Abstract
In flowering plants, penetration of the pollen tube through stigma, style, and transmitting tract is essential for delivery of sperm nuclei to the egg cells embedded deeply within female tissues. Despite its importance in plant reproduction, little is known about the underlying molecular mechanisms that regulate the navigation of the pollen tube through the stigma, style, and transmitting tract. Here, we report the identification and characterization of an Arabidopsis thaliana gene, VANGUARD1 (VGD1) that encodes a pectin methylesterase (PME)-homologous protein of 595 amino acids and is required for enhancing the growth of pollen tubes in the style and transmitting tract tissues. VGD1 was expressed specifically in pollen grain and the pollen tube. The VGD1 protein was distributed throughout the pollen grain and pollen tube, including the plasma membrane and cell wall. Functional interruption of VGD1 reduced PME activity in the pollen to 82% of the wild type and greatly retarded the growth of the pollen tube in the style and transmitting tract, resulting in a significant reduction of male fertility. In addition, the vgd1 pollen tubes were unstable and burst more frequently when germinated and grown on in vitro culture medium, compared with wild-type pollen tubes. Our study suggests that the VGD1 product is required for growth of the pollen tube, possibly via modifying the cell wall and enhancing the interaction of the pollen tube with the female style and transmitting tract tissues.
INTRODUCTION
The fertilization of flowering plants requires delivery of the sperm to the egg. The process begins with deposition of pollen grains on the stigmatic tissue. The compatible interaction between pollen and stigmatic cells triggers the hydration and germination of the pollen. The resulting pollen tube invades the stigmatic tissue, penetrates the style, navigates through the transmitting tract, and then is directed to the egg embedded in the ovule (Hülskamp et al., 1995b; Wilhelmi and Preuss, 1996; Ray et al., 1997; Lennon and Lord, 2000; Palanivelu and Preuss, 2000; Johnson and Preuss, 2002; Lord and Russell, 2002; Preuss, 2002; Kim et al., 2003; Palanivelu et al., 2003). Several studies have revealed that components such as lipids on pollen and stigma are important for transportation of water from stigmatic cells to pollen (Elleman et al., 1992; Preuss et al., 1993; Hülskamp et al., 1995a; Wolters-Arts et al., 1998; Zinkl et al., 1999; Fiebig et al., 2000). The morphological studies also show that a compatible interaction between pollen tubes and stigmatic cells is required for triggering degradation of the stigmatic and stylar cell walls (Atkinson et al., 1993; Hiscock et al., 1994; Johnson and Preuss, 2002; Lord and Russell, 2002). However, the genetic and molecular mechanisms that control the penetration of pollen tubes through stigmatic and stylar tissues are poorly understood.
In plant species such as lily (Lilium longiflorum) with a hollow stigma and style, adhesion between the pollen tube and transmitting tract epidermis is required for growth of the pollen tube toward ovule. The stylar matrix secreted by the transmitting tract epidermis also has been suggested to play an important role in such adhesion (Jauh et al., 1997; Lord, 2000, 2001; Lord and Russell, 2002; Kim et al., 2003; Park and Lord, 2003). Similarly, in species such as Arabidopsis thaliana with closed style, the intercellular space of the transmitting tract is filled completely with the nutrient-rich extracellular matrix (ECM). The pollen tube grows faster, longer, and more precisely through ECM than in vitro, indicating that the interaction between pollen tube and the transmitting tract ECM is important for the mobilization of pollen tubes in the transmitting tract (Lennon et al., 1998; Lennon and Lord, 2000; Lord, 2000). Nevertheless, despite their importance for the delivery of the sperm to the egg, little is known about the underlying molecular mechanisms that regulate the interaction of pollen tubes with female floral tissues.
Pectin methylesterases (PMEs) have been implicated to play roles in different physiological processes of plant development via modification of the cell wall (Bordenave and Goldberg, 1993; Kagan-Zur et al., 1995; Guglielmino et al., 1997; Wakeley et al., 1998; Futamura et al., 2000; Micheli et al., 2000; Ren and Kermode, 2000; Li et al., 2002). The plant cell wall is a polymeric network of crystalline cellulose microfibrils embedded in a hydrophilic matrix of hemicelluloses and pectin (Denés et al., 2000). PMEs contribute to cell development by regulating the mechanical and chemical properties of plant cell walls via demethylesterification of cell wall pectin. Changes in the mechanical strength and rigidity of the pollen tube wall also have been proposed to be important for the process of pollen tube elongation and its interaction with female floral tissues (Franklin-Tong et al., 1996; Holdaway-Clarke et al., 1997; Franklin-Tong, 1999). Furthermore, several studies have led to the identification of pollen tube–specific PMEs, indicating that plant PMEs may be involved in the processes of pollen tube development and its interaction with female floral tissues (Wakeley et al., 1998; Futamura et al., 2000; Li et al., 2002). However, the actual biological functions of the pollen tube–specific PMEs have not been elucidated. Here, we report the identification and characterization of a novel Arabidopsis PME mutation, vanguard1 (vgd1), that significantly retards growth of pollen tubes in the style and transmitting tract. The VGD1 gene encodes a PME-homologous protein of 595 amino acids and is expressed specifically in the pollen grain and pollen tube. This study suggests that VGD1 plays an important role in the growth of pollen tubes in the female floral tissues, possibly via modifying the cell wall and thus enhancing the interaction of the pollen tube with the female style and transmitting tract.
RESULTS
Isolation of the vgd1 Mutant
The vgd1 mutant was identified by its reduced fertility in a phenotypic screen of the enhancer-trap dissociation (Ds) insertion lines in Arabidopsis ecotype Landsberg erecta (Sundaresan et al., 1995). The homozygous vgd1 plant produced smaller and shorter siliques with fewer seeds (Figures 1A and 1B) compared wild-type plants (Figures 1C and 1D). No morphological abnormality in other floral and vegetative parts was observed (Figures 1E and 1F).
Figure 1.
Phenotype of the vgd1 Mutant Compared with the Wild Type.
(A) A vgd1 mutant plant with reduced fertility indicated by the smaller siliques with a few seeds.
(B) A vgd1 silique showing that the seeds were produced only in the upper part of the silique.
(C) A wild-type plant with full fertility indicated by the siliques with a full seed set.
(D) A wild-type silique showing the full seed set.
(E) A vgd1 flower with normal floral organs and pollen grains.
(F) A wild-type flower with normal floral organs and pollen grains.
sd, seeds. Bars = 1 cm in (A) and (C) and 1 mm in (B) and (D) to (F).
vgd1 Was a Male Gametophytic Mutation That Cosegregated with a Single Ds Insertion
To investigate if the vgd1 mutation affected male or female function, the vgd1 mutant plant was used as male or female to cross with wild-type plants. When a homozygous vgd1 plant was used as a female in a cross with a wild-type plant, nearly all ovules (449 out of 455) were able to produce viable seeds as those in the wild type, indicating that the vgd1 mutation did not affect female fertility. In a reciprocal cross, however, only a few ovules in the upper part of the silique were fertilized and developed into seeds. When a heterozygous vgd1 plant was used as a male in a cross with wild-type plants, the F1 seedlings segregated 1:40.8 (80:3261) kanamycin resistant to kanamycin sensitive instead of the expected 1:1 segregation. This result indicated that the vgd1 mutation led to a male gametophytic defect. In summary, the vgd1 mutation resulted in defects of male gametophytic function but did not affect female gametophytic function.
When a heterozygous vgd1 plant was used as a female in a cross with a wild-type plant, the F1 seedlings showed an average segregation ratio of approximate 1:1 (321:316) kanamycin resistant (Kanr) to kanamycin sensitive (Kans). All Kanr F1 seedlings gave rise to fully fertile plants. All F2 families resulting from the selfing of Kanr F1 plants showed segregation of the vgd1 mutant phenotype. When a homozygous vgd1 plant was used as a female in a cross with a wild-type plant, all F1 seedlings were resistant to kanamycin (Kanr) upon germination and fully fertile when matured. All resulting F2 families also showed segregation of the vgd1 mutant phenotype. These results showed tight linkage of a single recessive vgd1 mutation to the kanamycin selection marker carried by a single Ds element in the vgd1 genome. We further confirmed a single Ds insertion with DNA gel blotting hybridization using a 755-bp Ds 5′-end fragment as a probe (data not shown).
The vgd1 Mutation Retarded Growth of the Pollen Tube in the Style and Transmitting Tract
Genetic studies showed that only a few seeds were produced in the upper part of wild-type or vgd1 siliques when pollinated with the vgd1 pollen. To investigate the cause of the abnormal seed set, we compared the growth rate of vgd1 pollen tubes in the style and transmitting tract to that of wild-type pollen tubes using aniline blue staining and scanning electron microscopy techniques. As shown in Figure 2A, the vgd1 pollen tubes were able to germinate and grow on stigmatic cells similar to the wild type (Figure 2B). Therefore, it is unlikely that the vgd1 mutation affects the ability of pollen germination on female stigmatic tissue. Transmitting electronic microscopic (TEM) observations showed that the vgd1 pollen tubes also grew within stigmatic cell walls and intercellular spaces in the style and transmitting tract like wild-type pollen tubes (Figures 2C to 2F). This result indicated that the vgd1 mutant pollen tube still was able to burrow into the stigmatic cell wall as wild-type pollen tubes. However, the penetration rate of vgd1 pollen tubes was reduced compared with the wild type. Wild-type pollen tubes were able to pass through the style in 4 h after pollination (hap) (Figure 3A) and reached the base of the transmitting tract by 12 hap (Figure 3C). By contrast, the vgd1 pollen tubes were restricted within the stigmatic tissue at 4 hap (Figure 3B) and took ∼24 h to pass through the style (Figure 3D); they only reached approximately half a transmitting tract length at 48 hap (Figure 3E). This result was consistent with the fact that seeds were produced only in the upper part of a wild-type or vgd1 mutant silique when pollinated with the vgd1 pollens.
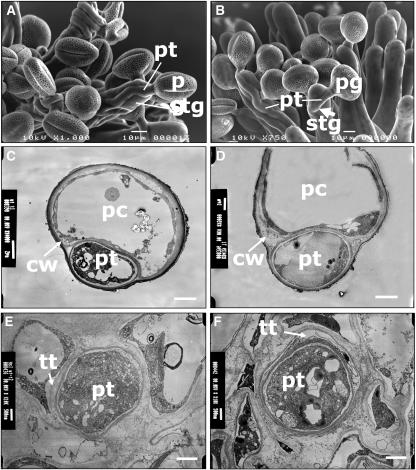
Figure 2.
Electronic Microscopic Observation of vgd1 Pollen Tubes Compared with the Wild-Type Pollen Tubes.
(A) Scanning electron microscopy of vgd1 pollen tubes on wild-type stigma.
(B) Scanning electron microscopy of wild-type pollen tubes on wild-type stigma. The arrow indicates the invasion of a pollen tube into a stigmatic cell.
(C) TEM of a papillary cell transversal section, showing a vgd1 pollen tube inside the cell wall of the papillary cell.
(D) TEM of a papillary cell transversal section, showing a wild-type pollen tube inside the cell wall of the papillary cell.
(E) TEM of a style transversal section, showing a vgd1 pollen tube inside the transmitting tract.
(F) TEM of a style transversal section, showing a wild-type pollen tube inside the transmitting tract.
cw, cell wall; pc, papillary cell; pg, pollen grain; pt, pollen tube; stg, stigmatic cell; tt, transmitting tract. Bars = 10 μm in (A) and (B), 2 μm in (C) and (D), and 1 μm in (E) and (F).
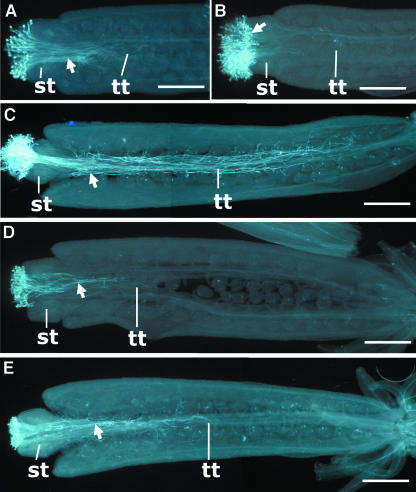
Figure 3.
Growth Patterns of the vgd1 Pollen Tubes in Female Floral Tissues Compared with Those of Wild-Type Pollen Tubes.
(A) Growth pattern of the wild-type pollen tubes in the wild-type female organs 4 hap, showing that the pollen tubes had penetrated through the style tissue and reached the upper end of transmitting tract.
(B) Growth pattern of the vgd1 pollen tubes in the wild-type female organs 4 hap, showing that the pollen tubes had not been able to penetrate through the style tissue.
(C) Growth pattern of the wild-type pollen tubes in the wild-type female organs 12 hap, showing that the pollen tubes almost had reached the base of the transmitting tract.
(D) Growth pattern of the vgd1 pollen tubes in the wild-type female organs 24 hap, showing that the pollen tubes had reached only approximately one-fifth of the transmitting tract length.
(E) Growth pattern of the vgd1 pollen tubes in the wild-type female organs 48 hap, showing that the pollen tubes had reached approximately half of the transmitting tract length.
st, style; tt, transmitting tract. The arrows indicate the pollen tubes. Bars = 200 μm.
The vgd1 Pollen Tube Was Unstable in Vitro
In vitro germination showed that the vgd1 pollen tubes were unstable when cultured in vitro. Eighty-four percent of wild-type pollen could germinate in a wide range of in vitro conditions. Wild-type pollen tubes grew straight on the medium surface and were able to elongate up to an average length of 350 μm after an overnight incubation (Figure 4D). By contrast, although ∼83% of vgd1 pollens could germinate in an optimized in vitro condition (see Methods), they were shorter, grew more slowly, and had an unusual shape (Figure 4C) compared with the wild-type pollen tubes germinated in the same conditions (Figure 4D). In all, 93.8% (334 out of 356) of germinating vgd1 pollen tubes burst (Figure 4E), indicating that the vgd1 pollen tubes were structurally unstable. By contrast, bursting of wild-type pollen tubes was only occasionally observed, at a very low rate of 3.7% (12 out of 328). These results suggested that VGD1 might be involved in stiffening the pollen tube wall and enhancing its interaction with the medium surface.
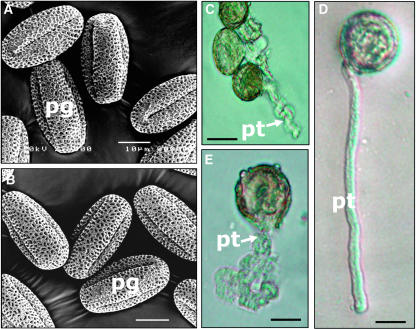
Figure 4.
Morphology and in Vitro Germination of vgd1 Pollen Compared with Wild-Type Pollen.
(A) Scanning electron microscopy of wild-type pollen grains.
(B) Scanning electron microscopy of vgd1 pollen grains.
(C) to (E) Growing vgd1 and wild-type pollen tubes.
(C) The unusual shape of the vgd1 pollen tube.
(D) A wild-type pollen tube.
(E) A burst of a vgd1 pollen tube.
pt, pollen tube; pg, pollen grain. Bars = 10 μm in (A) and (B) and 20 μm in (C) to (E).
The vgd1 Pollen Tube Is Morphologically Normal When Germinated on Stigma and Is Not Defective in Guidance
The scanning electronic microscopic observations showed that the vgd1 pollen appeared morphologically normal (Figures 4A and 4B) and had the same germination rate (>95%) on stigmatic cells as wild-type pollen. There were no morphological differences between the vgd1 and wild-type pollen tubes when germinated on stigmatic cells of both vgd1 and wild-type stigmas (Figures 2A and 2B). In addition, the scanning electron microscopy observations showed that the vgd1 pollen tubes were directed to ovules normally as wild-type pollen tubes (data not shown), indicating that the vgd1 mutation did not affect pollen tube guidance.
Molecular Cloning of the VGD1 Gene
To identify the VGD1 gene, we used thermal asymmetric interlaced (TAIL)-PCR (Liu et al., 1995; Grossniklaus et al., 1998) to obtain the genomic flanking sequences adjacent to both ends of the Ds element in vgd1. Sequencing the TAIL-PCR products revealed that a Ds element had inserted into the second exon of a predicted gene At2g47040 on chromosome II (BAC F14M4, AC004411) (Figure 5A) in the vgd1 genome.
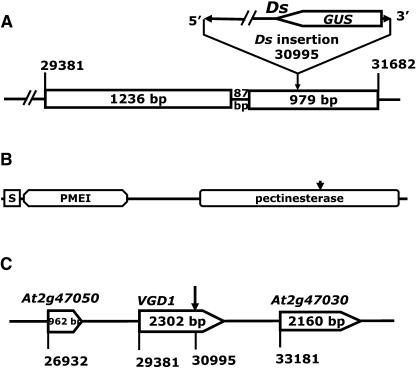
Figure 5.
The Genomic Organizations of VGD1 and the Structure of VGD1 Protein.
(A) Genomic organization of the VGD1 gene. The numbers over the vertical lines indicate the positions of the start and end nucleotides of the fragment in the BAC clone. The boxes indicate the positions of exons, and the intervening lines indicate the positions of introns. The numbers in the boxes indicate the sizes of the fragments. The arrow points out the Ds insertion site.
(B) The structure of VGD1, showing the positions of the secretive signal peptide (S), PMEI, and PME domains. The arrow indicates the Ds insertion site.
(C) Genomic organization of the VGD1 locus and the Ds insertion. The numbers under the vertical lines indicate the positions of start nucleotides of the fragment in BAC clone F14M4. The numbers in the boxes indicate the sizes of the transcribed regions.
To confirm the identification of VGD1, a 5.131-kb genomic DNA fragment, including the predicted promoter, transcripted region, and 3′-end nontranscribed region, was amplified by high-fidelity PCR and introduced into the vgd1 homozygous plants through Agrobacterium tumefaciens–mediated infiltration. Thirty-six independent transformants were obtained in a screen. Eighteen of the thirty-six T1 seedlings gave rise to fully fertile plants. The other transformants exhibited variable restorations of male fertility. In the T2 generation, all hygromycin-selected seedlings from the 18 fully fertile families gave rise to normal fully fertile plants, whereas nonselected T2 plants showed the segregation of the vgd1 phenotype with concordance of hygromycin-sensitive segregation. The results show that the 5.131-kb genomic fragment from chromosome II contained all the genetic information required for normal functioning of VGD1. A BLASTN search of the databases showed that an 1823-bp cDNA sequence (AJ250430) in the GenBank database matched the predicted 1952-bp mRNA sequence (AY091768) from gene At2g47040. We used RT-PCR to clone the full-length cDNA. The cloned cDNA (AY830948) was 1952 nucleotides in length, which was consistent in size to a band shown by RNA gel blot hybridization (Figure 7A). To confirm the genetic function of cloned VGD1 cDNA, the 1952-bp cDNA fragment was fused to the predicted VGD1 promoter (a 1513-bp fragment flanking the 5′-end of the VGD1 coding region) and VGD1 terminator (a 1786-bp fragment flanking the 3′-end of the VGD1 coding region). The resulting construct [PVGD1-VGD1(cDNA)-TVGD1] was subcloned into pCAMBIA1300 Ti-plasmid vector and introduced into the homozygous vgd1 mutant plants. Twenty-four independent transformants were obtained in a screen. Eight of the twenty-four T1 seedlings gave rise to fully fertile plants. Others exhibited a variable restoration of male fertility. In the T2 generation, all hygromycin-selected seedlings from the eight families gave rise to fully fertile plants, whereas nonselected T2 plants showed segregation of the vgd1 phenotype. These results showed that the 1952-bp mRNA sequence was sufficient to encode the functional VGD1 protein.
Figure 7.
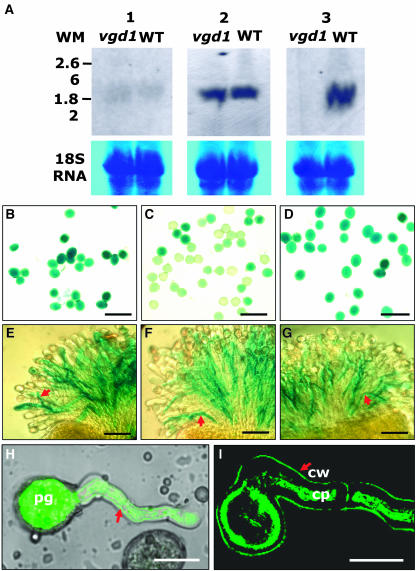
Promoter Activities of VGD1, At2g47030, and At3g62170 Monitored by GUS Activity and Subcellular Localization of VGD1-GFP.
(A) Expression of VGD1, At2g47030, and At3g62170 in the wild-type and vgd1 flowers revealed by RNA gel blot hybridization, showing that VGD1 was expressed only in wild-type floral tissue (3), whereas At2g47030 and At3g62170 were expressed in both vgd1 and wild-type floral tissues (1 and 2), respectively. Thirty micrograms of total RNA was loaded in each lane. The bottom panels are the loading controls showing the 18S RNA. WT, wild-type flower total RNAs; vgd1, vgd1 flower total RNA; WM, RNA molecular weight markers; 1, hybridized with At2g47030-specific probe; 2, hybridized with At3g62170-specific probe; 3, hybridized with VGD1 (At2g47040)-specific probe.
(B) The PVGD1-GUS-TNOS transgenic pollen grains, showing the GUS activity in the pollen grains.
(C) The PAt2g47030-GUS-TNOS transgenic pollen grains, showing the GUS stain in pollen grains.
(D) The PAt3g62170-GUS-TNOS transgenic pollen grains, showing the GUS stain in pollen grains.
(E) The PVGD1-GUS-TNOS transgenic pollen tubes, showing the GUS stain in the transgenic pollen tubes germinating in the wild-type stigmatic tissues.
(F) The PAt2g47030-GUS-TNOS transgenic pollen tubes, showing the GUS stain in the transgenic pollen tubes germinating in the wild-type stigmatic tissues.
(G) The PAt3g62170-GUS-TNOS transgenic pollen tubes, showing the GUS stain in the transgenic pollen tubes germinating in the wild-type stigmatic tissues.
(H) A transgenic pollen tube (arrow) showing that the VGD1-GFP fusion protein is distributed throughout the whole pollen tube. The picture was merged from a GFP signal and an image using transmitted light. pg, pollen grain.
(I) A section of a transgenic pollen tube showing the localization of VGD1-GFP fusion protein in the wall of the pollen tube after plasmolysis. The picture was scanned for GFP signal without light transmission. cw, cell wall; cp, cell plasma. The arrow indicates the pollen tube wall.
Bars = 100 μm in (B) to (G) and 25 μm in (H) and (I).
VGD1 Encodes a PME-Homologous Protein
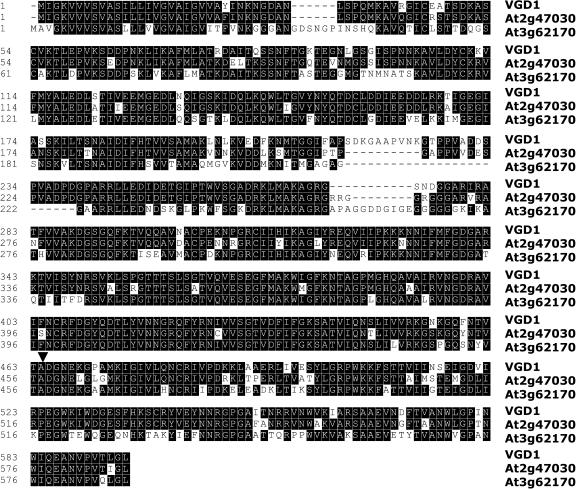
The VGD1 mRNA encodes a PME-homologous protein of 595 amino acids, which consists of a secretion-related transmembrane domain, a PME inhibitor (PMEI)-homologous domain, and a pectinesterase-homologous domain (Figure 5B). A BLASTP search was performed using the entire amino acid sequence of the predicted VGD1 protein and showed that there was a group of PME proteins in the Arabidopsis genome with >50% amino acid sequence identity to VGD1. Specifically, VGD1 had 85% (514/598) identity and 91% (550/598) similarity to the predicted At2g47030 gene product (T02184; T52330) and 69% (426/611) identity and 81% (503/611) similarity to the predicted At3g62170 gene product (Figure 6). In addition, VGD1 is located in a genomic locus consisting of three homologous genes, At2g47030, VGD1 (At2g47040), and At2g47050 (Figure 5C). The predicted At2g47050 protein (T02182) has only 216 amino acids and contains only one PMEI domain, which is likely to be a unique PMEI gene sharing a lower homology (27% identity) with the PMEI domain of VGD1.
Figure 6.
The Alignment of VGD1, At2g47030, and At3g62170 Proteins.
The black boxes indicate the identical amino acids. The arrowhead points out the Ds insertion site in VGD1.
The vgd1 Mutation Reduced the Total Pectin Demethylesterification Activity of PMEs in Pollen Grain
Molecular cloning shows that the VGD1 gene encoded a PME-homologous protein. PMEs belong to a family of cell wall enzyme proteins that act in the modification of cell walls via demethylesterification of cell wall pectin. To investigate whether the vgd1 mutation affected the PME activity in pollen and the pollen tube, we compared the total pectin demethylesterification activity of PMEs in the vgd1 pollen grain to that in wild-type pollen grain. The result showed that the total pectin demethylesterification activity of PMEs in the vgd1 pollen grain decreased ∼18% compared with that of wild-type pollen grain. This result indicated that VGD1 encoded a PME enzyme and that the retardation of pollen tube growth was caused by the loss of VGD1 PME activity in the pollen tubes.
VGD1 and Its Homologs, At2g47030 and At3g62170, All Were Expressed Specifically in the Pollen Grain and Pollen Tube
RNA gel blot hybridization showed that VGD1, At2g47030, and At3g62170 were expressed specifically in flowers (Figure 7A). We further investigated their expression in pollen and elongating pollen tubes using transgenic plants containing the PVGD1-β-glucuronidase (GUS) transcriptional fusion genes. Histochemical staining for GUS activity was used to monitor the activity of different promoters. The GUS staining patterns showed that all three genes were expressed specifically in pollen grains and pollen tubes (Figures 7B to 7G). Because they were expressed in pollen grains before pollination, the expressions of VGD1, At2g47030, and At3g62170 were unlikely to be induced by pollination. This finding showed that the expression of VGD1 is consistent with a function in pollen tube development.
Comparing the densities of GUS stains revealed that the expressional levels of three genes were very different. The expressional signal of VGD1 was the strongest, and that of At2g47030 was the weakest. We further used quantitative real-time PCR to compare the expression levels of VGD1 and its homologs, At2g47030 and At3g62170, in wild-type plants. The results showed that the level of VGD1 mRNA transcription was ∼2.7 times higher than that of At3g62170 and 21 times higher than that of At2g47030. These results were very suggestive that VGD1 may be functionally redundant to its homologs, At2g47030 and At3g62170. If so, VGD1 must be a main contributor to the process.
The At2g47030 Product Has the Same Biological Function as VGD1 Protein
As shown above, the VGD1 protein shares a very high sequence identity to At2g47030 and At3g62170 predicted proteins. To study whether these three genes are functionally redundant to each other, we overexpressed At2g47030 and At3g62170 in pollen and pollen tubes of homozygous vgd1 plants under the control of the VGD1 promoter. More than 40 independent transformants were obtained from each transformation. In the At2g47030 transgenic T1 population, three plants were fully fertile and the others exhibited variable restorations of the vgd1 phenotype. Further genetic and molecular analysis confirmed the complementation of vgd1 phenotype by overexpression of the At2g47030 coding region (data not shown). This result indicated that At2g47030 might have the same genetic function as VGD1. However, in the At3g62170 transgenic T1 population, all plants exhibited the typical vgd1 phenotype, indicating that At3g62170 may not have the same genetic function as VGD1.
The VGD1–Green Fluorescent Protein Fusion Was Distributed in the Whole Pollen Tube, Including the Plasma Membrane and Pollen Tube Wall
If VGD1 encodes a PME enzyme protein that acts in cell wall modification, it should be localized to the cell wall of the pollen tube. To investigate whether the subcellular localization of VGD1 protein was associated with the pollen tube wall, a green fluorescent protein (GFP) reporter protein was fused to the C terminus of VGD1 and expressed specifically in pollen and the pollen tube under the control of the VGD1 promoter in the wild-type background plants. In the transgenic plant, the GFP signal was first detected in the mature pollen (data not shown). After germination, the GFP signal was detected in whole pollen tube (Figure 7H). To investigate whether the GFP signal was located in the pollen tube wall, the germinating transgenic pollen tubes were treated with 40% sucrose buffer (see Methods) to separate the cell wall from the cytoplasm. The resulting plasmolysis image showed that the GFP signal was detected in the cell wall region of the pollen tube (Figure 7I). All these data suggest that VGD1 PME protein may be required all the time during growth of the pollen tube, and its subcellular localization is consistent with a function on the pollen tube wall.
DISCUSSION
VGD1 Is Important for Growth of the Pollen Tube in Female Floral Tissue
The vgd1 mutation we report here is a novel mutation that affects growth of pollen tubes in the Arabidopsis style and transmitting tract. Genetic studies indicated that the vgd1 mutation was a male gametophytic mutation and affected only the growth of pollen tubes. The vgd1 pollen tubes grew much more slowly than wild-type pollen in the style and transmitting tract, although they were able to germinate on the surface of the stigmatic cell and invaded into the stigmatic cell. Our observations also indicated that the vgd1 mutation did not obviously affect the growth rate of the pollen tube on the surface of stigmatic cells, and the retardation of pollen tube extension occurred mainly in the style and transmitting tract. Therefore, we conclude that the VGD1 product is required for enhancing the growth of the pollen tube in the style and transmitting tract.
The VGD1 protein was highly homologous to PMEs, a group of cell wall proteins. Consistently, shutting down of VGD1 function reduced PME activity in the pollen grain and indicated that VGD1 protein might function as a PME enzyme. In higher plants, different PME isoforms are encoded by multiple gene families (Richard et al., 1996; Micheli et al., 1998) and act in the demethylesterification of cell wall pectin, contributing to different processes of plant development (Micheli, 2001). Therefore, the VGD1 product may be involved in growth of the pollen tube by modification of the pollen tube wall. VGD1 was expressed specifically in pollen grains and pollen tubes. If VGD1 is a PME protein, it is a pollen- and pollen tube–specific PME because it is not expressed elsewhere.
The null vgd1 mutation did not block the growth of pollen tubes in the style and transmitting tract completely. A possible reason for this finding is that the VGD1 protein may act redundantly with At2g47030. The At2g47030 protein had a high amino acid sequence identity to VGD1 and exhibited the same expression pattern as VGD1. The complementation experiment also showed that the At2g47030 gene product had the same biological function as the VGD1 protein. Disruption of VGD1 function significantly reduced the growth rate of pollen tubes in the style and transmitting tract. If these two genes act redundantly, VGD1 must be the major contributor to the process.
As discussed above, the expression level of At2g47030 was 21 times lower than that of VGD1. Expression of At2g47030 at the same level as VGD1 using VGD1 promoter could complement the vgd1 mutant phenotype. This result indicates that different PMEs might have the same biological function when they are expressed at the same level and location.
The At3g62170 gene product also was highly homologous to VGD1 protein and had the same expression profile as VGD1. However, we failed to complement the vgd1 mutant by overexpression of At3g62170 protein using the VGD1 promoter. It is possible that At3g62170 might not have the same function as VGD1. This result showed that homologous genes with high sequence similarity and even the same location of expression might not unconditionally have the same biological function. We still do not know the actual biological function of the At3g62170 gene product. The At3g62170 protein also might have lost its function in pollen tube growth during evolution, although it still is expressed actively in the pollen tube.
The VGD1 Product May Be Involved in Building up the Strength of the Pollen Tube Wall
The in vitro germination study showed that the vgd1 pollen tube was very unstable when grown in the culture medium. Although it was able to germinate in vitro, the resulting pollen tubes often burst after germination. The result indicated that the vgd1 mutation altered the mechanical characteristics of the pollen tube wall. In other words, VGD1 is involved in the pollen tube wall.
Studies have shown that mature PMEs could have different modes of action. They may act either randomly or linearly along the pectin chain (Markovic and Kohn, 1984; Micheli, 2001). It is commonly believed that random demethylesterification of pectin depends on acidic PMEs, whereas linear demethylesterification of pectin requires basic PMEs (Micheli, 2001). The VGD1 protein exhibited a predicted basic isoelectric point of 8.9. Therefore, VGD1 may act by linear demethylesterification of pollen wall pectin. The linear demethylesterification on homogalacturonans by PME gives rise to blocks of free carboxyl groups that could interact with Ca2+, creating a pectate gel (Goldberg, 1996). The Ca2+ pectate gel is believed to contribute to cell wall stiffening and cell attachment by limitation of the action of endopolygalaturonases and formation of Ca2+ pectate gel lawn. In the vgd1 mutant, therefore, the loss of VGD1 function could lead to loss or reduction of formation of Ca2+ pectate gel lawn on the surface of the pollen tube wall, resulting in loss or reduction of strength of the pollen tube wall. As a result, the vgd1 pollen tube could have burst easily when grown in vitro.
Interestingly, we did not observe any burst pollen tubes on stigmatic cells. Possibly, the hydration expansion of pollen in vivo is milder or more controlled compared with our in vitro conditions. This finding implies that germination and development of pollen tubes are different in vivo and in vitro, and it is known that growth of pollen tubes in vivo is subject to intricate regulation. The interaction between pollen tubes and stigmatic cells may be important for the stabilization of the pollen tubes. The pollen tube grew within cell walls of stigmatic papillar cells; presumably the surrounding layer of the papillar cell wall also could provide additional physical support to the pollen tube wall.
The VGD1 Product May Involve the Interaction between the Pollen Tube and Female Floral Tissues via the Modification of Cell Walls
Microscopic observations showed that the vgd1 mutation did not affect the growth rate of pollen tubes on the surface of stigmatic cells; however, growth of pollen tube was retarded greatly in the style and transmitting tract. This finding indicated that the VGD1 product might be involved in the interaction between pollen tubes and female floral tissues via the modification of cell walls. Because VGD1 encodes a PME protein, it may act in the degradation of the papillary cell wall to promote the invasion of the pollen tube into the papillary and stylar cells and modification of the pollen tube wall to enhance its interaction with female floral tissues.
On one hand, the retardation of pollen tubes in the vgd1 mutant may result from the inefficient degradation of stigmatic and stylar cell walls. Studies have shown that many factors affect the mode of action of PMEs, such as pH, the initial degree of demethylesterification of pectins, and the presence of cations (Catoire et al., 1998; Denés et al., 2000). Some PME isoforms act randomly in one medium, but linearly in other medium. Therefore, VGD1 may act randomly or linearly depending on the environmental conditions. The VGD1-GFP fusion protein was found in the pollen tube wall, indicating that VGD1 protein might be secreted out of the pollen tube. It might diffuse to the cell walls of the female floral tissues, where the condition is suitable for random demethlesterification. The random demethylesterification of homogalacturonans releases protons, which promotes the action of endopolygalacturonases and contributes to the degradation or loosening of cell walls (Goldberg, 1996). In the vgd1 mutant, the loss of VGD1 function reduced significantly the PME activity in pollen, indicating that the vgd1 pollen tube also may have weaker PME activity. The weaker PME activity in extracellular space would reduce the efficiency of the degradation of the female tissue cell wall, resulting in retardation of pollen tube growth in female tissues.
On the other hand, the retardation of the pollen tube also could result from the inefficient interaction of pollen tubes with the ECM in the style and transmitting tract. As mentioned previously, the VGD1 protein exhibited a predicted basic isoelectric point of 8.9, which is advantageous to linear demetylesterification. The conditions in the transmitting tract also may be suitable for linear demeylesterification by VGD1. The linear demethylesterification of pectin can make a contribution to the formation of the Ca2+ pectate gel lawn that benefits the interaction between pollen tubes and ECM in the style and transmitting tract, promoting the elongation of the pollen tube in the style and transmitting tract. Loss of VGD1 function may reduce the efficiency of interaction of the pollen tube with the ECM in the style and transmitting tract, resulting in the retardation of the pollen tube in the transmitting tract.
In conclusion, we have described a novel vgd1 mutation that significantly retarded the navigation of the pollen tube through the style and transmitting tract, resulting in a significant reduction of male fertility. The VGD1 gene encoded a PME-homologous protein and was expressed specifically in pollen grain and the pollen tube. This study suggests that VGD1 plays an important role in growth of pollen tubes in female floral tissues, possibly via enhancing the interaction between the pollen tube and female floral tissues by modification of the cell walls.
METHODS
Plant Materials and Mutant Isolation
All Arabidopsis thaliana plants used in this study were in the Landsberg erecta background. The seeds were pregerminated on MS-salt agar plates with or without 50 μg/mL of kanamycin at 22°C under a light cycle of 16 h light/8 h dark. The plants were grown in soil at 22°C under the same light cycle as for germination. The generation of Ds insertion lines and screen of mutants were performed as described by Sundaresan et al. (1995). The selected mutant plants were backcrossed with wild-type plants to purify the vgd1 mutation. The F3 plants with a single Ds insertion linked to the vgd1 phenotype were selected for further phenotypic characterization.
Characterization of vgd1 Mutant Phenotype
To assay in vitro pollen germination, wild-type and vgd1 pollen grains were collected and cultured as described by Fan et al. (2001), with a modification by spreading several drops of stigma water extract onto the surface of agar plates containing different amounts of agar (0.8, 1.0, 1.2, and 1.5%, w/v) and sucrose (12, 16.6, and 20%, w/v). The pollen grains spread on the agar plates were cultured immediately at 23°C, 100% relative humidity, and cool fluorescence light at 30 μmol/m2s. The germinating pollen grains were counted under a microscope after overnight incubation. From each culture, at least 300 pollen grains were examined to calculate an average germination rate, and 20 pollen tubes were measured for the average pollen tube length.
Aniline blue staining of pollen tubes in pistils was performed as described by Sumie et al. (2001). The preemasculated mature wild-type flowers were pollinated either with wild-type or vgd1 pollen. The pollinated pistils were collected 4, 12, 24, and 48 hap and briefly fixed in fixing solution of ethanol:acetic acid (3:1) for 2 h at room temperature. The fixed pistils were washed three times with distilled water and treated in softening solution of 8 M NaOH overnight. Then, the pistil tissues were washed in distilled water and stained in aniline blue solution (0.1% aniline blue in 0.1 M K2HPO4-KOH buffer, pH 11) for 3 to 5 h in the dark. The stained pistils were observed and photographed with a Leica DMRA2 fluorescence microscope (Wetzlar, Germany).
The morphological observations of pollen grains and pollen tubes by scanning electronic microscopy and TEM were performed as described by Hülskamp et al. (1995b).
Genetic Analyses of vgd1
All crosses of vgd1 plants with wild-type plants were performed as described previously (Yang et al., 1999, 2003).
Molecular Cloning of the VGD1 Gene and DNA Sequencing
Isolation of the flanking sequences adjacent to the Ds element by TAIL-PCR (Liu et al., 1995; Grossniklaus et al., 1998) was performed as described previously (Yang et al., 1999, 2003) with the vgd1 genomic DNA and Ds3/AD2 or Ds5/AD4 primers. The full-length cDNAs of VGD1 (AY830948), At2g47030 (AY830949), and At3g62170 (AY830950) were amplified using the Access RT-PCR system (Promega, Madison, WI) with the gene-specific primer pairs listed in Table 1. All resulting DNA fragments were cloned into the pGEM-T vector or pGEM-T Easy vector (Promega). DNA Sequencing was performed using ABI PRISM Rhodamine Terminator thermal cycle sequencing ready reaction kit (PE-Applied Biosystems, Foster City, CA) with gene-specific primers or vector-derived M13 or T7 primers.
Table 1.
Primer Pairs Used for the Isolation of PVGD1, TVGD1, and cDNA Fragments
| Primer Sequences
|
||
|---|---|---|
| Gene Names | Forward Primers (Adaptor with Restriction Site) | Reverse Primers (Adaptor with Restriction Site) |
| VGD1 promoter | 5′-CTGCAGTGATGCTCCACATTCTGACG-3′ (PstI) | 5′-ACTAGTATTTTTTGCTCTCCCTCCGGT-3′ (SpeI) |
| VGD1 terminator | 5′-GCTAGCGGCGTATAAATCAAATCAAAT-3′ (NheI) | 5′-GAATTCCTATTGTGATGGTTACTGGAG-3′ (EcoRI) |
| VGD1 cDNA | 5′-ACTAGTACTGGCCCAAGTCATTCAACA-3′ (SpeI) | 5′-GCTAGCGATTTGATTTGATTTATACGC-3′ (NheI) |
| At2g47030 cDNA | 5′-ACTAGTATAAAGCCTCTCCCTCTCCGA-3′ (SpeI) | 5′-GCTAGCTTAAAAAGTTTTCATAAACCA-3′ (NheI) |
| At3g62170 cDNA | 5′-ACTAGTCCACCGGGCTCTGTTTCAATA-3′ (SpeI) | 5′-GCTAGCGAGACGACGTCGTATCGTTAC-3′ (NheI) |
Complementation Experiments
A 5.131-kb VGD1 genomic fragment was amplified by the ACCuTaq LA DNA polymerase PCR kit (Sigma-Aldrich, St. Louis, MO) with gene-specific primers 5′-GGATCCTGATGCTCCACATTCTGACGT-3′ and 5′-GAATTCTGGGTCAACGAATGGCTGAGA-3′ and cloned into the pGEM-T vector. After sequence verifications, the fragment was subcloned into pCAMBIA1300 Ti-derived binary vector (CAMBIA, Canberra, Australia; www.cambia.org.au).
For cDNA complementation experiments, the VGD1 promoter (PVGD1) and VGD1 terminator (TVGD1) fragments were amplified from wild-type genomic DNA using the primers listed in Table 1. VGD1, At2g47030, and At3g62170 cDNAs were amplified from the flower-specific cDNA pool using the gene-specific primers homologous to both end sequences of cDNAs (Table 1). The resulting fragments were cloned into pGEM-T vector and verified by DNA sequencing. Restriction enzyme pairs PstI and SpeI, SpeI and NheI, or NheI and EcoRI were used to excise the promoter or cDNAs or terminator from the pGEM-T vector, respectively. They were then subcloned into pCAMBIA1300 vector, resulting in the transcriptional fusion construct PVGD1-cDNA-TVGD1. All constructs in pCAMBIA vector for complementation experiments were introduced into the vgd1 homozygous plants using the Agrobacterium tumefaciens–mediated infiltration method. The transformants were selected using 20 mg/L of hygromycin and 50 mg/L of kanamycin.
Measurement of the Relative PME Activity in vgd1 Pollen Grain
PME activity assay was performed according to Hou and Lin (1998) and Ren and Kermode (2000). Approximately 50 mg of vgd1 or wild-type pollen grains were collected in an Eppendorf tube from newly mature anthers and frozen immediately in liquid nitrogen. Then, pollen grains were ground in ∼250 μL of PME extraction buffer (0.1 M citrate to 0.2 M Na2HPO4 buffer containing 1.0 M NaCl, pH 5.0). The homogenized slurry was centrifuged for 10 min at 14,000g at 2°C. The supernatants were collected and stored in −20°C temporarily. Exude protein was quantified using the Coomassie (Bradford) protein assay kit (Pierce, Rockford, IL) according to the supplier's instructions. Fifty micrograms of exude protein from each sample was added to 1 mL of 0.1% (w/v) 92% esterified pectin (Sigma-Aldrich) in 0.2 M Na2HPO4 buffer, pH 6.3. After overnight incubation at 37°C, 0.2 mL of 0.05% ruthenium red (Sigma-Aldrich) was added, mixed, and incubated for 10 min. Then, 0.5 mL of 0.6 M CaCl2 (Sigma-Aldrich) was added to precipitate the demethylesterified pectin that bound to ruthenium red. The mixture was centrifuged at 14,000g for 15 min to remove the precipitate. The supernatants of the samples and the control were measured for the absorbance at 534 nm. All measurements were repeated four times to calculate the average values.
Subcellular Localization of VGD1-GFP Fusion Protein in the Pollen Tube
The VGD1 coding cDNA fragment without the stop codon and VGD1 promoter fragments were amplified using the ACCuTaq LA DNA polymerase PCR kit (Sigma-Aldrich) with gene-specific primer pairs VGD1-cDNA-xba1 (5′-TCTAGAATGATTGGAAAAGTTGTGGTC-3′)/VGD1-cDNA-Kpn1 (5′-GGTACCTAATCCAAGCGTGACGGGAAC-3′) and VGD1-5′prom-pst1 (5′-CTGCAGGATGCTCCACATTCTGACGTA-3′)/VGD1-3′Prom-xba1 (5′-TCTAGAATTTTTTGCTCTCCCTCCGGT-3′), respectively. The resulting DNA fragments were cloned into pGEM-T Easy vector and verified by DNA sequencing. The VGD1 coding fragment was excised from pGEM-T Easy vector with XbaI and KpnI restriction enzymes and subcloned into pGFP-2 vector before the start codon of the GFP coding sequence, resulting in a VGD1-GFP fusion coding sequence. The VGD1 promoter fragment was excised with PstI and XbaI restriction enzymes. The VGD1-GFP fusion coding fragment was excised from the pGFP-2 vector with XbaI and SacI restriction enzymes. Then, both fragments were subcloned before the NOS terminator sequence in a modified pCAMBIA-1300 Ti-derived binary vector (CAMBIA), resulting in a PVGD1-VGD1-GFP-TNOS construct. The PVGD1-VGD1-GFP-TNOS construct was introduced into a wild-type Arabidopsis plant using the Agrobacterium-mediated infiltration method. The transformants were selected using 20 mg/L of hygromycin. For plasmolysis, the germinating pollen tubes on medium were soaked in 40% (w/v) sucrose solution for half an hour and then air-dried for 15 min. The subcellular localization of VGD1-GFP fusion protein in the pollen and pollen tubes was visualized under a confocal microscope (Zeiss, Jena, Germany or Bio-Rad, Hercules, CA).
RNA and DNA Gel Blot Hybridization
The total RNAs were extracted using a TRIzol reagent kit (Gibco BRL, Gaithersburg, MD) as described by the supplier. The extractions of the plant genomic DNAs were performed as described by Yang et al. (1999). The RNA and the restricted DNA samples were fractionated in 1% agarose gel. For the VGD1 gene-specific probe, a cDNA fragment was amplified by PCR using the primers 5′-GCCATGGAAGAAATTCTCTAC-3′ and 5′-GGTTTGATTTGATTTGATTTATCAG-3′. For the At2g47030 gene-specific probe, a cDNA fragment was amplified by PCR using the primers 5′-ACGTTGAGTACAACAACCGTGG-3′ and 5′-TCATAAACCAATTTCATACACCA-3′. For the At3g62170 gene-specific probe, a cDNA fragment was amplified by PCR using the primers 5′-AAACCAGAAGGATGGACCGAA-3′ and 5′-CTCTTACAATCCTAGTTGGAC-3′. For the Ds probe, a 755-bp 5′-terminal fragment of the Ds element was amplified from plasmid pWS32 (Sundaresan et al., 1995) using primers 5′-CTCACAGCACTTAGCAGTACA-3′ and 5′-CATACATCCGATGTGCACTTC-3′. The resulting DNA fragments were cloned into pGEM-T vector and verified via sequencing. All RNA and DNA probes were labeled using the DIG RNA labeling kit (Roche, Indianapolis, IN) or the PCR DIG probe synthesis kit (Roche) as described by the supplier. RNA and DNA gel blot hybridizations were performed following the instructions in the DIG system and DIG application manual provided by the supplier.
Analysis of Promoter Activity
The promoters of VGD1, At2g47030, and At3g62170 were amplified using the ACCuTaq LA DNA polymerase PCR kit (Sigma-Aldrich) with the gene-specific primer combinations listed in Table 2 and cloned into the pGEM-T vector. After sequence verification, the fragments were subcloned upstream of the GUS reporter gene in pCAMBIA1300 Ti-derived binary vector (CAMBIA). Then, they were introduced into the wild-type Arabidopsis plants. Plant transformation was performed as described above. GUS staining was performed as described previously (Sundaresan et al., 1995; Yang et al., 1999).
Table 2.
Primer Pairs Used for the PVGD1-GUS Transcriptional Fusion Constructs
| Primer Sequences
|
||
|---|---|---|
| Gene Fragment Names | Forward Primers | Reverse Primers |
| At2g47030 promoter | 5′-CTGCAGAATGTTCCCGTCACGCTTGGA-3′ | 5′-TCTAGATTTCTCTCCGATCCCTCCGGA-3′ |
| At3g62170 promoter | 5′-CTGCAGGATCGTATTAAGGGATTGGAT-3′ | 5′-TCTAGATGGTAGAGATTGTGGTGCATT-3′ |
| VGD1 promoter | 5′-GAATTCTGATGCTCCACATTCTGACGT-3′ | 5′-TCTAGATTTTTTGCTCTCCCTCCGGT-3′ |
Quantification of the mRNAs Transcribed from the VGD1, At2g47030, and At3g62170 Genes in Wild-Type Flower Tissue
Real-time PCR technology was used to quantify the mRNAs of VGD1, At2g47030, and At3g62170 in wild-type floral tissue. The poly(A)+ RNAs were purified using the Oligotex mRNA Midi kit (Qiagen, Valencia, CA) as described by the supplier and converted into single cDNAs with Stratagene cDNA synthesis kit (Stratagene, La Jolla, CA) following the instructions of the supplier. The single-strand cDNA pool was diluted to 50 ng/μL of single strand cDNA. Two microliters of diluted cDNA solution was used for each real-time PCR reaction. To prepare the standard template series, the cDNA fragments of the three genes were amplified by PCR using the gene-specific primer pairs listed in Table 3 and cloned into pGEM-T Easy vector, respectively. The plasmids containing the cDNA fragments were reproduced in Escherichia coli and recovered using the Qiagen plasmid mini preparation kit and then linearized with the restrictive enzyme NocI or SalI (Roche). The linearized plasmid DNAs were purified with Qiagen DNA spin columns and quantified with a UV spectrum photometer (UV-1601; Shimadzu, Columbia, MD). The template standards for each gene were prepared in a concentration series of 1010, 109, 108, 107, 106, 105, and 104 copies of the target cDNA molecules per microliter. The preparation of standard curves and quantification of each mRNA were performed using the Roche Lightcycler and FastStart DNA Master SYBR Green l kit following the instructions from the supplier (Roche).
Table 3.
Primer Pairs Used for mRNA Quantification
| Primer Sequences
|
||
|---|---|---|
| Gene Names | Forward Primers | Reverse Primers |
| VGD1-specific fragment (431 bp) | 5′-CAGAGATGCTATAACCCAATCA-3′ | 5′-AGCTGCTCCTTTGTCTGAAGGA-3′ |
| At2g47030-specific fragment (323 bp) | 5′-AATGAGCACCGAGATGGGAGATT-3′ | 5′-TCATAAACCAATTTCATACACCA-3′ |
| At3g62170-specific fragment (1077 bp) | 5′-CCAGCTGGCGGTGATGATGGT-3′ | 5′-CCAGAGACGACGTCGTATCGT-3′ |
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AY830948 (VGD1 full-length cDNA), AY830949 (At2g47030 full-length cDNA), and AY830950 (At3g62170 full-length cDNA).
Acknowledgments
This work was supported by research grants from the Agency for Science, Technology, and Research of Singapore. We thank Megan E. Griffith and Jinrong Peng for their critical comments on manuscript preparation.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: De Ye (yede@cau.edu.cn).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.027631.
References
- Atkinson, A., Heath, R., Simpson, R., Clarke, A., and Anderson, M. (1993). Proteinase inhibitors in Nicotiana alata stigmas are derived from a precursor protein which is processed into five homologous inhibitors. Plant Cell 5, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenave, M., and Goldberg, R. (1993). Purification and characterization of pectin methylesterases from mung bean hypocotyl cell walls. Phytochemistry 33, 999–1003. [Google Scholar]
- Catoire, L., Pierron, M., Morvan, C., du Penhoat, C.H., and Goldberg, R. (1998). Investigation of the action patterns of pectin methylesterase isoforms through kinetic analyses and NMR spectroscopy. J. Biol. Chem. 50, 33150–33156. [DOI] [PubMed] [Google Scholar]
- Denés, J.M., Naron, A., Renard, C.M., Pean, C., and Drilleau, J.F. (2000). Different action patterns for apple pectin methylesterase at pH 7.0 and 4.5. Carbohydr. Res. 327, 385–393. [DOI] [PubMed] [Google Scholar]
- Elleman, C.J., Franklin Tong, V., and Dickinson, H.G. (1992). Pollination in species with dry stigmas: The nature of the early stigmatic response and the pathway taken by pollen tubes. New Phytol. 121, 413–424. [DOI] [PubMed] [Google Scholar]
- Fan, L., Wang, Y., Wang, H., and Wu, W. (2001). In vitro Arabidopsis pollen germination and characterization of inward potassium currents in Arabidopsis pollen grain protoplasts. J. Exp. Bot. 52, 1603–1614. [PubMed] [Google Scholar]
- Fiebig, A., Mayfield, J.A., Miley, N.L., Chau, S., Fischer, R.L., and Preuss, D. (2000). Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems. Plant Cell 12, 2001–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin-Tong, V.E. (1999). Signaling and the modulation of pollen tube growth. Plant Cell 11, 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin-Tong, V.E., Drdbak, B.K., Allan, A.C., Watkins, P.A.C., and Trewavas, A.J. (1996). Growth of pollen tubes of Papaver rhoeas slow-moving calcium wave propagated by 1,4,5 trisphosphate is regulated by a inositol. Plant Cell 8, 1305–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futamura, N., Mori, H., Kouchi, H., and Shinohara, K. (2000). Male flower-specific expression of genes for polygalacturonase, pectin methylesterase and β-1,3-glucanase in a dioecious willow (Salix gilgliana Seemen). Plant Cell Physiol. 41, 16–26. [DOI] [PubMed] [Google Scholar]
- Goldberg, R. (1996). Methyle-esterification, de-esterification and gelation of pectins in the primary cell wall. In Pectins and Pectinases: Progress in Biotechnology, Vol. 14, J. Vesser and A.G.J. Voragen, eds (New York: Elsevier Science), pp. 151–172.
- Grossniklaus, U., Vielle-Calzada, J.P., Hoeppner, M.A., and Gagliano, W.B. (1998). Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science 280, 446–450. [DOI] [PubMed] [Google Scholar]
- Guglielmino, N., Liberman, M., Catesson, A.M., Mareck, A., Prat, R., Mutaftschiev, S., and Goldberg, R. (1997). Pectin immunolocalization and calcium visualization in differentiating derivatives from poplar cambium. Protoplasma 199, 151–160. [Google Scholar]
- Hiscock, S., Dewey, F., Coleman, J., and Dickinson, H. (1994). Identification and localization of an active cutinase in the pollen of Brassica napus L. Planta 193, 377–384. [Google Scholar]
- Holdaway-Clarke, T.L., Feijó, J.A., Hackett, G.R., Kunkel, J.G., and Hepler, P.K. (1997). Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9, 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, W.C., and Lin, Y.H. (1998). Activity staining of pectinesterase on polyacrylamide gels after acidic or sodium dodecyl sulfate electrophoresis. Electrophoresis 19, 692–694. [DOI] [PubMed] [Google Scholar]
- Hülskamp, M., Kopczak, S.D., Horejsi, T.F., Kihl, B.K., and Pruitt, R.E. (1995. a). Identification of genes required for pollen-stigma recognition in Arabidopsis thaliana. Plant J. 8, 703–714. [DOI] [PubMed] [Google Scholar]
- Hülskamp, M., Schneitz, K., and Pruitt, R.E. (1995. b). Genetic evidence for a long-range activity that directs pollen tube guidance in Arabidopsis. Plant Cell 7, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauh, G.Y., Eckard, K.J., Nothnagel, E.A., and Lord, E.M. (1997). Adhesion of lily pollen tubes on an artificial matrix. Sex. Plant Reprod. 10, 178–180. [Google Scholar]
- Johnson, M.A., and Preuss, D. (2002). Plotting a course: Multiple signals guide pollen tubes to their targets. Dev. Cell 2, 273–281. [DOI] [PubMed] [Google Scholar]
- Kagan-Zur, V., Tieman, D.M., Marlow, S.J., and Handa, A.K. (1995). Differential regulation of polygalacturonase and pectin methylesterase gene expression during and after heat stress in ripening tomato (Lycopersicon esculentum Mill.) fruits. Plant Mol. Biol. 29, 1101–1110. [DOI] [PubMed] [Google Scholar]
- Kim, S., Mollet, J.-C., Dong, J., Zhang, K., Park, S.-Y., and Lord, E.M. (2003). Chemocyanin, a small, basic protein from the lily stigma, induces pollen tube chemotropism. Proc. Natl. Acad. Sci. USA 100, 16125–16130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon, K., Roy, S., Hepler, P.K., and Lord, E.M. (1998). The structure of the transmitting tissue of Arabidopsis thaliana (L.) and the path of pollen tube growth. Sex. Plant Reprod. 11, 49–59. [Google Scholar]
- Lennon, K.A., and Lord, E.M. (2000). The in vivo pollen tube cell of Arabidopsis thaliana. I. Tube cell cytoplasm and wall. Protoplasma 214, 45–56. [Google Scholar]
- Li, Y.Q., Mareck, A., Faleric, C., Moscatelli, A., Liu, Q., and Cresti, M. (2002). Detection and localization of pectin methylesterase isoforms in pollen tubes of Nicotiana tabacum L. Planta 214, 734–740. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Mitsukawa, N., Oosumi, T., and Whitier, R.F. (1995). Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 8, 457–463. [DOI] [PubMed] [Google Scholar]
- Lord, E.M. (2000). Adhesion and cell movement during pollination: Cherches la femme. Trends Plant Sci. 5, 368–373. [DOI] [PubMed] [Google Scholar]
- Lord, E.M. (2001). Adhesion molecules in lily pollination. Sex. Plant Reprod. 14, 57–62. [Google Scholar]
- Lord, E.M., and Russell, S.D. (2002). Mechanisms of pollination and fertilization in plants. Annu. Rev. Cell Dev. Biol. 18, 81–105. [DOI] [PubMed] [Google Scholar]
- Markovic, O., and Kohn, R. (1984). Mode of pectin deesterification by Tricholderma reesei pectinesterase. Experientia 40, 842–843. [Google Scholar]
- Micheli, F. (2001). Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 6, 414–419. [DOI] [PubMed] [Google Scholar]
- Micheli, F., Holliger, C., Goldberg, R., and Richard, L. (1998). Characterization of the pectin methylesterase-like gene AtPME3: A new member of a gene family comprising at least 12 genes in Arabidopsis thaliana. Gene 220, 13–20. [DOI] [PubMed] [Google Scholar]
- Micheli, F., Sundberg, B., Goldberg, R., and Richard, L. (2000). Radial distribution pattern of pectin methylesterases across the cambial region of hybrid aspen at activity and dormancy. Plant Physiol. 124, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanivelu, R., Brass, L., Edlund, A.F., and Preuss, D. (2003). Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114, 47–59. [DOI] [PubMed] [Google Scholar]
- Palanivelu, R., and Preuss, D. (2000). Pollen tube targeting and axon guidance: Parallels in tip growth mechanisms. Trends Cell Biol. 10, 517–524. [DOI] [PubMed] [Google Scholar]
- Park, S.-Y., and Lord, E.M. (2003). Expression Studies of SCA in lily and confirmation of its role in pollen tube adhesion. Plant Mol. Biol. 51, 183–189. [DOI] [PubMed] [Google Scholar]
- Preuss, D. (2002). Sexual signaling on a cellular level: Lessons from plant reproduction. Mol. Biol. Cell 8, 1803–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss, D., Lemieux, B., Yen, G., and Davis, R.W. (1993). A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes Dev. 7, 974–985. [DOI] [PubMed] [Google Scholar]
- Ray, S.M., Park, S.S., and Ray, A. (1997). Pollen tube guidance by the female gametophyte. Development 124, 2489–2498. [DOI] [PubMed] [Google Scholar]
- Ren, C., and Kermode, A.R. (2000). An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiol. 124, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, L., Qin, L.X., and Goldberg, R. (1996). Clustered genes within the genome of Arabidopsis thaliana encoding pectin methylesterase-like enzymes. Gene 170, 207–211. [DOI] [PubMed] [Google Scholar]
- Sumie, I., Akiko, K., Junichi, U., Ikuo, N., and Kiyotaka, O. (2001). The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 10, 2191–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan, V., Springer, P., Volpe, T., Haward, S., Jones, J.D., Dean, C., Ma, H., and Martienssen, R. (1995). Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev. 9, 1797–1810. [DOI] [PubMed] [Google Scholar]
- Wakeley, P.R., Rogers, H.J., Rozychk, M., Greenland, A.J., and Hussey, P.J. (1998). A maize pectin methylesterase-gene, ZmC5, specifically expressed in pollen. Plant Mol. Biol. 37, 187–192. [DOI] [PubMed] [Google Scholar]
- Wilhelmi, L.K., and Preuss, D. (1996). Self-sterility in Arabidopsis due to defective pollen tube guidance. Science 274, 1535–1537. [DOI] [PubMed] [Google Scholar]
- Wolters-Arts, M., Lush, W.M., and Mariani, C. (1998). Lipids are required for directional pollen-tube growth. Nature 392, 818–821. [DOI] [PubMed] [Google Scholar]
- Yang, S.-L., Xie, L.-F., Mao, H.-Z., Puah, C.S., Yang, W.-C., Jiang, L., Sundaresan, V., and Ye, D. (2003). TAPETUM DERTERMINANT1 is required for cell specialization in the Arabidopsis anther. Plant Cell 15, 2792–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W.-C., Ye, D., Xu, J., and Sundaresan, V. (1999). The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev. 13, 2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkl, G.M., Zwiebel, B.I., Grier, D.G., and Preuss, D. (1999). Pollen-stigma adhesion in Arabidopsis: A species-specific interaction mediated by lipophilic molecules in the pollen exine. Development 126, 5431–5440. [DOI] [PubMed] [Google Scholar]