Abstract
Stereological methods were used to quantify secretin and gastric inhibitory peptide (GIP)-immunoreactivity (GIP-IR) in paraffin sections of the duodenum, jejunum and ileum of fetal and neonatal piglets. In addition, sections were processed for GLP-1-immunohistochemistry. The volume density of the tunica mucosa increased after birth, giving rise to a decreased volume density of the tela submucosa and tunica muscularis. Generally known region-specific morphological distinctions were reflected in differing volume densities of the various layers. The highest volume density of GIP-IR epithelial cells was observed in the jejunum of the neonate. In contrast, the volume density of secretin-IR epithelial cells was highest in the duodenum of both fetal and neonatal piglets. The volume occupied by GIP-IR and secretin-IR epithelial cells increased in the jejunum after birth. Additionally, ileal secretin-IR epithelial cells were more numerous in the neonatal piglet. In conclusion, the quantitative and qualitative presence of GIP-IR and secretin-IR epithelial cells agree with earlier reports of their presence and co-localization between GIP-IR and GLP-1-IR, in the pig small intestine. Furthermore, the differences suggest that age- and region-related functional demands are temporally and probably causally related with the morphological diversification of the intestine and its endocrine cells.
Keywords: development, endocrine, fetal, immunohistochemistry, neonatal
Introduction
Nutritional insufficiency, enterocolitis and diarrhoea are major causes of postnatal mortality in mammals, especially in pig and human (Argenzio, 1996). A detailed understanding of factors involved in perinatal development of the pig gastrointestinal tract is essential in order to target a reduction in postnatal morbidity and mortality.
Alumets et al. (1983) conducted a study concerning the developmental distribution of, among others, gastric inhibitory polypeptide (GIP, also known as glucose-dependent insulinotropic polypeptide) and secretin in the pig gastrointestinal tract in a semi-quantitative way. Both gastrointestinal hormones appear during the first half of gestation. Their number slowly increases during gestation and reaches the adult level by the end of gestation (Alumets et al. 1983).
The insulinotropic effect of GIP on the endocrine pancreas has led to the assumption that GIP analogues could be used therapeutically in the treatment of diabetes (Krarup & Groop, 1991; Morgan, 1996; Meier et al. 2002). Consequently, interest in this incretin hormone has again increased. The morphometric description of mucosal cells immunoreactive for glucagon-like peptide 1 (GLP-1), another important incretin with possible use in the treatment of diabetes, during intestinal development in the pig was the subject of an earlier study (Van Ginneken et al. 2002). To evaluate co-localization of GIP and GLP-1 in fetal and neonatal porcine small intestine, both peptides will be immunohistochemically detected in serial sections.
Besides stimulating insulin secretion from the endocrine pancreas, GIP increases lipogenesis (Krarup & Groop, 1991; Morgan, 1996; Meier et al. 2002). Possible effects on gastric functioning (inhibition of gastric acid secretion and gastric emptying) have not been fully clarified (Meier et al. 2002). For instance, unlike in dog (Pederson & Brown, 1972), GIP failed to inhibit gastric acid secretion in humans (Maxwell et al. 1980).
Secretin is part of the same family (VIP–secretin–glucagon family) and a potent secretagogue of the exocrine part of the pancreas, especially in combination with cholecystokin (Dockray, 1989). The most potent trigger for secretin release is the arrival of gastric acid in the proximal small intestine (Chey & Chang, 1989). Gastric acid secretion is found to increase during the last 15–20 days of gestation in the pig (Sangild et al. 1994). How this quantitatively relates to the presence of secretin-immunoreactive (IR) epithelial cells is not known.
The aim of this study is to describe qualitatively and quantitatively the distribution of two hormones, GIP and secretin, with effects on gastrointestinal functioning in the small intestine of the pig during two important periods in life (late in gestation and the first 4 days after birth) by using stereological methods in combination with immunohistochemistry.
Materials and methods
Tissue preparation
Animals
Fetal crossbred pigs (Piétrain × Large White) from the second half of gestation were obtained from a local slaughterhouse. The time interval between the death of the sow and the removal of the fetuses was approximately 15 min, by which time the fetuses had already died. The age of the fetuses was estimated by measuring the crown–rump length (Evans & Sack, 1973). Twelve fetuses, of ages ranging between 73 and 95 days of gestation (total time of gestation = 115 days; mean = 87.5 days of gestation), were examined.
Ten neonatal crossbred pigs (Piétrain × Large White), of ages ranging from 2 to 4 days (mean = 2.3 days postpartum), were obtained from a local farm. The neonates were killed by severing the carotid arteries under deep barbiturate anaesthesia (i.p., Nembutal®, Sanofi).
Fixation
The small intestinal segments (duodenum, jejunum, and ileum) were isolated. The duodenum encompassed the pyloric sphincter to the duodenojejunal flexure. The cranial duodenum was defined as the first third, whereas the caudal duodenum was defined as the last third of the duodenal segment. The jejunum ended at the attachment of the plica ileocecalis and the ileocecal opening marked the end of the ileal small intestinal segment. After rinsing in 0.01 m phosphate-buffered saline (PBS) solution, the intestinal segments were fixed for 2 h in 4% freshly prepared paraformaldehyde solution (0.1 m), pH 7.4. Fixation was carried out by immersion while maintaining a 10-cmH2O pressure. To obtain this pressure, one end of the intestinal tubes was ligated, and after filling the tubes with fixative, the opposite end was closed. Fixation under these conditions facilitated handling of the tissues and guaranteed ample fixation of the tissue during the short fixation period. After the 2-h fixation period, the intestinal tubes were opened and rinsed with PBS for 24 h.
Tissue sampling
Each intestinal segment (duodenum, jejunum, ileum) was divided into 3–6 pieces, depending on its initial length. A first biopsy (3–5 mm in diameter) and transverse tissue block (3 mm in length) was taken at a random position in a first piece of the intestinal segment. Subsequent biopsies and transverse tissue blocks were taken at a comparable position as in the first piece in the remaining pieces. Thus a total of 3–6 biopsies (3–5 mm in diameter) and 3–6 (3 mm in length) transverse tissue blocks were taken from each small intestinal segment at systematic random positions and routinely processed for paraffin embedding. Of these tissue blocks, 5-µm paraffin vertical (biopsies) and transverse (transverse tissue blocks) sections were made. The first section (both vertical and transverse) was collected randomly in a set of 20 serial sections. Subsequent sections were collected at a systematic distance of a random number of sections apart. The sections that were collected at systematic random positions were processed for immunohistochemistry (vertical sections) or conventionally stained with haematoxylin (transverse sections).
The linear shrinkage coefficient was estimated to be 25.6% (Van Ginneken et al. 2002).
Immunohistochemistry
Polyclonal GIP- and secretin-antiserum raised in rabbits was purchased from Milab (Malmö, Sweden). Both antisera were directed against pure porcine GIP and secretin, respectively. Polyclonal GLP-1 antiserum was raised in rabbits and purchased from Biogenesis (Poole, UK). All other immunoreagents were obtained from Dako (Glostrup, Denmark): Envision+™ (rabbit) and ready-to-use AEC+ solution (aminoethylcarbozol). The primary antisera were diluted (GIP: 1 : 3600; secretin: 1 : 2000; GLP-1: 1 : 800) in PBS enriched with 0.3% Triton-X-100, 0.1% bovine serum albumin and 0.03% NaN3.
After hydratation of the paraffin sections, endogenous peroxidase was blocked by a 30-min incubation in 3% H2O2 solution in PBS at room temperature. Following three rinses for 5 min each with PBS, the sections were incubated overnight at room temperature in the primary antiserum. After rinsing three times for 5 min with PBS, the sections were incubated for 30 min with 150 µL Envision+ solution at room temperature. Positive reactions were visualized by incubating for 7 min with the AEC+ solution at room temperature subsequent to three rinses for 5 min with PBS.
Additionally, the sections were counter-stained with haematoxylin.
For each immunoreagent, negative controls were carried out in which the primary antisera were left out of the incubation medium. In addition, non-immune rabbit antiserum was applied to detect non-specific staining. The negative controls revealed no positive immunoreactivity.
Morphometry
An Olympus BX50 microscope, equipped with a Sony CCD camera connected to a computer system running the software-program Cast-grid (Olympus, Denmark), was used for the quantitative analysis.
The vertical sections were used to estimate the volume densities (tunica mucosa, tela submucosa, tunica muscularis, tela serosa and IR epithelial cells) using a point-count stereological grid at magnification 20×. The volume densities were calculated as follows and are expressed in the text as percentages:
where ∑P(layer) is the number of test points falling on the region of interest and ∑P(reference volume) is the number of test points falling on the reference volume.
For clarity, the reference volume of the various intestinal layers was the intestinal wall and the reference volume of the immunoreactive epithelial cells was the tunica mucosa.
The volume of the intestinal segments was estimated using a point-counting method on transverse sections at a low magnification (Cavalieri, 1635). In order to yield the volume of the proximal and distal duodenal segment, the volume of the duodenal wall was divided by 3.
The stereological equation to calculate V(intestinal wall) was as follows:
where Δt is the distance between the transverse sections, ∑P is the sum of the number of test points that fall on the intestinal wall in each section and A(p) is the area associated with each test point of the point-count stereological grid. This volume was corrected for shrinkage using the linear shrinkage coefficient to calculate the areal shrinkage of the sections (55.7%).
The density of the stereological grid (number of points), the number of sections and the number of sample fields were chosen to give a coefficient of error (CE) of the estimation that was less than 0.05 (Gundersen & Jensen, 1987). The CE of the estimation can be approximated by using an equation for CE that was developed specifically for the use of a ratio estimator (Gundersen & Jensen, 1987). Gundersen & Jensen (1987) showed that a maximum of 200 points on approximately ten sections must be counted to give the required CE for the estimation of volume according to the method developed by Cavalieri (1635).
The absolute volumes were calculated by multiplying a volume density with the absolute volume of its reference space.
Statistical evaluation
The data that describe VV were arcsine-transformed according to the method described by Zar (1984) before using them in the statistical analyses. Inverse arcsine-transformation of mean and mean ± SEM of transformed data yielded the reported mean values and the 68% confidence interval. With the transformation, the data met the requirements for using parametric statistical methods.
To detect any significant effect of development (age) and region, repeated-measures anovas were performed. In order to clarify observed effects of age and/or region, post-hoc comparisons were carried out according to Bonferonni. A P value less than 0.05 was considered statistically significant.
Results
Qualitative observations
In general, the microscopic appearance of the small intestine was similar in fetal and neonatal piglets. Region-specific features such as submucosal glands in the duodenum, and long and slender villi in the jejunum and lymphoid tissue in the ileum could already be distinguished in the fetal piglets.
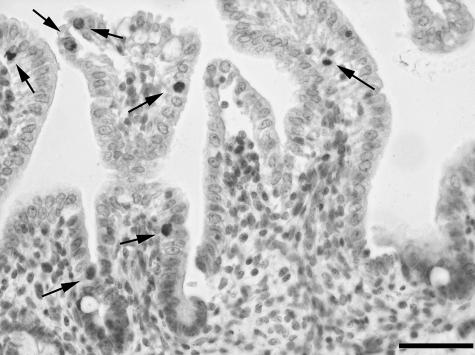
Irrespective of region, GIP-IR epithelial cells were located along the entire length of the villi and, but to a lower extent, in the crypts. GIP-IR epithelial cells had a slender apical process that reached the lumen (Figs 1 and 2).
Fig. 1.
GIP-IR epithelial cells (arrows) in the ileum of a 2-day-old neonatal piglet. One GIP-IR epithelial cell (arrowhead) clearly shows an apical process that reaches the lumen of the crypts. Scale bar, 50 µm.
Fig. 2.
GIP-IR epithelial cell in the jejunum of a 2-day-old neonatal piglet. The arrow indicates a GIP-IR cell that is also immunoreactive for GLP-1 (see Fig. 3). Scale bar, 50 µm.
In the duodenum, GLP-1-IR epithelial cells were occasionally seen and were located along the length of the villi. In the jejunum and ileum, GLP-1-IR epithelial cells were frequently encountered and were situated in the crypts and at the lower half of the villi (Fig. 3). Throughout the small intestine GLP-1-IR epithelial cells showed an apical process that reached the lumen. Co-localization between GIP and GLP-1-IR was seen in the jejunum (Figs 2 and 3).
Fig. 3.
GLP-1-IR epithelial cells (arrowhead) in the jejunum of a 2-day-old neonatal piglet. The arrow points to a GLP-1-IR cell that also is immunoreactive for GIP (see Fig. 2). Scale bar, 50 µm.
In the duodenum, secretin-IR epithelial cells were present along the entire length of the villi (Fig. 4). In the jejunum and ileum, secretin-IR epithelial cells were less frequently observed. Similar to GIP-IR epithelial cells, secretin-IR epithelial cells made contact with the lumen by means of an apical process, which gave them a pear-like shape.
Fig. 4.
Secretin-IR epithelial cells (arrow) in the duodenum of a 4-day-old neonatal piglet. Scale bar, 50 µm.
Quantitative observations
Volume densities and volumes of the intestinal layers (tunica muscosa, tela submucosa, tunica muscularis and tela serosa) (reference space: intestinal wall)
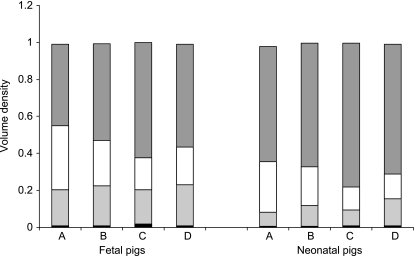
The volume density of the tunica mucosa (P < 0.0001) increased after birth. In contrast, the volume density of the tela submucosa (P = 0.0002) and the tunica muscularis (P < 0.0001) decreased, whereas the volume density of the tela serosa (P = 0.2) remained unchanged. These age-related changes were irrespective of small intestinal region (Fig. 5).
Fig. 5.
Mean volume densities of tunica mucosa (dark grey bar), tela submucosa (white bar), tunica muscularis (light grey bar) and tela serosa (black bar) according to small intestinal region (A: duodenum descendens; B: duodenum ascendens; C: jejunum and D: ileum) and age.
The qualitative region-specific differences were also reflected in the volume densities of the various layers. The volume density of the tunica mucosa was highest in the jejunum and lowest in the proximal part of the duodenum (P < 0.0001). The highest volume density of the tela submucosa was found (P < 0.0001) in the latter. The ileal small intestinal region comprised the highest percentage of muscle tissue when compared with the proximal part of the duodenum and jejunum (P = 0.0003). The volume density of the tela serosa did not show any region-related differences (P = 0.2) (Fig. 5).
The volumes of the intestinal regions and intestinal layers increased during development (Table 1).
Table 1.
Volume (V) of the small intestinal wall and of its layers according to small intestinal region and age (mean (SEM)) (mm3)
| Duodenum descendens | Duodenum ascendens | Jejunum | Ileum | ||
|---|---|---|---|---|---|
| V | Fetuses | 139 (18.8) | 139 (18.8) | 10 600 (13 500) | 200 (28.9) |
| Neonates | 568 (66.9) | 568 (66.9) | 63 200 (5950) | 1410 (128) | |
| V mucosa | Fetuses | 61.5 (8.30) | 74.2 (10.9) | 6 750 (1030) | 118 (21.9) |
| Neonates | 349 (36.1) | 380 (43.5) | 49 500 (5480) | 995 (98.9) | |
| V submucosa | Fetuses | 50.9 (8.75) | 35.1 (5.83) | 1 850 (277) | 38.7 (6.58) |
| Neonates | 164 (31.6) | 118 (12.5) | 7 560 (788) | 206 (37.5) | |
| V muscularis | Fetuses | 24.8 (2.23) | 28.4 (2.99) | 1 830 (194) | 40.9 (4.81) |
| Neonates | 45.6 (9.80) | 64.4 (14.0) | 5 540 (831) | 194 (12.9) |
Volume density and volume of GIP-IR epithelial cells (reference space: tunica mucosa)
The volume density of GIP-IR epithelial cells did not differ significantly between the fetal and neonatal piglets (P = 0.1) (Fig. 6). In contrast, the volume occupied by GIP-IR epithelial cells increased seven-fold in the jejunum (P = 0.003). In the other small intestinal regions the volume occupied by GIP-IR epithelial cells remained unaltered (Table 2).
Fig. 6.
Volume density of GIP-IR epithelial cells according to small intestinal region (A: duodenum descendens; B: duodenum ascendens; C: jejunum; D: ileum) in fetal (white box) and neonatal (black box) piglets (mean and 68% confidence interval).
Table 2.
Volume (V) of GIP and secretin-IR epithelial cells according to small intestinal region and age (mean (SEM)) (mm3).
| Duodenum descendens | Duodenum ascendens | Jejunum | Ileum | ||
|---|---|---|---|---|---|
| GIP | Fetuses | 0.33 (0.21) | 0.33 (0.27) | 30.5 (9.34) | 0.91 (0.22) |
| Neonates | 0.36 (0.25) | 0.31 (0.19) | 211 (43.4) | 1.53 (0.57) | |
| Secretin | Fetuses | 0.55 (0.08) | 0.52 (0.08) | 4.29 (1.44) | 0.05 (0.02) |
| Neonates | 3.17 (0.51) | 2.97 (0.34) | 91.6 (24.8) | 3.60 (1.65) |
Region-related differences only became apparent in the neonatal piglets (P = 0.001). In these piglets, the volume density of GIP-IR epithelial cells was significantly higher in the jejunum compared with in the duodenum and ileum (Fig. 6).
Volume density and volume of secretin-IR epithelial cells (reference space: tunica mucosa)
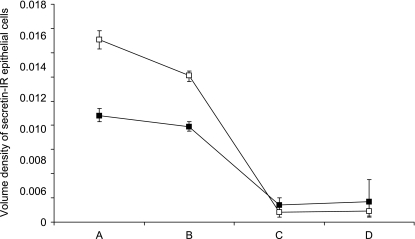
The volume density of secretin-IR epithelial cells did not differ significantly between the fetal and neonatal piglets (P = 0.3) (Fig. 7). In contrast, the volume occupied by secretin-IR epithelial cells increased tremendously in the jejunum (P = 0.009) and ileum (P = 0.008) (Table 2). Irrespective of age, regional differences were present. Post-hoc comparisons showed a significantly higher volume density of secretin-IR epithelial cells in both duodenal regions when compared with the jejunum and ileum (P < 0.0001) (Fig. 7).
Fig. 7.
Volume density of secretin-IR epithelial cells according to small intestinal region (A: duodenum descendens; B: duodenum ascendens; C: jejunum; D: ileum) in fetal (white box) and neonatal (black box) piglets (mean and 68% confidence interval).
Discussion
The various layers of the intestinal wall showed a morphology that was similar between the age groups. Nevertheless, the quantitative descriptions of the layers significantly differed between age groups and small intestinal regions. The volume densities of the tunica mucosa increased and the volume density of both the tela submucosa and the tunica muscularis decreased after birth. These changes agree with earlier observations of small intestinal morphology in the developing pig (Van Ginneken et al. 2001a); they found that the volume per serosal surface area of the tunica mucosa increased after birth. Thus, the differing volume density of the tunica mucosa in the perinatal pig relates to age- and birth-related changes. Birth coincides with major dietary changes. Sows’ milk contains various growth factors, such as epidermal growth factor and insulin-like growth factor-1, that have proven to influence cell proliferation and maturation in the pig small intestine (Cera et al. 1987; Jaeger et al. 1990; Tan et al. 1990; Odle et al. 1996; Houle et al. 1997; Burrin & Stoll, 2002). In addition, Grey & Morin (1990) and Grey et al. (1991) have found that luminal nutrition triggers the production of a growth-stimulating factor in the rat proximal small intestine. This suggests that dietary factors might, in addition to a direct effect, also indirectly modulate the proliferative capacity of the small intestine. Van Ginneken et al. (2001a) reported that increases of the volumes per serosal surface area of the tela submucosa and tunica muscularis only became apparent after weaning, indicating that changes of those layers are rather linked to changes with age and weaning than to birth-related events. Thus the reduced volume density of the tela submucosa and tunica muscularis observed in this study (after birth) is secondary to the increased volume density of the tunica mucosa.
The regionally differing volume densities of the intestinal layers match the general qualitative descriptions of the histology of the small intestine of the pig and relate to region-specific functions. The high, slender jejunal villi explain the high volume density of the tunica mucosa in the jejunum. Similarly, the duodenal glands cause the tela submucosa to occupy more volume in the duodenal segments than in other small intestinal regions. The tunica muscularis was thickest in the ileum. This thick muscle layer provides the ileum with the means to overcome the high resistance it experiences when it transfers the small intestinal content into the large intestine.
In conclusion, various layers of the intestinal wall significantly change after birth and/or display regional differences in the pig. These differences are temporally and, most probably, functionally related to dietary changes and to the maturation of gastrointestinal functioning.
Development-related changes of several gastrointestinal hormones have already been described in various mammals (Yanaihara et al. 1977; Alumets et al. 1983; Facer et al. 1989; Krause et al. 1989; Zabel et al. 1995; El-Salhy & Sandström, 1999; Lucini et al. 1999; Sandström & El-Salhy, 1999; Van Ginneken et al. 2001b, 2002). However, no stereological methods were used to obtain an unbiased estimate of the presence of GIP and secretin in the developing small intestine of the pig.
In the small intestine, open-typed GIP-IR epithelial cells were located along the entire length of the villi and, but to a lower extent, in the crypts. Apparently, this contradicts with earlier reports that found GIP-IR cells to predominate in the crypts (Alumets et al. 1983; Portela-Gomes et al. 1997; Sandström & El-Salhy, 1999). However, Alumets et al. (1983) pointed out that GIP-IR cells predominated in the crypts of the pig small intestine only after birth. Notwithstanding that GIP-IR mucosal cells were seen throughout the small intestine, statistical analysis revealed a high volume density of GIP-IR cells in the jejunum of the neonatal piglet. This accords with other reports describing the highest density in the jejunum of postnatal pigs (Mortensen et al. 2000, 2003). In this study as well as in Mortensen et al. (2003) the volume density and percentage of endocrine cells staining positive for GIP, respectively, were 4–5 times as high in the jejunum compared with in the proximal duodenal and ileal small intestinal segments. A similar distribution is encountered in rat (Aiken et al. 1994). By contrast, in human small intestine GIP displays the highest concentration in the duodenum and thereafter craniocaudally decreases (Sundler & Hakanson, 1984).
In addition, the present study confirmed the co-localization of GIP with GLP-1 in a proportion of GLP-1-IR epithelial cells in the distal half of the small intestine of the pig, which was first addressed by Mortensen et al. (2003). The presence of both GIP-IR and GLP-1-IR in the duodenum was too low to evaluate co-localization unambiguously.
The volume occupied by GIP-IR epithelial cells increased approximately seven-fold in the jejunum after birth. In rat intestine, GIP mRNA levels were found to increase markedly during the immediate postnatal period, suggesting a role for GIP in early postnatal developmental processes related to suckling (Higashimoto & Liddle, 1994). Knapper et al. (1995) have suggested that milk ingestion is capable of up-regulating GIP levels in suckling piglets.
In the duodenum, open-typed secretin-IR epithelial cells were present along the entire length of the villi. In the jejunum and ileum, secretin-IR epithelial cells were less frequently observed. These observations confirm earlier descriptions of secretin-IR mucosal cells in other mammals (Aiken & Roth, 1992; Portela-Gomes et al. 1997; Sandström & El-Salhy, 1999) including the pig (Larsson et al. 1977; Yanaihara et al. 1977; Alumets et al. 1983). Notwithstanding the fact that the highest functional impact of secretin resides in the duodenum, the volume occupied by secretin-IR epithelial cells increased tremendously after birth in both the jejunum (20-fold) and the ileum (70-fold). These increases cannot entirely be attributed to increased volumes of the tunica mucosa of the jejunal (seven-fold) and ileal (eight-fold) region. Thus the number of secretin-IR epithelial cells is increased in the small intestine. This increase is temporally related to the development of a mature gastric acid secretion during the first week after birth (Xu & Cranwell, 1990; Sangild et al. 1992).
In conclusion, the volume densities of GIP-IR and secretin-IR epithelial cells agree with earlier reports of their tissue distribution and content in pig small intestine (Larsson et al. 1977; Alumets et al. 1983; Krarup & Holst, 1984; Mortensen et al. 2003). Furthermore, immunohistochemistry on tissue sections in combination with stereological sampling and estimation yields reliable and useful quantitative data concerning the presence of the immunohistochemical target in addition to a description of its qualitative tissue distribution.
Acknowledgments
The technical assistance of Kathy Huybrechts and Walter Willems is gratefully acknowledged.
References
- Aiken KD, Roth KA. Temporal differentiation and migration of substance P, serotonin and secretin immunoreactive enteroendocrine cells in the mouse proximal small intestine. Dev. Dyn. 1992;194:303–310. doi: 10.1002/aja.1001940406. [DOI] [PubMed] [Google Scholar]
- Aiken KD, Yu W, Wright JR, Roth KA. Adaptation of enteroendocrine cells in response to jejunal–ileal transposition in the rat. Gastroenterology. 1994;106:1576–1583. doi: 10.1016/0016-5085(94)90413-8. [DOI] [PubMed] [Google Scholar]
- Alumets J, Hakanson R, Sundler F. Ontogeny of endocrine cells in porcine gut and pancreas. Gastroenterology. 1983;85:1359–1372. [PubMed] [Google Scholar]
- Argenzio RA. The pig as a model for studying the pathobiology of intestinal transport in infectious enteric disease. In: Tumbleson ME, Schook LB, editors. Advances in Swine in Biomedical Research. New York: Plenum Press; 1996. pp. 45–58. [Google Scholar]
- Burrin DG, Stoll B. Key nutrients and growth factors for the neonatal gastrointestinal tract. Clin. Perinatol. 2002;29:65–96. doi: 10.1016/s0095-5108(03)00065-4. [DOI] [PubMed] [Google Scholar]
- Cavalieri B. Geometria Indivisibilibus Continuorum. Typis Clemetis Feronij, Bononi. Torinese: Unione Tipgrafico-Editrice; 1635. [Google Scholar]
- Cera K, Mahan DC, Simmen FA. In vitro growth-promoting activity of porcine mammary secretions: initial characterization and relationship to known peptide growth factors. J. Anim. Sci. 1987;65:1149–1159. doi: 10.2527/jas1987.6541149x. [DOI] [PubMed] [Google Scholar]
- Chey WY, Chang T-M. Secretin. In: Schultz SG, Makhlouf GM, Rauner BB, editors. The Gastrointestinal System. Bethesda, MD: American Physiological Society; 1989. pp. 359–402. [Google Scholar]
- Dockray GJ. Comparative neuroendocrinology of gut peptides. In: Schultz SG, Makhlouf GM, Rauner BB, editors. The Gastrointestinal System. Bethesda, MD: American Physiological Society; 1989. pp. 133–170. [Google Scholar]
- El-Salhy M, Sandström O. How age changes the content of neuroendocrine peptides in the murine gastrointestinal tract. Gerontology. 1999;45:17–22. doi: 10.1159/000022050. [DOI] [PubMed] [Google Scholar]
- Evans HE, Sack WO. Prenatal development of domestic and laboratory animals: growth curves, external features and selected references. Anat. Histol. 1973;2:11–45. doi: 10.1111/j.1439-0264.1973.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Facer P, Bishop AA, Cole GA, et al. Developmental profile of chromogranin, hormonal peptides, and 5-hydroxytryptamine in gastrointestinal endocrine cells. Gastroenterology. 1989;97:48–57. doi: 10.1016/0016-5085(89)91414-5. [DOI] [PubMed] [Google Scholar]
- Grey VL, Morin CL. A growth-stimulating activity derived from the proximal small intestine is associated with an adaptive response. Can. J. Physiol. Pharmacol. 1990;68:645–649. doi: 10.1139/y90-095. [DOI] [PubMed] [Google Scholar]
- Grey VL, Seidman EG, Pham TN, Poullain MG, Morin CL. Detection of growth-stimulating activity in the proximal small intestine during weaning in the suckling rat. Biol. Neonate. 1991;59:37–45. doi: 10.1159/000243320. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Higashimoto Y, Liddle RA. Developmental expression of the glucose-dependent insulinotropic polypeptide gene in rat intestine. Biochem. Biophys. Res. Commun. 1994;201:964–972. doi: 10.1006/bbrc.1994.1796. [DOI] [PubMed] [Google Scholar]
- Houle VM, Schroeder EA, Odle J, Donovan SM. Small intestinal disaccharidase activity and ileal villus height are increased in piglets consuming formula containing recombinant human insulin-like growth factor-1. Pediatric Res. 1997;42:78–86. doi: 10.1203/00006450-199707000-00013. [DOI] [PubMed] [Google Scholar]
- Jaeger LA, Lamar CH, Cline TR, Cardona CJ. Effect of orally administered epidermal growth factor on the jejunal mucosa of weaned pigs. Am. J. Vet. Res. 1990;51:471–474. [PubMed] [Google Scholar]
- Knapper JM, Morgan LM, Fletcher JM, Marks V. Plasma and intestinal concentrations of GIP and GLP-1 (7–36) amide during suckling and after weaning in pigs. Horm. Metab. Res. 1995;27:485–490. doi: 10.1055/s-2007-980008. [DOI] [PubMed] [Google Scholar]
- Krarup T, Holst JJ. The heterogeneity of gastric inhibitory polypeptide in porcine and human gastrointestinal mucosa evaluated with five different antisera. Regul. Pept. 1984;9:35–46. doi: 10.1016/0167-0115(84)90005-3. [DOI] [PubMed] [Google Scholar]
- Krarup T, Groop P-H. Physiology and pathophysiology of GIP: a review. Scand. J. Clin. Lab. Invest. 1991;51:571–579. doi: 10.1080/00365519109104567. [DOI] [PubMed] [Google Scholar]
- Krause WJ, Yamada J, Cutts JH. Enteroendocrine cells in the developing opossum small intestine and colon. J. Anat. 1989;162:83–96. [PMC free article] [PubMed] [Google Scholar]
- Larsson LI, Sundler F, Alumets J, Hakanson R, Schaffalitzky de Muckadell OB, Fahrenkrug J. Distribution, ontogeny and ultrastructure of the mammalian secretin cell. Cell Tiss. Res. 1977;181:361–368. doi: 10.1007/BF00223111. [DOI] [PubMed] [Google Scholar]
- Lucini C, De Girolamo P, Coppola L, Piano G, Castalado L. Postnatal development of intestinal endocrine cell populations in the water buffalo. J. Anat. 1999;195:439–446. doi: 10.1046/j.1469-7580.1999.19530439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell V, Shulkes A, Brown JC, Solomon TE, Walsh JH. Effect of gastric inhibitory polypeptide on pentagastrin-stimulated acid secretion in man. Dig. Dis. Sci. 1980;24:113–116. doi: 10.1007/BF01308308. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Nauck MA, Schmidt WE, Gallwitz B. Gastric inhibitory polypeptide: the neglected incretin revisited. Regul. Pept. 2002;107:1–13. doi: 10.1016/s0167-0115(02)00039-3. [DOI] [PubMed] [Google Scholar]
- Morgan LM. The metabolic role of GIP: physiology and pathology. Biochem. Soc. Trans. 1996;24:585–591. doi: 10.1042/bst0240585. [DOI] [PubMed] [Google Scholar]
- Mortensen K, Petersen L, Orskov C. Colocalization of GLP-1 and GIP in the human and porcine small intestine. Ann. Ny Acad. Sci. 2000;921:469–472. doi: 10.1111/j.1749-6632.2000.tb07017.x. [DOI] [PubMed] [Google Scholar]
- Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul. Pept. 2003;114:189–196. doi: 10.1016/s0167-0115(03)00125-3. [DOI] [PubMed] [Google Scholar]
- Odle J, Zijlstra RT, Donovan SM. Intestinal effects of milkborn growth factors in neonates of agricultural importance. J. Anim. Sci. 1996;74:2509–2522. doi: 10.2527/1996.74102509x. [DOI] [PubMed] [Google Scholar]
- Pederson RA, Brown JC. Inhibition of histamine-, pentagastrin-, and insulin-stimulated canine gastric secretion by pure ‘gastric inhibitory polypeptide’. Gastroenterology. 1972;62:393–400. [PubMed] [Google Scholar]
- Portela-Gomes GM, Stridsberg M, Johansson H, Grimelius L. Complex co-localization of chromogranins and neurohormones in the human gastrointestinal tract. J. Histochem. Cytochem. 1997;45:815–822. doi: 10.1177/002215549704500606. [DOI] [PubMed] [Google Scholar]
- Sandström O, El-Salhy M. Ageing and endocrine cells of human duodenum. Mech. Ageing Dev. 1999;108:39–48. doi: 10.1016/s0047-6374(98)00154-7. [DOI] [PubMed] [Google Scholar]
- Sangild PT, Cranwell PD, Hilsted L. Ontogeny of gastric function in the pig: acid secretion and the synthesis and secretion of gastrin. Biol. Neonate. 1992;62:363–372. doi: 10.1159/000243892. [DOI] [PubMed] [Google Scholar]
- Sangild PT, Hilsted L, Nexø E, Fowden AL, Silver M. Secretion of acid, gastrin and cobalaminbinding proteins by the fetal pig stomach: developmental regulation by cortisol. Exp. Physiol. 1994;79:135–146. doi: 10.1113/expphysiol.1994.sp003747. [DOI] [PubMed] [Google Scholar]
- Sundler F, Hakanson R. Gastro-Entero-Pancreatic endocrine cells in higher mammals, with special reference to their ontogeny in the pig. In: Falmer S, Hakanson R, Sundler F, editors. Evolution and Tumour Pathology of the Neuroendocrine System. New York: Elsevier Science Publishers; 1984. pp. 111–135. [Google Scholar]
- Tan TJ, Schober DA, Simmen FA. Fibroblast mitogens in swine milk include an epidermal growth factor-related peptide. Regul. Pept. 1990;27:61–74. doi: 10.1016/0167-0115(90)90205-b. [DOI] [PubMed] [Google Scholar]
- Van Ginneken C, Van Meir F, Sys S, Weyns A. Stereologic description of the small intestine of the pig during normal development. Dig. Dis. Sci. 2001a;47:868–878. doi: 10.1023/a:1014768806773. [DOI] [PubMed] [Google Scholar]
- Van Ginneken C, Weyns A, Van Meir F. Stereologic evalution of the pig gastric wall of somatinergic and serotoninergic immunoreactive mucosal cells during perinatal development. Eur. J. Morph. 2001b;37:113–120. doi: 10.1076/ejom.39.2.113.7368. [DOI] [PubMed] [Google Scholar]
- Van Ginneken C, Verlinden K, Van Meir F, Sys S, Weyns A. A stereologic evaluation of glucagon-like peptide-1 (GLP-1) mucosal cells in the small intestine of the developing pig. Anat. Embryol. 2002;205:153–157. doi: 10.1007/s00429-002-0235-z. [DOI] [PubMed] [Google Scholar]
- Xu RJ, Cranwell PD. Development of gastric acid secretion in pigs from birth to thirty six days of age: the response to pentagastrin. J. Dev. Physiol. 1990;13:315–326. [PubMed] [Google Scholar]
- Yanaihara N, Sakagami M, Sato H, et al. Immunological aspects of secretin, subtance P and VIP. Gastroenterology. 1977;72:803–810. [PubMed] [Google Scholar]
- Zabel M, Surdyk-Zasada J, Lesisz I, et al. Immunocytochemical studies on endocrine cells of alimentary tract of the pig in the embryonic and fetal period of life. Folia Morph. (Warsz). 1995;54:69–90. [PubMed] [Google Scholar]
- Zar JH. The Arcsine transformation. In: Zar JH, editor. Biostatistical Analysis. New York: Prentice Hall International Editions; 1984. pp. 239–241. [Google Scholar]