Abstract
ABCG2/BCRP is a member of the ATP-binding cassette (ABC) transporter family and is expressed in intestine, kidney and liver where it modulates the absorption and excretion of xenobiotic compounds. ABCG2 is also expressed in hematopoietic stem cells and erythroid cells; however, little is known regarding its role in hematopoiesis. Abcg2 null mice have increased levels of protoporphyrin IX (PPIX) in erythroid cells; yet, the mechanism for this remains uncertain. We have found that Abcg2 mRNA expression was upregulated in differentiating erythroid cells, coinciding with increased expression of other erythroid specific genes. This expression pattern was associated with significant amounts of ABCG2 protein on the membrane of mature peripheral blood erythrocytes. Erythroid cells engineered to express ABCG2 had significantly lower intracellular levels of PPIX, suggesting the modulation of PPIX level by ABCG2. This modulating activity was abrogated by treatment with a specific ABCG2 inhibitor, Ko143, implying that PPIX may be a direct substrate for the transporter. Taken together, our results demonstrate that ABCG2 plays a role in regulating PPIX levels during erythroid differentiation and suggest a potential role for ABCG2 as a genetic determinant in erythropoietic protoporphyria.
Introduction
ABCG2, also known as BCRP/MXR/ABCP, is a member of the ATP-binding cassette (ATP) transporter superfamily. Like MDR1, a well-studied member of this family, ABCG2 is highly expressed in hepatic canalicular membranes, renal proximal tubules, and apical membranes of intestinal epithelium.1–4 Overexpression of ABCG2 in cell lines confers resistance to a variety of chemotherapeutic drugs,5–8 suggesting a role for ABCG2 expression in cancer cells as a mechanism of resistance to chemotherapy. We and others have shown expression of ABCG2 mRNA in hematopoietic stem cells (HSCs) and Ter119 positive erythrocytes;9;10 however, the function of ABCG2 in hematopoietic cells remains undefined.
Abcg2 null mouse models have been generated with no abnormalities in hematopoietic development observed.3;11 Abcg2 expression was required for the Side Population (SP) phenotype of HSCs and for protecting HSCs against mitoxantrone toxicity,9;11–13 suggesting a potential role for ABCG2 as a HSC marker and as a mechanism for protecting HSCs against naturally occurring toxins. Jonker et al found that Abcg2−/− mice had an elevated protoporphyrin IX (PPIX) level in red blood cells,3 a phenotype similar to the erythropoietic protoporphyria (EPP) caused by deficiency of ferrochelatase activity, but without clinical manifestations such as photosensitivity. The mechanism and significance for this accumulation of PPIX are unknown, nor has the expression pattern of ABCG2 during erythroid development been defined. In this study, we have examined expression of ABCG2 during erythroid maturation, and directly studied whether ABCG2 expression can decrease PPIX levels in several cellular systems. These results suggest a direct role of ABCG2 transporter in PPIX metabolism.
Materials and methods
Mice and cell lines
Abcg2−/− mice were generated in our lab and are on 129/C57BL6 mixed genetic background.11 Murine erythroleukemic MEL cells and human erythroleukemic K562 cells were cultured in DMEM medium containing 10% fetal bovine serum. K562 cells overexpressing ABCG2 (K562/ABCG2) were generated by transducing the cells with the HaBCRP retroviral vector pseudotyped with VSV-G envelope and subsequent sorting after staining with anti-ABCG2 antibody 5D3 (eBioscience, San Diego, CA),14 which recognizes an extracellular epitope of ABCG2, using fluorescent activated cell sorter. No drug selection was applied.
Staining of red blood cells with antibodies for flow cytometry
Peripheral blood samples were collected in heparinized tubes from healthy human donors after informed consent, from a 3 year-old rhesus macaque, and from 14-week old Abcg2−/− mice. For murine and rhesus monkey samples, 5ul red blood cells were washed with ice cold phosphate buffered saline (PBS) and fixed/permeabilized with cold acetone for 2 min on ice. Cells were then washed twice with ice cold PBS and labeled with 10ul anti-mouse Abcg2 antibody Bxp-53 (Monosan, The Netherlands) or 10ul of the anti-human ABCG2 antibody Bxp-21 (Kamiya biochemical company, Seattle, WA), which cross reacts with rhesus macaque ABCG2, for 20 min at room temperature. After washing, cells were incubated with 5ul Fluorescein Isothiocyanate (FITC) conjugated anti-Rat Ig’s (Camarillo, CA) or Phycoerythrin (PE) conjugated anti-mouse Ig’s (DAKO, Denmark), washed and analyzed in flow cytometry. 1ul human red blood cells were labeled with 1ug 5D3 for 20 min at RT, washed, and incubated with PE conjugated anti-mouse Ig’s. After washing, cells were analyzed in flow cytometry.
Induction of MEL cells
Murine leukemic cell line MEL was incubated with 2% DMSO for 4 days. RNA was extracted and analyzed by Northern blot using a full length mouse Abcg2 cDNA probe cloned from mouse kidney RNA by RT-PCR. A portion of cells were fixed/permeabilized with acetone and stained with Bxp-53 for protein expression analysis, or incubated with 2.5ug/ml Hoechst 33342 for 60min, and analyzed in a flow cytometry for Abcg2 function (LSR, Becton Dickinson).
PPIX Fluorescence assay
For PPIX efflux assays, cells were incubated with 10uM PPIX (SIGMA) in DMEM/10% FBS for 30min at 37 °C, washed once with medium and incubated at 37 °C for one hour. Ko143 (kindly provided by Balázs Sarkadi ) or 2-deoxyglucose, if used, was present during the entire procedure. Cells were spun down, resuspended into medium and analyzed in a flow cytometry (LSR, Becton Dickinson) for PPIX fluorescence using a 695/40nm filter after excitation by a 405nm UV light. ATP depletion was achieved by preincubating the cells in medium containing 50mM 2-deoxyglucose and 15mM sodium azide for 20min. Ko143 was used at 1uM. For endogenous PPIX efflux assays, cells were treated with 1mM δ-aminolevulinic acid (ALA) (SIGMA) for 21 hours, washed once with PBS and directly analyzed in flow cytometry for PPIX fluorescence.
HPLC measurement of PPIX
K562 or K562/ABCG2 cells were treated with 1mM δ-aminolevulinic acid (ALA) (SIGMA) at 5×105 cells/ml for 21 hours. The number of cells increased during the treatment, but no difference was seen between the two cell lines. Cells were washed once with PBS, and an aliquot was analyzed in flow cytometry using the same setting as for PPIX fluorescence analysis. 4×106 cells were pelleted, resuspended into 50ul DMEM medium. 20ul DMSO was added into the cells and vortexed vigorously for 5min. 200ul 60% methanol/40% 10mM potassium phosphate monobasic solution was then added and vortexed for 5min. The samples were spun down at 14,000g for 15min. 20ul supernatant was injected into HPLC system to quantitate PPIX. Separation was achieved by a Shimadzu Shim-Pack CLC-ODS 4.6 mm × 150 mm column using a gradient program. Mobile phase A was 10 mM potassium phosphate buffer, pH 4.6 and solution B was methanol. Detection was with a fluorescence detector with the absorbance set at 400 nm and emission at 620 nm. This reverse phase HPLC method is capable of separating seven porphyrin compounds including uroporphyrin, hexaporphyrin, heptaporphyrin, pentaporphyrin, coproporphyrin, mesoporphyrin, and protoporphyrin IX (PPIX) (data not shown). A PPIX standard curve over a concentration range of 0.1 μg/ml to 5 μg/ml was constructed for quantitation of PPIX in samples.
K562 or K562/ABCG2 cells were also treated with 1mM δ-aminolevulinic acid (ALA) (SIGMA) at 3×106 cells/ml for 7 hours. Cells were spun down, 20 μl of supernatants were directly injected into HPLC to quantitate the PPIX in the medium. Cell pellets were washed once with PBS, and 5 × 106 cells were pelleted and processed as above to quantitate PPIX of the cell pellet. Separation was achieved by a Shimadzu Shim-Pack CLC-ODS 4.6 mm × 150 mm column using an isocratic elution at 1.0 ml/min. Mobile phase was 90% methanol and 10% 10mM potassium phosphate buffer, pH 4.6. Two standard curves were constructed for quantification of the PPIX, one for the supernatant, which was not extracted but injected directly into the HPLC system (range 0.25–20.0 μg/mL), and another for the cell pellets, which were extracted using DMSO (range 0.1–5.0 μg/mL).
Microarray analysis of RNA expression
Day 16 fetal liver cells were obtained from homozygous Abcg2−/− mouse embryos or wild type mouse embryos. More than 87% of nucleated cells at this stage express the erythroid cell marker Ter119 when analyzed by flow cytometry. Friend virus-infected splenic erythroblasts were isolated as previously described,15 and cultured with erythropoietin for 48 hours. RNA was extracted, labeled and hybridized on Affymetrix Moe430A chip. The data were analyzed with MAS version 5.0 software (Affymetrix). A complete analysis of the microarray data sets will be published elsewhere.
Results
ABCG2 expression is upregulated during erythroid differentiation
In an earlier study, we found that Abcg2 mRNA was expressed in Ter119+ erythroid cells from murine bone marrow.9 Ter119 expression identifies erythroid cells in developmental stages spanning proerythroblast to mature red blood cells.16 To more precisely study the expression pattern of Abcg2 during erythroid differentiation, we treated murine erythroleukemia (MEL) cells with dimethyl sulfoxide (DMSO) for 4 days to induce erythroid differentiation. At the end of induction, more than 45 % of cells were hemoglobinized when analyzed by staining with benzidine (data not shown). We then evaluated Abcg2 mRNA levels using Northern Blot and protein levels using flow cytometry after staining with the anti-Abcg2 antibody Bxp-53. We found that Abcg2 mRNA was present at a relatively low level in non-induced cells, and significantly increased after induction with DMSO (4.6 fold, Fig 1A). This correlated with a significant increase in Abcg2 protein expression (Fig 1B). The Abcg2 protein expressed in induced MEL cells was functional as evidenced by the ability to efflux Hoechst 33342 dye, which is a known substrate for ABCG2 (Fig 1C).
Fig 1. Expression of Abcg2 in MEL cells before and after induction with DMSO, and in primary erythroblasts during differentiation.
(A) Non-induced MEL cells and MEL cells induced with 2% DMSO for 4 days were analyzed for Abcg2 mRNA by Northern blot, GAPDH probe served as loading control. (B) Flow cytometry analysis of MEL cells after staining with anti-Abcg2 antibody Bxp-53. (C) Hoechst 33342 fluorescence in cells analyzed by flow cytometry. Shaded area in B and C: non-induced MEL cells. Solid line in B and C: MEL cells induced with DMSO. (D) Murine splenic proerythroblast were cultured for differentiation, samples were taken at different time points and measured for Abcg2 mRNA using microarray.
We next examined the expression of Abcg2 mRNA during differentiation in primary erythroblasts. Murine erythroblasts infected with the anemia-inducing strain of Friend virus provide a model of erythroid differentiation. In the presence of erythropoietin, these cells differentiate from proerythroblasts to orthochromatic erythroblast over 48 hours. Using microarray expression analysis, we determined that Abcg2 mRNA was present at a low level initially, consistent with its low expression in MEL cells. Starting at four hours of culture, Abcg2 mRNA increased, and reached a peak level representing a 10 fold increase at the polychromatic erythroblast stage between 30–42 hours (Fig 1D), and then decreased at the orthochromatic erythroblast stage, when enzymes for heme synthesis, such as ALAS2 and ferrochelatase, reached their peak level (data not shown). These experiments demonstrate that Abcg2 expression increases during erythroid maturation in 2 different murine erythroid cell systems.
ABCG2 protein is expressed on mature red blood cells from mice, rhesus macaques and humans
We next tested whether ABCG2 protein was present on the membrane of mature peripheral red blood cells. We collected peripheral blood from mice, rhesus macaques and humans, and stained the cells with anti-ABCG2 monoclonal antibodies appropriate for each species. We used red blood cells from Abcg2−/− mice as negative controls for murine cells, and isotype antibody labeled cells as controls for rhesus and human samples. We found that ABCG2 was readily detected on mature red blood cells from all three species (Fig 2). These results indicate that expression of ABCG2 during erythroid maturation results in significant amounts of ABCG2 protein on the membrane of mature red blood cells.
Fig 2. Expression of ABCG2 protein on peripheral mature red blood cells.
Peripheral blood from mice, rhesus macaques and humans are collected, stained with primary antibodies recognizing respective ABCG2, and analyzed by flow cytometry. (A) red blood cells from Abcg2−/− mice (shaded area) or wild type mice (solid line) stained with anti-Abcg2 antibody Bxp-53. (B) rhesus macaque red blood cells stained with either isotype control antibody (shaded area) or anti-ABCG2 antibody Bxp-21 (solid line). (C) Human red blood cells stained with either isotype control antibody (shaded area) or anti-ABCG2 antibody 5D3 (solid line).
ABCG2 expression decreases exogenous PPIX accumulation in K562 cells
The accumulation of PPIX in red blood cells of Abcg2−/− mice and the marked upregulation of ABCG2 during erythroid maturation suggests that ABCG2 may function to decrease PPIX cellular levels, perhaps through a direct efflux mechanism. To test this hypothesis, we transduced K562 cells with our HaBCRP retroviral expression vector,9 and isolated a polyclonal pool of transduced cells (K562/ABCG2 cells). The expression of ABCG2 in the transduced cells was confirmed by staining with anti-ABCG2 antibody 5D314 (data not shown) and by assaying for Hoechst 33342 efflux activity (Fig 3A). This efflux activity was blocked by treatment with a specific ABCG2 functional inhibitor Ko14317 (Fig 3B).
Fig 3. Efflux of exogenous PPIX by ABCG2.
K562 cells or K562 cells engineered to overexpress ABCG2 (K562/ABCG2) are incubated with Hoechst 33342 (A, B) or PPIX (C, D). Cell were also coincubated with the ABCG2 inhibitor Ko143 (B, D). Shaded area: K562 cells. Solid line: K562/ABCG2 cells.
We then incubated the cells with PPIX and measured fluorescence of PPIX by flow cytometry after excitation with a 405nm UV light. The ABCG2 overexpressing cells (K562/ABCG2) had significantly lower PPIX fluorescence than parental K562 cells, consistent with PPIX efflux activity conferred by ABCG2 (Fig 3C). This activity was abrogated by treatment with Ko143 (Fig 3D). These results show that PPIX levels are decreased by expression of ABCG2.
ABCG2 decreases the levels of endogenously produced PPIX in erythroid cells
Although ABCG2 can reduce the accumulation of exogenous PPIX, it is unknown whether endogenous PPIX can be effluxed by ABCG2. PPIX is synthesized by protoporphyrinogen oxidase which is localized in the inner mitochondrial membrane with its active side oriented toward the intermembrane space.18 To determine whether endogenously synthesized PPIX could be effluxed by ABCG2, we treated K562 cells with δ-aminolevulinic acid (ALA),19;20 the second intermediate in the heme biosynthetic pathway, for 21 hours to increase the endogenous PPIX level. After this treatment, there was a 3 log increase of porphyrin fluorescence by flow cytometry (Fig 4A), indicating substantial induction of endogenous porphyrin synthesis. ALA treatment also increased the fluorescence in K562/ABCG2 cells, but to a significantly lesser extent than in parental K562 cells (Fig 4A). This activity on porphyrin fluorescence was specific for ABCG2 because when Ko143 was added, the porphyrin fluorescence of K562/ABCG2 cells became indistinguishable from that of parental K562 cells (Fig 4A). These results show that endogenous porphyrin levels can also be decreased by ABCG2 expression.
Fig 4. Efflux of endogenous PPIX by ABCG2.
(A) K562 cells or K562/ABCG2 cells were incubated with 1mM ALA for 21 hours to induce endogenous PPIX, with or without 1uM Ko143, and analyzed for PPIX fluorescence. K562+ALA (Blue line); K562/ABCG2+ALA (Red line); K562/ABCG2+ALA+Ko143 (Brown line); Non-treated K562 cells (Green line); Non-treated K562/ABCG2 cells (Black line). (B) MEL cells were incubated with 1mM ALA for 21 hours with or without 1uM Ko143 and analyzed in flow cytometry for PPIX fluorescence. MEL+ALA (Red line); MEL+ALA+Ko143 (Blue line); Non-treated MEL cells (Black line). (C) K562 or K562/ABCG2 cells were incubated with 1mM ALA for 21 hours and pelleted. The amount of PPIX in pelleted cells was measured in a HPLC assay. (D) An aliquot of cells was also analyzed in a flow cytometry for fluorescence. PPIX was undetectable in non-treated K562 cells as shown in the bottom panels.
Similar experiments were conducted using MEL cells, which express low level of Abcg2 even in the uninduced state (Fig 1A). As shown in Fig 4B, uninduced MEL cells treated with ALA showed an increase in PPIX fluorescence, consistent with an induction in endogenous PPIX synthesis with ALA. Treatment of ALA-induced MEL cells with Ko143 increased PPIX fluorescence even further, compared with cells that were treated with ALA alone. This shows that inhibition of endogenously expressed ABCG2 leads to accumulation of endogenously produced PPIX in erythroid cells.
To confirm that the fluorescence in ALA treated cells comes from PPIX, but not from other porphyrins of heme pathway, we directly measured PPIX level in these cells 21 hours after ALA treatment by HPLC. We found that PPIX was the only porphyrin detectable after ALA treatment. The amount of PPIX was 308.9±20.6ng per 1×106 K562 cells (n=3) and 28.7±1.8ng per 1×106 K562/ABCG2 cells, a 10.8 fold difference, which parallels the difference of fluorescence intensity measured by flow cytometry in these cells (759.9±8.9 vs 55.7±1.7, a 13.7 fold difference, n=3). A representative HPLC assay is shown in Fig 4C and Flow cytometry assay in Fig 4D. This result demonstrates that endogenous PPIX can be decreased by ABCG2 expression, and that fluorescence detection was a reliable measurement for relative PPIX level in these cells.
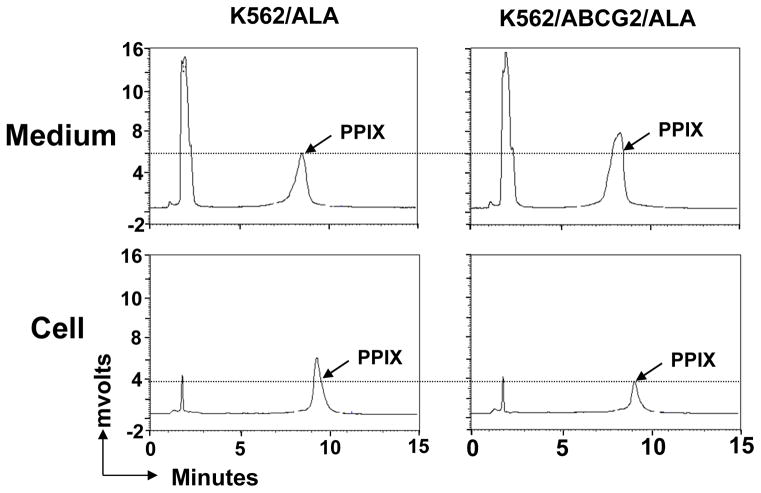
To confirm that ABCG2 can efflux PPIX into medium, we directly measured PPIX levels in both the medium and within cells 7 hours after ALA treatment by a sensitive and specific chromatographic method (Fig 5). We found that the amount of PPIX in the medium from K562/ABCG2 cell cultures was significantly higher compared to that of K562 cells (2220±160ng vs 1420±230ng per 1×106 cells, n=3, P=0.00058). In contrast, the amount of PPIX in the cell pellet from K562/ABCG2 cells was significantly lower than that of K562 cells (54±6ng vs 107±7 ng per 1×106 cells, n=3, P=0.0075). This result demonstrates that endogenous PPIX can be directly effluxed by ABCG2.
Fig 5. ABCG2 effluxes endogenous PPIX into medium.
K562 or K562/ABCG2 cells were incubated with 1mM ALA for 7 hours and the PPIX levels in both medium and within cells were measured by HPLC. The peak of PPIX is indicated in each panel. The peak on the left represents a component in the culture medium (not produced by cells) and serves as an internal control.
Mature red blood cells can efflux PPIX based on plasma membrane expression of ABCG2
In order to determine if endogenously expressed ABCG2 in mature peripheral red blood cells could decrease intracellular PPIX levels, we incubated murine peripheral red blood cells with PPIX, with or without the Abcg2 inhibitor Ko143. The PPIX fluorescence was significantly higher in the presence of Ko143 (Fig 6A), showing that the ABCG2 function on the plasma membrane was decreasing PPIX levels in primary, mature erythroid cells. This result was confirmed with another experiment in which cells were treated with 2-deoxyglucose to inhibit the synthesis of ATP, which is required for all ABC transporter function. As shown in Fig 6B, PPIX fluorescence was significantly increased in murine red blood cells treated with 2-deoxyglucose. These experiments confirm that the ABCG2 protein functions in mature red blood cells to decrease intracellular PPIX levels.
Fig 6. Red blood cells efflux PPIX due to expression of ABCG2.
(A) Murine peripheral red blood cells were incubated with PPIX, with Ko143 (solid line) or without Ko143 (shaded area) and analyzed for PPIX fluorescence. (B) Murine peripheral red blood cells were incubated with (solid line) or without (shaded area) 2-deoxyglucose and sodium azide and analyzed for PPIX fluorescence.
Expression of enzymes in heme biosynthesis pathway in erythroid cells from Abcg2−/− mice
One mechanism by which loss of ABCG2 could lead to increased levels of PPIX would be a secondary alteration in the level of enzymes involved in the heme biosynthesis pathway, especially the rate limiting enzymes ALA synthase and ferrochelatase. To rule out this possibility, we used microarray analysis to examine expression of these enzymes in erythroid cells from Abcg2−/− mice. We extracted RNA from day 16 fetal liver cells, which are primarily nucleated erythroid cells, and performed microarray analysis on Affymetrix MOE430A chips. Among the 22626 transcripts represented on the chip, 44% and 41 % are expressed from Abcg2−/− and wild type samples, respectively. Among the transcripts that are expressed in either genotype, 56 showed increased expression (2.1–8 fold), 26 showed decreased expression (2.1–10.6 fold) in Abcg2 −/− samples.
Among the eight enzymes directly involved in heme biosynthesis, seven showed no significant difference between the two genotypes. There was a 1.6 fold increase of one of the two transcripts representing coproporphyrinogen oxidase in Abcg2−/− sample (Table 1), which catalyzes the conversion of coproporphyrinogen to protoporyphyrinogen IX. However, we recognize that the microarray assay only measures relative changes in mRNA levels, and cannot rule out differences in protein levels of these enzymes. This is relevant because several of these enzymes may be regulated post-transcriptionally.
Table 1.
Microarray analysis of enzymes involved in heme synthesis
| Average signal intensity
|
P value | ||
|---|---|---|---|
| Wild Type | Abcg2−/− | ||
| Heme synthesis enzymes
| |||
| ALA synthase 1 | 799 | 675 | 0.02 |
| ALA Synthase 2 | 38528 | 33733 | 0.16 |
| Aminolevulinate dehydratase | 7880 | 6602 | 0.38 |
| Porphobilinogen deaminase | 17175 | 15458 | 0.5 |
| Uroporphyrinogen III synthase | 4078 | 4416 | 0.37 |
| Uroporphyrinogen decarboxylase | 8216 | 6404 | 0.03 |
| Coproporphyrinogen oxidase | 6045 | 9388 | 0.00002* |
| Protoporphyrinogen oxidase | 5138 | 5062 | 0.13 |
| Ferrochelatase | 7277 | 7111 | 0.5 |
difference considered significant
We also compared the expression of ABC transporter members in erythroid cells from wild type and Abcg2 −/− fetal liver cells. 37 out of 51 murine ABC transporters are represented on MOE430A chip, among them, 19 are expressed in wild type cells. Abcg2, Abcb10, Abca1, Abcb4, Abcb6, Abce1, Abcc5, Abcb7 as well as Abcg1 are expressed at relatively high levels, with signal intensities higher than 1000. ABCG2 and ABCB10 are the two most highly expressed transporters, ranked as top 6% and 7% of all the transcripts that are expressed in erythroid cells, with signal intensities of 5295 and 4676, respectively. Abcb10 is a mitochondrial transporter that has been shown to be highly expressed in maturing erythroid cells.21 Except for Abcg2, which was knocked out in Abcg2−/− mice, no alteration of expression of any other ABC transporters was noted when comparing wild type and Abcg2 −/− mice.
Discussion
Considering the established role of ABC transporters in human disease,22;23 it is important to ascertain the normal physiologic role of the ABCG2 transporter. Several observations suggest an important function for ABCG2 in hematopoiesis. Abcg2 mRNA is expressed in primitive HSCs and is then down-regulated during myeloid differentiation.9 However, there are no detectable hematopoietic abnormalities in Abcg2 null mice except for an increased level of PPIX in erythrocytes3;11. Therefore, we sought to better understand the expression pattern of Abcg2 during erythropoiesis and the mechanism by which loss of Abcg2 leads to PPIX accumulation.
In this study, we found that Abcg2 expression was sharply upregulated during erythroid differentiation, and was present on the plasma membrane of mature red blood cells. In fact, Abcg2 was one of the most highly induced genes in Friend virus infected primary erythroblasts. The induction of Abcg2mRNA in polychromatic erythroid precursors parallels the active biosynthesis of heme and hemoglobin. These findings are consistent with an important function in erythroid cells, most likely being modulation of products in the heme biosynthetic pathway.
The mechanism by which ABCG2 deletion leads to PPIX accumulation has not been clear; however the most straightforward explanation is that PPIX could be a direct substrate for the transporter. We now show that K562 cells that have been engineered to express high levels of ABCG2 have significant reduction in PPIX levels after incubation with PPIX in the medium. This effect was directly blocked by Ko143, an ABCG2 transporter inhibitor. Similar reduction was also seen when either K562 or MEL cells were induced with ALA to synthesize endogenous PPIX. Direct measurement of PPIX by HPLC showed that the PPIX level was significantly higher in the medium, but lower within the cells of K562/ABCG2 cell culture, compared to that of K562 cells. Moreover, an increase in intracellular PPIX was seen in primary mature red blood cells that were incubated with exogenous PPIX and ABCG2 inhibitors. These results demonstrate that PPIX is a direct substrate for the ABCG2 transporter.
An alternate possibility would be that loss of ABCG2 was resulting in secondary alterations in the levels of enzymes in the heme biosynthetic pathway. The expression of mRNAs for majority of these enzymes in fetal liver erythroblasts, including the rate limiting enzymes ALA synthase and ferrochelatase, showed no secondary alterations. We did find a modest increase in coproporphyrinogen oxidase mRNA associated with loss of Abcg2. While we cannot exclude the possibility that this could increase PPIX levels, it seems unlikely that this alone would cause the 10 fold increase of PPIX seen in red blood cells of Abcg2−/− mice. Moreover, the hematocrit and hemoglobin levels are comparable between wild type and Abcg2−/− mice,13 suggesting normal activity of heme synthesis enzymes. Another possibility would be that ABCG2 could modulate a factor that causes a secondary accumulation of endogenous PPIX. This putative factor could degrade PPIX or inhibit ferrochelatase, however no such factors have been identified.
Accumulation of PPIX in mature red blood cells that were incubated with ABCG2 inhibitors suggests that Abcg2 acts at the level of the plasma membrane to increase PPIX extrusion. PPIX is lipid soluble and can diffuse through the plasma membrane; however our data suggest that active transport mediated by ABCG2 can further decrease intracellular PPIX levels. This mechanism may be necessary to prevent cellular toxicity under pathological conditions in which PPIX accumulates. Increases in PPIX in erythrocytes is characteristic of the human disease of EPP, which is characterized by mild to moderate photosensitivity and in some cases hepatic manifestations, but no hematologic manifestations. Most of these cases have been associated with autosomal dominant mutations in the ferrochelatase gene, which catalyzes the insertion of iron into PPIX. While the EPP like phenotype in Abcg2−/− mice lacks the photosensitivity seen in EPP mouse models or the clinical manifestations seen in humans with ferrochelatase deficiency,24–26 it is possible that ABCG2 modulates the phenotype of ferrochelatase deficiency. It is important to note that the inheritance pattern of EPP due to ferrochelatase mutations shows highly variable degrees of penetrance,27 and that a few cases of EPP with recessive inheritance have been noted.28 Human polymorphisms of the ABCG2 gene have recently been described,29 and it is possible that these may represent mutations affecting PPIX levels in the face of ferrochelatase deficiency or in autosomal recessive cases. Another possible role for ABCG2 would be the control of PPIX levels during exposure to environmental toxins such as lead toxicity, which leads to marked elevation in cellular protoporphyrin levels. Both of the possibilities can now be tested with available mouse models.
Acknowledgments
Supported by NIH grant R01 HL67366 (to B.P.S), and by the American Lebanese Syrian Associated Charities.
We thank Flow Cytometry lab and Hartwell center for sample analysis, Geoffrey Neale for assistance in analysis of microarray data, and Taihe Lu for help with the Northern blot analysis.
References
- 1.Thiebaut F, Tsuruo T, Hamada H, et al. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonker JW, Smit JW, Brinkhuis RF, et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–1656. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- 3.Jonker JW, Buitelaar M, Wagenaar E, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci USA. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 5.Doyle LA, Yang W, Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH. The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res. 1999;59:4237–4241. [PubMed] [Google Scholar]
- 7.Knutsen T, Rao VK, Ried T, et al. Amplification of 4q21-q22 and the MXR gene in independently derived mitoxantrone-resistant cell lines. Genes Chromosomes Cancer. 2000;27:110–116. [PubMed] [Google Scholar]
- 8.Robey RW, Medina-Perez WY, Nishiyama K, et al. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7:145–152. [PubMed] [Google Scholar]
- 9.Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 10.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 11.Zhou S, Morris JJ, Barnes Y, et al. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci USA. 2002;99:12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 13.Zhou S, Zong Y, Lu T, Sorrentino BP. Hematopoietic cells from mice that are deficient in both Bcrp1/Abcg2 and Mdr1a/1b develop normally but are sensitized to mitoxantrone. Biotechniques. 2003;35:1248–1252. doi: 10.2144/03356ss04. [DOI] [PubMed] [Google Scholar]
- 14.Abbott BL, Colapietro AM, Barnes Y, et al. Low levels of ABCG2 expression in adult AML blast samples. Blood. 2002;100:4594–4601. doi: 10.1182/blood-2002-01-0271. [DOI] [PubMed] [Google Scholar]
- 15.Koury MJ, Sawyer ST, Bondurant MC. Splenic erythroblasts in anemia-inducing Friend disease: a source of cells for studies of erythropoietin-mediated differentiation. J Cell Physiol. 1984;121:526–532. doi: 10.1002/jcp.1041210311. [DOI] [PubMed] [Google Scholar]
- 16.Kina T, Ikuta K, Takayama E, et al. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br J Haematol. 2000;109:280–287. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- 17.Allen JD, van Loevezijn A, Lakhai JM, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- 18.Ferreira GC, Andrew TL, Karr SW, Dailey HA. Organization of the terminal two enzymes of the heme biosynthetic pathway. Orientation of protoporphyrinogen oxidase and evidence for a membrane complex. J Biol Chem. 1988;263:3835–3839. [PubMed] [Google Scholar]
- 19.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 20.Gederaas OA, Berg K, Romslo I. A comparative study of normal and reverse phase high pressure liquid chromatography for analysis of porphyrins accumulated after 5-aminolaevulinic acid treatment of colon adenocarcinoma cells. Cancer Lett. 2000;150:205–213. doi: 10.1016/s0304-3835(99)00399-7. [DOI] [PubMed] [Google Scholar]
- 21.Shirihai OS, Gregory T, Yu C, Orkin SH, Weiss MJ. ABC-me: a novel mitochondrial transporter induced by GATA-1 during erythroid differentiation. EMBO J. 2000;19:2492–2502. doi: 10.1093/emboj/19.11.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 23.Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33:453–458. doi: 10.1023/a:1012866803188. [DOI] [PubMed] [Google Scholar]
- 24.Tutois S, Montagutelli X, Da SV, et al. Erythropoietic protoporphyria in the house mouse. A recessive inherited ferrochelatase deficiency with anemia, photosensitivity, and liver disease. J Clin Invest. 1991;88:1730–1736. doi: 10.1172/JCI115491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magness ST, Maeda N, Brenner DA. An exon 10 deletion in the mouse ferrochelatase gene has a dominant-negative effect and causes mild protoporphyria. Blood. 2002;100:1470–1477. doi: 10.1182/blood-2001-12-0283. [DOI] [PubMed] [Google Scholar]
- 26.DeLeo VA, Poh-Fitzpatrick M, Mathews-Roth M, Harber LC. Erythropoietic protoporphyria. 10 years experience. Am J Med. 1976;60:8–22. doi: 10.1016/0002-9343(76)90528-3. [DOI] [PubMed] [Google Scholar]
- 27.Went LN, Klasen EC. Genetic aspects of erythropoietic protoporphyria. Ann Hum Genet. 1984;48 ( Pt 2):105–117. doi: 10.1111/j.1469-1809.1984.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 28.Lamoril J, Boulechfar S, de Verneuil H, et al. Human erythropoietic protoporphyria: two point mutations in the ferrochelatase gene. Biochem Biophys Res Commun. 1991;181:594–599. doi: 10.1016/0006-291x(91)91231-z. [DOI] [PubMed] [Google Scholar]
- 29.Zamber CP, Lamba JK, Yasuda K, et al. Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics. 2003;13:19–28. doi: 10.1097/00008571-200301000-00004. [DOI] [PubMed] [Google Scholar]