Abstract
Carotenoids play an integral and essential role in photosynthesis and photoprotection in plants and algae. A collection of Chlamydomonas reinhardtii mutants lacking carotenoids was characterized for pigment and tocopherol (vitamin E) composition, growth phenotypes under different light conditions, and the molecular basis of their mutant phenotype. The carotenoid-less mutants, or “white” mutants, were also deficient in chlorophylls but had approximately twice the tocopherol content of the wild type. White mutants grew in the dark but were unable to survive in the light, even under very low light conditions on acetate-containing medium. Genetic crosses and recombination tests revealed that all individual white mutants in the collection are alleles of a single gene, lts1, and the white phenotype was closely linked to a marker located in the phytoene synthase gene. DNA sequencing of the phytoene synthase gene from each of the mutants revealed nonsense, missense, frameshift, and splice site mutations. Transformation with a wild-type copy of the phytoene synthase gene was able to complement the lts1-210 mutation. Together, these results show that all the white mutants examined in this work are affected in the phytoene synthase gene.
CAROTENOIDS are a diverse group of C40 tetraterpene pigments that are important in many biochemical and biophysical processes of plants and algae (Cunningham and Gantt 1998). Carotenoids are essential for the structure and function of pigment-binding protein complexes and the prevention of photooxidative damage. In the chloroplast, the majority of carotenoids are located in pigment-binding proteins embedded in the thylakoid membrane. Here, carotenoids provide structural support to their associated proteins, participate in light-harvesting processes, absorbing at 450–570 nm, and dissipate excess light energy absorbed by antenna pigments (Herrin et al. 1992; Demmig-Adams et al. 1996; Baroli and Niyogi 2000). Carotenoids associated with reaction centers and antenna complexes play a critical role in protection of the photosynthetic apparatus from photooxidative damage by quenching triplet chlorophyll and singlet oxygen (Britton 1995; Demmig-Adams et al. 1996; Frank and Cogdell 1996). In addition, carotenoids are precursors of the plant growth hormone abscisic acid (Duckham et al. 1991; Rock and Zeevaart 1991) and are the pigments responsible for the yellow, orange, and red coloration of many flowers, fruits, vegetables, and roots.
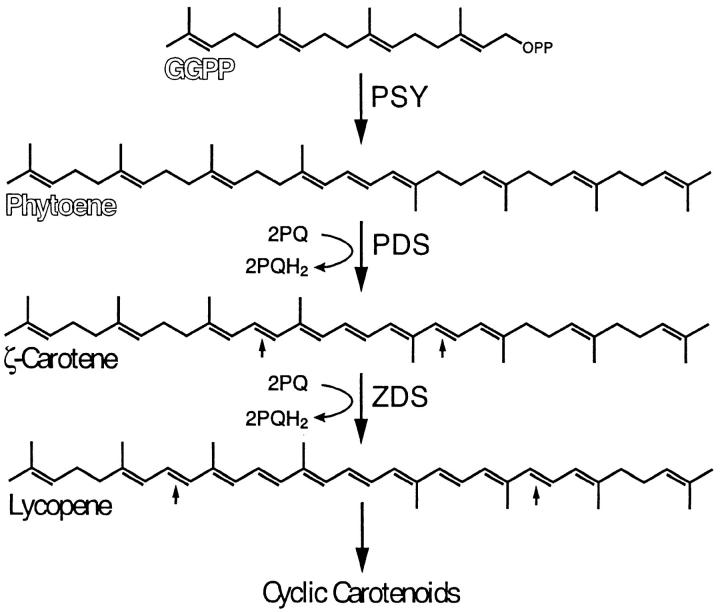
In plants and algae, carotenoids are synthesized via the biosynthetic pathway shown in Figure 1 (Cunningham and Gantt 1998). The formation of the colorless carotenoid phytoene from the condensation of two molecules of geranylgeranylpyrophosphate (GGPP) is the first committed step in the pathway and is catalyzed by the enzyme phytoene synthase (PSY). The next step, the conversion of phytoene to the first colored carotenoid in the pathway, ζ-carotene, by the enzyme phytoene desaturase (PDS), is a rate-limiting step of carotenogenesis (Chamovitz et al. 1993). PDS activity is inhibited by the bleaching herbicide norflurazon (Sandmann et al. 1989). ζ-carotene desaturase (ZDS), an enzyme related to PDS (Albrecht et al. 1995), converts ζ-carotene to lycopene. The electron carrier plastoquinone has been identified as an essential component for phytoene desaturation in higher plants (Norris et al. 1995). In addition, a recently identified carotenoid isomerase is necessary to generate all-trans lycopene (Isaacson et al. 2002; Park et al. 2002), which is the actual substrate for cyclization reactions leading to β-carotene, α-carotene, and xanthophylls. A mutant blocked in PSY, PDS, or plastoquinone biosynthesis would be expected to exhibit altered pigmentation, including a lack of colored carotenoids and reduced levels of chlorophylls.
Figure 1.—
Early steps of carotenoid biosynthesis in plants and algae. Intermediates labeled in chalkboard letters are colorless. Small arrows show the position of newly formed double bonds. GGPP, geranylgeranyl pyrophosphate; PSY, phytoene synthase; PDS, phytoene desaturase; ZDS, ζ-carotene desaturase; PQ, oxidized plastoquinone; PQH2, reduced plastoquinone (plastoquinol).
Four Chlamydomonas reinhardtii mutants lacking carotenoids have been described previously: lts1-30 (Chemerilova 1978), fn68 (Foster et al. 1984), w7 (Spreitzer and Mets 1981), and No. 95 (Sager and Zalokar 1958). The fn68 mutant was used to show that a rhodopsin photoreceptor controls the phototactic response in Chlamydomonas and that retinal, a derivative of carotenoids, constitutes the chromophore of the rhodopsin photoreceptor (Foster et al. 1984). Later experiments with lts1-30 indicated that all-trans retinal is the functional isomer of the rhodopsin chromophore (Hegemann et al. 1991). Studies of the light-induced expression of the glutamate 1-semialdehyde aminotransferase (GSA) gene in strain fn68 showed that a rhodopsin photoreceptor does not regulate GSA expression (Herman et al. 1999). Experiments examining chlorophyll apoprotein accumulation and expression in fn68 have shown that light-harvesting complexes do not correctly assemble in vivo without carotenoids, demonstrating a direct role for carotenoids and chlorophylls in the stabilization of certain chlorophyll apoproteins (Herrin et al. 1992). Because carotenoids play a critical structural role in pigment-binding protein complexes, it was shown that the low levels of chlorophyll observed in the carotenoid-less mutants are due to a lack of these complexes and a subsequent increased turnover of chlorophyll in the cell and not due to changes in chlorophyll biosynthesis (Herrin et al. 1992).
Although mutants lacking carotenoids have been used in numerous studies of Chlamydomonas biology, the biochemical and molecular basis for their phenotype has not been characterized. In this work, a collection of UV light-induced and chemically induced Chlamydomonas mutants that lack carotenoids, designated “white” because of their colorless appearance, were characterized for their growth phenotypes under the dark and various light conditions, pigment and tocopherol composition, and the genetic basis for their white mutant phenotype. Included in the collection are lts1-30, fn68, and w7, in addition to eight newly isolated mutants (Table 1).
TABLE 1.
White mutants used in this work
| Former/isolation name | New name | Reference |
|---|---|---|
| lts1-30 | lts1-30 | Chemerilova (1978) |
| w7 | lts1-201 | Spreitzer and Mets (1981) |
| fn68 | lts1-202 | Foster et al. (1984) |
| ltd1-1 | lts1-203 | This work |
| ltd1-2 | lts1-204 | This work |
| ltd1-3 | lts1-205 | This work |
| ltd1-4 | lts1-206 | This work |
| ltd1-5 | lts1-207 | This work |
| ltd1-6 | lts1-208 | This work |
| sm6-11 | lts1-209 | This work |
| sm6-27 | lts1-210 | This work |
MATERIALS AND METHODS
Strains and culture conditions:
Wild-type strains 4A+ (mt+) and 17D− (mt−) in the 137c strain background were obtained from Jean-David Rochaix (University of Geneva). Mutant strains lts1-30 mt− (CC-2359), fn68 (CC-2682), and w7 mt+ (CC-2843) were obtained from the Chlamydomonas Genetics Center (Duke University, Durham, NC). Additional mutants were generated by ethyl methanesulfonate (EMS) and UV mutagenesis of the 4A+ wild-type strain (Table 1). All strains were maintained at 25° on Tris-acetate-phosphate (TAP) agar medium (Harris 1989) in complete darkness. For experiments, cells were transferred to liquid TAP medium and grown in complete darkness with constant orbital shaking (100–120 rpm) at 25° until they reached a population density of 3–5 × 106 cells ml−1. Cell densities were measured with a hemocytometer. For growth experiments, light intensities were determined with a quantum meter (Li-Cor, Lincoln, NE).
Mutagenesis:
Liquid cultures of the wild-type strain were grown to a density of ∼5 × 106 cells ml−1. For chemical mutagenesis, cells were washed with and resuspended in 70 mm potassium phosphate (pH 6.9) at a concentration of 5 × 107 cells ml−1. Cells were incubated in 0.23, 0.27, or 0.30 m EMS (diluted with 70 mm potassium phosphate) in the dark at room temperature for 60 min with constant shaking. Samples were then washed once with 5% (w/v) sodium thiosulfate, twice with 70 mm potassium phosphate, and once with liquid TAP. For UV mutagenesis, 20 ml of cells (∼5 × 106 cells ml−1) in a 14-cm-diameter glass petri dish bottom were exposed to UV light ranging from 4 to 6 × 104 μJ cm−2 (Stratalinker; Stratagene, La Jolla, CA) and then incubated in the dark overnight with constant shaking. A series of dilutions of mutagenized cells was plated onto TAP agar medium, and the plates were kept in complete darkness until visible colonies formed (∼2 weeks). Colonies with a visible alteration in color were picked and restreaked, and single white mutant colonies were selected for further analysis.
Pigment and tocopherol analysis:
Liquid cultures were grown to a density of ∼5 × 106 cells ml−1 in complete darkness. Normalized quantities of cells were harvested by centrifugation at 20,000 × g for 5 min and the resulting pellet was extracted with 100 μl of acetone by vortexing for 30 sec. The extract was centrifuged at 20,000 × g for 1 min, and the supernatant was filtered (0.45-μm nylon filter) and saved. The pellet was extracted with a second 100 μl of acetone. The two extracts were pooled and immediately transferred to the dark. Twenty-five microliters of the filtered extract was subjected to HPLC and separated on a Spherisorb S5 ODS1 4.6- × 250-mm cartridge column (Waters, Milford, MA) at 30° using a method described previously (Müller-Moulé et al. 2003). Pigments were detected by absorbance at 445 nm (550 nm reference) by a diode array detector. Tocopherols were detected via fluorescence with excitation at 295 nm and emission at 325 nm. The concentrations of chlorophyll a and α-tocopherol were determined using standard curves of the purified compounds at known concentrations.
Genetic analysis:
Genetic crosses and tetrad analysis were performed according to established methods (Harris 1989). In crosses of white mutants to one another, homozygous white mutant zygospores were exposed to very low light (∼8 μmol photons m−2 sec−1) for 1–3 hr (instead of overnight) to initiate germination. The allelic arg7-1 and arg7-8 mutations exhibit intragenic complementation, allowing the selection of vegetative diploid strains on TAP medium lacking arginine in low light (∼80 μmol photons m−2 sec−1) for dominance tests (Harris 1989). For cosegregation analysis, an 891-bp DNA fragment of the PSY gene was amplified from progeny of crosses to the polymorphic wild-type strain S1D2 (mt−; Gross et al. 1988) using the primers PSYF2-2 (5′-AGGTCTGTGGTCCAACTGCT-3′) and PSYR2 (5′-GTGTCAGAAGGCCACCAAAAC-3′), and the PCR products were digested with HincII overnight.
Isolation of nucleic acids and DNA sequencing:
Nucleic acids were isolated from cells grown on TAP agar medium by lysing the cells in SDS-EB buffer containing 1% (w/v) SDS, 200 mm NaCl, 20 mm EDTA, and 50 mm Tris-HCl, pH 8.0. The solution was extracted twice with phenol:chlorofom:isoamyl alcohol (25:24:1) and once with chlorofom:isoamyl alcohol (24:1), and the DNA was precipitated with ethanol. Putative Chlamydomonas PSY and PDS cDNAs were identified by database searching of expressed sequence tags (ESTs; Asamizu et al. 2000). The cDNAs were obtained from the Kazusa DNA Research Institute (Kisarazu, Chiba, Japan) and completely sequenced. The cDNA sequences were used to identify the corresponding PSY and PDS genes in the Chlamydomonas nuclear genome sequence (version 1.0) on scaffolds 682 and 104, respectively (http://genome.jgi-psf.org/chlre1/chlre1.home.html). To sequence the PSY gene from the Chlamydomonas wild-type and mutant alleles, a series of synthetic primers was designed on the basis of both genomic and cDNA sequences. A 2576-bp fragment containing the PSY coding region was amplified with the primers PSYF1-1 (5′-CCAAGAGCATCTCCACCTTC-3′) and PSYR2. The same primer pair was used to amplify DNA from the polymorphic wild-type strain S1D2 to identify polymorphic markers for linkage tests. Sequencing of the PCR fragments on both strands was performed with the Big-Dye Terminator Version 3.0 kit (Applied Biosystems, Foster City, CA) and an ABI 3100 automated DNA sequencer (Applied Biosystems). The Lasergene MegAlign software package (DNASTAR, Madison, WI) was used to assemble DNA sequences into contigs and to align the PSY protein sequences.
Complementation with the wild-type phytoene synthase gene:
The plasmid (pSM1) used for transformation (Kindle 1990) contained a 6-kb fragment of genomic DNA (amplified with primer pairs T-PSYF1 5′-CCTTAATGCAGCGAATCCTT-3′ and PSYR2, and T-PSYF2 5′-GAGCGTGTGGGTTATCGTTC-3′ and T-PSYR1 5′-ATGCAGCTGTAGCAATGCAG-3′) inserted into the 3-kb pGEM-T Easy cloning vector (Promega, Madison, Wisconsin). The insert contained the 2262-bp PSY coding region, 500 bp upstream of the start codon, the 2021-bp 3′-UTR, and 1214 bp downstream of the 3′-UTR. Liquid cultures of lts1-210 were grown to a density of ∼5 × 106 cells ml−1 in complete darkness. Cells were harvested by centrifugation at 3000 × g for 3 min, resuspended in gamete autolysin (Harris 1989; 1/25 the original volume of the culture), and incubated for 90 min at room temperature. Autolysin-treated cells were collected by centrifugation at 1250 × g for 3 min and resuspended in liquid TAP at a density of 1.7 × 108 cells ml−1. The linearized pSM1 plasmid (300 ng), 300 μl (0.5 × 108) cells, and 100 μl of 20% (w/v) polyethylene glycol (PEG) were mixed and vortexed on high for 30 sec with 300 μg of glass beads (150–212 μm diameter). Cells were transferred to 10 ml liquid TAP and incubated overnight in the dark with constant shaking. Cells were then harvested by centrifugation at 1250 × g for 3 min, resuspended in 200 μl liquid TAP, and plated onto TAP agar medium. Plates were kept in the dark overnight and then gradually acclimated to very low light (∼8 μmol photons m−2 sec−1). Plates were scored for green transformants ∼2 weeks after exposure to low light.
RESULTS
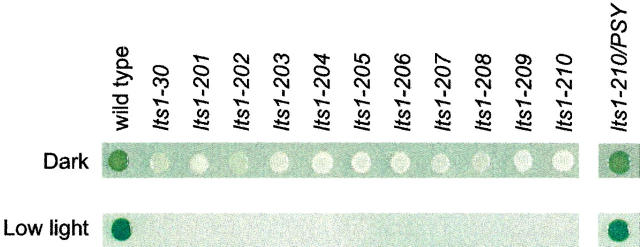
Eight new Chlamydomonas white mutants were isolated following UV or EMS mutagenesis and growth in the dark (Table 1). The phenotype of these eight mutants, along with the previously isolated lts1-30, fn68, and w7 mutants, was not completely colorless, but a very pale green (Figure 2). All white mutants exhibited extreme sensitivity to light. They were able to grow heterotrophically on acetate-containing (TAP) medium in the dark, but not on TAP medium in very low light (∼8 μmol photons m−2 sec−1) (Figure 2).
Figure 2.—
Growth phenotypes. Liquid cultures were spotted onto acetate-containing (TAP) agar medium and incubated in the dark or low light (∼8 μmol photons m−2 sec−1) for 1 week.
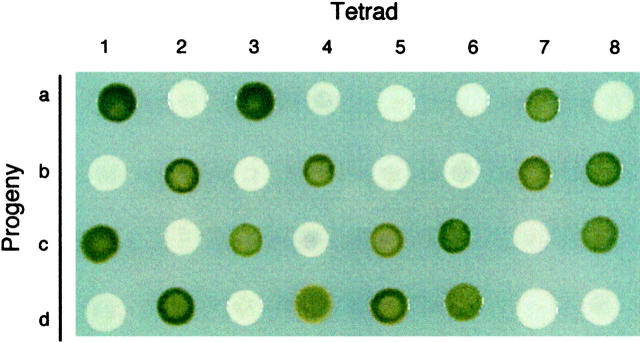
To determine the genetic basis for the white mutant phenotype, the eight new white mutants were crossed to a wild-type strain of opposite mating type, and the color of the resulting tetrad progeny was scored after growth in the dark (Figure 3, Table 2). Crosses of all eight mutants to the wild type showed a 2:2 (green:white) segregation in all tetrads and approximately a 1:1 segregation in total progeny (including progeny from incomplete tetrads; Table 2), indicating that a single nuclear gene is responsible for the white phenotype in each case. Mutant strains lts1-30 and w7 have been shown previously to be affected in a single locus (Chemerilova 1978; Spreitzer and Mets 1981).
Figure 3.—
Genetic analysis showing 2:2 segregation of the white phenotype. Tetrad analysis of a cross between lts1-203 and a wild-type strain (17D−) is shown. Progeny from eight tetrads were grown in complete darkness on TAP medium. Each vertical column represents a tetrad (labeled a–d).
TABLE 2.
Results of crosses of white mutants to the wild type
| Cross | Complete tetrads scored |
Total progeny scoreda |
Progeny with wild-type phenotype |
Progeny with white mutant phenotype |
|---|---|---|---|---|
| lts1-203 mt+ × mt− | 18 | 72 | 36 | 36 |
| lts1-204 mt+ × mt− | 4 | 62 | 31 | 31 |
| lts1-205 mt+ × mt− | 7 | 28 | 14 | 14 |
| lts1-206 mt+ × mt− | 3 | 28 | 15 | 13 |
| lts1-207 mt+ × mt− | 4 | 26 | 12 | 14 |
| lts1-208 mt+ × mt− | 14 | 73 | 40 | 33 |
| lts1-209 mt+ × mt− | 3 | 21 | 11 | 10 |
| lts1-210 mt+ × mt− | 9 | 36 | 18 | 18 |
Includes progeny from incomplete tetrads.
To test the dominance of the white mutation, the lts1-203 mutant was crossed to an arg7-8 strain, and a resulting lts1-203 arg7-8 progeny was mated with an arg7-1 strain. The allelic arg7-1 and arg7-8 mutations exhibit intragenic complementation, allowing the selection of vegetative diploid strains on medium lacking arginine. Heterozygous (lts1-203/LTS1) diploid colonies exhibited a wild-type green pigmentation (data not shown). PCR of genomic DNA from the green colonies with mating-type-specific primers (Werner and Mergenhagen 1998) showed both plus and minus mating-type bands, confirming that the green colonies were diploids (data not shown). This result shows that the lts1-203 mutation is recessive.
Insight into the number of genes defined by the white mutants in this collection was obtained by crossing white mutants to one another and scoring the resulting progeny for wild-type recombinants. White mutant diploid zygospores did not germinate (undergo meiosis) when subjected to low light (∼8 μmol photons m−2 sec−1) overnight. Shorter exposure to low light (1–3 hr of ∼8 μmol photons m−2 sec−1) resulted in a marked increase in germination rates (data not shown). In crosses between independently isolated white mutants, all resulting progeny were white, and no wild-type recombinants were observed (Table 3). These results show that lts1-30 and all eight new white mutants are alleles of a single gene, LTS1, and the new mutants have been designated lts1-203–lts1-210 (Table 1). Crosses to mutant strains fn68 and w7 did not yield zygospores; consequently recombination tests could not be performed on these mutants. Mutants lts1-30 and w7, however, have been shown previously to be alleles of the LTS1 locus on chromosome XI (Spreitzer and Mets 1981; Iroshnikova and Kvitko 1986). As described below, w7 and fn68 were both shown to contain mutations in the same gene as all the other lts1 alleles, so they were designated as lts1-201 and lts1-202, respectively.
TABLE 3.
Results of recombination tests between different white mutants
| Cross | Wild-type recombinants (green phenotype) |
Nonrecombinant progeny (white phenotype) |
|---|---|---|
| lts1-203 mt+ × lts1-30 mt− | 0 | 67 |
| lts1-203 mt+ × lts1-209 mt− | 0 | 30 |
| lts1-204 mt+ × lts1-209 mt− | 0 | 29 |
| lts1-205 mt+ × lts1-209 mt− | 0 | 63 |
| lts1-206 mt+ × lts1-209 mt− | 0 | 55 |
| lts1-210 mt+ × lts1-209 mt− | 0 | 40 |
HPLC analysis of extracts from dark-grown cells showed that white lts1 mutants lack carotenoids, but contain chlorophyll a and chlorophyll b in relatively low quantities (Figure 4), consistent with defects in PSY, PDS, or plastoquinone biosynthesis. White mutants showed up to 40 times less chlorophyll a per cell than the wild-type strain did (Table 4). The greatest variation in chlorophyll a between white mutants was observed in the lts1-30, lts1-201, and lts1-202 mutants, most likely due to variations in the strain backgrounds of these mutants. In addition, most white mutants contained at least twice as much α-tocopherol per cell as the wild type (lts1-207 contained only 1.6 times more α-tocopherol than the wild type; Table 4). Although it was reported previously that phytoene accumulates in lts1-202 (fn68; Herman et al. 1999), HPLC analysis (with detection by absorbance at 298 nm) did not show phytoene accumulation in any of the white mutants (data not shown). The absence of colored carotenoids and lack of phytoene accumulation suggested a defect in PSY rather than in PDS or plastoquinone biosynthesis.
Figure 4.—
Pigment analysis of wild type and a representative white mutant. Pigments from the wild-type strain 4A+ (A) and lts1-203 (B) were separated by HPLC. Each profile represents pigments extracted from an equal number of cells grown in complete darkness. N, neoxanthin; Lor, loroxanthin; V, violaxanthin; A, antheraxanthin; Lut, lutein; Cb, chlorophyll b; Ca, chlorophyll a; β, β-carotene.
TABLE 4.
Results of HPLC analysis
| Strain | Chlorophyll a (fmol cell−1) |
α-Tocopherol (fmol cell−1) |
|---|---|---|
| 4A+ | 45.5 ± 1.6 | 0.8 ± 0.1 |
| lts1-30 | 10.5 ± 1.0 | 2.2 ± 0.4 |
| lts1-201 | 3.4 ± 0.2 | 1.9 ± 0.2 |
| lts1-202 | 7.4 ± 1.8 | 2.5 ± 0.3 |
| lts1-203 | 2.8 ± 0.6 | 2.8 ± 0.8 |
| lts1-204 | 1.1 ± 0.2 | 1.8 ± 0.3 |
| lts1-205 | 1.2 ± 0.1 | 2.3 ± 0.1 |
| lts1-206 | 1.8 ± 0.3 | 2.8 ± 0.4 |
| lts1-207 | 1.4 ± 0.1 | 1.3 ± 0.2 |
| lts1-208 | 1.3 ± 0.2 | 2.0 ± 0.4 |
| lts1-209 | 2.5 ± 0.3 | 3.8 ± 0.3 |
| lts1-210 | 2.0 ± 0.1 | 1.6 ± 0.4 |
To facilitate the molecular analysis of the lts1 mutants, cDNAs with sequence similarity to known PSY and PDS genes were identified by searching the collection of Chlamydomonas EST sequences in GenBank (Asamizu et al. 2000). The cDNA sequences were used to identify the corresponding PSY and PDS genes in the Chlamydomonas nuclear genome sequence. Alignment of the deduced amino acid sequence of the PSY cDNA to the PSY proteins from other green algae and plants revealed that the cDNA was missing the 5′ region (including the start codon) of the PSY gene. The translation initiation site of the PSY gene shown in Figure 5 was therefore predicted from analysis of the genomic DNA sequence. The deduced amino acid sequence of the PSY cDNA showed the highest overall sequence similarity with PSY from the green alga Dunaliella bardawil (74% identity, 81% similarity; Figure 5A). The Chlamydomonas PSY protein is 382 amino acids in length and contains a putative amino-terminal chloroplast transit peptide of ∼50 amino acids (Figure 5A). Comparison of the cDNA and genomic DNA sequences revealed that the PSY gene is ∼4.3 kb in length and contains five exons (varying in length from 152 to 426 bp) and four introns (varying in length from 80 to 449 bp), with a 3′-UTR of 2021 bases (Figure 5B).
Figure 5.—
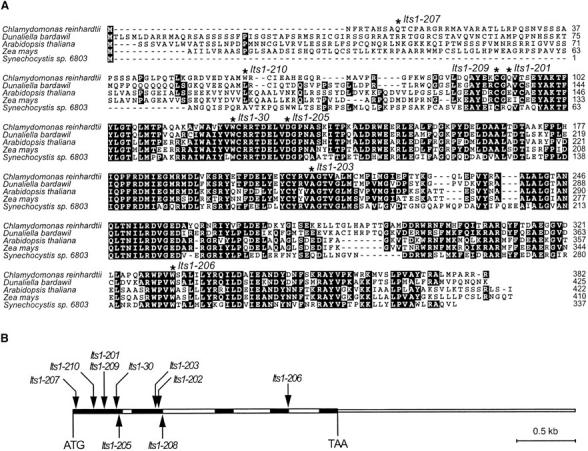
(A) Alignment of the amino acid sequences of the PSY protein from Chlamydomonas and selected algal and plant species. The aligned sequences are from another green alga (Dunaliella bardawil; GenBank accession no. U91900), Arabidopsis (Arabidopsis thaliana; GenBank accession no. BT002084), maize (Zea mays; GenBank accession no. AY455286), and a cyanobacterium (Synechocystis sp. 6803; GenBank accession no. X69172). Residues that are identical in at least three of the sequences are shaded in black. Mutation sites in lts1 alleles are indicated with asterisks. The mutation in lts1-202 causes a frameshift in the coding region, and the mutation in lts1-208 alters a splice site in the primary transcript. (B) Structure of the Chlamydomonas PSY gene and the positions of point mutations. The gene contains five exons (solid rectangles) and four introns (open rectangles). The 3′-untranslated region is indicated by a thinner box (the 5′-untranslated region is not yet determined). The positions of 10 mutant alleles are indicated by arrows.
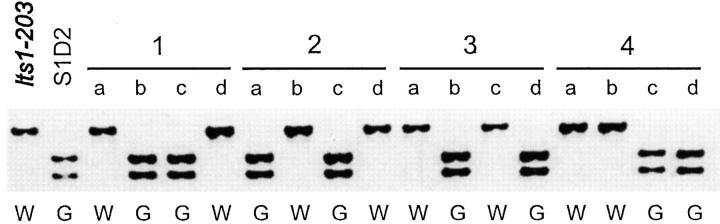
To determine whether a mutation in the PSY gene might be responsible for the white mutant phenotype, lts1-203 and lts1-209 were examined for cosegregation with the PSY gene in a cross. A single-nucleotide polymorphism was identified in the fourth intron of the PSY gene by sequencing the gene from a polymorphic wild-type strain (S1D2). In eight complete tetrads from a cross of lts1-203 to the polymorphic wild-type strain (four tetrads shown in Figure 6) and five complete tetrads from a cross of lts1-209 (data not shown), the white mutant phenotype cosegregated with the polymorphism in the PSY locus. These results show that lts1 is closely linked to the PSY locus, and it is likely that a mutation in the PSY gene is responsible for the white phenotype. In these crosses of the lts1-203 and lts1-209 mutants to S1D2, the white phenotype segregated independently of a marker located in the PDS locus (data not shown). The molecular markers CNA19 and GP49, located adjacent to the PSY gene in the Chlamydomonas nuclear genome sequence, map the PSY gene to linkage group XI. These findings are consistent with the previously determined map location of the LTS1 gene (Iroshnikova and Kvitko 1986). The molecular markers CNC53 and EYE2 map the PDS gene to linkage group XII/XIII.
Figure 6.—
Cosegregation of lts1 with a marker located in the PSY locus. Progeny from eight tetrads were obtained from a cross between lts1-203 and a polymorphic wild-type strain (S1D2), and a single-nucleotide polymorphism marker located in the fourth intron of the PSY gene was scored. A 891-bp fragment of the PSY gene was amplified from progeny, digested with HincII, and subjected to agarose gel electrophoresis. Both parental strains and progeny (labeled a–d) of four tetrads are shown. The pigmentation phenotype of each progeny is indicated by the letters G (green) or W (white).
DNA sequencing of the PSY gene from the white mutants identified mutations in all but one of the mutants (Table 5). In lts1-204, ∼200 bp of the PSY 5′-coding region could not be amplified, possibly because of a deletion, insertion, or rearrangement in this region of the gene that affected priming or amplification. Nine mutants generated by EMS, UV, or N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) mutagenesis contained a single-base-pair change from G/C to A/T, which corresponded to an amino acid change in the PSY protein (three alleles), a premature stop codon (five alleles), or a mutation affecting a splice site (one allele). One UV-induced mutant, lts1-202, contained both a base pair change and a base deletion in its PSY gene, the latter causing a frameshift in the open reading frame. The amino acid changes in the missense alleles (lts1-203, lts1-205, and lts1-209) are located in highly conserved regions and are likely to affect the function of the mature PSY protein (Figure 5A). Four out of five alleles with premature stop codons are located in the first half of the transcript, which would generate a severely truncated protein product (Figure 5A).
TABLE 5.
Sequences ofpsy mutant alleles
| Allele | Mutation | Effect of mutation | Mutagen |
|---|---|---|---|
| lts1-30 | G/C → A/T | Trp123 → Stop | MNNG |
| lts1-201 | G/C → A/T | Gln93 → Stop | EMS |
| lts1-202 | GCCCA → GTCA | Frameshift | UV |
| lts1-203 | G/C → A/T | Ala211 → Asp | EMS |
| lts1-204 | ND | ND | EMS |
| lts1-205 | G/C → A/T | Asp132 → Asn | UV |
| lts1-206 | G/C → A/T | Trp332 → Stop | UV |
| lts1-207 | G/C → A/T | Gln10 → Stop | UV |
| lts1-208 | G/C → A/T | Splice site | UV |
| lts1-209 | G/C → A/T | Cys91 → Tyr | EMS |
| lts1-210 | G/C → A/T | Trp60 → Stop | EMS |
ND, not determined.
A complementation experiment, in which the white mutant phenotype was rescued by transformation with a wild-type copy of the PSY gene, was performed to show conclusively that a defect in the PSY gene is responsible for the white phenotype of lts1 mutants. The lts1-210 mutant was transformed with a plasmid containing a 6-kb wild-type genomic DNA fragment. The genomic insert included the PSY gene, 500 bp upstream of the start codon, and 1214 bp downstream of the 3′-UTR. After 2 weeks exposure to very low light (∼8 μmol photons m−2 sec−1), green colonies were visible on the transformation plates, whereas no green revertants were observed on negative control plates (following mock transformation with no DNA). Growth phenotypes of the complemented line (lts1-210/PSY) in the dark and very low light (∼8 μmol photons m−2 sec−1) are shown in Figure 2. The presence of the wild-type PSY gene in the lts1-210/PSY strain was confirmed by PCR of genomic DNA with primers specific to the pSM1 plasmid (data not shown).
DISCUSSION
Several lines of genetic and molecular evidence show that defects in a single gene, PSY, are responsible for the carotenoid-less phenotype of all of the Chlamydomonas white mutants in our collection. Recombination tests between individual white mutants produced no wild-type recombinants (Table 3); the white mutant phenotype cosegregated with a marker located in the PSY locus (Figure 6); the PSY gene from each of the white mutants contains a mutation that causes a change in the resulting PSY protein (Figure 5A, Table 5); and transformation with a wild-type copy of the PSY gene was able to rescue the white phenotype (Figure 2). The similarity of the phenotype of the lts1 mutants (complete lack of carotenoids) and the predicted severity of the lesions in the PSY gene suggest that all the lts1 alleles are null mutations.
There appears to be some overlap in the spectrum of mutations caused by EMS and UV mutagenesis in Chlamydomonas. The chemical mutagen EMS alkylates guanine leading to the mispairing of guanine with thymidine, instead of cytosine. The resulting point mutations are mainly G/C to A/T transitions. Studies in yeast have shown that G/C to A/T transitions at dipyrimidine sites are the most common UV-induced mutation (Moshinsky and Wogan 1997). This is consistent with our findings, as four out of five UV-induced mutations in the PSY gene were G/C to A/T transitions, and all five mutations occurred at dipyrimidine sites (data not shown).
GGPP, the substrate of PSY, is also a precursor for the biosynthesis of several important molecules in algae and plants, including tocopherols, chlorophylls, and quinones. Therefore, GGPP may not accumulate in an lts1 mutant lacking PSY but instead may be diverted to one or more of these other biosynthetic pathways. In the fn68 mutant, it has been shown that chlorophyll is still synthesized, but it is rapidly turned over in the cell (Herrin et al. 1992). Therefore, chlorophyll biosynthesis could act as a sink for GGPP in lts1 mutants. HPLC analysis showed that the white lts1 mutants have on average approximately twice as much α-tocopherol per cell as wild type does. The increased tocopherol levels in the white lts1 mutants may be due to the diversion of excess GGPP into the tocopherol pathway, or, alternatively, to an upregulation of tocopherol biosynthesis in response to some other stimulus such as increased oxidative stress in the absence of carotenoids.
Chlamydomonas mutants defective in PDS or plastoquinone biosynthesis would be expected to exhibit a white phenotype similar to that of the lts1 mutants, so it is somewhat surprising that neither class of mutants was identified in our white mutant collection. Mutants affecting PDS or plastoquinone biosynthesis should accumulate phytoene, but phytoene accumulation was not detected in any of the white mutants, and the white phenotype did not cosegregate with a marker located in the PDS locus (data not shown). In Arabidopsis thaliana, two nonallelic albino mutations, pds1 and pds2, affecting PDS activity have been identified (Norris et al. 1995). The pds1 and pds2 mutants accumulate phytoene and lack plastoquinone, demonstrating that plastoquinone is an essential component of carotenoid biosynthesis in plants (Norris et al. 1995). The molecular basis for pds1, but not yet pds2, has been determined, and it was found that pds1 is a mutation in the gene encoding p-hydroxyphenylpyruvate dioxygenase (HPD) in Arabidopsis (Norris et al. 1998). Searching for an HPD homolog in the current draft of the Chlamydomonas nuclear genome revealed the presence of two tandemly linked HPD genes. Thus, the lack of a Chlamydomonas white mutant analogous to Arabidopsis pds1 is likely explained by genetic redundancy. Similarly, the presence of more than one functional PDS gene in the Chlamydomonas genome might provide an explanation for the inability to isolate a mutant affecting PDS; however, besides the putative PDS gene that we identified and a putative ZDS gene there does not appear to be an obvious additional homolog of PDS in Chlamydomonas. Alternatively, toxicity of phytoene accumulation or some other mechanism of lethality may prevent the isolation of mutants defective in PDS. However, the accumulation of phytoene in the pds1 and pds2 mutants of Arabidopsis (Norris et al. 1995) demonstrates that phytoene concentrations much higher than normal cellular levels are not lethal, at least in Arabidopsis. Isolation of a Chlamydomonas pds mutant by reverse genetics would provide insight into the viability of a mutant impaired in PDS, in addition to the possible redundancy of genes encoding enzymes that are capable of catalyzing phytoene desaturation.
The lts1 mutants grew heterotrophically in the dark using acetate as a carbon source, but they were unable to grow in the light, even under very low light conditions on acetate-containing medium (Figure 2). In contrast, the pds1 and pds2 mutants of Arabidopsis are able to grow to near maturity in the light on agar medium containing sucrose (Norris et al. 1995). A likely explanation for this difference in light sensitivity is a difference in chlorophyll accumulation. The Arabidopsis mutants completely lack colored carotenoids and chlorophylls (Norris et al. 1995), whereas the Chlamydomonas mutants are not actually completely white. The lts1 mutants accumulated easily detectable amounts of chlorophylls a and b (Figure 2, Figure 4, Table 4), which would act as potent photosensitizers of singlet oxygen formation in the absence of carotenoids (Demmig-Adams et al. 1996; Frank and Cogdell 1996; Baroli and Niyogi 2000), thereby leading to severe photooxidative stress.
In summary, we have characterized 11 lts1 mutations that affect the PSY gene of Chlamydomonas. The phenotype of lts1 mutants is easily scored, making it an ideal marker for mapping genes to linkage group XI. The lts1 mutants will also be useful in studying the relationships between the pathways downstream of GGPP, including the carotenoid, chlorophyll, tocopherol, and quinone biosynthetic pathways.
Acknowledgments
We thank Christoph Beck for helpful suggestions and Rachel Dent and Heidi Ledford for critical reading of this manuscript. We also thank the Department of Energy Joint Genome Institute for providing Chlamydomonas nuclear genome sequence information. This work was supported by grants to K.K.N. from the National Institutes of Health (GM58799) and the Torrey Mesa Research Institute/Syngenta Research and Technology.
References
- Albrecht, M., A. Klein, P. Hugueney, G. Sandmann and M. Kuntz, 1995. Molecular cloning and functional expression in E. coli of a novel plant enzyme mediating zeta-carotene desaturation. FEBS Lett. 372: 199–202. [DOI] [PubMed] [Google Scholar]
- Asamizu, E., K. Miura, K. Kucho, Y. Inoue, H. Fukuzawa et al., 2000. Generation of expressed sequence tags from low-CO2 and high-CO2 adapted cells of Chlamydomonas reinhardtii. DNA Res. 7: 305–307. [DOI] [PubMed] [Google Scholar]
- Baroli, I., and K. K. Niyogi, 2000. Molecular genetics of xanthophyll-dependent photoprotection in green algae and plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355: 1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton, G., 1995. Structure and properties of carotenoids in relation to function. FASEB J. 9: 1551–1558. [PubMed] [Google Scholar]
- Chamovitz, D., G. Sandmann and J. Hirschberg, 1993. Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J. Biol. Chem. 268: 17348–17353. [PubMed] [Google Scholar]
- Chemerilova, V. I., 1978. Investigation of pigmentation modifying mutations in Chlamydomonas reinhardtii strains of differing ploidy II. The lts1 mutation compounds and their use in obtaining triploid cultures. Genetika 14: 154–162. [Google Scholar]
- Cunningham, Jr., F. X., and E. Gantt, 1998. Genes and enzymes of carotenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 557–583. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams, B., A. M. Gilmore and W. W. Adams, III, 1996. In vivo function of carotenoids in higher plants. FASEB J. 10: 403–412. [DOI] [PubMed] [Google Scholar]
- Duckham, S. C., R. S. T. Linforth and I. B. Taylor, 1991. Abscisic-acid-deficient mutants at the aba gene locus of Arabidopsis thaliana are impaired in the epoxidation of zeaxanthin. Plant Cell Environ. 14: 601–606. [Google Scholar]
- Foster, K. W., J. Saranak, N. Patel, G. Zarilli, M. Okabe et al., 1984. A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas. Nature 311: 756–759. [DOI] [PubMed] [Google Scholar]
- Frank, H. A., and R. J. Cogdell, 1996. Carotenoids in photosynthesis. Photochem. Photobiol. 63: 257–264. [DOI] [PubMed] [Google Scholar]
- Gross, C. H., L. P. Ranum and P. A. Lefebvre, 1988. Extensive restriction fragment length polymorphisms in a new isolate of Chlamydomonas reinhardtii. Curr. Genet. 13: 503–508. [DOI] [PubMed] [Google Scholar]
- Harris, E. H., 1989 The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. Academic Press, San Diego. [DOI] [PubMed]
- Hegemann, P., W. Gaertner and R. Uhl, 1991. All-trans retinal constitutes the functional chromophore in Chlamydomonas rhodopsin. Biophys. J. 60: 1477–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, C. A., C. S. Im and S. I. Beale, 1999. Light-regulated expression of the gsa gene encoding the chlorophyll biosynthetic enzyme glutamate 1-semialdehyde aminotransferase in carotenoid-deficient Chlamydomonas reinhardtii cells. Plant Mol. Biol. 39: 289–297. [DOI] [PubMed] [Google Scholar]
- Herrin, D. L., J. F. Battey, K. Greer and G. W. Schmidt, 1992. Regulation of chlorophyll apoprotein expression and accumulation. Requirements for carotenoids and chlorophyll. J. Biol. Chem. 267: 8260–8269. [PubMed] [Google Scholar]
- Iroshnikova, G. A., and I. B. Kvitko, 1986. Investigation of pigmentation modifying mutations in Chlamydomonas reinhardtii strains of differring ploidy IV. Localization of LTS1 gene. Genetika 22: 761–766. [Google Scholar]
- Isaacson, T., G. Ronen, D. Zamir and J. Hirschberg, 2002. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of β-carotene and xanthophylls in plants. Plant Cell 14: 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle, K. L., 1990. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 87: 1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshinsky, D. J., and G. N. Wogan, 1997. UV-induced mutagenesis of human p53 in a vector replicated in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94: 2266–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Moulé, P., M. Havaux and K. K. Niyogi, 2003. Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol. 133: 748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, S. R., T. R. Barrette and D. DellaPenna, 1995. Genetic dissection of carotenoid synthesis in Arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell 7: 2139–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, S. R., X. Shen and D. DellaPenna, 1998. Complementation of the Arabidopsis pds1 mutation with the gene encoding p-hydroxyphenylpyruvate dioxygenase. Plant Physiol. 117: 1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H., S. S. Kreunen, A. J. Cuttriss, D. DellaPenna and B. Pogson, 2002. Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock, C. D., and J. A. D. Zeevaart, 1991. The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc. Natl. Acad. Sci. USA 88: 7496–7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager, R., and M. Zalokar, 1958. Pigments and photosynthesis in a carotenoid-deficient mutant of Chlamydomonas. Nature 182: 98–100. [DOI] [PubMed] [Google Scholar]
- Sandmann, G., H. Linden and P. Böger, 1989. Enzyme-kinetic studies on the interaction of norflurazon with phytoene desaturase. Z. Naturforsch. C 44: 787–790. [Google Scholar]
- Spreitzer, R. J., and L. Mets, 1981. Photosynthesis-deficient mutants of Chlamydomonas reinhardtii with associated light-sensitive phenotypes. Plant Physiol. 67: 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, R., and D. Mergenhagen, 1998. Mating type determination of Chlamydomonas reinhardtii by PCR. Plant Mol. Biol. Rep. 16: 295–299. [Google Scholar]