Abstract
Harmala alkaloids are endogenous substances, which are involved in neurodegenerative disorders such as M. Parkinson, but some of them also have neuroprotective effects in the nervous system.
While several sites of action at the cellular level (e.g. benzodiazepine receptors, 5-HT and GABAA receptors) have been identified, there is no report on how harmala alkaloids interact with voltage-gated membrane channels.
The aim of this study was to investigate the effects of harmaline and harmane on voltage-activated calcium- (ICa(V)), sodium- (INa(V)) and potassium (IK(V))-channel currents, using the whole-cell patch-clamp method with cultured dorsal root ganglion neurones of 3-week-old rats. Currents were elicited by voltage steps from the holding potential to different command potentials.
Harmaline and harmane reduced ICa(V), INa(V) and IK(V) concentration-dependent (10–500 μM) over the voltage range tested. ICa(V) was reduced with an IC50 of 100.6 μM for harmaline and by a significantly lower concentration of 75.8 μM (P<0.001, t-test) for harmane. The Hill coefficient was close to 1. Threshold concentration was around 10 μM for both substances.
The steady state of inhibition of ICa(V) by harmaline or harmane was reached within several minutes. The action was not use dependent and at least partly reversible.
It was mainly due to a reduction in the sustained calcium channel current (ICa(L+N)), while the transient voltage-gated calcium channel current (ICa(T)) was only partially affected.
We conclude that harmaline and harmane are modulators of ICa(V) in vitro. This might be related to their neuroprotective effects.
Keywords: Voltage-gated calcium currents, voltage-gated sodium currents, voltage-gated potassium currents, harmane, harmaline, dorsal root ganglion neurones, rat
Introduction
The β-carbolines 1-methyl-β-carboline (harmane), 1-methyl-7-methoxy-3,4-dihydro-β-carboline (harmaline) and 9H-pyrido[3,4-b]indole (norharmane) are naturally present in the human food chain and supposed to occur endogenously in normal body constituents. They are found in the blood plasma, heart, kidney, liver and also in brain tissue, where they have been proposed to be endogeneous ligands for benzodiazepine and imidazoline receptors (Rommelspacher et al., 1980; May et al., 1994; Hudson et al., 1999). Since high plasma levels of these compounds have been found in heavy smokers (Spijkerman et al., 2002), alcoholics (Rommelspacher et al., 1991), heroin-dependent humans (Stohler et al., 1996), patients with essential tremor (Louis et al., 2002) or Parkinson's disease (Kuhn et al., 1996), they are assumed to have a crucial role in the pathophysiology of various disorders of the CNS.
Speculations on the biological significance of β-carbolines comprised cytotoxic as well as neuroprotective properties. On the one hand, β-carbolines have been postulated to act as endogeneous neurotoxins because of their structural similarity to 1-methyl-4-phenyl-1-1,2,3,6-tetrahydropyridine (MPTP) (Albores et al., 1990), a compound which induced a parkinsonian-like syndrome in animals (Przedborski & Jackson-Lewis, 1998). Furthermore, harmaline has been shown to have a cytotoxic effect on PC-12 cells (Cobuzzi et al., 1994) and to cause degeneration of Purkinje cells in rat cerebellum. The latter finding may explain its tremor-evoking action (O'hearn & Molliver, 1993).
On the other hand, and, more recently, β-carbolines have been shown to protect neurones against the excitotoxic effects of dopamine and glutamate (Maher & Davis, 1996). Accordingly, a protective role of β-carbolines in the pathophysiology of Parkinson's disease has been suggested. Increasing evidence also hints to a protective effect of elevated endogeneous β-carbolines on oxidative neuronal damage. For example, β-carbolines were shown to depress the dopamine- or 6-hydroxydopamine-induced brain mitochondrial and synaptosomal dysfunctions, and also the loss of viability in PC-12 cells through a scavenging action on reactive oxygen species and maintenance of reduced thiols (Kim et al., 2001).
Apart from actions on benzodiazepine and imidazoline receptors, other effects of β-carbolines have been identified at the cellular level. These include an activation of 5-HT2A and 5-HT2C receptors, a potent inhibition of synaptosomal γ-hydroxybutyrate (GHB) re-uptake (McCormick & Tunnicliff, 1998), and an impairment of monoamine oxidase enzymes (MAO) and of sodium–hydrogen exchange (NHE) (Glennon et al., 2000; Anderson et al., 2003).
It appears surprising that, despite these manifold effects of harmale alkaloids on various targets, little information is available about their action on neuronal sodium, potassium and calcium channel currents and calcium homeostasis (Shi et al., 2001). Especially calcium – entering through membrane channels – acts as a major second messenger, whose intracellular concentration is tightly regulated. De-regulation results in reduced functionality of neurones, and a permanently increased calcium concentration within the cell could also result in malfunction and even cell death. Therefore, it might be possible that the opposing effects of harmala alkaloids described above may involve Ca2+i-homeostasis disturbances. In neurones, calcium enters the cell through different types of voltage-gated channels. The three main types of voltage-gated calcium channels which have originally been described and studied in dorsal root ganglion (DRG) neurones are L-, N- and T-type channels (Fox et al., 1987). They differ with respect to their voltage dependency and activation and inactivation properties (for details, see Hille, 1992). Most important for Ca2+i homeostasis are the noninactivating L-type channels, whose activation starts at a membrane potential of around −30 mV, and transient T-type channels, which are fast inactivating and open upon ‘small' depolarisations (in the range of −50 to −10 mV). In this study, we investigated the effects of harmane and harmaline on voltage-gated calcium channel currents (ICa(V)) in comparison to sodium (INa(V)) and potassium (IK(V)) channel currents. Using DRG neurones of adult rats and a concentration range of 10–500 μM harman or harmaline, we found that β-carbolines are indeed capable of reducing these channel currents.
A preliminary report has been published in abstract form (Splettstoesser et al., 2003).
Methods
Preparation of DRG neurones
DRG neurones were isolated from 3-week-old ‘Wistar' rats. Animals were deeply anaesthetised with Isofluran (Curamed), until punching the rat tail and feet revealed complete analgesia. Thereafter, the rats were decapitated and the vertebral column was opened by a dorsal approach, starting at the caudal end. Spinal cord was removed and dorsal root ganglia (DRG) were collected by fine forceps from both sides of the spinal column and transferred into ice-cooled F-12 medium (Sigma, Taufkirchen, Germany). Spinal nerves were cut off under optical control using fine scissors. Then, the ganglia were transferred into a mixture of 0.9 ml F12 medium plus 0.1 ml collagenase medium (2612.5 U ml−1, Sigma Type II) and digested in a humidified atmosphere (5% CO2) at 37°C for 45–55 min. In the next step, the collagenase was removed by washing the ganglia with F-12 medium for at least three times. Trypsination (2525 U trypsin per ml F-12 medium, Sigma Type IX) was carried out for another 2–3 min under the same conditions. After adding F-12 medium (final volume 0.7 ml), the DRG were titurated with a fire-polished Pasteur pipette (tip diameter 150 μM) until the ganglion capsules were opened, and the neurones were released from the ganglia. A portion of 50 μl of the resulting suspension was placed in the middle of small petri dishes (3 cm; Falcon ‘Easy Grip') and incubated for 2–4 h, allowing cells to adhere. Thereafter, 1 ml F-12 (with horse serum) was added to each petri dish. Cultures were used for electrophysiological recordings within the next 2 days.
Recording techniques and isolation of the different channel currents
With the whole-cell patch-clamp technique, membrane currents of DRG neurones were recorded using a HEKA EPC 9 amplifier and EPC screen software (HEKA). Microelectrodes were pulled from borosilicate glass with filament (o.d.: 1.5 mm and i.d.: 0.86 mm; Biomedical Instruments (BMI)) with a Sutter electrode puller (model P-87). Electrodes were fire polished with a Narashige micro forge (MF-830) to a final resistance of 3–4 MΩ and filled with the adequate internal solution (see Table 1; all chemicals for these solutions were obtained from Sigma-Aldrich, Taufkirchen, Germany). Special bath solutions were prepared to isolate the different types of voltage-activated channel currents (see Table 1). After a giga-ohm seal had been obtained and a stable access to the neurones had been established, membrane potential was routinely clamped at −80 mV. Voltage-activated channel currents were elicited by depolarising command pulses to 0 mV for 120 ms (80 ms when measuring current–voltage (I–V) relations) for ICa(V), 15 ms for INa(V) and 200 ms for IK(V). Data were sampled at 10 kHz and stored on hard disc. To obtain I–V relations (I–V curve), depolarisation steps were started at −80 mV and were increased stepwise by 10 mV, until a maximal depolarisation to +60 mV for calcium and potassium channel currents and +40 mV for sodium channel currents was reached.
Table 1.
Composition of external and internal solutions used to record ICa(V) (Calcium), INa(V) (Sodium) and IK(V) (Potassium)
| Calcium (mM) | Sodium (mM) | Potassium (mM) | |
|---|---|---|---|
| External solutions | |||
| NaCl | 145 | 145 | |
| KCl | 2.5 | 2.5 | |
| MgCl2 | 1 | 1.2 | |
| CaCl2 | |||
| CdCl2 | 0.05 | ||
| HEPES | 10 | 10 | 10 |
| TTX | 0.001 | 0.002 | |
| 4-AP | 1 | ||
| Glucose | 10 | 10 | 10 |
| BaCl2 | 10 | ||
| TEA-chloride | 130 | ||
| pH | 7.3 | 7.4 | 7.4 |
| Internal solutions | |||
| CsCl | 140 | 140 | |
| NaCl | 5 | ||
| MgCl2 | 4 | 4 | 4 |
| KCl | 140 | ||
| CaCl2 | 1 | ||
| HEPES | 10 | 10 | 10 |
| EGTA | 10 | 10 | 11 |
| Na-ATP | 2 | ||
| pH | 7.2 | 7.2 | 7.4 |
Harmaline and harmane (Sigma, Taufkirchen, Germany) were freshly dissolved in the respective bath solution (Table 1) at a concentration of 1 mM. Final dilutions (10–500 μM) were made immediately before use. Drugs were applied by a bath application system. To achieve total exchange of the bath saline, a volume of 10 ml was flushed through the bath (bath volume 1 ml), with a continuous flow of 5 ml min−1.
Data analysis
All currents were leak corrected on-line with a P/3 protocol and off-line normalised to the mean of five successive peak currents (−80 to 0 mV) obtained under control conditions.
Calcium channel currents used for calculation of time-course, I–V relations and dose–response curves were rundown-corrected. Assuming a linear rundown, all current values were extrapolated to the time point of drug application.
To compare the mean inhibitory effects (inclusive the standard error) of harmaline and harmane on sodium, potassium and calcium channel currents over the voltage range tested, all currents were normalised and expressed as a percentage of the maximum current obtained over the voltage range tested. An I–V relation was drawn from these data, and the relative effect of both substances was calculated over the voltage range. Analysis of calcium channel currents comprised a separate evaluation of sustained currents (plateau at the end of the depolarisation) and the peak currents. To separate the transient peak current (ICa(T)), the underlying sustained current (ICa(L+N)) was subtracted from the total peak current (ICa(V)). With this, the specific influence of harmaline and harmane on the transient current became evident.
Dose–response curves were obtained by calculating the mean percentage of action of the normalised data as well as the standard error for each concentration of harmaline and harmane. Data were fitted using the Langmuir equation: y=sch/(kh+ch), where c is the concentration, s the saturation (here 100), k the concentration at half-saturation and h the Hill coefficient.
Results
Both harmaline and harmane reduced the currents through voltage-activated calcium, sodium and potassium channels, but differed in efficiency and voltage dependence. The ICa(L+N) (sustained current of voltage-gated calcium channels) was reduced most efficiently, when activating the channels by a depolarisation to 0 mV. INa(V) (voltage-gated sodium channel currents) and IK(V) (voltage-gated potassium channel currents) were less affected by both substances when the channels were opened by the same depolarisation. Figure 1 illustrates the action of harmaline (a, c, e) and harmane (b, d, f) on voltage-activated calcium (a, b), sodium (c, d) and potassium channel currents (e, f) under control conditions and after a steady state of the effect had been reached. Raw traces of control currents and the currents after the application of 100 μM harmaline or 100 μM harmane are superimposed. Under control conditions, ICa(V) (a, b) reached a peak within 4.9 ms and declined by 30±5% over the period of activation (80 ms). Harmane was more effective in reducing the peak of the currents of these channels than harmaline, as it reduced these ICa(V) currents by 56.2±0.5%, while harmaline blocked the current by 37.1±6.8%. IK(V), also activated by a depolarisation to ±0 mV for 200 ms, were only weakly affected by harmaline or harmane (100 μM, each) (Figure 1e, f). This was also true for the INa(V), elicited by a voltage jump to ±0 mV for 15 ms (Figure 1c, d).
Figure 1.
Raw traces of voltage-activated calcium (a, b), sodium (c, d) and potassium channel currents (e, f) elicited by a depolarisation from the holding potential of −80 to 0 mV (upper trace). Currents under control conditions (black; lower traces) and after blockade of the channel currents (grey) by 100 μM harmaline (a, c, e) or 100 μM harmane (b, d, f) are superimposed.
Dose–response relationship
We next investigated the dose–response relationship for the peak currents through calcium channels elicited by a depolarisation from the holding potential of −80 to±0 mV under the influence of harmane or harmaline (Figure 2). Fitting all data points to the Langmuir equation, we found a threshold concentration for inhibiting the currents being lower than 10 μM. Total inhibition (>80%) was reached with concentrations above 250 μM for both substances. The IC50 value was calculated to be 75.8±1.1 μM for harmane and 100.6±5.3 μM for harmaline. The difference of 24.6±2.4 μM was highly significant (P<0.001, paired t-test). The Hill coefficient was close to 1 under all conditions, indicating a single binding site for either of the substances.
Figure 2.
Dose–response relationship for the block of ICa(V) by harmane (dashed line) and harmaline (black line) for a depolarising voltage jump from the holding potential of −80 to 0 mV for 100 ms. IC50's were 100.6 μM for harmaline and 75.8 μM for harmane. Threshold concentration was below 10 μM. For comparison, data for 100 and 500 μM harmane and harmaline on voltage-activated sodium and potassium channel currents are included (open diamonds are harmaline on potassium channel currents, open triangles are harmane on potassium channel currents, filled diamonds are harmaline on sodium currents and filled triangles are harmaline on sodium currents).
Higher concentrations of harmaline or harmane were needed to reduce the INa(V) and IK(V). A concentration of 100 μM harmaline reduced the INa(V) by 23.0±5.8% and IK(V) by 4.4±9.3%. A concentration of 500 μM blocked these currents by 75.4±3.1 and 28.1±2.3%, respectively. Similar results were obtained using 100 μM (500 μM) of harmane: the INa(V) was reduced by 4.2±1.7 (78.8±1.7%) and the IK(V) by 17.5±5.0 (75.1±5.7%). From this comparison, it became clear that harmaline and harmane most efficiently inhibited ICa(V).
Voltage dependence of the effect
ICa(V) (Figure 3a, b), INa(V) (Figure 3c, d) and IK(V) (Figure 3e, f) were differentially affected by harmaline (Figure 3a, c, e) or harmane (Figure 3b, d, f) (with 100 μM each) over the voltage range tested.
Figure 3.
Normalised I–V relation of the effect of harmaline (a, c, e) and harmane (b, d, f) on ICa(V) (a, b), INa(V) (c, d) and IK(V) (e, f). Control I–V's are represented by solid lines, effects of 100 μM of either substance are shown by dashed lines and the effects of 500 μM (for INa(V) and for IK(V)) by dotted lines.
Further analysis of the effects of both substances on the maximum current through ICa(V) showed that the channel currents were reduced by 40–80% over the entire voltage range from −60 to +40 mV (Figures 3a, b and 4a, b).
Figure 4.
Relative reduction of ICa(V) (a, b), INa(V) (c, d) and IK(V) (e, f) by harmaline (a, c, e) and harmane (b, d, e) (100 or 500 μM each) over the voltage range tested. Data were calculated from the I–V relations as shown in Figure 3.
A concentration of 100 μM of either substance revealed no obvious voltage dependence on INa(V), while harmaline was a little more effective in reducing the current (Figure 3c, d). There was also no clear difference in the effect of harmaline or harmane when 500 μM was applied (Figure 3c, d). In both cases, the reduction varied between 75 and 95% over the voltage range tested (Figure 4c, d).
Similarly, the reduction of IK(V) lacked a clear voltage dependence (Figure 3e, f). At excessive concentrations (500 μM), the effect of harmane exceeded that of harmaline (Figures 2 and 3e, f). While harmaline reduced the current by 25% (at lower voltages) to 45% (at more depolarised voltages) (Figure 4e), the effect of harmane increased from 50% at lower to 75% at higher depolarisations (Figure 4f).
As both substances were most efficient in reducing the ICa(V), in further experiments we focused on effects at these channels.
Time-course and use dependency of ICa(V)
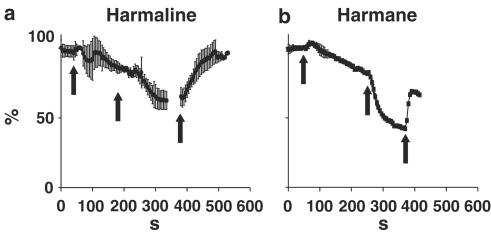
The time courses of reducing the peak ICa(V) by harmane or harmaline were not different. After application of either of the two drugs, a new lowered steady state of the reduction of the channel current was reached within 4–6 min (Figure 5a, b) subsequent to 25 and 100 μM harmaline (a) or harmane (b), respectively. This inhibition of ICa(V) was reversible (up to 85% of control values) with a similar time course (Figure 5) of recovery.
Figure 5.
Averaged time course with standard deviation of the effect of harmaline (a; n=8) and harmane (b; n=4) on peak ICa(V). Currents were elicited by a depolarisation from the holding potential of −80 to 0 mV. The first and second arrows in both panels mark the application of 25 and 100 μM of either substance, respectively. The third arrow indicates the beginning of wash-out of harmaline of harmane.
To test whether an open-channel state is necessary for the action of harmaline (a) and harmane (b), calcium channel activation was commenced 5 min after application of the substances. Figure 6 shows that the normalised peak channel currents, activated by a depolarisation to±0 mV, had reached a clear steady state and showed no further decline while the substances were still present (Figure 6). This indicates that neither of the two substances needed an open-channel state to reduce the ICa(V). As demonstrated in Figure 4, currents recovered were very similar during washout of the substances.
Figure 6.
Absence of a use-dependent reduction of ICa(V) by harmaline (A; 25 μM) and harmane (B; 100 μM). The voltage-driven activation of channel was interrupted for several minutes, while substances were applied (first arrow). When the activation was initiated after 350 s, a stable steady state had already been reached. Withdrawal of drugs (second arrow) resulted in a recovery of ICa(V).
Differential effects on ICa(L+N) and ICa(T)
The total peak channel current (ICa(V)) is composed of transient (ICa(T)) and sustained (ICa(L+N)) currents. While the sustained current (ICa(L+N)) at the end of the voltage jump (at 95 ms by depolarising to 0 mV) was largely reduced, there were only minor effects on the peak channel current (ICa(T)), indicating a different effect on these channels (Figure 1a, b).
This observation is underlined by the I–V relation. The ICa(L+N) was plotted over the whole voltage range before and after the application of harmaline and harmane (Figure 7a, b). Clearly, in the voltage range between −10 and +40 mV, the reduction of the current varies between 60 and up to 100% for more depolarised voltages. This is about 5–20% above the reduction of the total peak current, as shown in Figure 4(a, b), and further indicates that the ICa(L+N) is reduced to a degree higher than ICa(T). To isolate the transient component (ICa(T)) from the total calcium channel current (ICa(V)), we subtracted the ICa(L+N) from the total peak current. There was no clear indication for a reduction of this transient part of the current over most parts of the voltage range (Figure 7c, d). Therefore, the apparent reduction of the total peak current could be attributed mainly to the sustained component of the channel current.
Figure 7.
I–V relation for the sustained calcium channel current (ICa(L+N)), taken at the end of the depolarisation (a, b) and for the calculated transient peak current ICa(T) (c, d). Control curves (solid lines) and of the relative effect of harmaline (a, c) and harmane (b, d), both 100 μM (broken lines), are shown. Note that sustained and calculated peak voltage-activated calcium channel currents were differentially affected. While the sustained current was maximally reduced at depolarised voltages above −10 mV, the calculated peak current was mainly unaffected over the voltage range tested.
Discussion
Harmaline and harmane reduce ICa(V) voltage-activated calcium, sodium and potassium channel currents in rat DRG neurones over a wide voltage range and in a dose-dependent manner. Among these currents, ICa(L+N) turned out to be the most sensitive, being inhibited by 50% using 100.6 μM harmaline or 75.8 μM harmane, respectively. A concentration of 100 μM of either substance reduced the INa(V) only by 23.0±5.8 and 4.2±1.7%, respectively, and the IK(V) by 4.4±9.3 or 17.5±5.0% for a voltage step to 0 mV. For both substances, the effect on ICa(V) was reversible and was not use dependent. Furthermore, there was a clear specificity in reducing the sustained calcium channel current (L-/N-type calcium channel), while the transient current (T-type channel) was not significantly affected. This is partially supported by the findings of Shi et al. (2000; 2001), who used binding receptor assays to demonstrate an interaction of the related compound harmine with the 1,4-dihydropyridine-binding site of L-type calcium channels of cardiac cells.
While neurotoxicity of harmaline has been discussed in the literature over several years (O'Hearn & Molliver, 1993; Cobuzzi et al., 1994), neuroprotective effects of harmaline and harmane have also been described (Bonnet et al., 2000). Their possible mechanisms, however, remained uncertain. Our results may shed some light on the underlying mechanisms and at least two explanations shall be proposed: (1) reducing voltage-activated sodium channels, and (2) reducing the current through voltage-gated calcium channels. Each of these mechanisms by itself causes a reduced excitation of neurones, which might result in limiting excitotoxicity. However, considering that such a reduction of ICa(V) and INa(V) occurs simultaneously, it is most likely that the effectiveness of possible neuroprotective efficiency of harmaline and harmane will even increase.
Although the reduction of voltage-activated sodium channel currents by harmaline and harmane occurred at high concentrations (⩾100 μM), inhibition became evident at relatively small depolarisations (starting at −40 mV). Therefore, the threshold for the generation of action potentials might be shifted to less negative values. This in turn would result in less action potentials and therefore in a reduced firing activity of neurones, as was also described by Carpentier (1982).
While the fast inactivating, transient calcium channel current (T-type current) was not significantly affected by both substances, the sustained current (L-/N-type current) was obviously more sensitive, with a threshold concentration for harmaline and harmane below 10 μM. Voltage-gated calcium channels of the L-/N-type – which are mainly carrying the sustained current – are localised primarily at the postsynaptic terminal of neurones (Igelmund et al., 1996). Thus, the increase in the calcium concentration within these compartments is certainly a major intracellular signal for numerous processes and neuronal function. Consequently, harmaline and harmane have the potency to interfere also with synaptic transmission.
Recently, harmala alkaloids were hypothesised to reduce apoptosis by inhibition of mitochondrial membrane-transition pores (Lee et al., 2000). Apototic or necrotic neuronal death can be triggered by an increase in the intracellular calcium concentration (Han et al., 2001), which might be prevented by harmaline or harmane. Hence, the reduced rise of intracellular calcium, putatively occurring under the influence of harmaline and harmane, will make programmed cell death less likely. But our technique focuses on the actions of voltage-gated membrane channel currents and does not allow to specify any intracellular changes of the calcium level resulting from the calcium release from intracellular stores.
In summary, harmaline and harmane are able to lower voltage-gated calcium channel currents at concentrations that are likely to be sufficient for neuro-protective effects in vivo. This mechanism is likely to contribute to changes in excitability owing to β-carboline components.
Acknowledgments
We thank Professor Dr S. Cleveland at the Institute of Physiology at the University of Düsseldorf, for fitting the dose response data to the Langmuir equation. We also thank Mrs K. Göpelt for excellent technical assistance in preparing the cultures of dorsal root ganglion neurones.
Abbreviations
- BMI
Biomedical Instruments
- CNS
central nervous system
- DRG
dorsal root ganglion
- F-12
Hams F-12 medium
- GABAA
γ-aminobutyric acid a receptor subtype
- GHB
γ-hydroxybutyrate
- harmaline
1-methyl-β-carboline
- harmane
1-methyl-7-methoxy-3,4-dihydro-β-carboline
- 5-HT
5-hydroxytryptamin
- 5-HT2A
5-hydroxytryptamin 2A receptor
- 5-HT2C
5-hydroxytryptamin 2C receptor
- ICa(L+N)
sustained voltage-gated calcium channel current
- ICa(T)
transient voltage-gated calcium channel current
- ICa(V)
voltage-gated calcium channel current
- IK(V)
voltage-gated potassium channel current
- INa(V)
voltage-gated sodium channel current
- IC50
inhibitory concentration reducing 50%
- I–V curve
current–voltage relation curve
- MAO
monoaminoxidase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridin
- NHE
sodium–hydrogen exchange
- PC-12
rat adrenal pheochromocytoma
References
- ALBORES R., NEAFSEY E.J., DRUCKER G, FIELDS J.Z., COLLINS M.A. Mitochondrial respiratory inhibition by N-methylated beta-carboline derivatives structurally resembling N-methyl-4-phenylpyridine. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9368–9372. doi: 10.1073/pnas.87.23.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON N.J, ROBINSON E.S., HUSBANDS S.M., DELAGRANGE P., NUTT D.J., HUDSON A.L. Characterization of [(3)H]harmane binding to rat whole brain membranes. Ann. N. Y. Acad. Sci. 2003;1009:175–179. doi: 10.1196/annals.1304.020. [DOI] [PubMed] [Google Scholar]
- BONNET U., LENIGER T., WIEMANN M. Moclobemide reduces intracellular pH and neuronal activity CA3 neurones in guinea-pig hippocampal slices – implication for its neuroprotective properties. Neuropharmacology. 2000;39:2067–2074. doi: 10.1016/s0028-3908(00)00033-2. [DOI] [PubMed] [Google Scholar]
- CARPENTIER R.G. Effects of harmala alkaloids on transmembrane potentials of guinea-pig papillary muscles. Br. J. Pharmacol. 1982;75:207–212. doi: 10.1111/j.1476-5381.1982.tb08774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COBUZZI R.J., JR, NEAFSEY E.J., COLLINS M.A. Differential cytotoxicities of N-methyl-beta-carbolinium analogues of MPP+ in PC12 cells: insights into potential neurotoxicants in Parkinson's disease. J. Neurochem. 1994;62:1503–1510. doi: 10.1046/j.1471-4159.1994.62041503.x. [DOI] [PubMed] [Google Scholar]
- FOX A.P., NOWYCKY M.C., TSIEN R.W. Single-channel recordings of three types of calcium channels in chick sensory neurons. J. Physiol. 1987;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLENNON R.A., DUKAT M., GRELLA B., HONG S., COSTANTINO L., TEITLER M., SMITH C., EGAN C., DAVIS K., MATTSON M.V. Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors. Drug Alcohol Depend. 2000;60:121–132. doi: 10.1016/s0376-8716(99)00148-9. [DOI] [PubMed] [Google Scholar]
- HAN O.J., JOE K.H., KIM S.W., LEE H.S., KWON N.S., BAEK K.J., YUN H.Y. Involvement of p38 mitogen-activated protein kinase and apoptosis signal-regulating kinase-1 in nitric oxide-induced cell death in PC12 cells. Neurochem. Res. 2001;26:525–532. doi: 10.1023/a:1010917129951. [DOI] [PubMed] [Google Scholar]
- HILLE B. Ionic Channels of Excitable Membranes 1992Sunderland: Sinauer Associates Inc.2nd edn. [Google Scholar]
- HUDSON A.L., GOUGH R., TYACKE R., LIONE L., LALIES M., LEWIS J., HUSBANDS S., KNIGHT P., MURRAY F., HUTSON P., NUTT D.J. Novel selective compounds for the investigation of imidazoline receptors. Ann N. Y. Acad. Sci. 1999;881:81–91. doi: 10.1111/j.1749-6632.1999.tb09344.x. [DOI] [PubMed] [Google Scholar]
- IGELMUND P., ZHAO Y.Q., HEINEMANN U. Effects of T-type, L-type, N-type, P-type, and Q-type calcium channel blockers on stimulus-induced pre- and postsynaptic calcium fluxes in rat hippocampal slices. Exp. Brain Res. 1996;109:22–32. doi: 10.1007/BF00228623. [DOI] [PubMed] [Google Scholar]
- KIM D.H., JANG Y.Y., HAN E.S., LEE C.S. Protective effect of harmaline and harmalol against dopamine- and 6-hydroxydopamine-induced oxidative damage of brain mitochondria and synaptosomes, and viability loss of PC12 cells. Eur. J. Neurosci. 2001;13:1861–1872. doi: 10.1046/j.0953-816x.2001.01563.x. [DOI] [PubMed] [Google Scholar]
- KUHN W., MULLER T., GERLACH M., SOFIC E., FUCHS G., HEYE N., PRAUTSCH R., PRZUNTEK H. Depression in Parkinson's disease: biogenic amines in CSF of ‘de novo' patients. J. Neural Transm. 1996;103:1441–1445. doi: 10.1007/BF01271258. [DOI] [PubMed] [Google Scholar]
- LEE C.S., HAN E.S., JANG Y.Y., HAN J.H., HA H.W., KIM D.E. Protective effect of harmalol and harmaline on MPTP neurotoxicity in the mouse and dopamine-induced damage of brain mitochondria and PC12 cells. J. Neurochem. 2000;75:521–531. doi: 10.1046/j.1471-4159.2000.0750521.x. [DOI] [PubMed] [Google Scholar]
- LOUIS E.D., ZHENG W., JUREWICZ E.C., WATNER D., CHEN J., FACTOR-LITVAK P., PARIDES M. Elevation of blood beta-carboline alkaloids in essential tremor. Neurology. 2002;59:1940–1944. doi: 10.1212/01.wnl.0000038385.60538.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHER P., DAVIS J.B. The role of monoamine metabolism in oxidative glutamate toxicity. J. Neurosci. 1996;16:6394–6401. doi: 10.1523/JNEUROSCI.16-20-06394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY T., GREUBE A., STRAUSS S., HEINEKE D., LEHMANN J., ROMMELSPACHER H. Comparison of the in vitro binding characteristics of the beta-carbolines harmane and norharmane in rat brain and liver and in bovine adrenal medulla. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;349:308–317. doi: 10.1007/BF00169298. [DOI] [PubMed] [Google Scholar]
- MCCORMICK S.J., TUNNICLIFF G. Inhibitors of synaptosomal gamma-hydroxybutyrate transport. Pharmacology. 1998;57:124–131. doi: 10.1159/000028233. [DOI] [PubMed] [Google Scholar]
- O'HEARN E., MOLLIVER M.E. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience. 1993;55:303–310. doi: 10.1016/0306-4522(93)90500-f. [DOI] [PubMed] [Google Scholar]
- PRZEDBORSKI S., JACKSON-LEWIS V. Experimental developments in movement disorders: update on proposed free radical mechanisms. Curr. Opin. Neurol. 1998;11:338–339. doi: 10.1097/00019052-199808000-00009. [DOI] [PubMed] [Google Scholar]
- ROMMELSPACHER H., NANZ C., BORBE H.O., FEHSKE K.J., MULLER W.E., WOLLERT U. 1-Methyl-beta-carboline (harmane), a potent endogenous inhibitor of benzodiazepine receptor binding. Naunyn-Schmiedeberg's Arch. Pharmacol. 1980;314:97–100. doi: 10.1007/BF00498436. [DOI] [PubMed] [Google Scholar]
- ROMMELSPACHER H., SCHMIDT L.G., MAY T. Plasma norharman (beta-carboline) levels are elevated in chronic alcoholics. Alcohol. Clin. Exp. Res. 1991;15:553–559. doi: 10.1111/j.1530-0277.1991.tb00559.x. [DOI] [PubMed] [Google Scholar]
- SHI C.C., CHEN S.Y., WANG G.J., LIAO J.F., CHEN C.F. Vasorelaxant effect of harmane. Eur. J. Pharmacol. 2000;390:319–325. doi: 10.1016/s0014-2999(99)00928-0. [DOI] [PubMed] [Google Scholar]
- SHI C.C., LIAO J.F., CHEN C.F. Spasmolytic effects of three harmala alkaloids on guinea-pig isolated trachea. Pharmacol. Toxicol. 2001;89:259–264. doi: 10.1034/j.1600-0773.2001.d01-157.x. [DOI] [PubMed] [Google Scholar]
- SPIJKERMAN R., VAN DEN EIJNDEN R., VAN DE MHEEN D., BONGERS I., FEKKES D. The impact of smoking and drinking on plasma levels of norharman. Eur. Neuropharmacol. 2002;12:61–71. doi: 10.1016/s0924-977x(01)00143-2. [DOI] [PubMed] [Google Scholar]
- SPLETTSTOESSER F., BONNET U., WIEMANN M., BINGMANN D., BÜSSELBERG D. Modulation of voltage gated channel currents by harman and harmaline. Proc. Neurosci. 2003. p. 766.2. [DOI] [PMC free article] [PubMed]
- STOHLER R., HUG I., KNOLL B., MOHLER B., LADEWIG D. Initial results with withdrawal treatments of male and female participants in the diversified Janus opiate prescription project in Basel. Schweiz. Rundsch. Med. Prax. 1996;48:1537–1541. [PubMed] [Google Scholar]