaled by Gα13. Phosphorylation of JNK1/2 in vitro by DNA-PK, and Ku80/Ku70 interaction with JNK1/2 in human malignant glioblastoma cells upon DNA damage has been reported [34]. In extension of our previous studies that Gα13Q226L, mediates P19 cell differentiation by activating JNK1, more experiments need to be performed to prove if JNK1 and Ku80 are linked or separate signaling pathways make use of the two proteins to cause P19 cells to differentiate. Nonetheless, our results demonstrate that Ku80 is an indispensable protein downstream of Gα13Q226L signaling required for P19 cell differentiation process.
Acknowledgments
This work was supported by USPHS grant award DK30111 from the National Institutes of Health.
References
- 1.Dvir A, Peterson SR, Knuth MW, Lu H, Dynan WS. Ku autoantigen is the regulatory component of a template-associated protein kinase that phosphorylates RNA polymerase II. Proc Natl Acad Sci USA. 1992;89:11920–11924. doi: 10.1073/pnas.89.24.11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mimori T, Hardin JA, Steitz JA. Characterization of the DNA-binding protein antigen Ku recognized by autoantibodies from patients with rheumatic disorders. J Biol Chem. 1986;261:2274–2278. [PubMed] [Google Scholar]
- 3.Le Romancer ML, Reyl-Desmars F, Cherifi Y, Pigeon C, Bottari S, Meyer O, Lewin MJM. The 86-kDa subunit of autoantigen Ku is a somatostatin receptor regulating protein phosphatase-2A activity. J Biol Chem. 1994;269:7464–17468. [PubMed] [Google Scholar]
- 4.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheer U, Zentgraf H, pug A. In: The Cell Nucleus II. Busch H, Rothblum L, editors. Academic Press; New York: 1982. pp. 143–176. [Google Scholar]
- 6.Kuhn A, Stefanovsky V, Grummt I. The nucleolar transcription activator UBF relieves Ku antigen-mediated repression of mouse ribosomal gene transcription. Nucleic Acids Res. 1993;21:2057–2063. doi: 10.1093/nar/21.9.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oderwald H, Hughes MJ, Jost JP. Non-histone protein 1 (NHP1) is a member of the Ku protein family which is upregulated in differentiating mouse myoblasts and human promyelocytes. FEBS Lett. 1996;382:313–318. doi: 10.1016/0014-5793(96)00189-5. [DOI] [PubMed] [Google Scholar]
- 8.Malbon CC. Heterotrimeric G-proteins and development. Biochem Pharmacol. 1997;53:1–4. doi: 10.1016/s0006-2952(96)00662-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang HY, Watkins DC, Malbon CC. Antisense oligodeoxy-nucleotides to GS protein alpha-subunit sequence accelerate differentiation of fibroblasts to adipocytes. Nature. 1992;358:334–337. doi: 10.1038/358334a0. [DOI] [PubMed] [Google Scholar]
- 10.Su HL, Malbon CC, Wang HY. Increased expression of Gi alpha 2 in mouse embryo stem cells promotes terminal differentiation to adipocytes. Am J Physiol. 1993;265:C1729–C1735. doi: 10.1152/ajpcell.1993.265.6.C1729. [DOI] [PubMed] [Google Scholar]
- 11.Jones-Villeneuve EM, Rudnicki MA, Harris JF, McBurney MW. Retinoic acid-induced neural differentiation of embryonal carcinoma cells. Mol Cell Biol. 1983;3:2271–2279. doi: 10.1128/mcb.3.12.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones-Villeneuve EM, McBurney MW, Rogers KA, Kalnins VI. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982;94:253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jho EH, Davis RJ, Malbon CC. c-Jun amino-terminal kinase is regulated by Galpha12/Galpha13 and obligate for differentiation of P19 embryonal carcinoma cells by retinoic acid. J Biol Chem. 1997;272:24468–24474. doi: 10.1074/jbc.272.39.24468. [DOI] [PubMed] [Google Scholar]
- 14.Jho EH, Malbon CC. Galpha12 and Galpha13 mediate differentiation of P19 mouse embryonal carcinoma cells in response to retinoic acid. J Biol Chem. 1997;272:24461–24467. doi: 10.1074/jbc.272.39.24461. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Dong X, Stojanov L, Kimpel D, Satoh M, Reeves WH. Human autoantibodies stabilize the quaternary structure of Ku antigen. Arthritis Rheum. 1997;40:1344–1353. doi: 10.1002/1529-0131(199707)40:7<1344::AID-ART20>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 16.Pati UK. Novel vectors for expression of cDNA encoding epitope-tagged proteins in mammalian cells. Gene. 1992;114:285–288. doi: 10.1016/0378-1119(92)90589-h. [DOI] [PubMed] [Google Scholar]
- 17.Fanger GR, Johnson NL, Johnson GL. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42, EMBO. J. 1997;16:4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz LB, Roeder RG. Purification and subunit structure of deoxyribonucleic acid-dependent ribonucleic acid polymerase I from the mouse myeloma, MOPC 315. J Biol Chem. 1974;249:5898–5906. [PubMed] [Google Scholar]
- 19.Kabler RL, Srinivasan A, Taylor LJ, Mowad J, Rothblum LI, Cavanaugh AH. Androgen regulation of ribosomal RNA synthesis in LNCaP cells in rat prostate. J Steroid Biochem Mol Biol. 1996;59:431–439. doi: 10.1016/s0960-0760(96)00126-4. [DOI] [PubMed] [Google Scholar]
- 20.Chan AM, Fleming TP, McGovern ES, Chedid M, Miki T, Aaronson SA. Expression cDNA cloning of a transforming gene encoding the wild-type G alpha 12 gene product. Mol Cell Biol. 1993;13:762–768. doi: 10.1128/mcb.13.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang H, Wu D, Simon MI. The transforming activity of activated G alpha 12. FEBS Lett. 1993;3:319–322. doi: 10.1016/0014-5793(93)80896-3. [DOI] [PubMed] [Google Scholar]
- 22.Jones TL, Gutkind JS. Galpha12 requires acylation for its transforming activity. Biochemistry. 1998;37:3196–3202. doi: 10.1021/bi972253j. [DOI] [PubMed] [Google Scholar]
- 23.Kanungo J, Potapova I, Malbon CC, Wang HY. MEKK4 mediates differentiation in response to retinoic acid via activation of c-Jun N- terminal kinase in rat embryonal carcinoma P19 cells. J Biol Chem. 2000;275:24032–24039. doi: 10.1074/jbc.M002747200. [DOI] [PubMed] [Google Scholar]
- 24.Wang HY, Kanungo J, Malbon CC. Expression of Galpha 13 (Q226L) induces P19 stem cells to primitive endoderm via MEKK1, 2, or 4. J Biol Chem. 2002;277:3530–3536. doi: 10.1074/jbc.M107031200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labhart P. DNA-dependent protein kinase specifically represses promoter-directed transcription initiation by RNA polymerase I. Proc Natl Acad Sci USA. 1995;92:2934–9238. doi: 10.1073/pnas.92.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datta PK, Budhiraja S, Reichel RR, Jacob ST. Regulation of ribosomal RNA gene transcription during retinoic acid-induced differentiation of mouse teratocarcinoma cells. Exp Cell Res. 1997;231:198–205. doi: 10.1006/excr.1996.3446. [DOI] [PubMed] [Google Scholar]
- 27.Schnapp A, Pfleiderer C, Rosebauer H, Grummt I. A growth-dependent transcription initiation factor (TIF-IA) interacting with RNA polymerase I regulates mouse ribosomal RNA synthesis. EMBO J. 1990;9:2857–2863. doi: 10.1002/j.1460-2075.1990.tb07475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs FA, Bird RC, Sells BH. Differentiation of rat myoblasts. Regulation of turnover of ribosomal proteins and their mRNAs. Eur J Biochem. 1985;150:255–263. doi: 10.1111/j.1432-1033.1985.tb09015.x. [DOI] [PubMed] [Google Scholar]
- 29.Larson DE, Xie W, Glibetic M, O’Mahony D, Sells BH, Rothblum L. Coordinated decreases in rRNA gene transcription factors and rRNA synthesis during muscle cell differentiation. Proc Natl Acad Sci USA. 1993;90:7933–7936. doi: 10.1073/pnas.90.17.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michaelidis TM, Grummt I. Mechanism of inhibition of RNA polymerase I transcription by DNA-dependent protein kinase. Biol Chem. 2002;383:1683–1690. doi: 10.1515/BC.2002.189. [DOI] [PubMed] [Google Scholar]
- 31.Muller C, Monferran S, Gamp AC, Calsou P, Salles B. Inhibition of Ku heterodimer DNA end binding activity during granulocytic differentiation of human promyelocytic cell lines. Oncogene. 2001;20:4373–4382. doi: 10.1038/sj.onc.1204571. [DOI] [PubMed] [Google Scholar]
- 32.Satoh M, Wang J, Reeves WH. Role of free p70 (Ku) subunit in posttranslational stabilization of newly synthesized p80 during DNA-dependent protein kinase assembly. Eur J Cell Biol. 1995;66:127–135. [PubMed] [Google Scholar]
- 33.Li GC, Ouyang H, Li X, Nagasawa H, Little JB, Chen DJ, Ling CC, Fuks Z, Cordon-Cardo C. Ku70: a candidate tumor suppressor gene for murine T cell lymphoma. Mol Cell. 1998;2:1–8. doi: 10.1016/s1097-2765(00)80108-2. [DOI] [PubMed] [Google Scholar]
- 34.Park SJ, Oh EJ, Yoo MA, Lee SH. Involvement of DNA-dependent protein kinase in regulation of stress-induced JNK activation. DNA Cell Biol. 2001;20:637–645. doi: 10.1089/104454901753340622. [DOI] [PubMed] [Google Scholar]


