Abstract
To investigate the effects of persistent elevation of synaptic glycine at Schaffer collateral–CA1 synapses of the hippocampus, we studied the glutamatergic synaptic transmission in acute brain slices from mice with reduced expression of glycine transporter type 1 (GlyT1+/−) as compared to wild type (WT) littermates using whole-cell patch-clamp recordings of CA1 pyramidal cells. We observed faster decay kinetics, reduced ifenprodil sensitivity and increased zinc-induced antagonism in N-methyl-d-aspartate receptor (NMDAR) currents of GlyT1+/− mice. Moreover, the ratio α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR)/NMDAR was decreased in mutants compared to WT. Surprisingly, this change was associated with a reduction in the number of AMPARs expressed at the CA1 synapses in the mutants compared to WT. Overall, these findings highlight the importance of GlyT1 in regulating glutamatergic neurotransmission.
The N-methyl-d-aspartate receptor (NMDAR) plays a pivotal role in neural development, learning, memory, and synaptic plasticity (Malenka & Nicoll, 1999). Functional impairment of NMDARs has been implicated in a number of pathological conditions such as schizophrenia (for review, see Coyle & Tsai, 2004). NMDARs are heteromultimeric ligand-gated channels comprised of three different subunit families (NR1, NR2A–NR2D, NR3A and NR3B) (Dingledine et al. 1999). The NR1 subunit, in combination with at least one type of NR2 subunit (NR2A–NR2D), gives rise to receptor subtypes with distinct pharmacological profiles, gating properties, glycine affinity, and Mg2+ sensitivity (for reviews, see McBain & Mayer, 1994; Sucher et al. 1996; Danysz & Parsons, 1998). NMDAR activation requires two co-agonists acting at the glutamate and glycine recognition sites located on the NR2 and NR1 subunits, respectively (for review see Danysz & Parsons, 1998). Importantly, the type of NR2 subunit co-assembled with NR1 determines NMDAR affinity for glycine (Kutsuwada et al. 1992; Kew et al. 1998). Glycine has approximately a 10-fold higher affinity for NR2B-, NR2C-, or NR2D-containing than for NR2A-containing receptors (for review, see Danysz & Parsons, 1998).
Several investigators have raised some reservations about the in vivo role of glycine in physiological conditions (Danysz & Parsons, 1998). This is mainly due to the fact that the glycine concentration in cerebrospinal fluids (CSF) has been estimated to be in a low micromolar range (Westergren et al. 1994), while the affinity of glycine for the glycine modulatory site of the NMDAR is in the nanomolar range, suggesting that the site is fully saturated and therefore of little pharmacological relevance. However, the concentration of glycine in the synaptic cleft could be reduced well below 1 μm (150 nm; Roux & Supplisson, 2000) by glycine transporters (GlyT) strategically localized around the synaptic cleft (Smith et al. 1992; Zafra et al. 1995). Expression of glycine transporter type 1 (GlyT1) closely corresponds to the expression pattern of NMDARs (Smith et al. 1992). GlyT1 is localized on astroglia cells in close proximity to both excitatory and inhibitory synapses (Zafra et al. 1995). GlyT1 may be the main mechanism for regulating the glycine concentration at synapses (Bergeron et al. 1998). The GlyT1 expression levels determine the glycine concentration gradient (Vandenberg & Aubrey, 2001).
To gain further insight into the in vivo role of GlyT1 in regulating NMDAR neurotransmission, a genetic approach was used to inactivate the GlyT1 gene (Gomeza et al. 2003; Tsai et al. 2004). Homozygote knockout mice (GlyT1–/–) died within 24 h of birth due to failure to breathe (Gomeza et al. 2003). Consequently, all the experiments were performed in adult heterozygous (GlyT1+/−) and wild type (WT) mice. Data from real-time quantitative PCR and [3H]glycine uptake assays showed that the number of GlyT1 was significantly reduced by approximately 50% in GlyT1+/− mice (Tsai et al. 2004). Moreover, electrophysiological recordings in GlyT1+/− mice show that the glycine modulatory site is virtually fully occupied in GlyT1+/− mice (Tsai et al. 2004). In the present study, we investigated the CA1 hippocampal glutamatergic neurotransmission and found that persistent increased glycine levels in the synapse lead to major changes in glutamatergic synaptic transmission at CA1 synapses.
Methods
Genotyping
All genotyping was done using polymerase chain reaction (PCR) amplification of genomic DNA prepared from mouse tissue. Tissue samples from mice tails were incubated in proteinase K (0.5 mg ml−1; Sigma) at 50°C overnight. Samples were centrifuged (20 min at 16000 g) and the supernatant was added to a double volume of isopropanol to precipitate the genomic DNA. The supernatant was removed and the DNA was washed with 70% ethanol and allowed to dry. DNA was resuspended in 300 μl of water; 1 μl of DNA was added to the PCR mixture. PCR analysis was done using Taq DNA polymerase (Invitrogen). Reaction products were run on a 1% agarose gel and visualized using ethidium bromide. Primer sequences (5′–3′) were: Primer 1: GCCTTGGGAAAAGCGCCTCC; Primer 2: CCCCTACTTCATCATGCTGATC; Primer 3: CACCTACCAGTAGTTGCCTT. Cycling conditions (Perkin-Elmer GeneAmp 2400, Foster City, CA, USA) were 2 min at 95°C followed by 36 cycles of 94°C (melting) for 30 s, 57°C (annealing) for 30 s, 72°C (extention) for 1 min 40 s. The reaction solution contained Mg2+ (1.5 mm), dNTPs (0.2 mm each), oligonucleotide primers (0.625 μm for Primer 1 and 0.25 μm for Primer 3), Taq polymerase (2.5 units), reaction buffer (10X; 2 μl) and 1 μl of solubilized genomic DNA (20 μl final volume) in HPLC water. Each animal was genotyped using one reaction as previously shown (Tsai et al. 2004). WT animals showed a single band at 1.3 Kb and the heterozygous knockout animals an additional band at 1.0 Kb due to homologous recombination.
Preparation of hippocampal slices
Coronal brain slices (300 μm) containing the hippocampus were obtained from WT and GlyT1+/− mice (12–13 weeks old). Prior to decapitation, the animals were anaesthetized with isofluorane, in agreement with the guidelines of the Canadian Council of Animal Care. The brain was removed and placed in an oxygenated (95% O2–5% CO2) physiological solution, artificial cerebrospinal fluid (ACSF) at 4°C, containing (mm) 126 NaCl, 2.5 KCl, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2 and 10 glucose. The osmolarity of the ACSF was adjusted to 300 mosmol and the pH to 7.3. A block containing the region of interest was prepared, and sections (300 μm) were obtained with a vibrating microtome (Leica VT 1000S). The slices were stored for 1 h in an oxygenated chamber at room temperature prior to the experiments.
Data recording and analysis
In current-clamp experiments, whole-cell recordings were obtained with borosilicate pipettes filled with a solution containing (mm) 130 K-gluconate, 10 N-2-hydroxy-ethylpiperazine-N′-2-ethanesulphonic acid (Hepes), 10 KCl, 2 MgCl2, 2 ATP-Mg and 0.2 GTP-tris(hydroxy-methil) aminomethane (GTP). In voltage-clamp experiments, to further minimize current attenuation, we performed experiments with a solution containing (mm) 130 Cs+-methanesulphonate, 10 Hepes, 10 CsCl, 2 MgCl2, 2 ATP-Mg and 0.2 GTP, 5 lignocaine (lidocaine) N-ethyl bromide (QX-314), and 10 caesium-BAPTA. For recordings of spontaneous activity, 0.2 mm EGTA was used instead of 10 mm caesium-BAPTA in the intracellular solution. pH was adjusted to 7.3 and osmolarity to 280–290 mosmol. With this solution, the liquid junction potential was measured (10 mV) and the membrane potential (Vm) was corrected accordingly. The pipettes had a resistance of 3–6 MΩ when filled with these solutions. Recordings with series resistance higher than 20 MΩ were discarded. Bridge balance was monitored regularly during the recordings. To allow the drugs added in the pipette to induce their pharmacological action, a delay of 10–15 min was systematically observed prior to recording.
Current- and voltage-clamp recordings were obtained with a Multiclamp 700 A amplifier (Axon Instruments, Union City, CA, USA) under visual control using differential interference contrast and infrared video microscopy (IR-DIC; Leica DMLFSA, Germany). Electroresponsive properties of neurones were studied by applying 500 ms current pulses from rest. The amplitude of current pulses was varied in fixed increments of 10 pA. The input resistance (Rin) was estimated in the linear portion of current–voltage plots. Whole-cell currents were recorded at room temperature from individual pyramidal cells of the CA1 region of the hippocampus voltage-clamped at −70 mV.
The CA3 input to CA1 pyramidal cells, mediated by the Schaffer collaterals, is glutamatergic (Amaral & Witter, 1989). Postsynaptic responses were evoked by electrical stimulation of the Schaffer collaterals with a bipolar microelectrode positioned in the stratum radiatum. The stimulation intensity consisted of 100 μs current pulses (0.1–1 mA; 0.3–0.01 Hz) and was adjusted to evoke an EPSC amplitude in the range of 60–120 pA at Vm = −70 mV. The recordings were first obtained in normal ACSF.
To isolate the NMDAR-mediated component of evoked responses, we used ACSF containing a low concentration of MgCl2 (0.1 mm) with osmolarity maintained by CaCl2, and the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) antagonist 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulphonamide (NBQX), the GABAA receptor antagonist picrotoxin, the GABAB receptor antagonist 3-[[(3,4-dichlorophenyl)methyl]amino]propyl]diethoxymethyl)phosphinic acid (CGP 52432) and the glycine receptor antagonist strychnine. NBQX is highly selective for AMPARs and does not act at the glycine site of the NMDAR (Yu & Miller, 1995).
To isolate the AMPAR-mediated component of evoked responses, we used normal ACSF containing the NMDAR antagonist dl-2-amino-5-phosphonovaleric acid (AP5), picrotoxin, CGP 52432 and strychnine.
The concentrations of drugs applied in the perfusate were (μM) 20 NBQX, 50 picrotoxin, 10 CGP 52432, 0.5 strychnine, 50 AP5, 0.5 tetrodotoxin (TTX), 10 ifenprodil and 0.1 ZnCl2. All drugs were obtained from RBI (Natick, MA, USA), with the exception of CGP 52432 (Tocris, Bristol, UK). ZnCl2 solution (100 μm) was made fresh every day and prepared by progressive dilutions with bidistilled water of a solution containing 100 mm ZnCl2 and 10 mm HCl. We added 100 μl of 100 μm ZnCl2 per 100 ml of extracellular solution, to obtain a 100 nm concentration of zinc.
AMPAR/NMDAR ratios were computed by taking the average of EPSCs at +40 mV in ACSF containing picrotoxin, CGP 52432 and strychnine, in the absence and presence of NBQX. The average AMPAR-mediated current was calculated from subtraction of the average response obtained in the absence and presence of NBQX. The peak of AMPAR-mediated current was divided by the peak of NMDAR-mediated current to yield the AMPAR/NMDAR ratio.
To record NMDAR-mediated miniature postsynaptic currents (NMDAR mEPSCs), the pyramidal cells were held at +50 mV to relieve the Mg2+ block of the NMDAR in a normal ACSF containing picrotoxin, NBQX, CGP 52432, strychnine and TTX. To record miniature postsynaptic currents (mEPSCs), the cells were recorded at −70 mV in low Mg2+ ACSF containing picrotoxin, CGP 52432, strychnine and TTX. The subsequent addition of AP5 (50 μm) was used to isolate AMPAR-mediated miniature postsynaptic current (AMPAR mEPSCs). In all the cases, the recording solution was the Cs-methanesulphonate solution as described above. mEPSCs, NMDAR mEPSCs and AMPAR mEPSCs were analysed off-line using the mini analysis program by Justin Lee (Synaptosoft Inc.). Quantal sizes, which correspond to the response to a single quantum of transmitters released from individual synapses, were measured as the average amplitudes of mEPSCs, NMDAR mEPSCs and AMPAR mEPSCs.
Kinetic analysis was performed on averaged EPSCs (usually 20–25 consecutive traces). The rise times of NMDAR currents were measured at peak to the end. Their decays were fitted with the exponential functions: y=Afe−t/τf+Ase−t/τs for double and y=A1 exp−t/τ for single exponential decay, where A is the amplitude, τ is the time is the decay time constant, and the subscript f and s denote fast and slow components, respectively. Weighted time constants (τmean) were calculated using the equation: τmean=[Af/(Af+As)]τf+[As/(As+Af)]τs (Stocca & Vicini, 1998).
Data were collected using software pClamp 9 (Axon Instruments). Analyses were performed offline with the software IGOR (WaveMetrics Inc., Lake Oswego, OR, USA). Results are presented as means ± s.e.m. Data were compared statistically by either Student's t tests or Kolomogorov–Smirnov tests and significance was defined as P < 0.05.
Morphological identification of recorded cells
In some experiments, the recorded cells were infused with Lucifer Yellow (2 mm). The slices were removed from the chamber and fixed for 1–3 days in 0.1 m PBS, pH 7.4, containing 4% paraformaldahyde. Slices were then washed in dimethyl-sulfoxide (DMSO) for 1 h. Cells were then visualized with an LSM 510 confocal laser-scanning microscope (Zeiss, Germany) using 10X and 40X water immersion objectives. Three-dimensional reconstruction of the neurones was done from z-series data using the confocal system software.
Results
Evoked NMDAR currents have faster decay kinetics in GlyT1+/− CA1 pyramidal cells
It is known that the application of either glycine site agonists (glycine or d-serine) or GlyT1 antagonists increases the amplitude and slows down the decay time constant of the NMDAR current (Berger et al. 1998; Bergeron et al. 1998; Chen et al. 2003; Martina et al. 2003). To investigate if reduced expression of GlyT1 has an effect on the time course of NMDAR currents, we measured the rise times and decay time constants of evoked NMDAR currents in presence and absence of glycine in WT and GlyT1+/− CA1 pyramidal cells.
To evoke postsynaptic glutamatergic currents, the Schaffer collaterals were stimulated with a bipolar electrode while the postsynaptic CA1 pyramidal cells were held at Vm=−70 mV. The NMDAR-mediated component of the postsynaptic current was pharmacologically isolated in a low Mg2+ ACSF (see Methods) containing NBQX, picrotoxin, CGP 52432 and strychnine.
The decay of NMDAR currents was best fitted with a bi-exponential function (Table 1) and weighted time constants (τmean) were calculated (see Methods). NMDAR current rise times in WT and GlyT1+/− CA1 pyramidal cells were similar and did not change significantly in the presence of glycine. Application of glycine (10 μm) significantly increased the slow decay time course and τmean of NMDAR currents in WT CA1 pyramidal cells (Table 1, P < 0.005), while having no effect in GlyT1+/− cells (P > 0.05; Table 1; Fig. 1A and B). Interestingly, the τmean was significantly faster in GlyT1+/− (Table 1; Fig. 1B and C), compared to WT (Table 1; Fig. 1A and C; P < 0.005).
Table 1.
Rise and decay time constants of NMDAR currents in WT and GlyT1+/− CA1 pyramidal cells recorded in the absence and presence of glycine at −70 mV
| Low Mg 2+ ACSF* (–Glycine) | +Glycine† | |||||||
|---|---|---|---|---|---|---|---|---|
| Activation | Deactivation | Activation | Deactivation | |||||
| +70mV | τact(ms) | τf‡(ms) | τs‡(ms) | τmean‡(ms) | τact(ms) | τf(ms) | τs(ms) | τmean‡(ms) |
| WT | 7.44 ± 0.6 n = 27 | 56.73 ± 3.91 n = 27 | 433.02 ± 66.02 n = 27 | 142.48 ± 20.83 n = 27 | 9.97 ± 0.86 n = 5 | 67.68 ± 7.55 n = 5 | 512.65 ± 42.46 n = 5 | 182.65 ± 42.09 n = 5 |
| GlyT1+/− | 8.01 ± 0.9 n = 35 | 38.79 ± 4.12 n = 35 | 232.73 ± 23.95 n = 35 | 95.41 ± 5.98 n = 35 | 9.21 ± 1.76 n = 13 | 43.12 ± 7.27 n = 13 | 257.18 ± 37.48 n = 13 | 102.71 ± 6.41 n = 13 |
Values are mean ± s.e.m.
Electrically evoked NMDAR currents were recorded in a low Mg2+ ACSF (0.1 mm) in the presence of NBQX (20 μm), picrotoxin (50 μm), CGP 52432 (10 μm) and strychnine (0.5 μm). The fast and slow decay components are designated τf and τs, the weighted time constant by τmean.
Electrically evoked NMDAR currents recorded in low Mg2+ ACSF as in * plus glycine (10μm);
Significant difference between WT and GlyT1+/− mice (P < 0.05).
Figure 1. The administration of 10 μm glycine slows the decay time course of the evoked NMDAR currents in WT but not in GlyT1+/− CA1 pyramidal cells.
Responses were evoked by bipolar electrical stimuli at Vm = −70 mV in CA1 pyramidal cells of WT (A) and GlyT1+/− (B) mice. The NMDAR currents recorded in low Mg2+ ACSF (thin line), and during application of glycine (thick line), are superimposed. The application of glycine did not change the amplitude of the NMDAR currents in GlyT1+/− CA1 pyramidal cells (B). Fitting of the decay time course of NMDAR currents in low Mg2+ ACSF (thin broken line) and during application of glycine (thick broken line) are shown. The fitting traces were moved from the NMDAR current traces for reason of clarity. The τmean values were calculated (see Methods) and the values for the NMDAR currents shown. Note that the decay of the NMDAR currents in GlyT1+/− CA1 pyramidal cells is faster than in WT, before and during the application of glycine (10 μm). Each trace is an average of 20 traces. C, the NMDAR currents recorded in low Mg2+ ACSF, as shown in A and B, are scaled and the fitting traces shown.
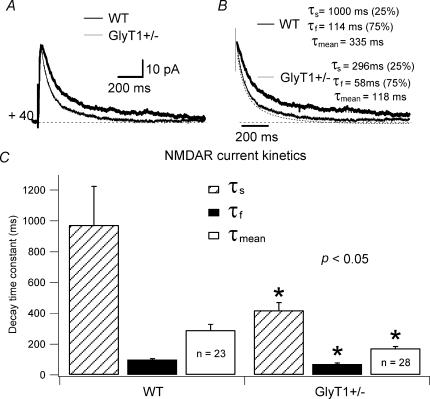
The difference in kinetics of NMDAR currents in WT and GlyT1+/− CA1 pyramidal cells was also observed in NMDAR currents isolated at +40 mV (Fig. 2). While activation kinetics were not significantly different in WT (16.63 ± 4.17 ms; n = 23) and GlyT1+/− (16.43 ± 4.21 ms, n = 28; P > 0.5), the deactivation kinetics of NMDAR currents were significantly faster in GlyT1+/− (τf= 69.85 ± 7.22 ms; τs= 418.42 ± 51.3 ms; τmean= 172.31 ± 12.16 ms; n = 28) than in WT (τf= 100.15 ± 5.98 ms; τs= 973.54 ± 251.42 ms; τmean= 290.08 ± 37.79 ms; n = 23; P < 0.05; Fig. 2B and C). Relative portions (Af and As) of decay time constants at this potential were not significantly different in the two types of mice.
Figure 2. NMDAR current deactivation time constants are faster in GlyT1+/− CA1 pyramidal cells.
Neurones were recorded at +40 mV to relieve the Mg2+ block of the NMDAR and outward currents were evoked by bipolar electrical stimuli. A, recorded NMDAR currents of one GlyT1+/− (thin line), and one WT CA1 pyramidal cell (thick line) are superimposed. B, the same traces as illustrated in A are scaled and the fitting traces shown. Each trace is an average of 20 traces. Values of τs, τf, and τmean for one WT and one GlyT1+/− CA1 pyramidal cell NMDAR current are given. C, histogram of the averaged τs, τf, and τmean for NMDAR currents of WT (n = 23) and GlyT1+/−(n = 28) CA1 pyramidal cells. *Significant difference between WT and GlyT1+/− mice (P < 0.05). Error bars are s.e.m.
Morphological and physiological properties of CA1 pyramidal cells in WT and GlyT1+/− mice
To rule out the possibility that the difference in kinetics of NMDAR currents could be due to differences in morphological (cable filter properties) or electrophysiological (resistance, capacitance, etc.) properties, we performed whole-cell recordings in current-clamp with Lucifer Yellow in the pipette solution. Cells were visually identified in slices with IR-DIC and selected for recording on the basis of their shape and localization in the stratum pyramidale of the CA1 region of the hippocampus. A total of 28 WT and 15 GlyT1+/− CA1 pyramidal cells were recorded, 5 WT and 5 GlyT1+/− CA1 pyramidal cells were morphologically characterized. The soma of the WT CA1 pyramidal cells were measured (longest axis 20.1 ± 1.59 μm by shortest axis 14.3 ± 1.58 μm; n = 5; Fig. 3A). The soma of GlyT1+/− (longest axis 19.8 ± 1.32 μm by shortest axis 15.1 ± 0.39 μm; n = 5; Fig. 3C) did not differ significantly from that of WT CA1 pyramidal cells. WT and GlyT1+/− CA1 pyramidal cells had spiny dendrites (Fig. 3B and D). The apical dendrites crossing the entire stratum radiatum and ending in the stratum lacunosum moleculare were similar in GlyT1+/− compared to WT (465 ± 55.65 μm, n = 5 WT; 561 ± 38.47, n = 5 GlyT1+/−; Fig. 3A and C). These pyramidal cells (WT, n = 28 and GlyT1+/−, n = 15) generated spike trains that exhibited frequency adaptation when depolarized (Fig. 3E and F). The physiological properties (Vm, Rin, spike amplitude, τ and spike duration at half amplitude) of WT and GlyT1+/− CA1 pyramidal cells were not significantly different (see Table 2). Therefore, the faster kinetics observed in GlyT1+/− CA1 pyramidal cells cannot be explained by difference in morphological or electrophysiological properties.
Figure 3. Morphological and physiological properties of WT and GlyT1+/− CA1 pyramidal cells. Confocal images of WT (A), and GlyT1+/− (C) CA1 pyramidal cells.
The cells were recorded in the CA1 stratum pyramidale of the hippocampus. B and D, confocal images of the boxed dendritic regions shown in A and C, respectively. The arrowheads indicate dendritic spines. E and F, the voltage responses of the same pyramidal cells as in A and C to a series of intracellular current pulses are shown. The current was applied at rest (−73 and −75 mV, respectively). Abbreviations: so, stratum oriens; sp., stratum pyramidale; sr, stratum radiatum; slm, stratum lacunosum moleculare.
Table 2.
Physiological properties of WT and GlyT1+/− CA1 pyramidal cells
| Genotype | Vm(mV) | Rin(mΩ) | Spike amplitude(mV) | τ(ms) | Spike duration at half amplitude(ms) |
|---|---|---|---|---|---|
| WT | −75.93 ± 0.77 n = 28 | 199.31 ± 7.93 n = 28 | 95.17 ± 1.93 n = 28 | 27.20 ± 1.94 n = 28 | 2.08 ± 0.08 n = 28 |
| GlyT1+/− | −78.25 ± 0.53 n = 15 | 183.07 ± 13.69 n = 15 | 99.54 ± 2.14 n = 15 | 22.10 ± 1.52 n = 15 | 1.89 ± 0.10 n = 15 |
Values are mean ±s.e.m.
Pharmacological evidence for a distinct functionality of NMDAR in GlyT1+/− CA1 pyramidal cells
The decay time constant of the NMDAR EPSC depends on several factors such as dissociation of glutamate from the NMDAR (Hestrin et al. 1990), channel kinetics (D'Angelo et al. 1990; Lester et al. 1990), NMDAR subunits composition (Monyer et al. 1994; Vicini et al. 1998), glycine-dependent and glycine-independent desensitization, and Ca2+/CaM-dependent channel inactivation (McBain & Mayer, 1994). It has also been reported that glycine decreases desensitization of the NMDAR (Mayer et al. 1989; Lerma et al. 1990) and that in conjunction with glutamate primes the receptor to be internalized by clathrin-mediated endocytosis (Nong et al. 2003).
To determine whether the faster kinetics of the NMDAR currents observed in GlyT1+/− CA1 pyramidal cells result from differences in pharmacological sensitivity, we used ifenprodil, a highly selective non-competitive antagonist of NR2B-containing NMDARs (Williams, 1993), and zinc that, in nanomolar concentration, is known to antagonize the NMDARs containing the subunit NR2A (Paoletti et al. 1997).
Data from cultured rat hippocampal neurones show that increasing glycine concentration reduces the inhibition produced by high concentrations of ifenprodil (100 μm), whereas inhibition caused by low concentrations of ifenprodil was glycine independent (Legendre & Westbrook, 1991). Since the glycine modulatory site is virtually saturated in GlyT1+/− pyramidal cells (Tsai et al. 2004), we used a low concentration of ifenprodil (10 μm). We found that NMDAR currents of GlyT1+/− CA1 pyramidal cells were less sensitive to ifenprodil than those from WT (P < 0.05; Fig. 4A and B). Indeed, ifenprodil reduced the amplitude of NMDAR currents by 68.2 ± 7.8% in WT (n = 4) and by only 44 ± 6.6% in GlyT1+/− CA1 pyramidal cells (n = 5) at +40 mV (Fig. 4C).
Figure 4. Differences in ifenprodil sensitivity of NMDAR currents in WT and GlyT1+/− CA1 pyramidal cells.
A and B, examples of averaged NMDAR current traces at +40 mV in the absence (thick line) and presence (thin line) of ifenprodil (10 μm) are shown for WT (A) and GlyT1+/− (B). C, summarized data from WT (n = 4) and GlyT1+/−(n = 5) CA1 pyramidal cells at +40 mV. Ifenprodil reduces the amplitude of the NMDAR currents by 68.2 ± 7.8% in WT and 44 ± 6.6% in GlyT1+/− 0. * Significant difference between WT and GlyT1+/− mice (P < 0.05). D, I–V relationships of NMDAR currents are shown for WT (•; n = 7) and GlyT1+/− (▪; n = 7). Error bars are s.e.m.
It has been established that zinc can inhibit NMDAR through a dual mechanism involving both voltage-dependent channel block and voltage-independent inhibition (Westbrook & Mayer, 1987; Legendre & Westbrook, 1990). The voltage-independent block depends on the type of NR2 subunit and on the NR1 splice variant that form the receptor (Paoletti et al. 1997). However, receptors consisting of the NR2A subunit are much more sensitive to zinc than NR2B-, NR2C-, or NR2D-containing receptors, being inhibited in the nanomolar range by as much as 70% (Paoletti et al. 1997). Contrary to ifenprodil, NMDAR currents of GlyT1+/− CA1 pyramidal cells were more sensitive to ZnCl2 (100 nm) than those from WT (P < 0.05). ZnCl2 reduced the amplitude of NMDAR currents by 43.02 ± 4.7% in WT (n = 9) and by 62.8 ± 4.2% in GlyT1+/− CA1 pyramidal cells (n = 9) at +40 mV (Fig. 5). Voltage-dependent properties of the NMDAR-mediated component of evoked EPSCs in WT and in GlyT1+/− CA1 pyramidal cells were also studied (Fig. 4D) and no differences were found.
Figure 5. Differences in zinc sensitivity of NMDAR currents in WT and GlyT1+/− CA1 pyramidal cells.
A and B, examples of averaged NMDAR current traces at +40 mV in the absence (thick line) and presence (thin line) of ZnCl2 (100 nm) are shown for WT (A) and GlyT1+/− (B). C, summarized data from WT (n = 9) and GlyT1+/−(n = 9) CA1 pyramidal cells at +40 mV. ZnCl2 reduces the amplitude of the NMDAR currents by 43.02 ± 4.7% in WT and 62.8 ± 4.2% in GlyT1+/−. D, effect of ZnCl2 on NMDAR currents as a function of time in WT (•) and GlyT1+/− (○) mice. * Significant difference between WT and GlyT1+/− mice (P < 0.05). Error bars are s.e.m.
These findings are consistent with the data showing faster kinetics of NMDAR currents in GlyT1+/− compared to WT CA1 pyramidal cells. Overall, our results suggest that GlyT1+/− and WT CA1 pyramidal cells may express NMDARs with different pharmacological properties.
AMPAR/NMDAR ratio is lower in GlyT1+/− CA1 pyramidal cells
It has been established that increasing the occupancy of the strychnine-insensitive glycine site increases NMDAR function (Wilcox et al. 1996; Bergeron et al. 1998; Chen et al. 2003; Martina et al. 2003). Since the AMPAR/NMDAR ratio expresses a measure of the postsynaptic sensitivity to glutamatergic input, we examined the AMPAR/NMDAR ratio at the Schaffer collateral–CA1 synapses in WT and GlyT1+/− mice to determine how a persistent saturating level of glycine can alter glutamatergic neurotransmission at these synapses.
WT and GlyT1+/− CA1 pyramidal cells were held at +40 mV in normal ACSF containing picrotoxin, CGP 52432 and strychnine, while the Schaffer collaterals were stimulated to evoke EPSCs in the amplitude range of 60–120 pA. Currents were then recorded in the absence and presence of NBQX (20 μm). The average response in the presence of NBQX (NMDAR-only response) was subtracted from that measured in its absence, and an average AMPAR current was then calculated. AMPAR/NMDAR ratios were obtained by dividing the peak of AMPAR current by the peak of NMDAR current.
We found that the AMPAR/NMDAR ratio was significantly smaller in GlyT1+/− (0.21 ± 0.02, n = 12) compared to WT (0.36 ± 0.08, n = 11; P < 0.05; Fig. 6), as previously reported (Tsai et al. 2004). These values are consistent with a previous report that showed that at +60/+40 mV, the major fraction of the Schaffer collateral EPSC is attributable to the NMDAR current (Otmakhova et al. 2002).
Figure 6. AMPAR/NMDAR ratio is smaller in GlyT1+/− CA1 pyramidal cells.
A and B, examples of NMDAR currents (thin line) and derived AMPAR currents (thick line) are shown for WT (A) and GlyT1+/− (B) CA1 pyramidal cells. (C) Histogram of the AMPAR/NMDAR ratios for WT (n = 9) and GlyT1+/−(n = 9) CA1 pyramidal cells. *The AMPAR/NMDAR ratio in GlyT1+/− is significantly smaller than in WT CA1 pyramidal cells (P < 0.05).
To investigate the possibility that a change in the properties of the AMPARs could influence the AMPAR/NMDAR ratio in GlyT1+/−, we studied the current–voltage relationship (I–V) and kinetics (activation and inactivation) of evoked AMPAR currents in WT (n = 5) and GlyT1+/−(n = 5) CA1 pyramidal cells. In the mammalian central nervous system, differential expression of each of the AMPAR subunits, GluR (1–4) and GluR (A–D), leads to profound differences in Ca2+ permeability and gating between cells (Geiger et al. 1995). Homomeric channels, assembled from GluR2 subunits, are not permeable to calcium and show outwardly rectifying current–voltage (I–V) relations (Burnashev et al. 1992a, b). In contrast, receptors assembled from GluR1, GluR3 and GluR4 subunits are highly permeable to calcium, and showed a doubly rectifying I–V relation (Hollmann et al. 1991; Verdoorn et al. 1991).
To evoke postsynaptic AMPAR currents, the Schaffer collaterals were stimulated with a bipolar electrode while the postsynaptic CA1 pyramidal cells were held at Vm=−70 mV in ACSF containing AP5, picrotoxin, CGP 52432 and strychnine. The rise times of the AMPAR currents were 4.18 ± 1.08 ms and 3.108 ± 0.163 ms, and the decay time constants were 19.28 ± 1.07 ms and 18.75 ± 1.66 ms in WT (n = 5; Fig. 7A) and GlyT1+/− (n = 5;Fig. 7B) CA1 pyramidal cells, respectively. No significant difference was found between these values (P > 0.1). The I–V relations were linear over the whole voltage range, with a reversal potential of 0 mV for both WT and mutant mice (Fig. 7C). These results suggest no difference in the expression of AMPARs at the Schaffer collateral–CA1 synapses in WT and GlyT1+/− mice.
Figure 7. AMPAR current properties in WT and GlyT1+/− CA1 pyramidal cells.
Pyramidal cells were recorded in ACSF containing picrotoxin (50 μm), CGP 52432 (10 μm), strychnine (0.5 μm) and AP5 (50 μm). A and B, examples of AMPAR current traces for WT (A) and GlyT1+/− (B) pyramidal cells. C, I–V relationship of AMPAR currents in WT (○; n = 5) and GlyT1+/− (•; n = 5) CA1 pyramidal cells. Error bars are s.e.m.
Although synaptic activity can regulate the sEPSC amplitude through a change in postsynaptic receptor number or function, we could not exclude the possibility of presynaptic change. To study the possibility of a difference in the probability of neurotransmitter release (Pr) that could influence the expression of AMPAR/NMDAR ratio in GlyT1+/− mice, we measured the degree of pair pulse facilitation (PPF) in the two types of mice. Paired pulses were delivered with different interpulse intervals: 50 ms, 100 ms and 150 ms. The second response showed facilitation in both WT and GlyT1+/− mice in normal and low Mg2+ ACSF. To induce a high Pr, we used a solution with low Mg2+ (high Ca2+/Mg2+ ratio) (Hsia et al. 1998). In GlyT1+/− mice, the stimulations induced two PSCs with ratios (peak2/peak1) of 2.06 ± 0.17, 1.74 ± 0.04, 1.52 ± 0.02, and 2.08 ± 0.36, 1.68 ± 0.17, 1.51 ± 0.11 for 50, 100 and 150 ms intervals in ACSF and low Mg2+ ACSF, respectively (n = 6; data not shown). These ratios were not significantly different from those in WT mice (1.84 ± 0.08, 1.58 ± 0.08, 1.44 ± 0.05 and 1.76 ± 0.13, 1.58 ± 0.12, 1.43 ± 0.09 for 50, 100 and 150 ms intervals in ACSF and low Mg2+ ACSF, respectively, n = 6; P > 0.05; data not shown).
Miniature postsynaptic current (mEPSC) quantal size is smaller in GlyT1+/− CA1 pyramidal cells
Since the smaller AMPAR/NMDAR ratio in GlyT1+/− could not be explained by a change in AMPAR channel rectification or in neurotransmitter release probability, it is possible that it could be due to a change in the NMDAR and/or AMPAR component of the spontaneous EPSC. To investigate the nature of the difference in AMPAR/NMDAR ratio between WT and GlyT1+/− mice, we recorded miniature postsynaptic currents (mEPSC) in WT and GlyT1+/− CA1 pyramidal cells at −70 mV in a low Mg2+ ACSF (see Methods) containing picrotoxin, CGP 52432, strychnine, and TTX.
We found that the average amplitude of the mEPSCs in GlyT1+/− mice was significantly smaller (9.83 ± 0.69 pA, n = 11) compared to WT (14.52 ± 2.02 pA, n = 8, P < 0.05, paired t test; Fig. 8), while the frequencies were not significantly different (0.243 ± 0.09 Hz, n = 11 GlyT1+/− and 0.224 ± 0.03 Hz, n = 8 WT; Fig. 8). The distribution of mEPSC amplitudes in WT was significantly different from that of GlyT1+/− mice (P < 0.05, Kolmogorov–Smirnov two-sample test; Fig. 8). The average amplitude of mEPSCs corresponds to response to a single quantum of transmitter released at an individual synapse. Consequently, the reduction of the mEPSC quantal size in GlyT1+/− mice might reflect either a reduction in the number of NMDARs and/or AMPARs.
Figure 8. Miniature postsynaptic current (mEPSC) quantal size is smaller in GlyT1+/− CA1 pyramidal cells.
A, cumulative amplitude distributions obtained from WT (n = 9, solid line) and GlyT1+/− CA1 pyramidal cells (n = 7; broken line). The threshold for peak detection was set between 5 and 10 pA. Data were binned in 1 pA intervals. B, bar graph showing mEPSC amplitude (quantal size). The mEPSC amplitude is larger in WT (11.12 ± 0.41 pA, n = 9) compared to GlyT1+/− CA1 pyramidal cells (7.95 ± 0.78 pA, n = 9; *P < 0.05, paired t test). C, cumulative frequency distributions from WT (n = 9, solid line) and GlyT1+/− (n = 7; broken line) CA1 pyramidal cells. D, histogram of the mEPSC frequency for WT (0.224 ± 0.03 Hz, n = 9) and GlyT1+/− (0.243 ± 0.09 Hz, n = 7). No significant difference was found. E, examples of averaged mEPSCs for WT (thick line; average of 74 events) and GlyT1+/− (thin line; average 150 events) are superimposed. Error bars are s.e.m.
NMDAR-mediated miniature postsynaptic current (NMDAR mEPSC) deactivation kinetics are faster in GlyT1+/− CA1 pyramidal cells
To investigate the basis of the reduction of the mEPSC quantal size in GlyT1+/− mice, we recorded NMDAR-mediated miniature postsynaptic currents (NMDAR mEPSCs) in WT and GlyT1+/− CA1 pyramidal cells. Since the duration of the evoked NMDAR currents in GlyT1+/− was shorter compared to WT CA1 pyramidal cells (Fig. 1C), we expected NMDAR mEPSCs to be shorter in GlyT1+/− compared to WT. We also expected to observe greater current amplitudes, since glycine is known to increase the amplitude of the NMDAR currents.
We recorded neurones held at +50 mV in a low Mg2+ ACSF containing the following drugs: NBQX, picrotoxin, CGP 52432, strychnine and TTX. The NMDAR mEPSCs were seen as outward currents (Fig. 9). The slow currents observed at +50 mV were completely blocked by AP5, indicating that these currents were solely NMDAR mediated (data not shown). Figure 9A shows sweeps of responses from individual WT and GlyT1+/− CA1 pyramidal cells, while representative averaged traces are shown in Fig. 9B (WT upper trace, GlyT1+/− lower trace). The frequency of occurrence of NMDAR mEPSCs was 0.350 ± 0.08 Hz for GlyT1+/−(n = 8), which was not significantly different from the frequency in WT, 0.279 ± 0.045 Hz for WT (n = 10; P > 0.1; Fig. 9E). Surprisingly, the NMDAR quantal size, calculated by the average amplitude of NMDAR mEPSCs, was not significantly different (20.44 ± 1.17 pA, n = 8, and 18.7 ± 2.2 pA, n = 10, for WT and GlyT1+/− mice, respectively, P > 0.1, paired t test; Fig. 9F), which suggests that the number of NMDARs present at the synapses is equivalent in WT and mutant mice. Moreover, the distribution of NMDAR mEPSCs in WT was indistinguishable from that of the GlyT1+/− mice (P > 0.1, Kolmogorov–Smirnov two-sample test; Fig. 9G). Deactivation kinetics were significantly faster in GlyT1+/−(τf= 13.5 ± 1.8, τs= 147.1 ± 40.9, τmean= 37.64 ± 5.07 ms; n = 8) compared to WT mice (τf= 26.3 ± 4.5, τs= 353.6 ± 49.8, τmean= 141.57 ± 34.13 ms; n = 10; P < 0.05, paired t test; Figs 9C and D), consistent with our data describing the kinetics of the evoked NMDAR currents.
Figure 9. NMDAR-mediated miniature postsynaptic current (NMDAR mEPSC) deactivation kinetics are faster in GlyT1+/− CA1 pyramidal cells.
NMDAR mEPSCs were recorded from WT and GlyT1+/− CA1 pyramidal cells. A, representative recording traces of NMDAR mEPSCs from WT and GlyT1+/− CA1 pyramidal cells. The threshold for peak detection was set between 10 and 20 pA. Data were binned in 1 pA intervals. B, averaged NMDAR mEPSCs for WT (upper trace; average of 74 events) and GlyT1+/− (lower traces; average of 120 events) CA1 pyramidal cells. C, the same traces as illustrated in B are scaled up and the NMDAR mEPSC deactivation τmean values are given. D–F, histograms showing the averaged τmean, frequency and amplitude of WT (n = 10) and GlyT1+/−(n = 8) CA1 pyramidal cells. *The τmean of NMDAR mEPSCs in WT is significantly slower than in GlyT1+/− CA1 pyramidal cells (P < 0.05, paired t test). G, cumulative probability histogram of NMDAR mEPSC amplitude of WT and GlyT1+/− CA1 pyramidal cells. Note the Kolmogorov–Smirnov two-sample test shows no difference in the distribution of the NMDAR mEPSC amplitudes in WT and GlyT1+/− mice (P > 0.1). Error bars are s.e.m.
AMPAR-mediated miniature postsynaptic current (AMPAR mEPSC) quantal size is smaller in GlyT1+/− CA1 pyramidal cells
Since the number of NMDARs present at the synapses is similar in GlyT1+/− and WT mice, we investigated whether the smaller mEPSC quantal size and AMPAR/NMDAR ratio in GlyT1+/− could be due to a lower number of AMPARs present at GlyT1+/− CA1 pyramidal cells, by studying the AMPAR-mediated miniature postsynaptic current (AMPAR mEPSC). AMPAR mEPSCs were measured at −70 mV in a low Mg2+ ACSF containing picrotoxin, CGP 52432, strychnine, AP5 and TTX. The average amplitude of the AMPAR mEPSCs in GlyT1+/− mice was significantly smaller (9.39 ± 0.68 pA, n = 8) compared to WT (13.06 ± 0.58 pA, n = 13, P < 0.05, paired t test; Fig. 10B), while the frequencies were not significantly different (0.171 ± 0.03 Hz, n = 10 GlyT1+/− and 0.126 ± 0.02 Hz, n = 13 WT; P > 0.05, paired t test). The distribution of AMPAR mEPSC amplitudes was significantly different in GlyT1+/− and WT mice (Fig. 10A; P < 0.05, Kolmogorov–Smirnov two-sample test). The rise time and the decay time constants of the AMPAR mEPSCs were also measured and no significant difference was found (τact= 2.68 ± 0.28 ms and τdeact= 13.28 ± 0.95 ms in WT, n = 13; τact= 2.6 ± 0.4 ms and τdeact= 14.16 ± 1.57 ms in GlyT1+/−; n = 8; P > 0.1). Altogether these results indicate that the reduced AMPAR/NMDAR ratio as well as the reduced mEPSC amplitude in GlyT1+/− compared to WT mice could be due to a reduced number of AMPARs present at the CA1 synapses in GlyT1+/− mice.
Figure 10. AMPAR-mediated miniature postsynaptic current (AMPAR mEPSC) quantal size is smaller in GlyT1+/− CA1 pyramidal cells.
A, cumulative probability histogram of AMPAR mEPSC amplitude of WT (n = 13) and GlyT1+/−(n = 8) CA1 pyramidal cells. Note the Kolmogorov–Smirnov two-sample test shows a difference in the distribution of the AMPAR mEPSC amplitudes in WT and GlyT1+/− mice (P < 0.05). B, histogram showing the averaged AMPAR mEPSC amplitude in WT (n = 13) and GlyT1+/−(n = 8) CA1 pyramidal cells. *The amplitude of AMPAR mEPSCs in WT is significantly larger than in GlyT1+/− CA1 pyramidal cells (P < 0.05, paired t test). C, averaged AMPAR mEPSCs for WT (thick line) and GlyT1+/− (thin line) CA1 pyramidal cells. Error bars are s.e.m.
Discussion
In the present study, we used GlyT1+/− mice to investigate the effect of reduced GlyT1 expression on glutamatergic neurotransmission in CA1 hippocampal pyramidal cells. We found that the deactivation kinetics of NMDAR currents were faster in GlyT1+/− compared to WT CA1 pyramidal cells. This was further confirmed by the reduced ifenprodil sensitivity and increased zinc-induced antagonism of the NMDAR currents in GlyT1+/− CA1 pyramidal cells, suggesting a difference in the pharmacological profile of NMDARs at the synapses. We observed a reduced number of AMPARs present at the CA1 synapses in GlyT1+/− mice that could explain the lower AMPAR/NMDAR ratio observed in GlyT1+/− CA1 pyramidal cells (Tsai et al. 2004).
Our observations show that application of glycine significantly increased the slow decay time course and τmean of NMDAR currents in WT, while having no effect in GlyT1+/− CA1 pyramidal cells. Glycine is responsible for a form of desensitization observed during both outside-out and whole-cell recording (McBain & Mayer, 1994). However, it has been reported that glycine-dependent desensitization of NMDARs occurs at a low concentration of glycine (<1 μm; Mayer et al. 1989), while a higher level of glycine decreases desensitization of NMDARs (Mayer et al. 1989; Lerma et al. 1990). Consequently, application of glycine would reduce the glycine-dependent desensitization and increase the decay time constant of NMDAR current in WT CA1 pyramidal cells, while having no effect on the decay time constant of the NMDAR current in the GlyT1+/−, where the glycine appears to be virtually fully saturated. Our experiments on NMDAR currents in the presence of exogenous glycine support these assumptions.
We observed that the decay time course of NMDAR EPSCs in GlyT1+/− was faster than that in WT CA1 pyramidal cells. The decay time constant of the NMDAR EPSC depends on several factors such as dissociation of glutamate from the NMDAR (Hestrin et al. 1990), channel kinetics (D'Angelo et al. 1990; Lester et al. 1990), NMDAR subunits composition (Monyer et al. 1994; Vicini et al. 1998), glycine-dependent, and glycine-independent desensitization, and Ca2+/CaM-dependent channel inactivation (McBain & Mayer, 1994). The Ca2+-dependent inactivation of NMDARs requires an elevation of intracellular Ca2+ concentration. It is unlikely that Ca2+-dependent inactivation of NMDARs may play a role in the difference of kinetics observed in the two types of mice, because of the presence of 10 mm BAPTA (a Ca2+ chelator) in the recording electrode. On the other hand, the glycine-independent desensitization occurs following the binding of glutamate at a supramaximal concentration of glycine in Ca2+-free medium (Sather et al. 1990). A prominent characteristic of this type of desensitization is the time-dependent increase in the speed and extent of desensitization during the time of recording (Sather et al. 1990). In addition it is controlled by the nature of the NR2 subunits that form the receptors (Krupp et al. 1996). We can exclude this form of desensitization because of the lack of time-dependent increase in the speed of the NMDAR kinetics and the extent of desensitization during our recordings. Consequently, the difference observed in the NMDAR kinetics of the two types of mice does not seem to involve desensitization but rather, points to a different functionality of these receptors.
We used ifenprodil, a highly selective non-competitive antagonist of NR2B-containing NMDARs (Williams, 1993), and zinc, a potent and highly selective antagonist in nanomolar concentration of the NMDARs containing the subunit NR2A (Paoletti et al. 1997), to determine the difference contribution of the two types of subunit in the NMDAR kinetics of GlyT1+/− and WT CA1 pyramidal cells. Our results show that NMDAR currents in GlyT1+/− have a lower and a higher sensitivity to ifenprodil and ZnCl2, respectively, compared to WT CA1 pyramidal cells. It is known that changes in the NMDAR EPSC duration are likely to result from alteration of the NMDAR itself (Hestrin, 1992). Studies of mRNA expression suggest that the decay times of NMDAR currents, observed on pyramidal neurones, are conferred by the NR2A and NR2B subunits (Monyer et al. 1994). A reduction in the contribution of NR2B-containing NMDARs occurs during development and is associated with an increase in expression of NR2A subunits (Flint et al. 1997). Replacement of NR2B by NR2A subunits during postnatal development accounts for acceleration of the NMDAR EPSCs' decay in neocortical neurones (Monyer et al. 1994; Flint et al. 1997). Similar changes in functional and pharmacological properties of NMDAR EPSCs have been described in hippocampal pyramidal cells (Kirson et al. 1999). These cells display a change in NMDAR EPSC kinetics and ifenprodil sensitivity, which is consistent with a decreased contribution of NR2B subunits and an increase of NR2A (Kirson et al. 1999). However, preliminary Western blot analysis for NR2A and NR2B on the hippocampus of WT and GlyT1+/− mice shows no difference between the protein levels of the two types of mice (unpublished observation). The lower sensitivity to ifenprodil and the higher zinc-induced antagonism observed in GlyT1+/− should not necessary imply a difference in the levels of expression of the two types of subunits, but rather a possible difference between the NMDARs expressed at the synapses and those internalized.
Zinc accumulates at some nerve terminals in specific brain regions and is released into synaptic clefts in a Ca2+-dependent manner during neuronal activity (Smart et al. 1994). The sensitivities of NR2 subunit species to high-affinity voltage-independent zinc inhibition have been reported to be variable. The NR1/NR2A channel has the highest sensitivity to voltage-independent zinc inhibition with an IC50 value of 5 ± 80 nm, whereas the IC50 values of the NR1/NR2B, NR1/NR2C and NR1/NR2D channels are 0.5 ± 10, 14 ± 38 and 14 mm, respectively (Williams, 1996; Chen et al. 1997; Paoletti et al. 1997; Traynelis et al. 1998). The sensitivity to zinc of the NR1/NR2A channel is high enough to allow ambient zinc (0.1 mm; Bogden et al. 1977) to tonically inhibit this channel. However, in our experiments a component of the NMDAR current is still present after the application of ifenprodil, suggesting that a percentage of NR2A-containing receptors are not blocked by the ambient zinc. We added ZnCl2 to the extracellular solution without chelating the ambient zinc, to be able to compare the sensitivity of the NMDAR currents not blocked by the ambient zinc, as in the case of the experiments performed with ifenprodil, in WT and GlyT1+/− mice. Our finding that NMDAR currents of GlyT1+/− CA1 pyramidal cells are more sensitive to ZnCl2 (100 nm) than those from WT, cannot, however, exclude a difference in the percentage of NMDARs blocked by the ambient zinc in WT and GlyT1+/− mice.
We found that the AMPAR/NMDAR ratio was lower in GlyT1+/− CA1 pyramidal cells compared to WT, suggesting a difference in the postsynaptic sensitivity to glutamatergic input in the two types of mice. We ruled out a change in AMPAR channel rectification or in neurotransmitter release probability as cause for the smaller AMPAR/NMDAR ratio in GlyT1+/− because the current-voltage relationship and kinetics of evoked AMPAR currents as well as the pair pulse facilitation were the same in WT and mutant mice. The analysis of the mEPSC showed a significant reduction of the mEPSC quantal size in GlyT1+/− mice compared to WT. Since the average amplitude of mEPSCs corresponds to the response to a single quantum of transmitter released at an individual synapse, this might reflect either a reduction in the number of NMDARs and/or AMPARs.
It is known that AMPAR and NMDAR synaptic components of glutamatergic synapses develop on different timescales, with the NMDAR EPSC developmentally preceding the AMPAR EPSC (Wu et al. 1996; Hsia et al. 1998; Liao et al. 1999). The higher level of glycine present in the GlyT1+/− mice could modify the NMDAR activity and, via some form of feedback, result in altered expression of the receptor at the synapses. Surprisingly, the analysis of the NMDAR mEPSCs showed that the NMDAR quantal size, calculated by the average amplitude of the NMDAR mEPSCs, was not significantly different between WT and GlyT1+/− mice, suggesting that the persistent high level of glycine did not influence the number of NMDARs present at the synapses of GlyT1+/− CA1 pyramidal cells. Indeed, it has been established that the NMDAR quantal size stays stable during development, suggesting that synaptogenesis can account entirely for the increase in NMDAR mEPSC amplitude (Gomperts et al. 2000). These data are also confirmed by the Western blot analysis (Gomeza et al. 2003) showing no difference in the expression of the NMDAR NR1 subunit of GlyT1+/− and WT mice.
The smaller AMPAR/NMDAR ratio in GlyT1+/− mice could be explained by a lower number of AMPARs. Change in NMDAR properties may have a large impact on experience-dependent regulation of AMPAR-mediated response (Philipot et al. 2001). AMPAR-mediated transmission is potentiated as a consequence of a rise of the intracellular calcium during strong NMDAR activation during long-term potentiation (LTP; Malenka & Nicoll, 1999). Conversely, modest increases in calcium due to weak NMDAR activation can trigger long-term depression (LTD) of synaptic transmission (Feldman et al. 1998). Change in the subunit structure of the NMDAR, as observed during development (Monyer et al. 1994; Flint et al. 1997; Kirson et al. 1999), can substantially modify the calcium current that occurs in response to repetitive stimulation, and may play a role in diminishing plasticity of AMPARs (Carmignoto & Vicini, 1992; Crair & Malenka, 1995; Feldman et al. 1998). We found that the AMPAR mEPSC amplitudes were significantly smaller in GlyT1+/− compared to WT mice, suggesting a reduced number of AMPARs present at the CA1 synapses in GlyT1+/− mice.
In GlyT1+/− mice, the glycine binding site of the NMDAR is saturated, and the NMDAR mEPSC quantal size is unchanged. Nong et al. (2003) reported that glycine and glutamate site activation of NMDARs together are necessary for the receptor to be endocytosed. It is known that the NR2A subunit has a lower affinity for glycine compared to NR2B; therefore NR2B-containing receptors would be more sensitive to internalization than NR2A-containing receptors (Lavezzari et al. 2004). If indeed the NR2B-containing receptors are internalized in GlyT1+/− mice, while the NMDAR mEPSC quantal size is unchanged, then a larger number of NR2A-containing receptors will be observable at these synapses. This is supported by the faster kinetics of the NMDAR currents, by the lower sensitivity to ifenprodil and by the higher zinc-induced antagonism observed in the GlyT1+/− mice. However, in the presence of hypothetical ambient glycine concentrations (300 nm to 1 μm; Supplisson & Bergman, 1997), the NMDARs with a relatively low affinity for glycine (microscopic dissociation constant (mKD) =∼800 nm; Kew et al. 1998) are only occupied in a range from ∼20% to ∼65%. With these glycine concentrations, almost every high-affinity receptor (KD= 100–500 nm; Buller et al. 1994; Laurie & Seeburg, 1994; Matsui et al. 1995; Priestley et al. 1995) would be saturated. The addition of exogenous glycine could enhance the NMDAR responses by acting on NMDARs not occupied by the ambient glycine. Indeed, it has been published that exogenous addition of glycine and d-serine increased the amplitude of the NMDAR mEPSC and slowed down their kinetics (Berger et al. 1998). In addition, our results showed that application of glycine increases the amplitude of evoked NMDAR currents in WT but not in GlyT1+/− mice. Consequently, the NMDAR mEPSC in WT, in the presence of glycine, would be larger than that in GlyT1+/− CA1 pyramidal cells. We hypothesize that a compensatory mechanism involving levels of glycine and clathrin-dependent endocytosis might take place in the mutant mice to keep the NMDAR activity to an efficient point for synaptic transmission. In WT mice, the NMDAR activity is tightly regulated by the level of glycine present in the synaptic cleft. This level is kept below the ‘set point’ of the NMDAR internalization priming mechanism by the presence of a certain number of GlyT1s. In GlyT1+/− mice, the saturating level of glycine can cause a hyperactivity of the NMDAR, on one hand, and prime the NMDAR for internalization on the other, rebalancing the NMDAR activity at an optimal point of efficiency.
Tsai et al. (2004) have shown that whereas motor function and rate of acquisition of a spatial learning task did not differ between GlyT1+/− and WT mice, the GlyT1+/− outperformed WT mice in spatial retention. It is known that a reduction of the NMDAR time course could influence the duration of the response to glutamatergic inputs. Indeed, the early component of the EPSCs depends largely on activation of the non-NMDA receptors, while the late component is due to the NMDAR (Hestrin et al. 1990). Because the kinetics of NMDAR currents in GlyT1+/− are faster than those in WT CA1 pyramidal cells, the duration of the EPSC is shorter in GlyT1+/−. Consequently, this reduces the time interval during which temporal summation of synaptic potential can occur in GlyT1+/−. The reduced NMDAR time course of GlyT1+/− CA1 pyramidal cells provides a reduced time window for coincidence detection, which can influence the efficacy to exhibit experience-dependent synaptic plasticity. However, very recently, it has been reported that distinct NMDAR subunit types are critical factors that determine the polarity of synaptic plasticity (LTP versus LTD) in the CA1 region of the hippocampus (Liu et al. 2004). Liu et al. (2004) have suggested that LTD and LTP require the activation of NR2B-containing and NR2A-containing NMDARs, respectively. The altered balance between NR2A and NR2B subunits expressed in GlyT1+/− mice may be responsible of a shift in the equilibrium LTP–LTD, causing the better spatial retention observed in GlyT1+/− mice (Tsai et al. 2004).
Overall, our results highlight the importance of GlyT1 expression and glycine levels at the synapse in regulating the glutamatergic neurotransmission.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR) and by the National Alliance for Research on Depression and Schizophrenia. M. Martina is recipient of a postdoctoral fellowship from CIHR. We thank C. Metivier for technical assistance.
References
- Amaral DG, Witter MP. The three dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Dieudonne S, Ascher P. Glycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. J Neurophysiol. 1998;80:3336–3340. doi: 10.1152/jn.1998.80.6.3336. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-d-aspartate receptor function by glycine transport. Proc Natl Acad Sci U S A. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogden JD, Troiano RA, Joselow MM. Copper, zinc, magnesium, and calcium in plasma and cerebrospinal fluid of patients with neurological diseases. Clin Chem. 1977;23:485–489. [PubMed] [Google Scholar]
- Buller AM, Larson HC, Schneider BE, Beaton JA, Morrisett RA, Monaghan DT. The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci. 1994;14:5471–5484. doi: 10.1523/JNEUROSCI.14-09-05471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, Monyer H, Seeburg PH, Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992b;256:1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992a;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Chen N, Moshaver A, Raymond LA. Differential sensitivity of recombinant N-methyl-d-aspartate receptor subtypes to zinc inhibition. Mol Pharmacol. 1997;51:1015–1023. doi: 10.1124/mol.51.6.1015. [DOI] [PubMed] [Google Scholar]
- Chen L, Muhlhauser M, Yang CR. Glycine transporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol. 2003;89:691–703. doi: 10.1152/jn.00680.2002. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G. NMDA receptor function, neuroplasticity and the pathophysiology of schizophrenia. Int Rev Neurobiol. 2004;59:491–515. doi: 10.1016/S0074-7742(04)59019-0. [DOI] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- D'Angelo E, Rossi P, Garthwaite J. Dual-component NMDA receptor currents at a single central synapse. Nature. 1990;346:467–470. doi: 10.1038/346467a0. 10.1038/346467a0. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons AC. Glycine and N-methyl-d-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC, Isaac JT. Long-term depression at thalamocortical synapses in developing rat somatosensory cortex. Neuron. 1998;21:347–357. doi: 10.1016/s0896-6273(00)80544-9. 10.1016/S0896-6273(00)80544-9. [DOI] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakman B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Hulsmann S, Ohno K, Eulenburg V, Szoke K, Richter D, Betz H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron. 2003;40:785–796. doi: 10.1016/s0896-6273(03)00672-x. 10.1016/S0896-6273(03)00672-X. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Carroll R, Malenka RC, Nicoll RA. Distinct roles for ionotropic and metabotropic glutamate receptors in the maturation of excitatory synapses. J Neurosci. 2000;20:2229–2237. doi: 10.1523/JNEUROSCI.20-06-02229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–699. doi: 10.1038/357686a0. 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Nicoll RA, Perkel DJ, Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Malenka RC, Nicoll RA. Development of excitatory circuitry in the hippocampus. J Neurophysiol. 1998;79:2013–2024. doi: 10.1152/jn.1998.79.4.2013. [DOI] [PubMed] [Google Scholar]
- Kew JN, Richards JG, Mutel V, Kemp JA. Development changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons. J Neurosci. 1998;18:1935–1943. doi: 10.1523/JNEUROSCI.18-06-01935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson ED, Schirra C, Konnerth A, Yaari Y. Early postnatal switch in magnesium sensitivity of NMDA receptors in rat CA1 pyramidal cells. J Physiol. 1999;521:99–111. doi: 10.1111/j.1469-7793.1999.00099.x. 10.1111/j.1469-7793.1999.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Heinemann SF, Westbrook GL. Calcium-dependent inactivation of recombinant N-methyl-d-aspartate receptors in NR2 subunit specific. Mol Pharmac. 1996;50:1680–1688. [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH. Ligand affinities at recombinant N-methyl-d-aspartate receptors depend on subunit composition. Eur J Pharmacol. 1994;268:335–345. doi: 10.1016/0922-4106(94)90058-2. 10.1016/0922-4106(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Westbrook GL. The inhibition of N-methyl-d-aspartate-activated channels by zinc ions on cultured rat neurones. J Physiol. 1990;429:429–449. doi: 10.1113/jphysiol.1990.sp018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Westbrook GL. Ifenprodil blocks N-methyl-d-aspartate receptors by a two-component mechanism. Mol Pharmacol. 1991;40:289–298. [PubMed] [Google Scholar]
- Lerma J, Zukin RS, Bennett MV. Glycine decreases desensitization of N-methyl-d-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Proc Natl Acad Sci U S A. 1990;87:2354–2358. doi: 10.1073/pnas.87.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RA, Clements JD, Westbrook GL, Jahr CE. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990;346:565–567. doi: 10.1038/346565a0. 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza M, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation: a decade of progress. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Martina M, Krasteniakov NV, Bergeron R. d-serine differently modulates NMDA receptor function in rat CA1 hippocampal pyramidal cells and interneurons. J Physiol. 2003;548:411–423. doi: 10.1113/jphysiol.2002.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Sekiguchi M, Hashimoto A, Tomita U, Nishikawa T, Wada K. Functional comparison of d-serine and glycine in rodents: the effect on cloned NMDA receptors and the extracellular concentration. J Neurochem. 1995;65:454–458. doi: 10.1046/j.1471-4159.1995.65010454.x. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Vyklicky L, Clements J. Regulation of NMDAR desensitization in mouse by glycine. Nature. 1989;338:425–427. doi: 10.1038/338425a0. 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-d-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–307. doi: 10.1038/nature01497. 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Otmakhov N, Lisman JE. Pathway-specific properties of AMPA and NMDA-mediated transmission in CA1 hippocampal pyramidal cells. J Neurosci. 2002;22:1199–1207. doi: 10.1523/JNEUROSCI.22-04-01199.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. 10.1016/S0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Myers J, Le Bourdelles B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-d-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/s0896-6273(00)80901-0. 10.1016/S0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Sather W, Johnson JW, Henderson G, Asher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990;4:725–731. doi: 10.1016/0896-6273(90)90198-o. 10.1016/0896-6273(90)90198-O. [DOI] [PubMed] [Google Scholar]
- Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog Neurobiol. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Smith KE, Borden LA, Hartig PR, Branchek T, Weinshank RL. Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron. 1992;8:927–935. doi: 10.1016/0896-6273(92)90207-t. 10.1016/0896-6273(92)90207-T. [DOI] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher NJ, Awobuluyi M, Choi YB, Lipton SA. NMDA receptors: from genes to channels. Trends Pharmacol Sci. 1996;17:348–355. 10.1016/S0165-6147(96)10046-8. [PubMed] [Google Scholar]
- Supplisson S, Bergman C. Control of NMDA receptor activation by a glycine transporter co-expressed in Xenopus oocytes. J Neurosci. 1997;17:4580–4590. doi: 10.1523/JNEUROSCI.17-12-04580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Burgess MF, Zheng F, Lyuboslavsky P, Powers JL. Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. J Neurosci. 1998;18:6163–6175. doi: 10.1523/JNEUROSCI.18-16-06163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Ralph-Williams RJ, Martina M, Bergeron R, Berger-Sweeney J, Dunham KS, Jiang Z, Caine SB, Coyle JT. Gene knockout of the glycine transporter 1: characterization of the behavioural phenotype. Proc Natl Acad Sci U S A. 2004;101:8485–8490. doi: 10.1073/pnas.0402662101. 10.1073/pnas.0402662101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg RJ, Aubrey KR. Glycine transport inhibitors as potential antipsychotic drugs. Expert Opin Ther Targets. 2001;5:507–518. doi: 10.1517/14728222.5.4.507. [DOI] [PubMed] [Google Scholar]
- Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakman B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991;252:1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-d-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Westbrook GL, Mayer ML. Micromolar concentration of Zn2+ antagonize NMDA and GABA responses in hippocampal neurons. Nature. 1987;328:640–643. doi: 10.1038/328640a0. 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Westergren I, Nyström B, Hamberger A, Nordborg C, Johansson BB. Concentrations of amino acids in extracellular fluid after opening of the blood–brain barrier by intracarotid infusion of protamine sulfate. J Neurochem. 1994;62:159–165. doi: 10.1046/j.1471-4159.1994.62010159.x. [DOI] [PubMed] [Google Scholar]
- Wilcox KS, Fitzsimonds RM, Johnson B, Dichter MA. Glycine regulation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol. 1996;76:3415–3424. doi: 10.1152/jn.1996.76.5.3415. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminate subtypes of the N-methyl-d-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Williams K. Separating dual effects of zinc at recombinant N-methyl-d-aspartate receptors. Neurosci Lett. 1996;215:9–12. doi: 10.1016/s0304-3940(96)12924-4. 10.1016/S0304-3940(96)12924-4. [DOI] [PubMed] [Google Scholar]
- Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- Yu W, Miller RF. NBQX, an improved non-NMDA antagonist studied in retinal ganglion cells. Brain Res. 1995;692:190–194. doi: 10.1016/0006-8993(95)00665-d. 10.1016/0006-8993(95)00665-D. [DOI] [PubMed] [Google Scholar]
- Zafra F, Aragón C, Oliveres L, Danbolt NC, Gimenez C, Storm-Methisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]